-
PDF
- Split View
-
Views
-
Cite
Cite
Christian Kuehn, Stefan Ruemke, Philipp Rellecke, Artur Lichtenberg, Dominik Joskowiak, Christian Hagl, Mohamed Hassan, Rainer G Leyh, Stefan Erler, Jens Garbade, Sandra Eifert, Philippe Grieshaber, Andreas Boening, Torsten Doenst, Ilia Velichkov, Tomas Madej, Michael Knaut, Andreas Hain, Heiko Burger, Wearable cardioverter defibrillator multicentre experience in a large cardiac surgery cohort at transient risk of sudden cardiac death, European Journal of Cardio-Thoracic Surgery, Volume 61, Issue 5, May 2022, Pages 1031–1040, https://doi.org/10.1093/ejcts/ezac086
Close - Share Icon Share
Abstract
The wearable cardioverter defibrillator (WCD) is an established, safe, effective solution, protecting patients at risk of sudden cardiac death. We specifically investigated WCD use in cardiac surgery patients since data for this patient group are rare.
Retrospective data analysis in 10 German cardiac surgery centres was performed. Cardiac surgery patients with left ventricular ejection fraction (LVEF) ≤35% or after implantable cardioverter defibrillator (ICD) explantation who received WCD between 2010 and 2020 were assessed using LifeVest Network data.
A total of 1168 patients with a median age of 66 years [interquartile range (IQR) 57–73] were enrolled; 87% were male. Clinical indications included coronary artery bypass grafting (43%), valve surgery (16%), combined coronary artery bypass graft/valve surgery (15%), ICD explantation (24%) and miscellaneous (2%). The median wear time of WCD was 23.4 h/day (IQR 21.7–23.8). A total of 106 patients (9.1%) exhibited ventricular tachycardia. A total of 93.2% of episodes occurred within the first 3 months. Eighteen patients (1.5%) received 26 adequate shocks. The inadequate shock rate was low (8 patients, 0.7%). LVEF improved from a median of 28% (IQR 22–32%) before WCD prescription to 35% (IQR 28–42%) during follow-up. Excluding ICD explant patients, 37% of patients received an ICD.
The risk of sudden cardiac death is substantial within the first 3 months after cardiac surgery. Patients were protected effectively by WCD. Due to significant LVEF improvement, the majority did not require ICD implantation after WCD use. Compliance was high despite sternotomy. This multicentre experience confirms existing data regarding effectiveness, safety and compliance. Therefore, WCD should be considered in cardiac surgery patients with severely reduced LVEF.
INTRODUCTION
Sudden cardiac death (SCD) is one of the leading causes of death in Europe, with ∼600 000 deaths annually [1]. Over 50% are due to life-threatening ventricular tachyarrhythmia (VT)/ventricular fibrillation (VF), often with underlying cardiac disease [2]. The vast majority of patients have reduced left ventricular ejection fraction (LVEF) [3, 4]. A correlation between the occurrence of life-threatening VT/VF and reduced LVEF has been demonstrated, making severely reduced LVEF one of the most important predictors for SCD [3, 4].
Cardiac surgery patients often demonstrate severely reduced LVEF even preoperatively. Advances in surgical therapeutic techniques have reduced the operative risk [5]. However, there is still a high peri- and postoperative mortality risk, mostly due to VT/VF [6, 7] This risk is highest in the first 3 months postoperatively and decreases significantly over time [6–9]. Recovery correlates with regeneration of the heart during this time, which leads to a substantial LVEF improvement in a significant proportion of cases. Guideline-directed medical therapy is recommended for at least 3 months for optimal adjustment [10, 11]. In cardiac surgery patients, significant LVEF improvement has been observed up to 6 months postoperatively [12–14].
Implantable cardioverter defibrillators (ICD) can effectively terminate VT/VF. However, current guidelines of the European Society of Cardiology and the American Heart Association recommend a final decision on ICD implantation not earlier than 3 months after the diagnosis of severe heart failure and exclusion of reversible causes [10, 11, 15].
To close the gap between hospital discharge and ICD decision, guidelines recommend the temporary use of a wearable cardioverter defibrillator (WCD) in the vulnerable phase to protect patients at risk from SCD [10, 11, 16, 17]. The WCD is a non-invasive device, which can detect and terminate haemodynamically relevant VT/VFs [18, 19]. The first randomized controlled trial (VEST) showed a significant overall mortality reduction, although a significant arrhythmic mortality reduction was only found in the per-protocol and as-treated analyses [20]. Results underscore the need for clear and direct patient education about the risks of SCD and the benefits of wearing a WCD to ensure good compliance [21]. Several large real-life studies consistently confirmed safety, effectiveness and high WCD compliance [18–27]. Based on these data, the German Society for Thoracic and Cardiovascular Surgery (DGTHG) recently published a position paper recommending WCD use [17]. So far, only a small number of cardiac surgery patients have been included in studies and, thus far, have never been selectively investigated in a larger patient population. Therefore, the aim of this study was to investigate safety, effectiveness and compliance of the WCD, as well as the incidence of VT/VFs, the development of LVEF and potential differences between patients who underwent certain types of cardiac surgery or ICD explantation.
METHODS
Ethics statement
According to the Ethics Committee of the Hannover Medical School (No. 1673-2013), Hannover, Germany, a retrospective analysis like ours handling with anonymized patient data does not require a specific assessment by the ethics committee. Patient data were anonymized.
Study design and patient population
In this retrospective study, 10 German cardiac surgery centres included 1168 patients discharged with a WCD (LifeVest® Wearable Cardioverter Defibrillator; ZOLL, Pittsburgh, PA, USA) between 2010 and 2020. Based on current guidelines, cardiac surgery patients with postoperative LVEF ≤35% or after ICD explantation due to infection were prescribed with a WCD. Diagnosis and therapy strategy was assessed by a heart team of surgeons, clinical cardiologists and electrophysiologists, whenever possible, to obtain optimal decision-making.
Materials (wearable cardioverter defibrillator and LifeVest Network)
The WCD is a non-invasive external defibrillator, first introduced in the USA and Germany as early as 2001. It consists of 4 electrocardiogram electrodes for continuous rhythm analysis and 3 shock electrodes as well as a monitoring system, which reliably detects and treats life-threatening VT/VF without bystander intervention. All detected arrhythmias, as well as several other parameters, such as heart rate, electrocardiogram, arrhythmic events and wear time, are documented and transferred to the LifeVest Network (LVN). Based on this information, the physician can adjust therapy and medication as needed. Patients are trained to press 2 response buttons when conscious during an initiated shock sequence, thereby avoiding inadequate and unnecessary shocks. Components and detailed functionality of the WCD are described elsewhere [28].
Data availability statement
All relevant data are within the manuscript and its supporting information files. Further information can be accessed via the corresponding author.
Data collection and study end points
Clinical data were retrospectively analysed by the study centres. The ethics committee approved the study [decision of the ‘Ethics commitee Medizinische Hochschule Hannover (MHH)’, August 2019].
Data were collected on age, sex, type of cardiac surgery, time of surgery, diagnosis, LVEF at the time of prescription and at follow-up (FU) after 3 months, as well as previous atrial fibrillation (AF) events. In addition, data were collected on WCD wear time, VT events, time of event occurrence and WCD treatments. The LVN was used for assessment and analysis, which automatically processes and stores data.
The main aim of the study was to examine the outcome of cardiac surgery patients wearing a WCD, including follow-up from time of surgery until end of WCD therapy.
We thought to investigate the occurrence of VT/VF, appropriate WCD treatments and their respective temporal relationship to surgery, as well as the LVEF improvement and its influence on a subsequent ICD indication. Furthermore, compliance for the WCD was assessed.
Statistical analysis
Continuous variables were reported as median with interquartile range, and categorical variables were reported as numbers and percentages for each group and combined groups, respectively. Kruskal–Wallis rank-sum test followed by pairwise Wilcoxon rank-sum tests (adjusted by the Bonferroni method) was applied to test group differences. Kaplan–Meier curves and log-rank tests were performed by the R-package ‘Survival’. For all statistical analyses, the statistical software R-4.1.1 (R Core Team, 2021) was used (R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2021. https://www.R-project.org/).
RESULTS
We identified 1168 patients, discharged with a WCD after cardiac surgery intervention between 2010 and 2020. The median age in this patient cohort was 66 years [interquartile range (IQR) 57–73]. A total of 1016 (87%) were male and 152 (13%) female. A total of 678 patients received coronary artery bypass graft (CABG) surgery (172 in combination with heart valve intervention, and 506 as isolated coronary revascularization). In addition, 186 patients underwent isolated valve surgery. In 280 patients, ICD explantation was performed (see Table 1).
| . | Overall . | CABG . | CABG with valve . | Valve surgery . | ICD explantation . | Misc . |
|---|---|---|---|---|---|---|
| n | 1168 | 506 | 172 | 186 | 280 | 24 |
| Age (years), median [IQR] | 66.0 [57.0, 73.0] | 66.0 [59.0, 73.0] | 67.0 [59.0, 74.0] | 64.0 [54.0, 73.0] | 65.0 [55.0, 74.0] | 52.5 [35.5, 60.8] |
| Gender = female, n (%) | 152 (13.0) | 51 (10.1) | 13 (7.6) | 38 (20.4) | 45 (16.1) | 5 (20.8) |
| Days in hospital, median [IQR] | 15.0 [11.0, 20.0] | 14.0 [11.0, 18.0] | 17.0 [13.0, 21.0] | 17.0 [13.0, 23.0] | 14.0 [8.0, 26.0] | 14.0 [11.0, 25.0] |
| General ward, median [IQR] | 9.0 [5.0, 13.0] | 9.0 [5.0, 12.0] | 9.0 [5.0, 13.0] | 12.0 [7.0, 15.0] | 8.0 [3.0, 15.0] | 9.0 [5.0, 11.5] |
| Intermediate care, median [IQR] | 2.0 [0.0, 6.0] | 3.0 [0.0, 6.0] | 4.0 [1.0, 8.0] | 3.0 [0.0, 7.0] | 0.0 [0.0, 2.0] | 1.0 [0.0, 3.5] |
| Intensive care, median [IQR] | 1.0 [0.0, 3.0] | 1.0 [0.0, 3.0] | 1.0 [0.0, 3.0] | 1.0 [1.0, 4.0] | 0.0 [0.0, 2.0] | 0.5 [0.0, 6.0] |
| VT_pre-OP, n (%) | 73 (8.6) | 25 (6.7) | 8 (6.5) | 3 (2.2) | 33 (15.7) | 4 (28.6) |
| VF_pre-OP, n (%) | 63 (7.4) | 35 (9.4) | 5 (4.1) | 4 (3.0) | 14 (6.7) | 5 (33.3) |
| nsVT_pre-OP, n (%) | 37 (4.4) | 17 (4.7) | 3 (2.5) | 2 (1.5) | 14 (6.9) | 1 (7.7) |
| VT_post-OP, n (%) | 81 (8.3) | 34 (8.1) | 12 (8.5) | 18 (11.0) | 16 (7.0) | 1 (6.2) |
| VF_post-OP, n (%) | 49 (5.0) | 20 (4.8) | 12 (8.4) | 13 (7.9) | 2 (0.9) | 2 (11.8) |
| nsVT_ post-OP, n (%) | 45 (4.6) | 14 (3.3) | 8 (5.6) | 12 (7.3) | 11 (4.8) | 0 (0.0) |
| AF history, n (%) | 220 (18.8) | 73 (14.4) | 38 (22.1) | 33 (17.7) | 71 (25.4) | 5 (20.8) |
| WCD usage time per day (h)/ compliance, median [IQR] | 23.4 [21.7, 23.8] | 23.4 [21.6, 23.8] | 23.5 [22.4, 23.9] | 23.4 [22.5, 23.8] | 23.4 [20.2, 23.8] | 22.2 [20.6, 23.7] |
| Usage period (days), median [IQR] | 65.0 [34.0, 90.0] | 74.0 [38.0, 92.0] | 69.5 [42.5, 93.0] | 74.0 [40.2, 91.0] | 48.0 [27.0, 74.2] | 41.0 [26.8, 64.8] |
| . | Overall . | CABG . | CABG with valve . | Valve surgery . | ICD explantation . | Misc . |
|---|---|---|---|---|---|---|
| n | 1168 | 506 | 172 | 186 | 280 | 24 |
| Age (years), median [IQR] | 66.0 [57.0, 73.0] | 66.0 [59.0, 73.0] | 67.0 [59.0, 74.0] | 64.0 [54.0, 73.0] | 65.0 [55.0, 74.0] | 52.5 [35.5, 60.8] |
| Gender = female, n (%) | 152 (13.0) | 51 (10.1) | 13 (7.6) | 38 (20.4) | 45 (16.1) | 5 (20.8) |
| Days in hospital, median [IQR] | 15.0 [11.0, 20.0] | 14.0 [11.0, 18.0] | 17.0 [13.0, 21.0] | 17.0 [13.0, 23.0] | 14.0 [8.0, 26.0] | 14.0 [11.0, 25.0] |
| General ward, median [IQR] | 9.0 [5.0, 13.0] | 9.0 [5.0, 12.0] | 9.0 [5.0, 13.0] | 12.0 [7.0, 15.0] | 8.0 [3.0, 15.0] | 9.0 [5.0, 11.5] |
| Intermediate care, median [IQR] | 2.0 [0.0, 6.0] | 3.0 [0.0, 6.0] | 4.0 [1.0, 8.0] | 3.0 [0.0, 7.0] | 0.0 [0.0, 2.0] | 1.0 [0.0, 3.5] |
| Intensive care, median [IQR] | 1.0 [0.0, 3.0] | 1.0 [0.0, 3.0] | 1.0 [0.0, 3.0] | 1.0 [1.0, 4.0] | 0.0 [0.0, 2.0] | 0.5 [0.0, 6.0] |
| VT_pre-OP, n (%) | 73 (8.6) | 25 (6.7) | 8 (6.5) | 3 (2.2) | 33 (15.7) | 4 (28.6) |
| VF_pre-OP, n (%) | 63 (7.4) | 35 (9.4) | 5 (4.1) | 4 (3.0) | 14 (6.7) | 5 (33.3) |
| nsVT_pre-OP, n (%) | 37 (4.4) | 17 (4.7) | 3 (2.5) | 2 (1.5) | 14 (6.9) | 1 (7.7) |
| VT_post-OP, n (%) | 81 (8.3) | 34 (8.1) | 12 (8.5) | 18 (11.0) | 16 (7.0) | 1 (6.2) |
| VF_post-OP, n (%) | 49 (5.0) | 20 (4.8) | 12 (8.4) | 13 (7.9) | 2 (0.9) | 2 (11.8) |
| nsVT_ post-OP, n (%) | 45 (4.6) | 14 (3.3) | 8 (5.6) | 12 (7.3) | 11 (4.8) | 0 (0.0) |
| AF history, n (%) | 220 (18.8) | 73 (14.4) | 38 (22.1) | 33 (17.7) | 71 (25.4) | 5 (20.8) |
| WCD usage time per day (h)/ compliance, median [IQR] | 23.4 [21.7, 23.8] | 23.4 [21.6, 23.8] | 23.5 [22.4, 23.9] | 23.4 [22.5, 23.8] | 23.4 [20.2, 23.8] | 22.2 [20.6, 23.7] |
| Usage period (days), median [IQR] | 65.0 [34.0, 90.0] | 74.0 [38.0, 92.0] | 69.5 [42.5, 93.0] | 74.0 [40.2, 91.0] | 48.0 [27.0, 74.2] | 41.0 [26.8, 64.8] |
AF: atrial fibrillation; CABG: coronary artery bypass graft; ICD: implantable cardioverter defibrillator; IQR: interquartile range; OP: surgery; VF: ventricular fibrillation; VT: ventricular tachycardia; WCD: wearable cardioverter defibrillator.
| . | Overall . | CABG . | CABG with valve . | Valve surgery . | ICD explantation . | Misc . |
|---|---|---|---|---|---|---|
| n | 1168 | 506 | 172 | 186 | 280 | 24 |
| Age (years), median [IQR] | 66.0 [57.0, 73.0] | 66.0 [59.0, 73.0] | 67.0 [59.0, 74.0] | 64.0 [54.0, 73.0] | 65.0 [55.0, 74.0] | 52.5 [35.5, 60.8] |
| Gender = female, n (%) | 152 (13.0) | 51 (10.1) | 13 (7.6) | 38 (20.4) | 45 (16.1) | 5 (20.8) |
| Days in hospital, median [IQR] | 15.0 [11.0, 20.0] | 14.0 [11.0, 18.0] | 17.0 [13.0, 21.0] | 17.0 [13.0, 23.0] | 14.0 [8.0, 26.0] | 14.0 [11.0, 25.0] |
| General ward, median [IQR] | 9.0 [5.0, 13.0] | 9.0 [5.0, 12.0] | 9.0 [5.0, 13.0] | 12.0 [7.0, 15.0] | 8.0 [3.0, 15.0] | 9.0 [5.0, 11.5] |
| Intermediate care, median [IQR] | 2.0 [0.0, 6.0] | 3.0 [0.0, 6.0] | 4.0 [1.0, 8.0] | 3.0 [0.0, 7.0] | 0.0 [0.0, 2.0] | 1.0 [0.0, 3.5] |
| Intensive care, median [IQR] | 1.0 [0.0, 3.0] | 1.0 [0.0, 3.0] | 1.0 [0.0, 3.0] | 1.0 [1.0, 4.0] | 0.0 [0.0, 2.0] | 0.5 [0.0, 6.0] |
| VT_pre-OP, n (%) | 73 (8.6) | 25 (6.7) | 8 (6.5) | 3 (2.2) | 33 (15.7) | 4 (28.6) |
| VF_pre-OP, n (%) | 63 (7.4) | 35 (9.4) | 5 (4.1) | 4 (3.0) | 14 (6.7) | 5 (33.3) |
| nsVT_pre-OP, n (%) | 37 (4.4) | 17 (4.7) | 3 (2.5) | 2 (1.5) | 14 (6.9) | 1 (7.7) |
| VT_post-OP, n (%) | 81 (8.3) | 34 (8.1) | 12 (8.5) | 18 (11.0) | 16 (7.0) | 1 (6.2) |
| VF_post-OP, n (%) | 49 (5.0) | 20 (4.8) | 12 (8.4) | 13 (7.9) | 2 (0.9) | 2 (11.8) |
| nsVT_ post-OP, n (%) | 45 (4.6) | 14 (3.3) | 8 (5.6) | 12 (7.3) | 11 (4.8) | 0 (0.0) |
| AF history, n (%) | 220 (18.8) | 73 (14.4) | 38 (22.1) | 33 (17.7) | 71 (25.4) | 5 (20.8) |
| WCD usage time per day (h)/ compliance, median [IQR] | 23.4 [21.7, 23.8] | 23.4 [21.6, 23.8] | 23.5 [22.4, 23.9] | 23.4 [22.5, 23.8] | 23.4 [20.2, 23.8] | 22.2 [20.6, 23.7] |
| Usage period (days), median [IQR] | 65.0 [34.0, 90.0] | 74.0 [38.0, 92.0] | 69.5 [42.5, 93.0] | 74.0 [40.2, 91.0] | 48.0 [27.0, 74.2] | 41.0 [26.8, 64.8] |
| . | Overall . | CABG . | CABG with valve . | Valve surgery . | ICD explantation . | Misc . |
|---|---|---|---|---|---|---|
| n | 1168 | 506 | 172 | 186 | 280 | 24 |
| Age (years), median [IQR] | 66.0 [57.0, 73.0] | 66.0 [59.0, 73.0] | 67.0 [59.0, 74.0] | 64.0 [54.0, 73.0] | 65.0 [55.0, 74.0] | 52.5 [35.5, 60.8] |
| Gender = female, n (%) | 152 (13.0) | 51 (10.1) | 13 (7.6) | 38 (20.4) | 45 (16.1) | 5 (20.8) |
| Days in hospital, median [IQR] | 15.0 [11.0, 20.0] | 14.0 [11.0, 18.0] | 17.0 [13.0, 21.0] | 17.0 [13.0, 23.0] | 14.0 [8.0, 26.0] | 14.0 [11.0, 25.0] |
| General ward, median [IQR] | 9.0 [5.0, 13.0] | 9.0 [5.0, 12.0] | 9.0 [5.0, 13.0] | 12.0 [7.0, 15.0] | 8.0 [3.0, 15.0] | 9.0 [5.0, 11.5] |
| Intermediate care, median [IQR] | 2.0 [0.0, 6.0] | 3.0 [0.0, 6.0] | 4.0 [1.0, 8.0] | 3.0 [0.0, 7.0] | 0.0 [0.0, 2.0] | 1.0 [0.0, 3.5] |
| Intensive care, median [IQR] | 1.0 [0.0, 3.0] | 1.0 [0.0, 3.0] | 1.0 [0.0, 3.0] | 1.0 [1.0, 4.0] | 0.0 [0.0, 2.0] | 0.5 [0.0, 6.0] |
| VT_pre-OP, n (%) | 73 (8.6) | 25 (6.7) | 8 (6.5) | 3 (2.2) | 33 (15.7) | 4 (28.6) |
| VF_pre-OP, n (%) | 63 (7.4) | 35 (9.4) | 5 (4.1) | 4 (3.0) | 14 (6.7) | 5 (33.3) |
| nsVT_pre-OP, n (%) | 37 (4.4) | 17 (4.7) | 3 (2.5) | 2 (1.5) | 14 (6.9) | 1 (7.7) |
| VT_post-OP, n (%) | 81 (8.3) | 34 (8.1) | 12 (8.5) | 18 (11.0) | 16 (7.0) | 1 (6.2) |
| VF_post-OP, n (%) | 49 (5.0) | 20 (4.8) | 12 (8.4) | 13 (7.9) | 2 (0.9) | 2 (11.8) |
| nsVT_ post-OP, n (%) | 45 (4.6) | 14 (3.3) | 8 (5.6) | 12 (7.3) | 11 (4.8) | 0 (0.0) |
| AF history, n (%) | 220 (18.8) | 73 (14.4) | 38 (22.1) | 33 (17.7) | 71 (25.4) | 5 (20.8) |
| WCD usage time per day (h)/ compliance, median [IQR] | 23.4 [21.7, 23.8] | 23.4 [21.6, 23.8] | 23.5 [22.4, 23.9] | 23.4 [22.5, 23.8] | 23.4 [20.2, 23.8] | 22.2 [20.6, 23.7] |
| Usage period (days), median [IQR] | 65.0 [34.0, 90.0] | 74.0 [38.0, 92.0] | 69.5 [42.5, 93.0] | 74.0 [40.2, 91.0] | 48.0 [27.0, 74.2] | 41.0 [26.8, 64.8] |
AF: atrial fibrillation; CABG: coronary artery bypass graft; ICD: implantable cardioverter defibrillator; IQR: interquartile range; OP: surgery; VF: ventricular fibrillation; VT: ventricular tachycardia; WCD: wearable cardioverter defibrillator.
| . | Overall . | CABG . | CABG with valve . | Valve surgery . | ICD explantation . | Misc . |
|---|---|---|---|---|---|---|
| n . | 1168 . | 506 . | 172 . | 186 . | 280 . | 24 . |
| Patients with VT/VF (%) | 106 (9.1) | 36 (7.1) | 19 (11.0) | 17 (9.1) | 34 (12.1) | 0 (0.0) |
| Appropriate shock, n (%) | 18 (1.5) | 7 (1.4) | 1 (0.6) | 2 (1.1) | 8 (2.9) | 0 (0.0) |
| Withholding response, n (%) | 17 (1.5) | 3 (0.6) | 7 (4.1) | 4 (2.2) | 3 (1.1) | 0 (0.0) |
| Non-sustained VT, n (%) | 63 (5.4) | 20 (3.9) | 11 (6.4) | 10 (5.3) | 22 (7.8) | 0 (0.0) |
| Below detection rate, n (%) | 7 (0.6) | 5 (1.0) | 0 (0.0) | 1 (0.5) | 1 (0.4) | 0 (0.0) |
| No. VT/VF episodes, n (%) | 250 (21.4) | 81 (16.0) | 75 (43.6) | 28 (15.1) | 66 (23.6) | 0 (0.0) |
| No. appropriate shocks, n (%) | 26 (2.2) | 11 (2.2) | 3 (1.7) | 2 (1.1) | 10 (3.6) | 0 (0.0) |
| AF newly detected, n (%) | 92 (7.9) | 34 (6.7) | 21 (12.2) | 24 (12.9) | 13 (4.6) | 0 (0.0) |
| . | Overall . | CABG . | CABG with valve . | Valve surgery . | ICD explantation . | Misc . |
|---|---|---|---|---|---|---|
| n . | 1168 . | 506 . | 172 . | 186 . | 280 . | 24 . |
| Patients with VT/VF (%) | 106 (9.1) | 36 (7.1) | 19 (11.0) | 17 (9.1) | 34 (12.1) | 0 (0.0) |
| Appropriate shock, n (%) | 18 (1.5) | 7 (1.4) | 1 (0.6) | 2 (1.1) | 8 (2.9) | 0 (0.0) |
| Withholding response, n (%) | 17 (1.5) | 3 (0.6) | 7 (4.1) | 4 (2.2) | 3 (1.1) | 0 (0.0) |
| Non-sustained VT, n (%) | 63 (5.4) | 20 (3.9) | 11 (6.4) | 10 (5.3) | 22 (7.8) | 0 (0.0) |
| Below detection rate, n (%) | 7 (0.6) | 5 (1.0) | 0 (0.0) | 1 (0.5) | 1 (0.4) | 0 (0.0) |
| No. VT/VF episodes, n (%) | 250 (21.4) | 81 (16.0) | 75 (43.6) | 28 (15.1) | 66 (23.6) | 0 (0.0) |
| No. appropriate shocks, n (%) | 26 (2.2) | 11 (2.2) | 3 (1.7) | 2 (1.1) | 10 (3.6) | 0 (0.0) |
| AF newly detected, n (%) | 92 (7.9) | 34 (6.7) | 21 (12.2) | 24 (12.9) | 13 (4.6) | 0 (0.0) |
AF: atrial fibrillation; CABG: coronary artery bypass graft; ICD: implantable cardioverter defibrillator; VF: ventricular fibrillation; VT: ventricular tachycardia.
| . | Overall . | CABG . | CABG with valve . | Valve surgery . | ICD explantation . | Misc . |
|---|---|---|---|---|---|---|
| n . | 1168 . | 506 . | 172 . | 186 . | 280 . | 24 . |
| Patients with VT/VF (%) | 106 (9.1) | 36 (7.1) | 19 (11.0) | 17 (9.1) | 34 (12.1) | 0 (0.0) |
| Appropriate shock, n (%) | 18 (1.5) | 7 (1.4) | 1 (0.6) | 2 (1.1) | 8 (2.9) | 0 (0.0) |
| Withholding response, n (%) | 17 (1.5) | 3 (0.6) | 7 (4.1) | 4 (2.2) | 3 (1.1) | 0 (0.0) |
| Non-sustained VT, n (%) | 63 (5.4) | 20 (3.9) | 11 (6.4) | 10 (5.3) | 22 (7.8) | 0 (0.0) |
| Below detection rate, n (%) | 7 (0.6) | 5 (1.0) | 0 (0.0) | 1 (0.5) | 1 (0.4) | 0 (0.0) |
| No. VT/VF episodes, n (%) | 250 (21.4) | 81 (16.0) | 75 (43.6) | 28 (15.1) | 66 (23.6) | 0 (0.0) |
| No. appropriate shocks, n (%) | 26 (2.2) | 11 (2.2) | 3 (1.7) | 2 (1.1) | 10 (3.6) | 0 (0.0) |
| AF newly detected, n (%) | 92 (7.9) | 34 (6.7) | 21 (12.2) | 24 (12.9) | 13 (4.6) | 0 (0.0) |
| . | Overall . | CABG . | CABG with valve . | Valve surgery . | ICD explantation . | Misc . |
|---|---|---|---|---|---|---|
| n . | 1168 . | 506 . | 172 . | 186 . | 280 . | 24 . |
| Patients with VT/VF (%) | 106 (9.1) | 36 (7.1) | 19 (11.0) | 17 (9.1) | 34 (12.1) | 0 (0.0) |
| Appropriate shock, n (%) | 18 (1.5) | 7 (1.4) | 1 (0.6) | 2 (1.1) | 8 (2.9) | 0 (0.0) |
| Withholding response, n (%) | 17 (1.5) | 3 (0.6) | 7 (4.1) | 4 (2.2) | 3 (1.1) | 0 (0.0) |
| Non-sustained VT, n (%) | 63 (5.4) | 20 (3.9) | 11 (6.4) | 10 (5.3) | 22 (7.8) | 0 (0.0) |
| Below detection rate, n (%) | 7 (0.6) | 5 (1.0) | 0 (0.0) | 1 (0.5) | 1 (0.4) | 0 (0.0) |
| No. VT/VF episodes, n (%) | 250 (21.4) | 81 (16.0) | 75 (43.6) | 28 (15.1) | 66 (23.6) | 0 (0.0) |
| No. appropriate shocks, n (%) | 26 (2.2) | 11 (2.2) | 3 (1.7) | 2 (1.1) | 10 (3.6) | 0 (0.0) |
| AF newly detected, n (%) | 92 (7.9) | 34 (6.7) | 21 (12.2) | 24 (12.9) | 13 (4.6) | 0 (0.0) |
AF: atrial fibrillation; CABG: coronary artery bypass graft; ICD: implantable cardioverter defibrillator; VF: ventricular fibrillation; VT: ventricular tachycardia.
| . | Overall . | CABG . | CABG with valve . | Valve surgery . | ICD explantation . | Misc . |
|---|---|---|---|---|---|---|
| n . | 1168 . | 506 . | 172 . | 186 . | 280 . | 24 . |
| LVEF | ||||||
| LVEF_pre-OP, median [IQR] | 29.0 [20.5, 37.0] | 29.0 [23.5, 35.0] | 29.0 [22.0, 40.0] | 29.5 [20.0, 40.0] | 27.5 [20.0, 45.0] | 20.0 [15.0, 21.5] |
| LVEF_post-OP, median [IQR] | 28.0 [22.0, 32.0] | 28.0 [24.0, 30.0] | 27.0 [20.0, 30.0] | 26.0 [21.0, 30.0] | 30.0 [22.0, 40.5] | 27.0 [20.0, 32.0] |
| LVEF_end of use, median [IQR] | 35.0 [28.0, 42.0] | 35.0 [30.0, 41.0] | 36.0 [30.0, 40.8] | 40.0 [30.0, 41.0] | 31.0 [25.0, 45.0] | 27.5 [23.8, 41.2] |
| End of WCD use | ||||||
| Improvement in LVEF, n (%) | 471 (42.2) | 265 (54.4) | 78 (47.9) | 103 (58.2) | 22 (8.3) | 3 (13.0) |
| ICD (re)implantation, n (%) | 546 (48.6) | 184 (37.7) | 70 (42.9) | 59 (32.8) | 217 (80.4) | 16 (69.6) |
| Miscellaneous,an (%) | 124 (10.6) | 49 (9.7) | 21 (12.2) | 22 (11.8) | 29 (10.4) | 3 (12.5) |
| No data | 27 | 8 | 3 | 2 | 12 | 2 |
| . | Overall . | CABG . | CABG with valve . | Valve surgery . | ICD explantation . | Misc . |
|---|---|---|---|---|---|---|
| n . | 1168 . | 506 . | 172 . | 186 . | 280 . | 24 . |
| LVEF | ||||||
| LVEF_pre-OP, median [IQR] | 29.0 [20.5, 37.0] | 29.0 [23.5, 35.0] | 29.0 [22.0, 40.0] | 29.5 [20.0, 40.0] | 27.5 [20.0, 45.0] | 20.0 [15.0, 21.5] |
| LVEF_post-OP, median [IQR] | 28.0 [22.0, 32.0] | 28.0 [24.0, 30.0] | 27.0 [20.0, 30.0] | 26.0 [21.0, 30.0] | 30.0 [22.0, 40.5] | 27.0 [20.0, 32.0] |
| LVEF_end of use, median [IQR] | 35.0 [28.0, 42.0] | 35.0 [30.0, 41.0] | 36.0 [30.0, 40.8] | 40.0 [30.0, 41.0] | 31.0 [25.0, 45.0] | 27.5 [23.8, 41.2] |
| End of WCD use | ||||||
| Improvement in LVEF, n (%) | 471 (42.2) | 265 (54.4) | 78 (47.9) | 103 (58.2) | 22 (8.3) | 3 (13.0) |
| ICD (re)implantation, n (%) | 546 (48.6) | 184 (37.7) | 70 (42.9) | 59 (32.8) | 217 (80.4) | 16 (69.6) |
| Miscellaneous,an (%) | 124 (10.6) | 49 (9.7) | 21 (12.2) | 22 (11.8) | 29 (10.4) | 3 (12.5) |
| No data | 27 | 8 | 3 | 2 | 12 | 2 |
Miscellaneous (multiple answers possible) = died (18, 1.5%), refuses ICD (16, 1.4%), non-compliance (23, 2.0%), discomfort (6, 0.5%), refuses WCD (15, 1.3%), and others (51, 4.4%).
CABG: coronary artery bypass graft; ICD: implantable cardioverter defibrillator; IQR: interquartile range; LVEF: left ventricular ejection fraction; OP: surgery; WCD: wearable cardioverter defibrillator.
| . | Overall . | CABG . | CABG with valve . | Valve surgery . | ICD explantation . | Misc . |
|---|---|---|---|---|---|---|
| n . | 1168 . | 506 . | 172 . | 186 . | 280 . | 24 . |
| LVEF | ||||||
| LVEF_pre-OP, median [IQR] | 29.0 [20.5, 37.0] | 29.0 [23.5, 35.0] | 29.0 [22.0, 40.0] | 29.5 [20.0, 40.0] | 27.5 [20.0, 45.0] | 20.0 [15.0, 21.5] |
| LVEF_post-OP, median [IQR] | 28.0 [22.0, 32.0] | 28.0 [24.0, 30.0] | 27.0 [20.0, 30.0] | 26.0 [21.0, 30.0] | 30.0 [22.0, 40.5] | 27.0 [20.0, 32.0] |
| LVEF_end of use, median [IQR] | 35.0 [28.0, 42.0] | 35.0 [30.0, 41.0] | 36.0 [30.0, 40.8] | 40.0 [30.0, 41.0] | 31.0 [25.0, 45.0] | 27.5 [23.8, 41.2] |
| End of WCD use | ||||||
| Improvement in LVEF, n (%) | 471 (42.2) | 265 (54.4) | 78 (47.9) | 103 (58.2) | 22 (8.3) | 3 (13.0) |
| ICD (re)implantation, n (%) | 546 (48.6) | 184 (37.7) | 70 (42.9) | 59 (32.8) | 217 (80.4) | 16 (69.6) |
| Miscellaneous,an (%) | 124 (10.6) | 49 (9.7) | 21 (12.2) | 22 (11.8) | 29 (10.4) | 3 (12.5) |
| No data | 27 | 8 | 3 | 2 | 12 | 2 |
| . | Overall . | CABG . | CABG with valve . | Valve surgery . | ICD explantation . | Misc . |
|---|---|---|---|---|---|---|
| n . | 1168 . | 506 . | 172 . | 186 . | 280 . | 24 . |
| LVEF | ||||||
| LVEF_pre-OP, median [IQR] | 29.0 [20.5, 37.0] | 29.0 [23.5, 35.0] | 29.0 [22.0, 40.0] | 29.5 [20.0, 40.0] | 27.5 [20.0, 45.0] | 20.0 [15.0, 21.5] |
| LVEF_post-OP, median [IQR] | 28.0 [22.0, 32.0] | 28.0 [24.0, 30.0] | 27.0 [20.0, 30.0] | 26.0 [21.0, 30.0] | 30.0 [22.0, 40.5] | 27.0 [20.0, 32.0] |
| LVEF_end of use, median [IQR] | 35.0 [28.0, 42.0] | 35.0 [30.0, 41.0] | 36.0 [30.0, 40.8] | 40.0 [30.0, 41.0] | 31.0 [25.0, 45.0] | 27.5 [23.8, 41.2] |
| End of WCD use | ||||||
| Improvement in LVEF, n (%) | 471 (42.2) | 265 (54.4) | 78 (47.9) | 103 (58.2) | 22 (8.3) | 3 (13.0) |
| ICD (re)implantation, n (%) | 546 (48.6) | 184 (37.7) | 70 (42.9) | 59 (32.8) | 217 (80.4) | 16 (69.6) |
| Miscellaneous,an (%) | 124 (10.6) | 49 (9.7) | 21 (12.2) | 22 (11.8) | 29 (10.4) | 3 (12.5) |
| No data | 27 | 8 | 3 | 2 | 12 | 2 |
Miscellaneous (multiple answers possible) = died (18, 1.5%), refuses ICD (16, 1.4%), non-compliance (23, 2.0%), discomfort (6, 0.5%), refuses WCD (15, 1.3%), and others (51, 4.4%).
CABG: coronary artery bypass graft; ICD: implantable cardioverter defibrillator; IQR: interquartile range; LVEF: left ventricular ejection fraction; OP: surgery; WCD: wearable cardioverter defibrillator.
In 106 (9.1%) patients, 250 VT/VF episodes occurred (Table 2). Among these, 26 episodes in 18 patients (1.5%) were appropriately defibrillated by the WCD. All shocks were successful. Eight (0.7%) patients received a total number of 9 inadequate shocks, of whom 4 patients had supraventricular tachycardias with rapid transition. In 1 patient, the supraventricular tachycardia was of haemodynamic relevance. Four inadequate shocks were caused by artefact. A period of asystole was documented in 7 (0.6%) patients.
Of 506 patients with isolated CABG, 36 (7.1%) patients developed 81 VT/VF episodes. Of these, 7 (1.4%) patients received 11 adequate WCD shocks, whereas in 3 patients (0.6%) the response buttons to withhold a shock during consciousness were pressed. Twenty (3.9%) patients had non-sustained ventricular tachycardias (nsVTs). In the combined CABG and valve replacement group (n = 172), 19 (11%) patients developed 75 VT/VF episodes, of whom 1 (0.6%) patient received 3 adequate shocks, 7 (4.1%) patients pressed the response buttons to suppress impending defibrillation and 11 (6.4%) patients had nsVTs. Patients with isolated valve surgery (n = 186) developed 28 VT/VF episodes in 17 (9.1%) cases. Among these, 2 (1.1%) patients received adequate shocks due to VT/VF, whereas 4 (2.2%) patients pressed the response buttons. Ten (5.3%) patients had nsVTs. Of 280 patients undergoing ICD explantation, 34 patients (12.1%) had 66 VT/VF episodes, of whom 8 (2.9%) patients received 10 adequate shocks and 3 (1.1%) used the response buttons to prevent defibrillation. Moreover, 22 (7.8%) patients had nsVTs. No VT/VF episode was detected in the group of miscellaneous surgical procedures. The prevalence of VT/VF episodes in relation to the time of surgical intervention is shown in Fig. 1. The probability of WCD shock delivery depending on the surgical method is displayed in Fig. 2. The estimated survival probability without wearable cardioverter defibrillator is shown in Fig. 3 and Supplementary Material.

The prevalence of ventricular tachycardia/ventricular fibrillation episodes in relation to the time of surgical intervention.
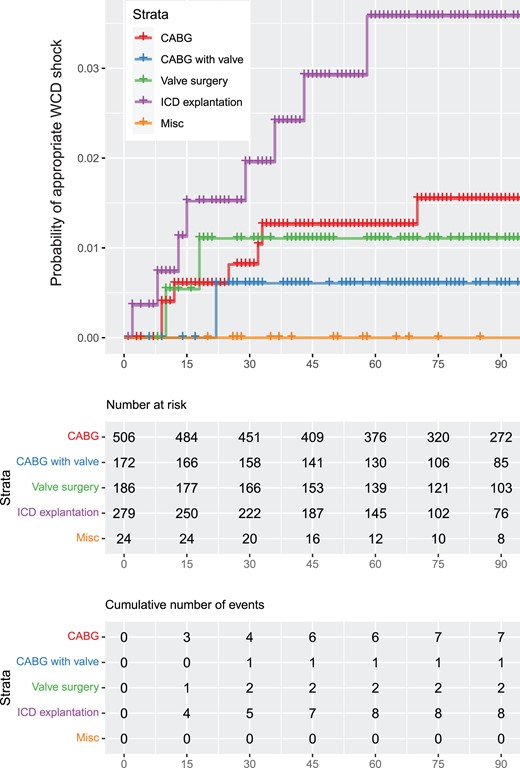
Probability of wearable cardioverter defibrillator shock depending on the surgical method.
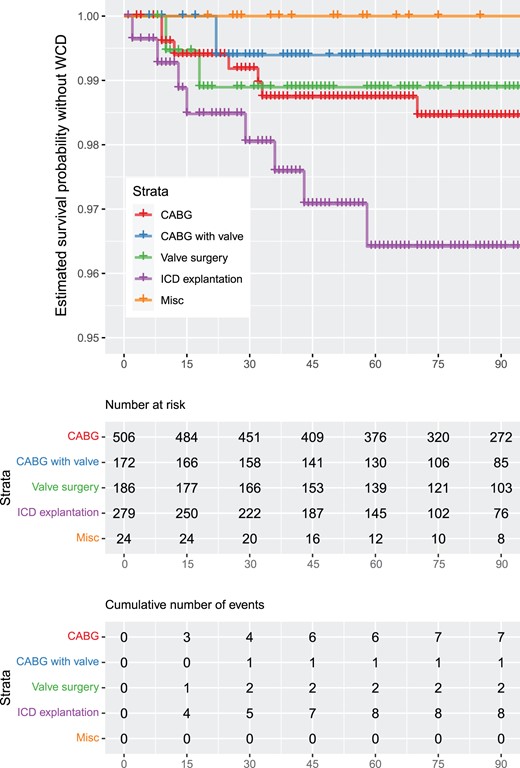
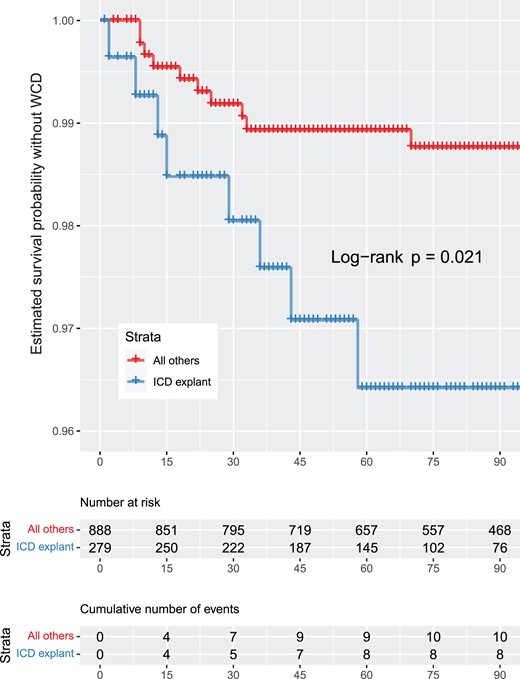
(A) Estimated survival probability without wearable cardioverter defibrillator per procedure. The vertical axis was cut for better readability of the data curves. (B) Estimated survival probability without wearable cardioverter defibrillator of implantable cardioverter defibrillator explant patients versus all other patients. The vertical axis was cut for better readability of the data curves.
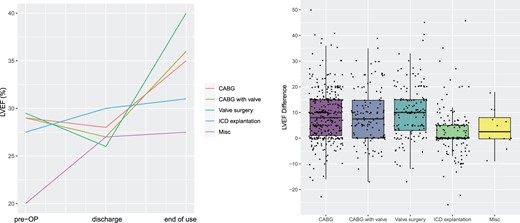
(A) Left ventricular ejection fraction development per surgical procedure. Shown are medians previous to surgery, at discharge, and at end of wearable cardioverter defibrillator use. (B) Box plots. Left ventricular ejection fraction differences from discharge (0) to end of wearable cardioverter defibrillator use per procedure.
The median time to first VT/VF episode with and without shock was 17 days (IQR 6–40) after start of WCD therapy and 29 days (IQR 17–51) after surgery.
A total of 220 patients (18.8%) had a prior history of AF. In addition, the WCD detected AF for the first time in 92 patients (7.9%). New diagnosis of AF most frequently occurred in the groups of isolated valve surgery (n = 21, 12.2%) and combined CABG and valve surgery (n = 24, 12.9%).
The median wearing period was 65 days (IQR: 34–90). The longest wearing period was after valve surgery with a median of 74 days (IQR: 40–91) and after isolated CABG with a median of 74 days (IQR: 38–92). ICD explant patients showed the shortest wearing periods with 48 days (IQR: 27–74). Daily wear time in the entire patient cohort was a median of 23.4 h per day (IQR: 21.7–23.8). There were no significant differences in daily wear time depending on clinical procedure nor sex.
Postoperatively, the median LVEF of the total population was 28% (IQR: 22–32) and improved to a median of 35% (IQR: 28–42) at FU examination (Table 3). Patients in the CABG, valve and CABG plus valve groups displayed a significant median LVEF difference of between +8% and 10%. The ICD explant group did not show LVEF increase in median. However, LVEF improvement seemed to be quite individual. While patients in the ICD explant and miscellaneous cohort showed an LVEF increase in median from pre-OP to post-OP and end of FU, the LVEF of patients in the CABG, valve and combined CABG with valve groups decreased from pre-OP to post-OP with a pronounced improvement to FU. The left ventricular ejection fraction development per surgical procedure is shown in Fig. 4.
After WCD use, ICD implantation was performed in 546 (48.6%) patients of the entire population. In 471 (42.2%) patients, an ICD implantation could be avoided due to an improved LVEF.
In the ICD explant patient cohort, reimplantation was necessary in 217 (80.4%) patients, while ICD reimplantation could be avoided due to LVEF improvement in 22 (8.3%) individuals.
In the cohort without ICD explantation, 329 (37%) patients finally received an ICD, while further device-based prevention prior to SCD was no longer necessary in 449 (50.6%) patients.
Most ICDs could be avoided after isolated CABG and isolated valve surgery. Here, WCD therapy could be terminated due to an LVEF improvement in 265 (54.4%) and 103 (58.2%) patients. ICD implantation was still performed in 184 (37.7%) and 59 (32.8%) patients.
Most patients could be allocated to the end-of-WCD-use reasons ICD implantation or LVEF improvement. However, 124 (10.6%) patients had different reasons and for 27 patients, no data were available for this parameter. Reasons for "Miscellaneous end of use" included patient refuses WCD, death, non-compliance, discomfort, still active use, end of prescription and others. Furthermore, a reason for a not implanted ICD was refusal of an ICD by the patient.
A total of 18 patients died during the observation period. All patients were in hospital, not wearing the WCD at the time of death. Mortality was significantly higher for explant patients compared to all other patients.
DISCUSSION
This retrospective, multicentre study presents WCD experience in a large, strictly cardiac surgery patient population. Effectiveness, safety and high WCD compliance in nonsurgical patients have been consistently demonstrated in recent years. However, direct transferability of the favourable clinical results in mixed patient populations to a purely cardiac surgery collective does not appear to be imperative because of the specific periprocedural circumstances such as sternotomy or the use of a heart–lung machine and the need for rapid rehabilitation. We therefore investigated the extent to which the available evidence can be transferred to cardiac surgery patients.
Ventricular arrhythmias and shocks
The rate of patients developing VT/VFs is substantial (250 ventricular arrhythmias; 9.1%). The highest proportion of patients with VT/VF occurred in the ICD explant group, followed by CABG with valve, valve surgery alone and CABG alone. The most patients with appropriate shocks were found in the ICD explant group, followed by CABG alone, valve surgery and CABG with valve.
All recorded VT/VFs occurred within the first 180 postoperative days and could be detected from the first day of wearing the WCD. However, the majority of arrhythmic events occurred within the first 90 days.
The rate of 1.5% of adequately treated patients found in our collective aligns with current European studies in various large patient collectives. Wäßnig (n = 6043), Garcia (n = 1157) and Veltmann (n = 781) reported similar adequate shock rates in mixed patient populations (1.6%; 1.6%; 1.3%, respectively). Our study documented an inadequate shock rate of 0.7%, which is also comparable to previous studies (0.4%, 0.7% and 0.3%, respectively) [19, 22, 23].
The cause of inadequate shocks in 4 of 8 patients was supraventricular arrhythmias and artificial rhythm detection in the remaining 4. More recent WCD studies show a trend towards decreasing rates of inadequate shock delivery, which could be attributed to an update of the WCD detection algorithm. According to the manufacturer ZOLL, this was accomplished in 2018 to reduce artefacts that led to misinterpretation of the detected heart rhythm.
Wearing time
The daily wearing time of our collective was overall very good in all patient cohorts, with a median of 23.4 h per day. This is especially remarkable as sternotomy was frequent and wound-healing processes were still ongoing. Similarly, patients participated in rehabilitation activities that did not prevent them from wearing the WCD except when swimming. No difference was observed between sexes. The total median wearing time of 65 days did not vary noticeably between the subgroups. However, there was an exception for patients who underwent ICD explantation. Depending on an already existing preoperatively ICD indication, patients received an ICD much more quickly and therefore showed a shorter WCD usage with a median of 48 days. Overall, recorded total wearing times and in particular daily wearing times were in line with previous study results in non-surgical or mixed patient populations, ranging from a median of 22.6–23.5 h per day [19, 23–25].
Thus, it can be concluded that the WCD shows a good acceptance in cardiac surgery patients, which does not differ from other patient collectives despite specific postoperative challenges. In general, detailed patient education and training is crucial. Online patient management with the LVN can be helpful to ensure compliance.
Development of left ventricular ejection fraction and follow-up care after wearable cardioverter defibrillator use
Postoperatively, many cardiac surgery patients show severely reduced LVEF, which is associated with elevated risk of SCD. In our study, the initial median LVEF of 28% improved during ∼3 months of WCD use to a median of 35% (28–42%). Except for patients with ICD explantation, LVEF subgroup analyses did not show significant differences in the increase of pump function between the various clinical procedures. Box plot analyses showed LVEF differences between discharge and FU of +8–10% for patients with CABG and valve surgery. While patients with ICD explant and miscellaneous interventions increased their LVEF from pre- to post-surgery and FU, patients with CABG and valve surgery experienced an LVEF decrease post-surgery with a steep and prominent increase to FU.
Remarkably, a substantial proportion of all patients recovered above the threshold value of an LVEF >35%, which represents a criterion for permanent ICD indication. Excluding the ICD explantation cohort, ICD implantation was no longer indicated in 50.6% of patients. This is accomplished by drug therapy, which must be slowly adjusted over several months, and by the progressive regenerative processes of the heart itself. The results in terms of LVEF recovery are also consistent with previous studies [22–24].
The high burden of VT/VF within the first months might raise the question of early ICD implantation. However, our study results clearly underline the recommended waiting times required by current guidelines as well as the reliable exclusion of reversible causes prior to permanent ICD implantation. In particular, patients who recover after CABG or valve surgery show a significant improvement in LVEF within 3–6 months and at the same time confirm their willingness to wear a WCD with a high therapy compliance. Therefore, wearing the device during this temporary high-risk phase can provide safe and effective protection from SCD and simultaneously avoid permanent ICD implantation in a relevant proportion of patients. Prematurely implanted ICDs may lose their justification after the end of the recovery period and, if there is no indication, only result in complications such as device infections or lead failure. Thus, the use of a WCD can overall improve the patient's quality of life and reduce the financial burden on health care systems. A recent health technology assessment on WCD demonstrated cost-effectiveness in patients after myocardial infarction and cost-savings after ICD explantation [29].
CONCLUSION
In conclusion, this study demonstrates that the risk of VT and life-threatening tachyarrhythmias is highest within the first 3 months in cardiac surgery patients with LVEF ≤35%, and patients with ICD explant, regardless of the procedure. These patients can be protected with a WCD. During the observation period, LVEF improved significantly in non-ICD explant patients, avoiding permanent ICD implantation in a substantial part of patients. Furthermore, compliance was very good despite sternotomy.
Available clinical evidence consistently demonstrates high efficacy, safety and compliance with a WCD in patients at increased transient risk of SCD due to severely impaired LVEF. This particularly applies to the postoperative care of cardiac surgery patients for whom, depending on the ventricular function and the individual risk of SCD, temporary WCD protection should be recommended.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Conflict of interest: Christian Kuehn reports honorarium fees from ZOLL. The other authors report no conflict of interest.
Author contributions
Christian Kuehn: Conceptualization; Formal analysis; Writing—original draft. Stefan Ruemke: Data curation; Investigation; Writing—review & editing. Philipp Rellecke: Data curation; Formal analysis; Investigation; Writing—review & editing. Artur Lichtenberg: Formal analysis; Investigation; Writing—review & editing. Dominik Joskowiak: Data curation; Formal analysis; Investigation; Writing—review & editing. Christian Hagl: Formal analysis; Investigation; Writing—review & editing. Mohamed Hassan: Data curation; Writing—review & editing. Rainer G. Leyh: Formal analysis; Investigation; Writing—review & editing. Stefan Erler: Data curation; Formal analysis; Writing—review & editing. Jens Garbade: Conceptualization; Investigation; Validation; Writing—review & editing. Sandra Eifert: Data curation; Investigation; Writing—review & editing. Philippe Grieshaber: Data curation; Investigation; Writing—review & editing. Andreas Boening: Conceptualization; Investigation; Supervision; Writing—review & editing. Torsten Doenst: Conceptualization; Investigation; Supervision; Writing—review & editing. Ilia Velichkov: Data curation; Writing—review & editing. Tomas Madej: Data curation; Writing—review & editing. Michael Knaut: Conceptualization; Data curation; Supervision; Writing—review & editing. Andreas Hain: Data curation; Investigation; Validation; Writing—review & editing. Heiko Burger: Conceptualization; Investigation; Validation; Writing—original draft.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Pradeep Narayan, Ulrich Otto von Oppell and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Presented at the 49th Annual Meeting of the German Society for Thoracic and Cardiovascular Surgery, Wiesbaden, Germany, 29 February–3 March 2020.
REFERENCES
ABBREVIATIONS
- AF
Atrial fibrillation
- CABG
Coronary artery bypass graft
- FU
Follow-up
- ICD
Implantable cardioverter defibrillator
- IQR
Interquartile range
- LVEF
Left ventricular ejection fraction
- LVN
LifeVest Network
- SCD
Sudden cardiac death
- nsVTs
Non-sustained ventricular tachycardias
- VF
Ventricular fibrillation
- VT
Ventricular tachycardia
- WCD
Wearable cardioverter defibrillator
- left ventricular ejection fraction
- coronary artery bypass surgery
- tachycardia, ventricular
- sudden cardiac death
- cardiac surgery procedures
- implantable defibrillators
- follow-up
- shock
- ventricular arrhythmia
- wearable automatic external defibrillator
- implantable defibrillator insertion
- heart valve surgery





