-
PDF
- Split View
-
Views
-
Cite
Cite
Takayuki Shijo, Hitoshi Matsuda, Koki Yokawa, Yosuke Inoue, Yoshimasa Seike, Kyokun Uehara, Mitsuyoshi Takahara, Hiroaki Sasaki, The impact of vascularized tissue flap coverage on aortic graft infection with and without infected graft excision, European Journal of Cardio-Thoracic Surgery, Volume 60, Issue 5, November 2021, Pages 1043–1050, https://doi.org/10.1093/ejcts/ezab179
Close - Share Icon Share
Abstract
Aortic graft infection (AGI) is a serious condition associated with a high mortality rate. However, optimal surgical options have not been identified. Therefore, we retrospectively reviewed AGI cases, including those in the thoracic and abdominal regions, with or without fistula formation, to investigate the various options for better outcomes.
We reviewed 50 patients who underwent surgical interventions for AGI out of 97 patients with arterial infective disease. The mean patient age was 67 ± 17 years. Fourteen patients (28%) had a fistula with the gastrointestinal tract or lung. A combination of graft excision and vascularized tissue flap coverage was performed in 25 cases (50%). Tissue flap alone, graft excision alone and cleansing alone were performed in 9 (18%), 10 (20%), and 6 cases (12%), respectively.
Total in-hospital mortality rate was 32% (n = 16). In-hospital mortalities in patients with and without fistulas were 43% (6/14) and 28% (10/36), respectively (P = 0.33). Subgroup analysis among patients without fistula demonstrated that the in-hospital mortality rate of the patients with vascularized tissue flap (3/21, 14%) was significantly lower than that of the patients without vascularized tissue flap (7/14, 50%, P = 0.026). Overall 1- and 5-year survival rates were 66% and 46%, respectively. In multivariable analysis, an independent factor associated with in-hospital mortality was vascularized tissue flap (odds ratio 0.20, P = 0.024).
Vascularized tissue flaps could provide better outcomes for AGI. Graft preservation with vascularized tissue flaps could be a useful option for AGI without fistula.
INTRODUCTION
For the past several decades, despite improvements in the management of aortic pathologies, surgical treatment of aortic graft infection (AGI) remains challenging. Although the reported incidence rate of AGI is as low as 1–3% [1–3], the mortality is extremely high, ranging from 25% to 75% [4–6]. Classically, AGI is managed through the excision of the infected aortic graft and extensive debridement of surrounding tissue. However, the infected graft excision is sometimes highly invasive for patients exhausted from the infection. Some authors reported that vascularized tissue flap has better outcomes in terms of controlling aorta-related infection, including omental and muscular flaps [7–9]. However, the optimal treatment option for AGI remains unclear because it is a rare condition and occurs under heterogeneous conditions (for example, it can occur with or without a fistula with the gastrointestinal or respiratory tracts). Therefore, we retrospectively reviewed 50 cases of AGI in our institution, including those in the thoracic and abdominal regions, with or without gastrointestinal or respiratory fistula formation, to investigate the various options for better outcomes.
PATIENTS AND METHODS
Patients
Between January 2001 and December 2019, 97 patients underwent surgical intervention for arterial infective disease at our institution. In this study, we excluded 19 cases of peripheral arterial graft infection and 28 cases of mycotic aortic aneurysm. Finally, we reviewed the cases of 50 patients with AGI who had histories of graft replacement or endografting for aortic disease (mean age 67 ± 17 years, male 39). There were 29 cases of thoracic or thoraco-abdominal aortic disease and 21 cases of abdominal aortic disease. The median duration from previous graft replacement to the main surgical intervention of the AGI was 17 months [interquartile range (IQR) 3–54]. The preoperative comorbidities and other patient’s characteristics are listed in Table 1. Median follow-up duration was 46 months (IQR 22–77 months). When the study closing date was defined as the end of 2019, the median follow-up index was 0.88 (IQR 0.43–1) [10].
| . | Total (n = 50) . | With fistula (n = 14) . | Without fistula (n = 36) . | P-value . |
|---|---|---|---|---|
| Age (years), mean ± SD | 67 ± 17 | 75 ± 9 | 64 ± 18 | 0.031* |
| Male:female | 40:10 | 12:2 | 28:8 | 0.70 |
| Comorbidity, n (%) | ||||
| Hypertension | 37 (74) | 12 (86) | 25 (69) | 0.30 |
| Dyslipidaemia | 11 (22) | 3 (21) | 8 (22) | 1.00 |
| Diabetes mellitus | 7 (14) | 0 | 7 (19) | 0.17 |
| Chronic kidney disease | 15 (30) | 5 (36) | 10 (28) | 0.73 |
| Cerebrovascular disease | 10 (20) | 2 (14) | 8 (22) | 0.70 |
| Chronic obstructive pulmonary disease | 7 (14) | 2 (14) | 5 (14) | 1.00 |
| Chronic heart failure | 6 (12) | 1 (7) | 5 (14) | 0.66 |
| Coronary artery disease | 12 (24) | 6 (43) | 6 (17) | 0.07 |
| Thoracic:abdominal | 29:21 | 6:8 | 23:13 | 0.21 |
| Non-dissection:dissection | 28:22 | 11:3 | 17:19 | 0.06 |
| Endograft infection, n (%) | 8 (16) | 3 (21) | 5 (14) | 0.67 |
| Preoperative C-reactive protein (mg/dl) | 5.4 (IQR: 1.7–10.7) | 4.0 (IQR: 1.6–9.6) | 6.5 (IQR: 1.6–13) | 0.23 |
| Positive blood culture, n (%) | 21 (42) | 4 (29) | 17 (47) | 0.34 |
| Positive local specimen culture, n (%) | 37 (74) | 11 (79) | 26 (72) | 0.73 |
| . | Total (n = 50) . | With fistula (n = 14) . | Without fistula (n = 36) . | P-value . |
|---|---|---|---|---|
| Age (years), mean ± SD | 67 ± 17 | 75 ± 9 | 64 ± 18 | 0.031* |
| Male:female | 40:10 | 12:2 | 28:8 | 0.70 |
| Comorbidity, n (%) | ||||
| Hypertension | 37 (74) | 12 (86) | 25 (69) | 0.30 |
| Dyslipidaemia | 11 (22) | 3 (21) | 8 (22) | 1.00 |
| Diabetes mellitus | 7 (14) | 0 | 7 (19) | 0.17 |
| Chronic kidney disease | 15 (30) | 5 (36) | 10 (28) | 0.73 |
| Cerebrovascular disease | 10 (20) | 2 (14) | 8 (22) | 0.70 |
| Chronic obstructive pulmonary disease | 7 (14) | 2 (14) | 5 (14) | 1.00 |
| Chronic heart failure | 6 (12) | 1 (7) | 5 (14) | 0.66 |
| Coronary artery disease | 12 (24) | 6 (43) | 6 (17) | 0.07 |
| Thoracic:abdominal | 29:21 | 6:8 | 23:13 | 0.21 |
| Non-dissection:dissection | 28:22 | 11:3 | 17:19 | 0.06 |
| Endograft infection, n (%) | 8 (16) | 3 (21) | 5 (14) | 0.67 |
| Preoperative C-reactive protein (mg/dl) | 5.4 (IQR: 1.7–10.7) | 4.0 (IQR: 1.6–9.6) | 6.5 (IQR: 1.6–13) | 0.23 |
| Positive blood culture, n (%) | 21 (42) | 4 (29) | 17 (47) | 0.34 |
| Positive local specimen culture, n (%) | 37 (74) | 11 (79) | 26 (72) | 0.73 |
IQR: interquartile range; SD: standard deviation.
Significant value; P < 0.05.
| . | Total (n = 50) . | With fistula (n = 14) . | Without fistula (n = 36) . | P-value . |
|---|---|---|---|---|
| Age (years), mean ± SD | 67 ± 17 | 75 ± 9 | 64 ± 18 | 0.031* |
| Male:female | 40:10 | 12:2 | 28:8 | 0.70 |
| Comorbidity, n (%) | ||||
| Hypertension | 37 (74) | 12 (86) | 25 (69) | 0.30 |
| Dyslipidaemia | 11 (22) | 3 (21) | 8 (22) | 1.00 |
| Diabetes mellitus | 7 (14) | 0 | 7 (19) | 0.17 |
| Chronic kidney disease | 15 (30) | 5 (36) | 10 (28) | 0.73 |
| Cerebrovascular disease | 10 (20) | 2 (14) | 8 (22) | 0.70 |
| Chronic obstructive pulmonary disease | 7 (14) | 2 (14) | 5 (14) | 1.00 |
| Chronic heart failure | 6 (12) | 1 (7) | 5 (14) | 0.66 |
| Coronary artery disease | 12 (24) | 6 (43) | 6 (17) | 0.07 |
| Thoracic:abdominal | 29:21 | 6:8 | 23:13 | 0.21 |
| Non-dissection:dissection | 28:22 | 11:3 | 17:19 | 0.06 |
| Endograft infection, n (%) | 8 (16) | 3 (21) | 5 (14) | 0.67 |
| Preoperative C-reactive protein (mg/dl) | 5.4 (IQR: 1.7–10.7) | 4.0 (IQR: 1.6–9.6) | 6.5 (IQR: 1.6–13) | 0.23 |
| Positive blood culture, n (%) | 21 (42) | 4 (29) | 17 (47) | 0.34 |
| Positive local specimen culture, n (%) | 37 (74) | 11 (79) | 26 (72) | 0.73 |
| . | Total (n = 50) . | With fistula (n = 14) . | Without fistula (n = 36) . | P-value . |
|---|---|---|---|---|
| Age (years), mean ± SD | 67 ± 17 | 75 ± 9 | 64 ± 18 | 0.031* |
| Male:female | 40:10 | 12:2 | 28:8 | 0.70 |
| Comorbidity, n (%) | ||||
| Hypertension | 37 (74) | 12 (86) | 25 (69) | 0.30 |
| Dyslipidaemia | 11 (22) | 3 (21) | 8 (22) | 1.00 |
| Diabetes mellitus | 7 (14) | 0 | 7 (19) | 0.17 |
| Chronic kidney disease | 15 (30) | 5 (36) | 10 (28) | 0.73 |
| Cerebrovascular disease | 10 (20) | 2 (14) | 8 (22) | 0.70 |
| Chronic obstructive pulmonary disease | 7 (14) | 2 (14) | 5 (14) | 1.00 |
| Chronic heart failure | 6 (12) | 1 (7) | 5 (14) | 0.66 |
| Coronary artery disease | 12 (24) | 6 (43) | 6 (17) | 0.07 |
| Thoracic:abdominal | 29:21 | 6:8 | 23:13 | 0.21 |
| Non-dissection:dissection | 28:22 | 11:3 | 17:19 | 0.06 |
| Endograft infection, n (%) | 8 (16) | 3 (21) | 5 (14) | 0.67 |
| Preoperative C-reactive protein (mg/dl) | 5.4 (IQR: 1.7–10.7) | 4.0 (IQR: 1.6–9.6) | 6.5 (IQR: 1.6–13) | 0.23 |
| Positive blood culture, n (%) | 21 (42) | 4 (29) | 17 (47) | 0.34 |
| Positive local specimen culture, n (%) | 37 (74) | 11 (79) | 26 (72) | 0.73 |
IQR: interquartile range; SD: standard deviation.
Significant value; P < 0.05.
This retrospective record review was approved by the institutional review board of our hospital (M30-057). Informed consent was waived because of the retrospective nature of the study. All patients were followed up in the outpatient department of our institution. Follow-up clinical status was obtained through medical records from the outpatient clinic.
Diagnosis and intervention
We comprehensively diagnosed AGI based on clinical symptoms (fever, pain), blood test (white blood cell count, C-reactive protein), blood culture, radiographical findings (computed tomography, gallium-scintigraphy), intraoperative findings and the culture of specimen. Pre- and postoperatively, all patients received antibiotic therapy. After the microbe was identified, antibiotic spectra were narrowed. Antibiotic therapy was continued for 6 weeks intravenously and for >6 months orally.
As the first step for the treatment, the patients with thoracic AGI underwent exploration of the infected prosthetic graft, debridement of surrounding abnormal tissue and cleansing with saline. Then, debridement and continuous or intermittent cleansing were implemented every 1–2 days until the main surgical intervention was performed. From 2009, negative pressure wound therapy was adopted for thoracic AGI. Wounds were filled with polyurethane sponge to protect organs and the anastomotic site, using a generated negative pressure of 75–125 mmHg. Negative pressure wound therapy was applied for the patients with AGI of the descending and thoraco-abdominal aorta as well as for those with mediastinal AGI. After repetitive cleansing in combination with negative pressure wound therapy, the decision to perform re-replacement was made. If the patient’s condition was improved, as observed through negative culture and reduced dirty exudate, the graft was preserved, and the patient underwent vascularized tissue flap coverage. Omentum was harvested by dissecting it from the greater curvature of the stomach, and it was pedicled by the right gastroepiploic artery. When the AGI was located at the mediastinum, the space around the aortic graft was filled with omentum via a midline incision. When the AGI was located in the left thoracic cavity, the aortic graft was wrapped in omentum via the trans-left diaphragm route. If the dead space was extremely narrow, vascularized tissue flap coverage was not performed. When the appearance around the aortic graft or culture was not improved, graft excision was promptly performed. When there was pseudoaneurysm or bleeding, we performed graft replacement or repair at the first intervention. The infected endograft was initially removed, and graft replacement was performed for the radical debridement of the thrombosed aneurysm. For patients with fistulas with the gastrointestinal tract or lung, the prosthetic graft was excised, and the fistulized organ was resected or repaired at the first instance. Details of the fistula repairs are shown in Supplementary Material, Table S2. The median duration from initial graft exposure to the main surgical intervention for thoracic AGI was 13 days (IQR 0–22), excluding the patients with pseudoaneurysm, fistula and endograft infection. In patients with abdominal AGI, repair in a single stage or staged procedures with short intervals were preferred because fluid management is difficult in patients treated with prolonged open abdomens. After first-stage radical debridement and cleansing, the infected graft was removed or preserved with or without omental flap coverage to fill the dead space.
In patients with abdominal graft replacement, expanded polytetrafluoroethylene grafts were used in 16 patients, and rifampicin-soaked Dacron grafts were used in 3 patients. With regard to thoracic graft replacement (n = 14), Dacron graft was used in 12 patients (including 7 rifampicin-soaked grafts) and allografts were used in 2 patients. Regarding final wound closure, vascularized tissue flaps were implanted in 70% of patients (35/50); omental flaps in 33 of 50 patients (66%) and muscular flaps were implanted in 2 patients (the pectoralis major in 1 patient, the rectus abdominis in the other).
These treatment options (i.e. the main surgical interventions) were categorized as follows: (i) combination of prosthetic graft excision and vascularized tissue flap implantation (excision + flap; 25/50, 50%); (ii) prosthetic graft excision alone (excision; 9/50, 18%); (iii) vascularized tissue flap implantation alone (flap; 10/50, 20%); and (iv) debridement and cleansing alone (cleansing; 6/50, 12%). For patients who underwent vascularized tissue flap implantation following prosthetic graft excision, we defined the date of main surgical intervention as the date of graft excision.
Definitions of the end points and statistical analysis
All statistical analyses were performed using JMP Pro 13.0.0 (SAS Institute Inc., Cary, NC, USA). Continuous variables were presented as medians with IQR or means with standard deviation. Categorical variables were presented as counts and percentages. At first, we reviewed total early and late outcomes of AGI. Remission of AGI was defined as stable inflammatory reaction and discharge from hospital. Recurrence of AGI was defined as an elevated inflammatory reaction, which was difficult to explain except as AGI. Kaplan–Meier estimates were used to evaluate late outcomes.
Thereafter, we divided patients into 2 groups depending on the presence or absence of fistulas with the gastrointestinal or respiratory tracts because we needed to resect or repair the fistulized organ in addition to the treatment of the infected aortic graft. Intergroup comparisons were performed using a Mann–Whitney U-test for continuous variables and Fisher’s exact test for categorical variables. Factors associated with in-hospital death were determined using a univariable logistic regression analysis. A multivariable logistic regression analysis was performed using 2 covariates, which were vascularized tissue flap and infected graft excision. To verify the assumption of the logistic regression, the estimated proportion of in-hospital mortality of each treatment option (excision + flap, excison, flap and cleansing) was calculated from the developed logistic regression model and was compared with the observed proportion.
RESULTS
Early and late outcomes
Table 1 shows patient characteristics. We identified microorganisms in 42/50 patients (84%). Blood culture and focal specimen culture was positive in 21/50 patients (42%) and 37/50 patients (74%), respectively. The major causative microorganism was methicillin-resistant Staphylococcus aureus (n = 14, 28%). Three patients suffered from Candida albicans infection. One patient recovered after graft excision with omental flap. The other patient with abdominal AGI treated by cleansing alone died in hospital due to multiple organ failure. The remaining patient with thoracic AGI was treated by omental flap coverage alone and achieved remission. However, she developed a recurrence of the infection 10 months after intervention and died of multiple organ failure. Treatment selection and in-hospital death are shown in Fig. 1. In-hospital mortality rate was 23% (8/35 patients) and 35% (12/34 patients) for patients who underwent vascularized tissue flap and those who underwent graft excision, respectively. Table 2 displays early outcomes. The in-hospital mortality rate was 32% (n = 16). A major cause of in-hospital death was multiple organ failure (n = 13). The median duration of hospital stay and intensive care unit stay after the main surgical intervention was 48 (IQR 38–72) days and 5 (IQR 2–12) days, respectively. The rate of cardiopulmonary bypass usage in surgery for thoracic AGI was 52% (13/25 patients) because the remaining patients did not undergo graft excision. Whereas, 19 of 21 patients (90%) with abdominal graft infection underwent graft excision. In-hospital mortality was not significantly different between thoracic and abdominal AGI, which was found in 10 of 29 patients (34%) and 6 of 21 patients (29%), respectively (P = 0.76).
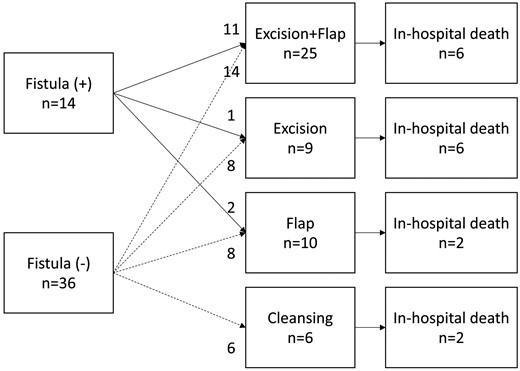
Treatment selection and in-hospital death. Excision + flap: combination of prosthetic graft excision and vascularized tissue flap; excision: prosthetic graft excision alone; flap: vascularized tissue flap alone; cleansing: debridement and cleansing alone.
Total early outcome and inter-group comparison between the patients with fistula and without fistula
| . | Total (n = 50) . | With fistula (n = 14) . | Without fistula (n = 36) . | P-value . |
|---|---|---|---|---|
| Time of main surgical intervention (min) | 468 (IQR: 224–596) | 586 (IQR: 448–703) | 445 (IQR: 186–569) | 0.023* |
| Extracorporeal circulation, n (%) | 14 (28) | 6 (43) | 8 (22) | 0.17 |
| Definitive intervention, n (%) | ||||
| Excision + flap | 25 (50) | 11 (79) | 14 (39) | 0.004* |
| Excision | 9 (18) | 1 (7) | 8 (22) | 0.41 |
| Flap | 10 (20) | 2 (14) | 8 (22) | 0.41 |
| Cleansing | 6 (12) | 0 | 6 (17) | 0.17 |
| Graft used | ||||
| Dacron | 15 (RFN: 10) | 7 (RFN: 5) | 8 (RFN: 5) | |
| ePTFE | 16 | 5 | 11 | |
| Allograft | 2 | 0 | 2 | |
| Mortality, n (%) | ||||
| Operative death | 6 (12) | 3 (21) | 3 (8) | 0.33 |
| In-hospital death | 16 (32) | 6 (43) | 10 (28) | 0.33 |
| Morbidities, n (%) | ||||
| Cerebral infarction | 1 (2) | 0 | 1 (3) | 1.00 |
| CRRT | 5 (10) | 3 (21) | 2 (6) | 0.13 |
| Tracheostomy | 9 (18) | 5 (36) | 4 (11) | 0.09 |
| . | Total (n = 50) . | With fistula (n = 14) . | Without fistula (n = 36) . | P-value . |
|---|---|---|---|---|
| Time of main surgical intervention (min) | 468 (IQR: 224–596) | 586 (IQR: 448–703) | 445 (IQR: 186–569) | 0.023* |
| Extracorporeal circulation, n (%) | 14 (28) | 6 (43) | 8 (22) | 0.17 |
| Definitive intervention, n (%) | ||||
| Excision + flap | 25 (50) | 11 (79) | 14 (39) | 0.004* |
| Excision | 9 (18) | 1 (7) | 8 (22) | 0.41 |
| Flap | 10 (20) | 2 (14) | 8 (22) | 0.41 |
| Cleansing | 6 (12) | 0 | 6 (17) | 0.17 |
| Graft used | ||||
| Dacron | 15 (RFN: 10) | 7 (RFN: 5) | 8 (RFN: 5) | |
| ePTFE | 16 | 5 | 11 | |
| Allograft | 2 | 0 | 2 | |
| Mortality, n (%) | ||||
| Operative death | 6 (12) | 3 (21) | 3 (8) | 0.33 |
| In-hospital death | 16 (32) | 6 (43) | 10 (28) | 0.33 |
| Morbidities, n (%) | ||||
| Cerebral infarction | 1 (2) | 0 | 1 (3) | 1.00 |
| CRRT | 5 (10) | 3 (21) | 2 (6) | 0.13 |
| Tracheostomy | 9 (18) | 5 (36) | 4 (11) | 0.09 |
Cleansing: debridement and cleansing alone; CRRT: continuous renal replacement therapy; ePTFE: expanded polytetrafluoroethylene; excision + flap: combination of prosthetic graft excision and vascularized tissue flap; excision: prosthetic graft excision alone; flap: vascularized tissue flap alone; IQR: interquartile range; RFN: rifampicin-soaked Dacron.
Significant value; P < 0.05.
Total early outcome and inter-group comparison between the patients with fistula and without fistula
| . | Total (n = 50) . | With fistula (n = 14) . | Without fistula (n = 36) . | P-value . |
|---|---|---|---|---|
| Time of main surgical intervention (min) | 468 (IQR: 224–596) | 586 (IQR: 448–703) | 445 (IQR: 186–569) | 0.023* |
| Extracorporeal circulation, n (%) | 14 (28) | 6 (43) | 8 (22) | 0.17 |
| Definitive intervention, n (%) | ||||
| Excision + flap | 25 (50) | 11 (79) | 14 (39) | 0.004* |
| Excision | 9 (18) | 1 (7) | 8 (22) | 0.41 |
| Flap | 10 (20) | 2 (14) | 8 (22) | 0.41 |
| Cleansing | 6 (12) | 0 | 6 (17) | 0.17 |
| Graft used | ||||
| Dacron | 15 (RFN: 10) | 7 (RFN: 5) | 8 (RFN: 5) | |
| ePTFE | 16 | 5 | 11 | |
| Allograft | 2 | 0 | 2 | |
| Mortality, n (%) | ||||
| Operative death | 6 (12) | 3 (21) | 3 (8) | 0.33 |
| In-hospital death | 16 (32) | 6 (43) | 10 (28) | 0.33 |
| Morbidities, n (%) | ||||
| Cerebral infarction | 1 (2) | 0 | 1 (3) | 1.00 |
| CRRT | 5 (10) | 3 (21) | 2 (6) | 0.13 |
| Tracheostomy | 9 (18) | 5 (36) | 4 (11) | 0.09 |
| . | Total (n = 50) . | With fistula (n = 14) . | Without fistula (n = 36) . | P-value . |
|---|---|---|---|---|
| Time of main surgical intervention (min) | 468 (IQR: 224–596) | 586 (IQR: 448–703) | 445 (IQR: 186–569) | 0.023* |
| Extracorporeal circulation, n (%) | 14 (28) | 6 (43) | 8 (22) | 0.17 |
| Definitive intervention, n (%) | ||||
| Excision + flap | 25 (50) | 11 (79) | 14 (39) | 0.004* |
| Excision | 9 (18) | 1 (7) | 8 (22) | 0.41 |
| Flap | 10 (20) | 2 (14) | 8 (22) | 0.41 |
| Cleansing | 6 (12) | 0 | 6 (17) | 0.17 |
| Graft used | ||||
| Dacron | 15 (RFN: 10) | 7 (RFN: 5) | 8 (RFN: 5) | |
| ePTFE | 16 | 5 | 11 | |
| Allograft | 2 | 0 | 2 | |
| Mortality, n (%) | ||||
| Operative death | 6 (12) | 3 (21) | 3 (8) | 0.33 |
| In-hospital death | 16 (32) | 6 (43) | 10 (28) | 0.33 |
| Morbidities, n (%) | ||||
| Cerebral infarction | 1 (2) | 0 | 1 (3) | 1.00 |
| CRRT | 5 (10) | 3 (21) | 2 (6) | 0.13 |
| Tracheostomy | 9 (18) | 5 (36) | 4 (11) | 0.09 |
Cleansing: debridement and cleansing alone; CRRT: continuous renal replacement therapy; ePTFE: expanded polytetrafluoroethylene; excision + flap: combination of prosthetic graft excision and vascularized tissue flap; excision: prosthetic graft excision alone; flap: vascularized tissue flap alone; IQR: interquartile range; RFN: rifampicin-soaked Dacron.
Significant value; P < 0.05.
Overall survival rates at 1 and 5 years were 66% and 46%, respectively. Among the patients in remission from AGI (n = 34), rates of freedom from AGI recurrence at 1 and 5 years were 88% and 81%, respectively.
Comparison of the patients with and without fistula
Fistulized organs were the duodenum (10/14), oesophagus (3/14) and lung (1/14). The mean age of the patients with fistulas (75 ± 9 years) was higher than that of the patients without fistulas (64 ± 18 years, P = 0.031). Other characteristics were similar between the groups (Table 1). The rate of positive blood culture was not significantly different between the groups: 29% (4/14) for the patients with fistula and 47% (17/36) for the patients without fistula (P = 0.34). The mean operation time of the main surgical intervention for the patients with fistula was significantly longer than that of the patients without fistula (P = 0.023). In-hospital mortality rates in the patients with fistula and without fistula were 43% (6/14) and 28% (10/36), respectively (P = 0.33).
With regard to the patients with a fistula (Fig. 2A), 12 patients underwent graft excision, resulting in 5 in-hospital deaths (42%). Similarly, 5 of 13 patients who underwent a vascularized tissue flap (38%) died in-hospital. Aorto-oesophageal fistula occurred in 3 cases, which resulted in 1 in-hospital death. On the other hand, 4 of 10 patients with aorto-duodenal fistula died in-hospital. Regarding the patients without fistula (Fig. 2B), the in-hospital mortality rate of the patients with vascularized tissue flap (3/21, 14%) was significantly lower than that of the patients without vascularized tissue flap (7/14, 50%, P = 0.026). However, the in-hospital mortality rates in those with and without graft excision were similar (P = 0.71). The mid-term outcome of each group was described with a Kaplan–Meier curve (Fig. 3). The overall survival rate of the patients with fistula at 1 and 5 years was 56% and 38%, respectively. The overall survival rate of the patients without fistula at 1 and 5 years was 69% and 48%, respectively. The freedom rate from recurrence of the patients with fistula and without fistula at 5 years was 73% and 80%, respectively.
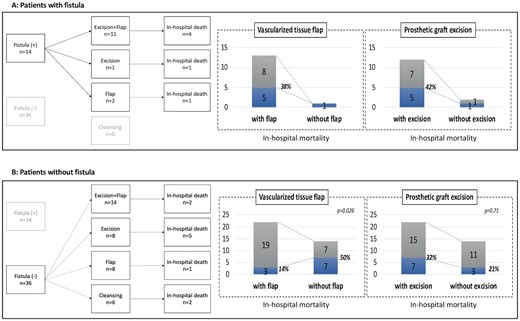
Treatment selection and in-hospital death. (A) Patients with fistula. (B) Patients without fistula. Excision + flap: combination of prosthetic graft excision and vascularized tissue flap; excision: prosthetic graft excision alone; flap: vascularized tissue flap alone; cleansing: debridement and cleansing alone.
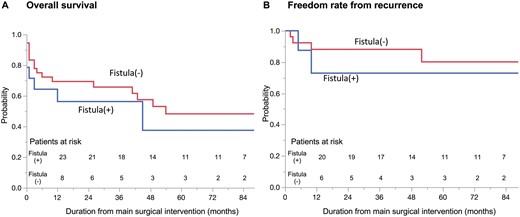
Kaplan–Meier survival curves for patients with and without fistula. (A) Overall survival. (B) Rate of freedom from aortic graft infection recurrence.
Assessment of the factors associated with in-hospital death
According to the univariable logistic regression analysis, vascularized tissue flap was a significant factor to improve in-hospital mortality, of which odds ratio (OR) and 95% confidence interval (CI) were 0.26 and 0.07–0.94 (P = 0.040) (Table 3). Significant risk factors associated with in-hospital death were age >75 years, positive blood culture, and operation time >10 h. On the other hand, endograft infection, fistula and infected graft excision were not significant factors. According to the multivariable logistic regression analysis, vascularized tissue flap was an independent factor improving in-hospital mortality (OR; 0.20, CI; 0.05–0.81, P = 0.024). The proportions estimated from the logistic regression model were close to the observed ones (Supplementary Material, Table S1).
| . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Male | 2.15 (0.40–11.56) | 0.37 | ||
| Age >75 years | 4.00 (1.14–14.00) | 0.030* | ||
| Diabetes mellitus | 0.83 (0.14–4.65) | 0.83 | ||
| Chronic kidney disease | 1.09 (0.30–3.96) | 0.89 | ||
| Cerebral vascular disease | 1.56 (0.37–6.53) | 0.55 | ||
| Chronic obstructive pulmonary disease | 0.80 (0.14–4.65) | 0.80 | ||
| Chronic heart failure | 1.07 (0.17–6.56) | 0.94 | ||
| Coronary artery disease | 1.08 (0.27–4.31) | 0.91 | ||
| Thoracic aortic infection | 1.32 (0.39–4.45) | 0.66 | ||
| Dissection | 2.08 (0.62–6.93) | 0.23 | ||
| Fistula | 1.95 (0.54–7.05) | 0.31 | ||
| Positive blood culture | 5.28 (1.45–19.16) | 0.011* | ||
| Positive local specimen culture | 1.08 (0.28–4.23) | 0.91 | ||
| MRSA | 0.70 (0.18–2.66) | 0.60 | ||
| Endograft infection | 1.22 (0.21–7.12) | 0.64 | ||
| Operation time >10 h | 4.51 (1.15–17.75) | 0.031* | ||
| Extracorporeal circulation | 3.00 (0.82–10.91) | 0.10 | ||
| Vascularized tissue flap | 0.26 (0.07–0.94) | 0.040* | 0.20 (0.05–0.81) | 0.024* |
| Infected graft excision | 1.64 (0.16–2.32) | 0.47 | 3.46 (0.70–17.07) | 0.13 |
| . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Male | 2.15 (0.40–11.56) | 0.37 | ||
| Age >75 years | 4.00 (1.14–14.00) | 0.030* | ||
| Diabetes mellitus | 0.83 (0.14–4.65) | 0.83 | ||
| Chronic kidney disease | 1.09 (0.30–3.96) | 0.89 | ||
| Cerebral vascular disease | 1.56 (0.37–6.53) | 0.55 | ||
| Chronic obstructive pulmonary disease | 0.80 (0.14–4.65) | 0.80 | ||
| Chronic heart failure | 1.07 (0.17–6.56) | 0.94 | ||
| Coronary artery disease | 1.08 (0.27–4.31) | 0.91 | ||
| Thoracic aortic infection | 1.32 (0.39–4.45) | 0.66 | ||
| Dissection | 2.08 (0.62–6.93) | 0.23 | ||
| Fistula | 1.95 (0.54–7.05) | 0.31 | ||
| Positive blood culture | 5.28 (1.45–19.16) | 0.011* | ||
| Positive local specimen culture | 1.08 (0.28–4.23) | 0.91 | ||
| MRSA | 0.70 (0.18–2.66) | 0.60 | ||
| Endograft infection | 1.22 (0.21–7.12) | 0.64 | ||
| Operation time >10 h | 4.51 (1.15–17.75) | 0.031* | ||
| Extracorporeal circulation | 3.00 (0.82–10.91) | 0.10 | ||
| Vascularized tissue flap | 0.26 (0.07–0.94) | 0.040* | 0.20 (0.05–0.81) | 0.024* |
| Infected graft excision | 1.64 (0.16–2.32) | 0.47 | 3.46 (0.70–17.07) | 0.13 |
CI: confidence interval; MRSA: methicillin-resistant Staphylococcus aureus; OR: odds ratio.
Significant value; P < 0.05.
| . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Male | 2.15 (0.40–11.56) | 0.37 | ||
| Age >75 years | 4.00 (1.14–14.00) | 0.030* | ||
| Diabetes mellitus | 0.83 (0.14–4.65) | 0.83 | ||
| Chronic kidney disease | 1.09 (0.30–3.96) | 0.89 | ||
| Cerebral vascular disease | 1.56 (0.37–6.53) | 0.55 | ||
| Chronic obstructive pulmonary disease | 0.80 (0.14–4.65) | 0.80 | ||
| Chronic heart failure | 1.07 (0.17–6.56) | 0.94 | ||
| Coronary artery disease | 1.08 (0.27–4.31) | 0.91 | ||
| Thoracic aortic infection | 1.32 (0.39–4.45) | 0.66 | ||
| Dissection | 2.08 (0.62–6.93) | 0.23 | ||
| Fistula | 1.95 (0.54–7.05) | 0.31 | ||
| Positive blood culture | 5.28 (1.45–19.16) | 0.011* | ||
| Positive local specimen culture | 1.08 (0.28–4.23) | 0.91 | ||
| MRSA | 0.70 (0.18–2.66) | 0.60 | ||
| Endograft infection | 1.22 (0.21–7.12) | 0.64 | ||
| Operation time >10 h | 4.51 (1.15–17.75) | 0.031* | ||
| Extracorporeal circulation | 3.00 (0.82–10.91) | 0.10 | ||
| Vascularized tissue flap | 0.26 (0.07–0.94) | 0.040* | 0.20 (0.05–0.81) | 0.024* |
| Infected graft excision | 1.64 (0.16–2.32) | 0.47 | 3.46 (0.70–17.07) | 0.13 |
| . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Male | 2.15 (0.40–11.56) | 0.37 | ||
| Age >75 years | 4.00 (1.14–14.00) | 0.030* | ||
| Diabetes mellitus | 0.83 (0.14–4.65) | 0.83 | ||
| Chronic kidney disease | 1.09 (0.30–3.96) | 0.89 | ||
| Cerebral vascular disease | 1.56 (0.37–6.53) | 0.55 | ||
| Chronic obstructive pulmonary disease | 0.80 (0.14–4.65) | 0.80 | ||
| Chronic heart failure | 1.07 (0.17–6.56) | 0.94 | ||
| Coronary artery disease | 1.08 (0.27–4.31) | 0.91 | ||
| Thoracic aortic infection | 1.32 (0.39–4.45) | 0.66 | ||
| Dissection | 2.08 (0.62–6.93) | 0.23 | ||
| Fistula | 1.95 (0.54–7.05) | 0.31 | ||
| Positive blood culture | 5.28 (1.45–19.16) | 0.011* | ||
| Positive local specimen culture | 1.08 (0.28–4.23) | 0.91 | ||
| MRSA | 0.70 (0.18–2.66) | 0.60 | ||
| Endograft infection | 1.22 (0.21–7.12) | 0.64 | ||
| Operation time >10 h | 4.51 (1.15–17.75) | 0.031* | ||
| Extracorporeal circulation | 3.00 (0.82–10.91) | 0.10 | ||
| Vascularized tissue flap | 0.26 (0.07–0.94) | 0.040* | 0.20 (0.05–0.81) | 0.024* |
| Infected graft excision | 1.64 (0.16–2.32) | 0.47 | 3.46 (0.70–17.07) | 0.13 |
CI: confidence interval; MRSA: methicillin-resistant Staphylococcus aureus; OR: odds ratio.
Significant value; P < 0.05.
DISCUSSION
Extensive debridement of infective tissue and excision of the infected graft are the classical principles of AGI treatment. However, radical graft excision is sometimes highly invasive and requires extensive re-replacement of the aortic graft in the context of sepsis. The indispensability of infected graft excision is still debated [11–13]. In a meta-analysis by Kahlberg et al. [14] involving thoracic and thoraco-abdominal AGI, graft excision showed a favourable result in terms of 1-year mortality (OR = 0.3, P = 0.06). Okita et al. [15] recommended aggressive surgical treatment of thoracic AGI, including radical infected tissue debridement and in situ graft replacement with vascularized tissue flap; the reported in-hospital mortality was 19% (5/26). These studies included patients with aorto-oesophageal and aorto-bronchial fistulas. We reported similar outcomes in the present study (30-day death: 12%, in-hospital death: 32%, 5-year survival: 46%). Although AGI was still associated with significant mortality, the rate of recurrent infection was around 20% at 5 years once remission was achieved.
Our current treatment strategy for thoracic AGI involves a staged approach. The first step is repetitive debridement and cleansing around the infected graft. For easier wound management, we use negative pressure wound therapy, which enables continuous irrigation and evacuation of excessive exudate. Second, we perform a definitive procedure, including in situ graft replacement and/or vascularized tissue flap. In cases of pseudoaneurysm and fistula formation, we replace the infected graft or repair the fistulized organs during the first stage, followed by covering the graft with a vascularized tissue flap. Recently, use of a bridging endograft has become an option for stabilizing a patient’s condition in emergency cases, such as active bleeding and pseudoaneurysm [16].
While preserving a graft, a major concern is the regrowth of microorganisms. Nakajima et al. [17] reported the ‘in situ preservation’ method for thoracic AGI treatment, which was originally used by us. Infection was controlled in all 6 patients using a two-step graft salvaging approach. First, the infected graft was exposed, and debridement and cleansing were performed. In the second stage, the prosthetic graft was wrapped with omentum, and the wound was closed. According to a review article on ascending/aortic arch prosthetic graft infections, the early survival rate of the prosthetic graft preserving strategy was 95% in 77 cases [13]. However, this excellent outcome was the sum of successful small cases. Furthermore, these reports did not include fistula cases.
Vascularized tissue flaps fill the space lost due to infection and debridement. It also provides rich blood flow and brings antibiotics to the area around the infected graft. Furthermore, the omentum generates vascular endothelial growth factor, which supports neovascularization and granulation. In addition, a recent study revealed that omental adipocytes produce various proinflammatory cytokines and antibacterial peptides, which play the role of primary defense against bacterial infection [18, 19]. However, only a few reports included statistical analysis regarding perioperative factors affecting survival rate [20, 21]. In a multicentre retrospective study, Oda et al. [21] reviewed 68 cases of thoracic AGI treated by median sternotomies. The overall in-hospital mortality rate was 35%, and multivariable analysis showed that the use of a flap was effective in controlling thoracic AGI (hazard ratio: 0.24, P = 0.001). In the present study, vascularized tissue flap was also a significant factor for reducing in-hospital death (OR; 0.09, CI; 0.01–0.68, P = 0.02), although our cohort included not only mediastinal AGI cases but also cases with AGI after thoracotomy and abdominal AGI. However, additional analysis of freedom rate from recurrence showed no significant difference between the patients with tissue flap and without tissue flap (log-rank P = 0.62) (Supplementary Material, Fig. S1). Further analysis is essential to identify the optimal treatment option for AGI.
Our subgroup analysis of patients without fistula revealed relatively lower in-hospital mortality rate with the use of vascularized tissue flaps, and infected graft excision did not influence in-hospital mortality. This suggests that, in fistula-free conditions, in situ graft preservation with vascularized tissue flap could be a useful option to control AGI. Although we aggressively performed resection or repair of the fistulized organ and in situ graft replacement with vascularized tissue flap, the in-hospital mortality rate was considerable. Radical surgery for the AGI with fistula required long, complicated procedures, which probably exhausted elderly ill patients. Using the same aggressive strategy, some authors reported better outcomes with regard to aorto-oesophageal fistula, of which in-hospital mortality was 22–25% [22, 23].
We did not assess the type of graft material used and its impact on AGI in this study. Commonly, biological material such as autologous veins, homografts and bovine pericardial tubes show a lower incidence of re-infection than prosthetic grafts do [16]. Autologous vein grafts demonstrate the lowest re-infection rate. When a patient’s condition is stable and there is no anatomical complexity, an autologous vein graft—using, for example a superficial femoral vein—might be an ideal solution. However, the harvesting of autologous vein requires additional surgical intervention, which prolongs the duration of the operation. Although allografts show low re-infection rates, the availability of material of suitable length and calibre is limited, and there is no commercially available allograft material in Japan. In addition, long-term imaging follow-up of allografts would be essential due to the possibility of graft degeneration. Autologous vein grafts and allografts are not suitable for emergency situations. Bovine pericardium tubes are another biological option for grafts. Although clinical data are limited, a low re-infection rate was reported with this material [24]. The advantage of using bovine pericardium tube material for grafts is that it can be sourced easily and tailored to the anatomy of individual patients.
Limitations
Our study was retrospective in nature and consisted of a heterogenous small population. Conservatively treated patients could not be included. In addition, the regimen of antibiotics was unclear. Also, other potential factors affecting survival rate include the choice of graft material. These could be intrinsic biases. However, AGI is a rare and heterogeneous condition, making it difficult to collect a large number of patients. Therefore, our study focusing on various treatment options for AGI included a relatively larger number of patients and had better statistical power compared to those of previously published reports.
CONCLUSION
AGI is associated with considerable in-hospital mortality. Long-term infection control was feasible among the patients who achieved AGI remission. The use of vascularized tissue flaps is probably integral to improving the survival rate of AGI treatment. Graft preservation with vascularized tissue flaps could be a useful option for AGI without fistula.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Conflict of interest: none declared.
Presented at the 34th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 8–10 October 2020.
Author contributions
Takayuki Shijo: Data curation; Investigation; Methodology; Visualization; Writing—original draft. Hitoshi Matsuda: Conceptualization; Supervision; Writing—review & editing. Koki Yokawa: Data curation. Yosuke Inoue: Data curation. Yoshimasa Seike: Data curation. Kyokun Uehara: Data curation. Mitsuyoshi Takahara: Formal analysis; Methodology. Hiroaki Sasaki: Data curation; Supervision.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Maximilian Kreibich, Yutaka Okita, Jürg Schmidli and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.





