-
PDF
- Split View
-
Views
-
Cite
Cite
Armin Peivandi, Henryk Welp, Mirela Scherer, Jürgen R Sindermann, Nana-Maria Wagner, Angelo M Dell’Aquila, An external validation study of the Utah Bleeding Risk Score, European Journal of Cardio-Thoracic Surgery, Volume 62, Issue 1, July 2022, ezab572, https://doi.org/10.1093/ejcts/ezab572
Close - Share Icon Share
Abstract
Gastrointestinal bleeding in patients with continuous-flow left ventricular assist devices (CF-LVAD) impairs quality of life and increases hospitalization rate. The Utah Bleeding Risk Score (UBRS) has been created to predict gastrointestinal bleeding (GIB) in patients on left ventricular assist device. We here aimed to externally validate UBRS on our cohort of CF-LVAD patients.
Utah Bleeding Risk Score was calculated, GIB events summarized on follow-up and patients stratified into 3 risk groups. Predictive ability of UBRS was examined at 3 years and during whole support time and person time incidence rates correlated to UBRS. In a sub-analysis, single effects of UBRS variables on freedom from GIB were assessed.
A total of 111 CF-LVAD patients were included. The median UBRS was 2 (3–1). Receiver operating characteristic curve analysis showed an area under the curve of 0.519 (P = 0.758, 95% confidence interval = 0.422–0.615) at 3 years and an area under the curve of 0.515 (P = 0.797, 95% confidence interval = 0.418–0.611) during whole support time. No significant difference was observed in UBRS between bleeders and non-bleeders (P = 0.80). No significant difference in freedom from GIB was observed (P3 years = 0.7; Psupport-time = 0.816) and no independent significance regarding the freedom from bleeding at 3 years for any variable was observed. Coronary artery disease was associated with higher risk of GIB beyond the 3rd year (P = 0.048).
UBRS was not able to predict GIB and therefore not applicable in our cohort of patients. Coronary artery disease could lead to a higher risk for GIB during support time. An additional validation in a larger cohort is advisable.
INTRODUCTION
Heart failure is nowadays considered a global pandemic that currently affects >26 million people worldwide [1]. The incidence of heart failure is rapidly increasing because of a growing and ageing population and has begun to exceed the predicted annual incidence already in the year 2000 [2, 3]. For patients with end-stage heart failure, heart transplantation is often the treatment of choice. However, the number of heart transplantations, 668 in 2019 in Eurotransplant countries, is still low due to a lack of donor organs [4]. This makes the importance of assist devices, either as bridge to transplantation or as destination therapy, continuously growing. In particular, magnetically levitated cardiac pumps have improved clinical outcomes [5]. With >95% of implanted pumps, continuous-flow left ventricular assist devices (CF-LVAD) have by now almost replaced pulsatile pumps and are currently the most commonly used assist devices in adults. According to the latest INTERMACS report, survival rates are >80% at 1 year and 70% at 2 years follow-up [6].
Despite their better survival and durability, continuous-flow devices are associated with significant bleeding complications. In particular, gastrointestinal bleeding (GIB) occurs at an incidence of 15–61% and a pooled prevalence of 23% in CF-LVAD patients and is thus far more often encountered than in patients with earlier pulsatile pumps [7, 8]. GIB events are the leading cause for hospital readmissions in LVAD patients [9], requiring 2–4 units of packed red blood cell transfusions per admission [10]. The occurrence of GIB in CF-LVAD patients is associated with significant quality of life impairment, increased morbidity, mortality and hospital costs. Thus, validated tools for the risk stratification of patients on CF-LVAD for GIB are of particular importance for patient selection, informed consent and clinical management post implantation.
Recently, the Utah Bleeding Risk Score (UBRS) was introduced as a predictive risk-assessing tool for GIB in patients on CF-LVAD. This score might potentially either influence patient’s selection before LVAD implantation or optimize medical therapy while on LVAD. Validated in 351 patients, it includes 7 preoperative variables: age >54 years (1 point), history of previous bleed (2 points), coronary artery disease (1 point), chronic kidney disease (1 point), severe right ventricular (RV) dysfunction (1 point), mean pulmonary artery pressure <18 mmHg (2 points) and glucose >107 mg/dl (1 point). Patients with 0–1 points rank low risk, 2–4 points lead to a stratification in the intermediate-risk group and patients with 5–9 points are high risk [11]. The objective of our study was to externally validate the UBRS on our cohort of CF-LVAD patients.
MATERIALS AND METHODS
This external validation study is a single-centre retrospective data analysis. Patients receiving an CF-LVAD at our institution from July 2009 to February 2020 were included in this study. Patients who did not survive hospital stay after implantation as well as emergency VAD implantations were excluded from the analysis. In the rare case of VAD replacement (n = 2), each VAD case was analysed as a separate patient. The outcome assessed was GIB during the first 3 years after LVAD implantation and during whole support time. UBRS includes 7 standard preoperative variables that are all assessed before elective CF-LVAD implantation at our institution. Therefore, there were no missing data for score calculation in included patients. The standard anticoagulation scheme of our CF-LVAD patients consists of phenprocoumon with a target INR of 2.5 as well as acetylsalicylic acid 100 mg per day.
Ethics statement
The Institutional Review Board and Ethics Committee of the University Hospital Münster (2021-062-f-S) approved this retrospective study. According to the approval, individual informed consent was not required in this retrospective analysis and therefore waived.
Statistical analysis
UBRS was calculated. Statistical and graphical analyses were conducted using MedCalc® Version 19.4.0 (MedCalc Software Ltd, Ostend, Belgium). Continuous variables were presented with median and interquartile range. Man–Whitney test was used to compare non-normally distributed continuous variables. ROC curve analysis was used to assess the discrimination of the UBRS. Freedom from GIB at 3 years and during whole support time was performed using the Kaplan–Meier analysis. Patients were stratified according to their UBRS into low-risk (0–1), medium-risk (2–4) and high-risk (5–9) groups in line with the original publication [11]. Log-rank test compared freedom from GIB over time of the different risk groups. Last, Cox proportional hazards regression analysis was applied to ascertain the effect of every single variable included in the UBRS. For calibration, Hosmer–Lemeshow test was used.
RESULTS
Patient characteristics
A total of 111 patients (86 males and 25 females, median age 55 years) who received an LVAD were included. The median age of our cohort was 55 years (62.75–45.25). Twenty-four patients received a HeartMate II, 21 patients received a HeartMate III and 66 patients received a HeartWare LVAD. Underlying disease in 62 patients was dilatative cardiomyopathy. Thirty-nine patients presented with ischaemic cardiomyopathy. Toxic cardiomyopathy was present in 6 patients. Amyloidosis, hypertrophic obstructive cardiomyopathy, congenital heart failure and myocarditis accounted for 1 case each (Table 1).
| Patients, n | 111 |
| Gender, n (%) | |
| Female | 25 (22.5) |
| Male | 86 (77.5) |
| Age (years), median (IQR) | 55 (45.3–62.8) |
| Implanted CF-LVAD device, n (%) | |
| HeartWare | 66 (59.5) |
| HeartMate II | 24 (21.6) |
| HeartMate III | 21 (18.9) |
| Underlying disease, n (%) | |
| Dilatative cardiomyopathy | 62 (55.9) |
| Ischaemic cardiomyopathy | 39 (35.1) |
| Toxic cardiomyopathy | 6 (5.4) |
| Amyloidosis | 1 (0.9) |
| Hypertrophic obstructive cardiomyopathy | 1 (0.9) |
| Congenital heart failure | 1 (0.9) |
| Myocarditis | 1 (0.9) |
| UBRS variables, n (%) | |
| Age >54 years | 57 (51.4) |
| History of previous bleed | 6 (5.4) |
| Coronary artery disease | 39 (35.1) |
| Chronic kidney disease | 43 (38.7) |
| Severe right ventricular dysfunction | 45 (40.5) |
| Mean pulmonary artery pressure <18 mmHg | 10 (9) |
| Glucose >107 mg/dl | 27 (24.3) |
| Patients, n | 111 |
| Gender, n (%) | |
| Female | 25 (22.5) |
| Male | 86 (77.5) |
| Age (years), median (IQR) | 55 (45.3–62.8) |
| Implanted CF-LVAD device, n (%) | |
| HeartWare | 66 (59.5) |
| HeartMate II | 24 (21.6) |
| HeartMate III | 21 (18.9) |
| Underlying disease, n (%) | |
| Dilatative cardiomyopathy | 62 (55.9) |
| Ischaemic cardiomyopathy | 39 (35.1) |
| Toxic cardiomyopathy | 6 (5.4) |
| Amyloidosis | 1 (0.9) |
| Hypertrophic obstructive cardiomyopathy | 1 (0.9) |
| Congenital heart failure | 1 (0.9) |
| Myocarditis | 1 (0.9) |
| UBRS variables, n (%) | |
| Age >54 years | 57 (51.4) |
| History of previous bleed | 6 (5.4) |
| Coronary artery disease | 39 (35.1) |
| Chronic kidney disease | 43 (38.7) |
| Severe right ventricular dysfunction | 45 (40.5) |
| Mean pulmonary artery pressure <18 mmHg | 10 (9) |
| Glucose >107 mg/dl | 27 (24.3) |
CF-LVAD: continuous-flow left ventricular assist devices; IQR: interquartile range; UBRS: Utah Bleeding Risk Score.
| Patients, n | 111 |
| Gender, n (%) | |
| Female | 25 (22.5) |
| Male | 86 (77.5) |
| Age (years), median (IQR) | 55 (45.3–62.8) |
| Implanted CF-LVAD device, n (%) | |
| HeartWare | 66 (59.5) |
| HeartMate II | 24 (21.6) |
| HeartMate III | 21 (18.9) |
| Underlying disease, n (%) | |
| Dilatative cardiomyopathy | 62 (55.9) |
| Ischaemic cardiomyopathy | 39 (35.1) |
| Toxic cardiomyopathy | 6 (5.4) |
| Amyloidosis | 1 (0.9) |
| Hypertrophic obstructive cardiomyopathy | 1 (0.9) |
| Congenital heart failure | 1 (0.9) |
| Myocarditis | 1 (0.9) |
| UBRS variables, n (%) | |
| Age >54 years | 57 (51.4) |
| History of previous bleed | 6 (5.4) |
| Coronary artery disease | 39 (35.1) |
| Chronic kidney disease | 43 (38.7) |
| Severe right ventricular dysfunction | 45 (40.5) |
| Mean pulmonary artery pressure <18 mmHg | 10 (9) |
| Glucose >107 mg/dl | 27 (24.3) |
| Patients, n | 111 |
| Gender, n (%) | |
| Female | 25 (22.5) |
| Male | 86 (77.5) |
| Age (years), median (IQR) | 55 (45.3–62.8) |
| Implanted CF-LVAD device, n (%) | |
| HeartWare | 66 (59.5) |
| HeartMate II | 24 (21.6) |
| HeartMate III | 21 (18.9) |
| Underlying disease, n (%) | |
| Dilatative cardiomyopathy | 62 (55.9) |
| Ischaemic cardiomyopathy | 39 (35.1) |
| Toxic cardiomyopathy | 6 (5.4) |
| Amyloidosis | 1 (0.9) |
| Hypertrophic obstructive cardiomyopathy | 1 (0.9) |
| Congenital heart failure | 1 (0.9) |
| Myocarditis | 1 (0.9) |
| UBRS variables, n (%) | |
| Age >54 years | 57 (51.4) |
| History of previous bleed | 6 (5.4) |
| Coronary artery disease | 39 (35.1) |
| Chronic kidney disease | 43 (38.7) |
| Severe right ventricular dysfunction | 45 (40.5) |
| Mean pulmonary artery pressure <18 mmHg | 10 (9) |
| Glucose >107 mg/dl | 27 (24.3) |
CF-LVAD: continuous-flow left ventricular assist devices; IQR: interquartile range; UBRS: Utah Bleeding Risk Score.
URBS is unable to predict GIB
Thirty out of 111 patients (27%) showed GIB during whole support time and 28 (25%) within the first 3 years after CF-LVAD implantation.
The median UBRS of the whole cohort was 2 (3–1). The median UBRS of bleeders and non-bleeders was equally 2 (3–1) (Table 2). There was no significant difference between the score of bleeders and non-bleeders in the Man–Whitney test (P = 0.802) (Fig. 1). In the ROC curve analysis, UBRS was not able to predict GIB at 3 years after LVAD implantation (3 years) as well as for bleeding events during the whole support time (st) [AUC3years = 0.519 (P = 0.758, 95% confidence interval = 0.422–0.615), AUCst = 0.515 (Pst=0.797, 95% confidence interval 0.418–0.611)] (Fig. 2). Calibration test showed a P-value of 0.089 when considering GIB within first 3 years. UBRS was not significantly associated with bleeding. In addition, the pump type did not have a significant impact on bleeding.
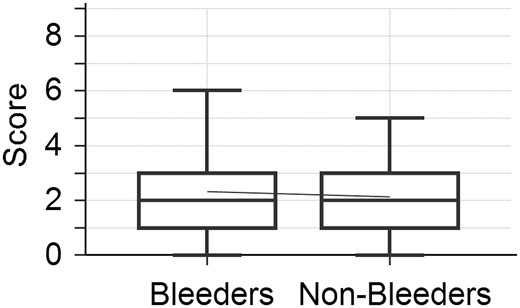
Box plot showing Utah Bleeding Risk Score of bleeders and non-bleeders. There was no significant difference in the Utah Bleeding Risk Score of bleeders and non-bleeders.
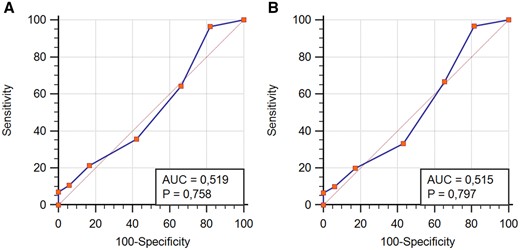
ROC curve analysis showing predictive ability of Utah Bleeding Risk Score within 3 years after implantation (A) and during whole support time (B): Utah Bleeding Risk Score was not able to predict gastrointestinal bleeding events in our cohort of patients.
| Bleeders, n (%) | |
| Within 3 years after CF-LVAD implantation (3 years) | 28 (25) |
| During whole support time (st) | 30 (27) |
| Utah Bleeding Risk Score, median (IQR) | |
| Bleeders | 2 (3-1) |
| Non-bleeders | 2 (3-1) |
| Bleeders, n (%) | |
| Within 3 years after CF-LVAD implantation (3 years) | 28 (25) |
| During whole support time (st) | 30 (27) |
| Utah Bleeding Risk Score, median (IQR) | |
| Bleeders | 2 (3-1) |
| Non-bleeders | 2 (3-1) |
CF-LVAD: continuous-flow left ventricular assist devices; IQR: interquartile range.
| Bleeders, n (%) | |
| Within 3 years after CF-LVAD implantation (3 years) | 28 (25) |
| During whole support time (st) | 30 (27) |
| Utah Bleeding Risk Score, median (IQR) | |
| Bleeders | 2 (3-1) |
| Non-bleeders | 2 (3-1) |
| Bleeders, n (%) | |
| Within 3 years after CF-LVAD implantation (3 years) | 28 (25) |
| During whole support time (st) | 30 (27) |
| Utah Bleeding Risk Score, median (IQR) | |
| Bleeders | 2 (3-1) |
| Non-bleeders | 2 (3-1) |
CF-LVAD: continuous-flow left ventricular assist devices; IQR: interquartile range.
Group-based risk stratification does not estimate freedom from GIB
According to the original article of the UBRS, patients were divided into 3 risk groups according to their score (0–1: low-risk group; 2–4: medium-risk group; 5–9: high-risk group). In the 3 risk groups, there was no significant difference in freedom from GIB (P3 years = 0.7; Pst = 0.816) (Fig. 3).
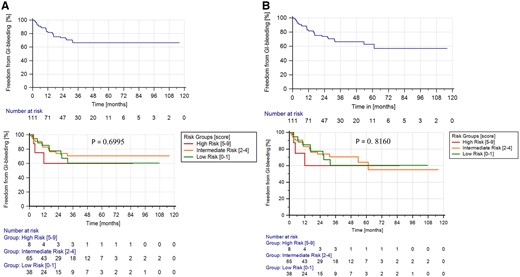
Kaplan–Meier curves within 3 years after implantation (A) and during whole support time (B). There was no significant difference between the 3 risk groups.
Only coronary artery disease predicts bleeding during whole support time
At 3 years after LVAD implantation, Cox proportional hazards regression analysis did not show any significant influence of the variables included in the score on freedom from bleeding. Including the whole support time, only the variable coronary artery disease turned out to be significant (Pst = 0.048) (Table 3). This can be equally depicted in the Kaplan–Meier survival curves of patients with and without coronary artery disease, compared by the log-rank test (Fig. 4).
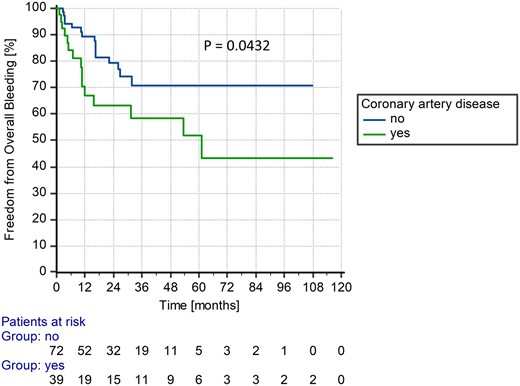
Kaplan–Meier curve of patients with and without coronary artery disease during whole support time.
Univariable Cox regression analysis of the variables included in Utah Bleeding Risk Score
| Score variable . | First 3 years after LVAD implantation (P-value) . | HR with 95% CI . | Whole support time (P-value) . | HR with 95% CI . |
|---|---|---|---|---|
| Age >54 years | 0.357 | – | 0.343 | – |
| Coronary artery disease | 0.092 | – | 0.048 | 2.0709 (1.0069–4.2592) |
| Chronic kidney disease | 0.389 | – | 0.314 | – |
| Glucose >107 mg/dl | 0.529 | – | 0.449 | – |
| MPAP <18 mmHg | 0.777 | – | 0.807 | – |
| History of previous bleed | 0.958 | – | 0.955 | – |
| Severe RV dysfunction | 0.427 | – | 0.611 | – |
| Score variable . | First 3 years after LVAD implantation (P-value) . | HR with 95% CI . | Whole support time (P-value) . | HR with 95% CI . |
|---|---|---|---|---|
| Age >54 years | 0.357 | – | 0.343 | – |
| Coronary artery disease | 0.092 | – | 0.048 | 2.0709 (1.0069–4.2592) |
| Chronic kidney disease | 0.389 | – | 0.314 | – |
| Glucose >107 mg/dl | 0.529 | – | 0.449 | – |
| MPAP <18 mmHg | 0.777 | – | 0.807 | – |
| History of previous bleed | 0.958 | – | 0.955 | – |
| Severe RV dysfunction | 0.427 | – | 0.611 | – |
CI: confidence interval; HR: hazard ratio; LVAD: left ventricular assist devices; MPAP: mean pulmonary artery pressure; RV: right ventricular; Significant values are highlighted in bold print.
Univariable Cox regression analysis of the variables included in Utah Bleeding Risk Score
| Score variable . | First 3 years after LVAD implantation (P-value) . | HR with 95% CI . | Whole support time (P-value) . | HR with 95% CI . |
|---|---|---|---|---|
| Age >54 years | 0.357 | – | 0.343 | – |
| Coronary artery disease | 0.092 | – | 0.048 | 2.0709 (1.0069–4.2592) |
| Chronic kidney disease | 0.389 | – | 0.314 | – |
| Glucose >107 mg/dl | 0.529 | – | 0.449 | – |
| MPAP <18 mmHg | 0.777 | – | 0.807 | – |
| History of previous bleed | 0.958 | – | 0.955 | – |
| Severe RV dysfunction | 0.427 | – | 0.611 | – |
| Score variable . | First 3 years after LVAD implantation (P-value) . | HR with 95% CI . | Whole support time (P-value) . | HR with 95% CI . |
|---|---|---|---|---|
| Age >54 years | 0.357 | – | 0.343 | – |
| Coronary artery disease | 0.092 | – | 0.048 | 2.0709 (1.0069–4.2592) |
| Chronic kidney disease | 0.389 | – | 0.314 | – |
| Glucose >107 mg/dl | 0.529 | – | 0.449 | – |
| MPAP <18 mmHg | 0.777 | – | 0.807 | – |
| History of previous bleed | 0.958 | – | 0.955 | – |
| Severe RV dysfunction | 0.427 | – | 0.611 | – |
CI: confidence interval; HR: hazard ratio; LVAD: left ventricular assist devices; MPAP: mean pulmonary artery pressure; RV: right ventricular; Significant values are highlighted in bold print.
DISCUSSION
GIB after LVAD implantation drives the frequency of hospital readmissions, healthcare costs and patient mortality. The UBRS identified 7 variables independently associated with the occurrence of GIB [11]. GIB originates mostly from the upper GI tract (∼47%), but also from the lower GI tract (22%), or the midgut (15%) [12]. From a pathophysiological point of view, the UBRS variables relate to the pre-existing entity of vascular disease (indicated by advanced age, insufficient glycaemic control, history of previous bleeds, coronary artery disease), with vascular disease progression further fuelled by congestion most likely from right heart failure, indicated by low mean pulmonary artery pressure, chronic kidney disease and hepatic congestion [11]. However, the identified weighed composition of these variables that is central to the UBRS score was unable to neither predict nor significantly influence GIB occurrence in our patient collective of 111 patients post-LVAD implantation. This suggests that either additional or more specific variables related to the underlying pathophysiology of vascular pathology in LVAD patients may require consideration for the composition of a valid risk prediction tool for GIB.
In patients with LVAD, pump-associated high shearing effects lead to acquired von Willebrand factor deficiency. Degradation of von Willebrand factor results in the reduced competence of primary haemostasis and the upregulation of mediators promoting vascular instability [13]. In the study population analysed for UBRS identification, 79% of patients bled from arterio-venous malformations (AVMs) in the upper GI tract. AVMs are in addition to peptic bleeding encountered in the majority of patients with LVAD [14, 15] and are particularly hard to treat [12]. AVMs have recently been particularly associated with RV enlargement in a collective of 398 patients with LVAD [16], which is of particular interest to this study as the incidence of RV dysfunction (40.5% in our collective) displays almost the only noteworthy difference in the clinical characteristics of our patient collective compared to the collective of patients used to establish the UBRS [11]. AVM-associated bleeds have been correlated to increased measures of pro-angiogenic mediators such as tumour necrosis factor alpha, vascular endothelial growth factor, transforming growth factor-beta and reduced concentrations of angiopoietin-1 that is associated with vascular stability [17]. Recent reports identify generalized capillary functional impairment (i.e. identified in the sublingual microcirculation) in addition to a decreased capillary glycocalyx thickness in patients with CF-LVAD as an independent risk factor for GIB [18]. Interestingly, these changes in LVAD patients were not statistically different from those identified by systematic assessment of microvascular integrity in patients with congestive heart failure but without LVAD support, suggesting that measures of pre-existing vascular pathology (i.e. microvascular integrity or biomarkers involved in vascular homeostasis) could contribute in addition to clinical parameters to the risk stratification of potential LVAD recipients prior to device implantation.
In addition to pre-existing pathology, pump-specific alterations in haemodynamics have been suggested to contribute to modulating GIB risk. Patients who had received pulsatile-flow LVAD exhibited reduced GIB incidence in comparison to patients with CF-LVAD [19]. This supported the notion that non-pulsatile flow is a potent inducer of vascular remodelling that contributes to the development of angiodysplasia and vascular frailty in patients with CF-LVAD. Among the CV-LVADs that are associated with improved outcome and greater durability, pulsatile flow through the pump depends on pump speed with increasing left ventricular contraction, resulting in increased left ventricular filling, left ventricular pressure and thus increased pump flow during diastole [20]. This is described by a higher pulsatility index indicating less support provided by the pump. Higher pulsatility index values were associated with reduced risk from GIB [21]. Although a previous report suggested a correlation of GIB with left ventricular contractility of >30% [22], recent reports again highlight the protective effects of pulsatility, aortic valve opening and also Lavare Cycle in a study population of 595 patients implanted with HM II (HeartMate II), HMIII (HeartMate III) and HVAD (HeartWare LVAD) devices [23]. In contrast, no difference in the incidence of GIB was found when patients who had received axial pumps versus centrifugal pumps were compared [24]. This is of particular importance to our results since the number of patients with centrifugal pumps differs from that in the publication by Yin et al. (60% of patients had received HeartWare centrifugal pumps in our collective vs 32% in [11]). Thus, technical pump specifications as well as characteristics of the actual haemodynamics may add additional value to composite scores for individual risk stratification but are unlikely to explain the discrepancy of score validity in our patient collective.
As a variety of parameters could contribute to the design of improved scores in the future, various variables may have contributed to the irreproducibility of the predictive value of the variables of the UBRS in our cohort of patients. In any case and based on our retrospective, single-centre analysis of 111 patients post-LVAD implantation, we cannot confirm the uniform validity of the UBRS for the prediction of bleeding complications. We suggest the implementation of additional or more specific variables when developing improved prediction scores for GI bleeding after CF-LVAD implantation to ultimately decrease hospitalization rate and improve the quality of life on patients on the device.
Limitations
This study excluded patients who died during primary hospital stay, as the majority of these patients were in INTERMACS 1, i.e. cardiac shock. In this state, there is a big fluctuation of many parameters included in the score. Moreover, GIB is a complication occurring on mid-term-follow-up, as arterio-venous malformations (the most common cause for GIB) develop as a consequence of an absence of pulsatility, i.e. continuous-flow device. The number of patients in this validation study was limited to the patients receiving CF-LVAD at our institution. In this cohort, UBRS was not able to predict GIB. Additional multicentre validation analysis on a larger scale is advisable.
Conflict of interest: none declared.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Armin Peivandi: Formal analysis; Funding acquisition; Investigation; Methodology; Software; Visualization; Writing—original draft; Writing—review & editing. Henryk Welp: Investigation; Methodology; Resources; Software; Writing—review & editing. Mirela Scherer: Supervision; Writing—review & editing. Jürgen R. Sindermann: Supervision; Writing—review & editing. Nana-Maria Wagner: Supervision; Writing—original draft; Writing—review & editing. Angelo M. Dell’Aquila: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Tom R. Karl, Daniel Y. Loisance and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
https://www.eurotransplant.org/wp-content/uploads/2020/06/Annual-Report-2019.pdf (24 December 2021, date last accessed).
ABBREVIATIONS
- AVMs
Arterio-venous malformations
- CF-LVAD
Continuous-flow left ventricular assist devices
- GIB
Gastrointestinal bleeding
- RV
Right ventricular
- st
Support time
- UBRS
Utah Bleeding Risk Score
Author notes
Armin Peivandi, Henryk Welp contributed equally to this work.
Nana-Maria Wagner, AngeloM. Dell’Aquila contributed equally to this work.





