-
PDF
- Split View
-
Views
-
Cite
Cite
Mikael Kastengren, Peter Svenarud, Göran Källner, Anders Franco-Cereceda, Jan Liska, Isak Gran, Magnus Dalén, Minimally invasive versus sternotomy mitral valve surgery when initiating a minimally invasive programme, European Journal of Cardio-Thoracic Surgery, Volume 58, Issue 6, December 2020, Pages 1168–1174, https://doi.org/10.1093/ejcts/ezaa232
Close - Share Icon Share
Abstract
An increasing number of mitral valve operations are performed using minimally invasive procedures. The initiation of a minimally invasive mitral valve surgery programme constitutes a unique opportunity to study outcome differences in patients with similar characteristics operated on through a sternotomy versus a minimally invasive procedure. The goal of this study was to compare short-term outcomes of patients undergoing mitral valve surgery before versus those having surgery after the introduction of a minimally invasive programme.
The single-centre study included mitral valve procedures performed through a sternotomy or with a minimally invasive approach between January 2012 and May 2019. Propensity score matching was performed to reduce selection bias.
A total of 605 patients (294 sternotomy, 311 minimally invasive) who underwent mitral valve surgery were included in the analysis. Propensity score matching resulted in 251 matched pairs. In the propensity score-matched analysis, minimally invasive procedures had longer extracorporeal circulation duration (149 ± 52 vs 133 ± 57 min; P = 0.001) but shorter aortic occlusion duration (97 ± 36 vs 105 ± 40 min, P = 0.03). Minimally invasive procedures were associated with a lower incidence of reoperation for bleeding (2.4% vs 7.2%; P = 0.012), lower need for transfusion (19.1% vs 30.7%; P = 0.003) and shorter in-hospital stay (5.0 ± 2.7 vs 7.2 ± 4.6 days; P < 0.001). The 30-day mortality was low in both groups (0.4% vs 0.8%; P = 0.56).
Minimally invasive mitral valve surgery was associated with short-term outcomes comparable to those with procedures performed through a sternotomy. Initiating a minimally invasive mitral valve programme with a limited number of surgeons and a well-executed institutional selection strategy did not confer an increased risk for adverse events.
INTRODUCTION
Mitral valve surgery is the fastest growing category of cardiac surgery in the USA [1]. It is prognosed to continue increasing in accordance with guidelines that recommend early referral of asymptomatic patients with mitral regurgitation [2]. An increasing proportion of patients with mitral valve disease undergo minimally invasive mitral valve surgery (MIMVS) instead of mitral valve surgery through a sternotomy [3]. In Germany, MIMVS increased from 13.1% in 2004 to 50.2% in 2017 [4].
In several previous studies, MIMVS has been associated with reduced postoperative bleeding, decreased postoperative pain, shorter length of stay, lower incidence of deep wound infection and improved cosmesis, compared to sternotomy [5–10]. However, some are concerned that limited surgical access might prolong procedure times and negatively affect the long-term durability of mitral valve repairs [11]. Furthermore, MIMVS has been associated with a significant learning curve that has led to the suggestion that the initiation of an MIMVS programme could result in an increased short-term risk for procedure-related complications [12, 13].
Patients undergoing minimally invasive versus sternotomy mitral valve surgery in existing comparative observational studies often have widely different preoperative characteristics due to selection bias, whereas patients with more comorbidity tend to undergo mitral valve surgery through a sternotomy [3, 8, 14]. The initiation of an MIMVS programme (where a minimally invasive approach is chosen primarily for all patients undergoing mitral valve surgery) constitutes a unique possibility to study outcome differences in patients with similar characteristics operated on through a sternotomy versus a minimally invasive approach.
The aim of this study was to compare short-term outcomes between patients undergoing mitral valve surgery before versus after the introduction of a minimally invasive programme.
METHODS
This single-centre study included patients undergoing mitral valve procedures between January 2012 and May 2019 at the Karolinska University Hospital, Stockholm, Sweden. MIMVS had been performed in a few selected patients since 2013, but in February 2016 a minimally invasive mitral valve programme was introduced whereby a minimally invasive approach was chosen as the primary treatment for all patients undergoing mitral valve surgery (with or without a concomitant procedure possible to perform via a right-sided minithoracotomy, i.e. tricuspid valve surgery, patent foramen ovale closure or ablation). Patients with mitral regurgitation as the primary indication for surgery included those with various types of mitral valve diseases such as isolated posterior or anterior mitral leaflet prolapse, bileaflet mitral valve prolapse and Barlow’s mitral valve disease.
All procedures that were performed or could potentially have been performed via a minimally invasive approach were included. Therefore, we excluded concomitant surgery not performed via a right-sided minithoracotomy at our centre, such as coronary artery bypass grafting, aortic valve procedures and severe mitral annular calcification that could require patch reconstruction of the posterior annulus. Also, in order to achieve a homogeneous study population, patients who had previously had cardiac surgery, had active endocarditis or were in a critical preoperative state were excluded from the analysis.
Demographic, preoperative, intraoperative and postoperative data were collected prospectively and retrospectively. Patients underwent postoperative transthoracic echocardiography 2–4 days after surgery and a surgeon outpatient visit 4–6 weeks after surgery.
Ethics statement
The study was approved by the regional human research ethics committee, Stockholm, Sweden (registration no. 2017/934-31 and 2018/909-32, date of approval 31 May 2017 and 2 May 2018, respectively). Informed consent was waived.
Outcomes
Early postoperative outcomes included reoperation for bleeding, stroke, perioperative myocardial infarction, new-onset dialysis, invasive ventilation >48 h, pacemaker implantation, new-onset atrial fibrillation, red blood cell transfusion, antibiotic treatment for chest wound infection or pneumonia, postoperative mitral regurgitation >grade I/IV, reoperation during hospital stay for mitral regurgitation, intensive care unit stay, in-hospital stay and 30-day mortality.
Surgical technique
MIMVS was performed through a right minithoracotomy with the patient in a supine position with the right hemithorax elevated. A 4- to 7-cm skin incision was placed at the 4th intercostal space. Four skin incisions of ∼0.5–1 cm were placed next to the thoracotomy, thereby allowing for a camera with CO2 supply, cross‐clamp, drainage and atrial roof retractor. Cannulation for extracorporeal circulation was performed peripherally in the right femoral vein and artery either percutaneously or under direct vision through a 3-cm vertical skin incision. Transoesophageal echocardiography was used to confirm the location of the venous cannulation of the right atrium with the tip in the superior vena cava. The pericardium was incised anterior to the phrenic nerve. The ascending aorta was occluded using a transthoracic cross-clamp, and cardioplegia was administered directly into the aortic root.
The left atrium was incised anteriorly to the right pulmonary veins for access to the mitral valve.
The median sternotomy mitral valve procedure was performed through a vertical midline skin incision from the suprasternal notch to the tip of the xiphoid, and the full length of the sternum was cut. The pericardium was fully opened in the midline. Cannulation to extracorporeal circulation was performed through central venous and arterial cannulation. The ascending aorta was occluded using a cross-clamp; blood cardioplegia was administered into the aortic root. The heart was rotated, and the left atrium was incised anteriorly to the right pulmonary veins for access to the mitral valve.
Statistical analyses
Variables are described using frequencies and percentages for categorical variables. Variables are described using means and standard deviations for continuous variables or medians and interquartile range for variables with skewed distributions. In the overall cohort, outcomes were compared by the independent samples t-test and the χ2 test for binary and categorical variables, and analysis of variance for continuous variables. The analysis was performed on an intention-to-treat basis, meaning that patients who underwent conversion from a minimally invasive approach to sternotomy were analysed in the minimally invasive cohort. To reduce selection bias, a propensity score was calculated with the minimally invasive approach/sternotomy as the dependent variable. In the propensity score-matched cohort, outcomes were compared by univariate conditional logistic regression for binary and categorical variables and by a paired samples t-test for continuous variables. The propensity score-matched cohort was constructed by nearest neighbour matching without replacement. The following variables were included as covariates: age, gender, left ventricular ejection fraction, European System for Cardiac Operative Risk Evaluation (EuroSCORE) II and indication for surgery (mitral regurgitation or stenosis). We calculated standardized differences for variables to investigate post-match balance. A standardized difference <0.1 was considered to indicate adequate balance between variables of the intervention cohorts. A two‐sided P-value of <0.05 was considered to indicate statistical significance. Analyses were performed using Stata v.15.1 statistical software (StataCorp LP, College Station, TX, USA).
RESULTS
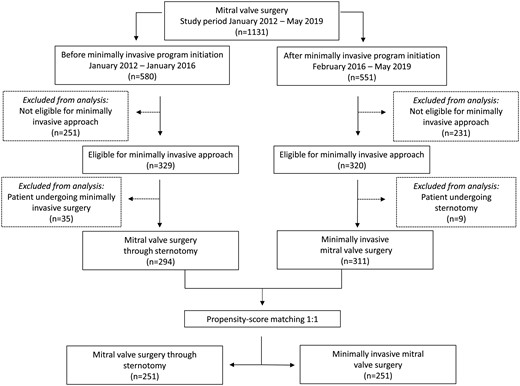
A total of 1131 consecutive mitral valve procedures were performed during the study period. The study flow chart is presented in Fig. 1; the reasons for exclusion from analysis due to ineligibility for MIMVS are given in Supplementary Material, Table S1 and the distribution of MIMVS versus mitral valve surgery through sternotomy at our clinic from January 2012 to May 2019 is given in Supplementary Material, Fig. S1. A total of 482 patients were excluded from analysis because of contraindications for MIMVS: of these, 251 patients were in the sternotomy group before the initiation of the MIMVS programme and 231 patients were in the MIMVS group after the initiation of the programme. In the sternotomy group, 329 patients were eligible for minimally invasive surgery and 320 patients were eligible for minimally invasive surgery in the MIMVS group. Of these, 35 patients underwent minimally invasive surgery before the initiation of the programme and 9 patients underwent full sternotomy after the initiation of the programme and were excluded, leaving a total cohort for analysis of 605 patients: 294 patients in the sternotomy group and 311 patients in the MIMVS group. Three surgeons performed the minimally invasive procedures and 8 surgeons performed the sternotomy mitral valve procedures. The mean age of the patients was 62.7 ± 12.0 years; 28.4% were female; the mean EuroSCORE II was 2.51 ± 2.51. In the unmatched analysis, left ventricular function, gender, atrial fibrillation, elective surgery and EuroSCORE II differed slightly, but the remaining preoperative characteristics were similar between the groups. Propensity score matching resulted in 251 matched pairs with well-balanced baseline characteristics. Patient characteristics are listed in Table 1.
| . | Overall cohort . | Propensity score-matched cohort . | ||||
|---|---|---|---|---|---|---|
| . | Sternotomy (n = 294) . | Minimally invasive procedure (n = 311) . | Standardized difference . | Sternotomy (n = 251) . | Minimally invasive procedure (n = 251) . | Standardized difference . |
| Age (years), mean ± SD | 63.1 ± 12.2 | 62.3 ± 11.8 | 0.0674 | 62.4 ± 12.6 | 61.1 ± 12.4 | 0.1050 |
| Female gender, n (%) | 96 (32.7) | 76 (24.4) | 0.1824 | 70 (27.9) | 72 (28.7) | −0.0177 |
| Height (cm), mean ± SD | 174 ± 10 | 176 ± 10 | −0.2215 | 175 ± 10 | 176 ± 10 | −0.0926 |
| Weight (kg), mean ± SD | 77 ± 15 | 78 ± 14 | −0.0385 | 78 ± 15 | 77 ± 15 | 0.1075 |
| Creatinine preoperatively (µmol/l), mean ± SD | 87 ± 22 | 87 ± 18 | −0.0081 | 86 ± 21 | 87 ± 19 | −0.0523 |
| Hypertension, n (%) | 111 (37.8) | 117 (37.6) | 0.0028 | 93 (37.1) | 91 (36.3) | 0.0165 |
| Prior stroke, n (%) | 16 (5.4) | 24 (7.7) | −0.0917 | 11 (4.4) | 16 (6.4) | −0.0882 |
| Extracardiac arteriopathy, n (%) | 6 (2.0) | 2 (0.6) | 0.1215 | 4 (1.6) | 2 (0.8) | 0.0732 |
| Diabetes mellitus, n (%) | 17 (5.8) | 12 (3.9) | 0.0898 | 16 (6.4) | 8 (3.2) | 0.1495 |
| Chronic lung disease, n (%) | 23 (7.8) | 16 (5.1) | 0.1088 | 17 (6.8) | 13 (5.2) | 0.0671 |
| Recent myocardial infarction, n (%) | 1 (0.3) | 1 (0.3) | 0.0032 | 1 (0.4) | 1 (0.4) | 0 |
| Atrial fibrillation, n (%) | 0.1849 | 0.1186 | ||||
| No | 163 (55.4) | 200 (64.3) | 143 (57.0) | 157 (62.5) | ||
| Paroxysmal | 59 (20.1) | 53 (17.0) | 50 (19.9) | 46 (18.3) | ||
| Non-paroxysmal | 72 (24.5) | 58 (18.6) | 58 (23.1) | 48 (19.1) | ||
| Prior percutaneous coronary intervention, n (%) | 10 (3.4) | 10 (3.2) | 0.0104 | 8 (3.2) | 9 (3.6) | −0.0220 |
| Left ventricular ejection fraction, n (%) | 0.3476 | 0.0822 | ||||
| >50% | 157 (53.4) | 217 (69.8) | 152 (60.6) | 157 (62.5) | ||
| 30–50% | 126 (42.9) | 89 (28.6) | 96 (38.2) | 89 (35.5) | ||
| <30% | 11 (3.7) | 5 (1.6) | 3 (1.2) | 5 (2.0) | ||
| Elective surgery, n (%) | 266 (90.5) | 297 (95.5) | −0.1973 | 232 (92.4) | 237 (94.4) | −0.0803 |
| EuroSCORE II, mean ± SD | 2.92 ± 2.83 | 2.13 ± 2.09 | 0.3144 | 2.39 ± 2.30 | 2.37 ± 2.25 | 0.0093 |
| Primary indication for surgery, n (%) | 0.0607 | −0.0827 | ||||
| Mitral regurgitation | 275 (93.5) | 286 (92.0) | 233 (92.8) | 238 (94.8) | ||
| Mitral stenosis | 19 (6.5) | 25 (8) | 18 (7.2) | 13 (5.2) | ||
| . | Overall cohort . | Propensity score-matched cohort . | ||||
|---|---|---|---|---|---|---|
| . | Sternotomy (n = 294) . | Minimally invasive procedure (n = 311) . | Standardized difference . | Sternotomy (n = 251) . | Minimally invasive procedure (n = 251) . | Standardized difference . |
| Age (years), mean ± SD | 63.1 ± 12.2 | 62.3 ± 11.8 | 0.0674 | 62.4 ± 12.6 | 61.1 ± 12.4 | 0.1050 |
| Female gender, n (%) | 96 (32.7) | 76 (24.4) | 0.1824 | 70 (27.9) | 72 (28.7) | −0.0177 |
| Height (cm), mean ± SD | 174 ± 10 | 176 ± 10 | −0.2215 | 175 ± 10 | 176 ± 10 | −0.0926 |
| Weight (kg), mean ± SD | 77 ± 15 | 78 ± 14 | −0.0385 | 78 ± 15 | 77 ± 15 | 0.1075 |
| Creatinine preoperatively (µmol/l), mean ± SD | 87 ± 22 | 87 ± 18 | −0.0081 | 86 ± 21 | 87 ± 19 | −0.0523 |
| Hypertension, n (%) | 111 (37.8) | 117 (37.6) | 0.0028 | 93 (37.1) | 91 (36.3) | 0.0165 |
| Prior stroke, n (%) | 16 (5.4) | 24 (7.7) | −0.0917 | 11 (4.4) | 16 (6.4) | −0.0882 |
| Extracardiac arteriopathy, n (%) | 6 (2.0) | 2 (0.6) | 0.1215 | 4 (1.6) | 2 (0.8) | 0.0732 |
| Diabetes mellitus, n (%) | 17 (5.8) | 12 (3.9) | 0.0898 | 16 (6.4) | 8 (3.2) | 0.1495 |
| Chronic lung disease, n (%) | 23 (7.8) | 16 (5.1) | 0.1088 | 17 (6.8) | 13 (5.2) | 0.0671 |
| Recent myocardial infarction, n (%) | 1 (0.3) | 1 (0.3) | 0.0032 | 1 (0.4) | 1 (0.4) | 0 |
| Atrial fibrillation, n (%) | 0.1849 | 0.1186 | ||||
| No | 163 (55.4) | 200 (64.3) | 143 (57.0) | 157 (62.5) | ||
| Paroxysmal | 59 (20.1) | 53 (17.0) | 50 (19.9) | 46 (18.3) | ||
| Non-paroxysmal | 72 (24.5) | 58 (18.6) | 58 (23.1) | 48 (19.1) | ||
| Prior percutaneous coronary intervention, n (%) | 10 (3.4) | 10 (3.2) | 0.0104 | 8 (3.2) | 9 (3.6) | −0.0220 |
| Left ventricular ejection fraction, n (%) | 0.3476 | 0.0822 | ||||
| >50% | 157 (53.4) | 217 (69.8) | 152 (60.6) | 157 (62.5) | ||
| 30–50% | 126 (42.9) | 89 (28.6) | 96 (38.2) | 89 (35.5) | ||
| <30% | 11 (3.7) | 5 (1.6) | 3 (1.2) | 5 (2.0) | ||
| Elective surgery, n (%) | 266 (90.5) | 297 (95.5) | −0.1973 | 232 (92.4) | 237 (94.4) | −0.0803 |
| EuroSCORE II, mean ± SD | 2.92 ± 2.83 | 2.13 ± 2.09 | 0.3144 | 2.39 ± 2.30 | 2.37 ± 2.25 | 0.0093 |
| Primary indication for surgery, n (%) | 0.0607 | −0.0827 | ||||
| Mitral regurgitation | 275 (93.5) | 286 (92.0) | 233 (92.8) | 238 (94.8) | ||
| Mitral stenosis | 19 (6.5) | 25 (8) | 18 (7.2) | 13 (5.2) | ||
EuroSCORE: European System for Cardiac Operative Risk Evaluation; SD: standard deviation.
| . | Overall cohort . | Propensity score-matched cohort . | ||||
|---|---|---|---|---|---|---|
| . | Sternotomy (n = 294) . | Minimally invasive procedure (n = 311) . | Standardized difference . | Sternotomy (n = 251) . | Minimally invasive procedure (n = 251) . | Standardized difference . |
| Age (years), mean ± SD | 63.1 ± 12.2 | 62.3 ± 11.8 | 0.0674 | 62.4 ± 12.6 | 61.1 ± 12.4 | 0.1050 |
| Female gender, n (%) | 96 (32.7) | 76 (24.4) | 0.1824 | 70 (27.9) | 72 (28.7) | −0.0177 |
| Height (cm), mean ± SD | 174 ± 10 | 176 ± 10 | −0.2215 | 175 ± 10 | 176 ± 10 | −0.0926 |
| Weight (kg), mean ± SD | 77 ± 15 | 78 ± 14 | −0.0385 | 78 ± 15 | 77 ± 15 | 0.1075 |
| Creatinine preoperatively (µmol/l), mean ± SD | 87 ± 22 | 87 ± 18 | −0.0081 | 86 ± 21 | 87 ± 19 | −0.0523 |
| Hypertension, n (%) | 111 (37.8) | 117 (37.6) | 0.0028 | 93 (37.1) | 91 (36.3) | 0.0165 |
| Prior stroke, n (%) | 16 (5.4) | 24 (7.7) | −0.0917 | 11 (4.4) | 16 (6.4) | −0.0882 |
| Extracardiac arteriopathy, n (%) | 6 (2.0) | 2 (0.6) | 0.1215 | 4 (1.6) | 2 (0.8) | 0.0732 |
| Diabetes mellitus, n (%) | 17 (5.8) | 12 (3.9) | 0.0898 | 16 (6.4) | 8 (3.2) | 0.1495 |
| Chronic lung disease, n (%) | 23 (7.8) | 16 (5.1) | 0.1088 | 17 (6.8) | 13 (5.2) | 0.0671 |
| Recent myocardial infarction, n (%) | 1 (0.3) | 1 (0.3) | 0.0032 | 1 (0.4) | 1 (0.4) | 0 |
| Atrial fibrillation, n (%) | 0.1849 | 0.1186 | ||||
| No | 163 (55.4) | 200 (64.3) | 143 (57.0) | 157 (62.5) | ||
| Paroxysmal | 59 (20.1) | 53 (17.0) | 50 (19.9) | 46 (18.3) | ||
| Non-paroxysmal | 72 (24.5) | 58 (18.6) | 58 (23.1) | 48 (19.1) | ||
| Prior percutaneous coronary intervention, n (%) | 10 (3.4) | 10 (3.2) | 0.0104 | 8 (3.2) | 9 (3.6) | −0.0220 |
| Left ventricular ejection fraction, n (%) | 0.3476 | 0.0822 | ||||
| >50% | 157 (53.4) | 217 (69.8) | 152 (60.6) | 157 (62.5) | ||
| 30–50% | 126 (42.9) | 89 (28.6) | 96 (38.2) | 89 (35.5) | ||
| <30% | 11 (3.7) | 5 (1.6) | 3 (1.2) | 5 (2.0) | ||
| Elective surgery, n (%) | 266 (90.5) | 297 (95.5) | −0.1973 | 232 (92.4) | 237 (94.4) | −0.0803 |
| EuroSCORE II, mean ± SD | 2.92 ± 2.83 | 2.13 ± 2.09 | 0.3144 | 2.39 ± 2.30 | 2.37 ± 2.25 | 0.0093 |
| Primary indication for surgery, n (%) | 0.0607 | −0.0827 | ||||
| Mitral regurgitation | 275 (93.5) | 286 (92.0) | 233 (92.8) | 238 (94.8) | ||
| Mitral stenosis | 19 (6.5) | 25 (8) | 18 (7.2) | 13 (5.2) | ||
| . | Overall cohort . | Propensity score-matched cohort . | ||||
|---|---|---|---|---|---|---|
| . | Sternotomy (n = 294) . | Minimally invasive procedure (n = 311) . | Standardized difference . | Sternotomy (n = 251) . | Minimally invasive procedure (n = 251) . | Standardized difference . |
| Age (years), mean ± SD | 63.1 ± 12.2 | 62.3 ± 11.8 | 0.0674 | 62.4 ± 12.6 | 61.1 ± 12.4 | 0.1050 |
| Female gender, n (%) | 96 (32.7) | 76 (24.4) | 0.1824 | 70 (27.9) | 72 (28.7) | −0.0177 |
| Height (cm), mean ± SD | 174 ± 10 | 176 ± 10 | −0.2215 | 175 ± 10 | 176 ± 10 | −0.0926 |
| Weight (kg), mean ± SD | 77 ± 15 | 78 ± 14 | −0.0385 | 78 ± 15 | 77 ± 15 | 0.1075 |
| Creatinine preoperatively (µmol/l), mean ± SD | 87 ± 22 | 87 ± 18 | −0.0081 | 86 ± 21 | 87 ± 19 | −0.0523 |
| Hypertension, n (%) | 111 (37.8) | 117 (37.6) | 0.0028 | 93 (37.1) | 91 (36.3) | 0.0165 |
| Prior stroke, n (%) | 16 (5.4) | 24 (7.7) | −0.0917 | 11 (4.4) | 16 (6.4) | −0.0882 |
| Extracardiac arteriopathy, n (%) | 6 (2.0) | 2 (0.6) | 0.1215 | 4 (1.6) | 2 (0.8) | 0.0732 |
| Diabetes mellitus, n (%) | 17 (5.8) | 12 (3.9) | 0.0898 | 16 (6.4) | 8 (3.2) | 0.1495 |
| Chronic lung disease, n (%) | 23 (7.8) | 16 (5.1) | 0.1088 | 17 (6.8) | 13 (5.2) | 0.0671 |
| Recent myocardial infarction, n (%) | 1 (0.3) | 1 (0.3) | 0.0032 | 1 (0.4) | 1 (0.4) | 0 |
| Atrial fibrillation, n (%) | 0.1849 | 0.1186 | ||||
| No | 163 (55.4) | 200 (64.3) | 143 (57.0) | 157 (62.5) | ||
| Paroxysmal | 59 (20.1) | 53 (17.0) | 50 (19.9) | 46 (18.3) | ||
| Non-paroxysmal | 72 (24.5) | 58 (18.6) | 58 (23.1) | 48 (19.1) | ||
| Prior percutaneous coronary intervention, n (%) | 10 (3.4) | 10 (3.2) | 0.0104 | 8 (3.2) | 9 (3.6) | −0.0220 |
| Left ventricular ejection fraction, n (%) | 0.3476 | 0.0822 | ||||
| >50% | 157 (53.4) | 217 (69.8) | 152 (60.6) | 157 (62.5) | ||
| 30–50% | 126 (42.9) | 89 (28.6) | 96 (38.2) | 89 (35.5) | ||
| <30% | 11 (3.7) | 5 (1.6) | 3 (1.2) | 5 (2.0) | ||
| Elective surgery, n (%) | 266 (90.5) | 297 (95.5) | −0.1973 | 232 (92.4) | 237 (94.4) | −0.0803 |
| EuroSCORE II, mean ± SD | 2.92 ± 2.83 | 2.13 ± 2.09 | 0.3144 | 2.39 ± 2.30 | 2.37 ± 2.25 | 0.0093 |
| Primary indication for surgery, n (%) | 0.0607 | −0.0827 | ||||
| Mitral regurgitation | 275 (93.5) | 286 (92.0) | 233 (92.8) | 238 (94.8) | ||
| Mitral stenosis | 19 (6.5) | 25 (8) | 18 (7.2) | 13 (5.2) | ||
EuroSCORE: European System for Cardiac Operative Risk Evaluation; SD: standard deviation.
Procedural characteristics are presented in Table 2. In patients with mitral valve regurgitation, the rate of mitral valve repair was lower in the sternotomy group compared with the MIMVS group though the results were not statistically significant (propensity score-matched cohort: 81.5% vs 87.0%; P = 0.11). Sternotomy patients were significantly more likely to undergo concomitant surgery [tricuspid annuloplasty (P < 0.001), left atrial appendage closure (P < 0.001) and ablation (P = 0.01)]. In the propensity score-matched analysis, patients who had MIMVS had significantly longer time on extracorporeal circulation (133 ± 57 vs 149 ± 52 min; P = 0.001) but significantly shorter aortic cross-clamp time (105 ± 40 vs 97 ± 36 min; P = 0.030). Conversion from MIMVS to sternotomy was performed in 12 (3.9%) patients, usually due to bleeding complications (n = 6) and pulmonary adhesions (n = 3).
| . | Overall cohort . | Propensity score-matched cohort . | ||||
|---|---|---|---|---|---|---|
| Sternotomy (n = 294) . | Minimally invasive procedure (n = 311) . | P-value . | Sternotomy (n = 251) . | Minimally invasive procedure (n = 25)1 . | P-value . | |
| Type of procedure, n (%) | 0.38 | 0.15 | ||||
| Repair | 223 (75.9) | 248 (79.7) | 190 (75.7) | 207 (82.5) | ||
| Biological prosthesis | 38 (12.9) | 38 (12.2) | 33 (13.1) | 26 (10.4) | ||
| Mechanical prosthesis | 33 (11.2) | 25 (8.0) | 28 (11.2) | 18 (7.2) | ||
| Repair in case of mitral regurgitation, n (%) | 223 (81.1) | 248 (86.7) | 0.070 | 190 (81.5) | 207 (87.0) | 0.11 |
| Type of mitral valve repair, n (%) | ||||||
| Annuloplasty | 223 (100) | 248 (100) | 0.25 | 190 (100) | 207 (100) | 0.062 |
| Artificial chordae | 170 (76.2) | 242 (97.6) | <0.001 | 146 (76.8) | 201 (97.1) | <0.001 |
| Resection | 67 (30.0) | 2 (0.8) | <0.001 | 58 (30.5) | 2 (1.0) | <0.001 |
| Suture | 23 (10.3) | 48 (19.4) | 0.004 | 19 (10) | 37 (17.9) | 0.011 |
| Concomitant procedures, n (%) | ||||||
| Tricuspid annuloplasty | 73 (24.8) | 21 (6.8) | <0.001 | 57 (22.7) | 20 (8.0) | <0.001 |
| Left atrial appendage closure | 67 (22.8) | 22 (7.1) | <0.001 | 55 (21.9) | 18 (7.2) | <0.001 |
| Patent foramen ovale closure | 30 (10.2) | 28 (9.0) | 0.62 | 21 (8.4) | 27 (10.8) | 0.36 |
| Ablation | 82 (27.9) | 48 (15.4) | <0.001 | 68 (27.1) | 44 (17.5) | 0.010 |
| Conversion to full sternotomy, n (%) | na | 12 (3.9) | na | na | 11 (4.4) | na |
| Extracorporeal circulation duration (min), mean ± SD | 133 ± 56 | 149 ± 52 | <0.001 | 133 ± 57 | 149 ± 52 | 0.001 |
| Aortic occlusion duration (min), mean ± SD | 105 ± 40 | 97 ± 36 | 0.020 | 105 ± 40 | 97 ± 36 | 0.030 |
| . | Overall cohort . | Propensity score-matched cohort . | ||||
|---|---|---|---|---|---|---|
| Sternotomy (n = 294) . | Minimally invasive procedure (n = 311) . | P-value . | Sternotomy (n = 251) . | Minimally invasive procedure (n = 25)1 . | P-value . | |
| Type of procedure, n (%) | 0.38 | 0.15 | ||||
| Repair | 223 (75.9) | 248 (79.7) | 190 (75.7) | 207 (82.5) | ||
| Biological prosthesis | 38 (12.9) | 38 (12.2) | 33 (13.1) | 26 (10.4) | ||
| Mechanical prosthesis | 33 (11.2) | 25 (8.0) | 28 (11.2) | 18 (7.2) | ||
| Repair in case of mitral regurgitation, n (%) | 223 (81.1) | 248 (86.7) | 0.070 | 190 (81.5) | 207 (87.0) | 0.11 |
| Type of mitral valve repair, n (%) | ||||||
| Annuloplasty | 223 (100) | 248 (100) | 0.25 | 190 (100) | 207 (100) | 0.062 |
| Artificial chordae | 170 (76.2) | 242 (97.6) | <0.001 | 146 (76.8) | 201 (97.1) | <0.001 |
| Resection | 67 (30.0) | 2 (0.8) | <0.001 | 58 (30.5) | 2 (1.0) | <0.001 |
| Suture | 23 (10.3) | 48 (19.4) | 0.004 | 19 (10) | 37 (17.9) | 0.011 |
| Concomitant procedures, n (%) | ||||||
| Tricuspid annuloplasty | 73 (24.8) | 21 (6.8) | <0.001 | 57 (22.7) | 20 (8.0) | <0.001 |
| Left atrial appendage closure | 67 (22.8) | 22 (7.1) | <0.001 | 55 (21.9) | 18 (7.2) | <0.001 |
| Patent foramen ovale closure | 30 (10.2) | 28 (9.0) | 0.62 | 21 (8.4) | 27 (10.8) | 0.36 |
| Ablation | 82 (27.9) | 48 (15.4) | <0.001 | 68 (27.1) | 44 (17.5) | 0.010 |
| Conversion to full sternotomy, n (%) | na | 12 (3.9) | na | na | 11 (4.4) | na |
| Extracorporeal circulation duration (min), mean ± SD | 133 ± 56 | 149 ± 52 | <0.001 | 133 ± 57 | 149 ± 52 | 0.001 |
| Aortic occlusion duration (min), mean ± SD | 105 ± 40 | 97 ± 36 | 0.020 | 105 ± 40 | 97 ± 36 | 0.030 |
na: not applicable; SD: standard deviation.
| . | Overall cohort . | Propensity score-matched cohort . | ||||
|---|---|---|---|---|---|---|
| Sternotomy (n = 294) . | Minimally invasive procedure (n = 311) . | P-value . | Sternotomy (n = 251) . | Minimally invasive procedure (n = 25)1 . | P-value . | |
| Type of procedure, n (%) | 0.38 | 0.15 | ||||
| Repair | 223 (75.9) | 248 (79.7) | 190 (75.7) | 207 (82.5) | ||
| Biological prosthesis | 38 (12.9) | 38 (12.2) | 33 (13.1) | 26 (10.4) | ||
| Mechanical prosthesis | 33 (11.2) | 25 (8.0) | 28 (11.2) | 18 (7.2) | ||
| Repair in case of mitral regurgitation, n (%) | 223 (81.1) | 248 (86.7) | 0.070 | 190 (81.5) | 207 (87.0) | 0.11 |
| Type of mitral valve repair, n (%) | ||||||
| Annuloplasty | 223 (100) | 248 (100) | 0.25 | 190 (100) | 207 (100) | 0.062 |
| Artificial chordae | 170 (76.2) | 242 (97.6) | <0.001 | 146 (76.8) | 201 (97.1) | <0.001 |
| Resection | 67 (30.0) | 2 (0.8) | <0.001 | 58 (30.5) | 2 (1.0) | <0.001 |
| Suture | 23 (10.3) | 48 (19.4) | 0.004 | 19 (10) | 37 (17.9) | 0.011 |
| Concomitant procedures, n (%) | ||||||
| Tricuspid annuloplasty | 73 (24.8) | 21 (6.8) | <0.001 | 57 (22.7) | 20 (8.0) | <0.001 |
| Left atrial appendage closure | 67 (22.8) | 22 (7.1) | <0.001 | 55 (21.9) | 18 (7.2) | <0.001 |
| Patent foramen ovale closure | 30 (10.2) | 28 (9.0) | 0.62 | 21 (8.4) | 27 (10.8) | 0.36 |
| Ablation | 82 (27.9) | 48 (15.4) | <0.001 | 68 (27.1) | 44 (17.5) | 0.010 |
| Conversion to full sternotomy, n (%) | na | 12 (3.9) | na | na | 11 (4.4) | na |
| Extracorporeal circulation duration (min), mean ± SD | 133 ± 56 | 149 ± 52 | <0.001 | 133 ± 57 | 149 ± 52 | 0.001 |
| Aortic occlusion duration (min), mean ± SD | 105 ± 40 | 97 ± 36 | 0.020 | 105 ± 40 | 97 ± 36 | 0.030 |
| . | Overall cohort . | Propensity score-matched cohort . | ||||
|---|---|---|---|---|---|---|
| Sternotomy (n = 294) . | Minimally invasive procedure (n = 311) . | P-value . | Sternotomy (n = 251) . | Minimally invasive procedure (n = 25)1 . | P-value . | |
| Type of procedure, n (%) | 0.38 | 0.15 | ||||
| Repair | 223 (75.9) | 248 (79.7) | 190 (75.7) | 207 (82.5) | ||
| Biological prosthesis | 38 (12.9) | 38 (12.2) | 33 (13.1) | 26 (10.4) | ||
| Mechanical prosthesis | 33 (11.2) | 25 (8.0) | 28 (11.2) | 18 (7.2) | ||
| Repair in case of mitral regurgitation, n (%) | 223 (81.1) | 248 (86.7) | 0.070 | 190 (81.5) | 207 (87.0) | 0.11 |
| Type of mitral valve repair, n (%) | ||||||
| Annuloplasty | 223 (100) | 248 (100) | 0.25 | 190 (100) | 207 (100) | 0.062 |
| Artificial chordae | 170 (76.2) | 242 (97.6) | <0.001 | 146 (76.8) | 201 (97.1) | <0.001 |
| Resection | 67 (30.0) | 2 (0.8) | <0.001 | 58 (30.5) | 2 (1.0) | <0.001 |
| Suture | 23 (10.3) | 48 (19.4) | 0.004 | 19 (10) | 37 (17.9) | 0.011 |
| Concomitant procedures, n (%) | ||||||
| Tricuspid annuloplasty | 73 (24.8) | 21 (6.8) | <0.001 | 57 (22.7) | 20 (8.0) | <0.001 |
| Left atrial appendage closure | 67 (22.8) | 22 (7.1) | <0.001 | 55 (21.9) | 18 (7.2) | <0.001 |
| Patent foramen ovale closure | 30 (10.2) | 28 (9.0) | 0.62 | 21 (8.4) | 27 (10.8) | 0.36 |
| Ablation | 82 (27.9) | 48 (15.4) | <0.001 | 68 (27.1) | 44 (17.5) | 0.010 |
| Conversion to full sternotomy, n (%) | na | 12 (3.9) | na | na | 11 (4.4) | na |
| Extracorporeal circulation duration (min), mean ± SD | 133 ± 56 | 149 ± 52 | <0.001 | 133 ± 57 | 149 ± 52 | 0.001 |
| Aortic occlusion duration (min), mean ± SD | 105 ± 40 | 97 ± 36 | 0.020 | 105 ± 40 | 97 ± 36 | 0.030 |
na: not applicable; SD: standard deviation.
Postoperative outcomes are presented in Table 3. In the propensity score-matched analysis, MIMVS patients had a significantly reduced risk for reoperation due to bleeding (7.2% vs 2.4%; P = 0.012) as well as a significantly lower need for 1 or more red blood cell transfusions (30.7% vs 19.1%; P = 0.003). Reoperation for mitral valve regurgitation during hospital stay was uncommon in both groups but significantly reduced in patients who had MIMVS (propensity score-matched cohort: 2.4% vs 0%; P = 0.014). Intensive care unit stay was short in both groups and did not differ (median 1, quartile 1:1, quartile 3:1; P = 0.76). However, total in-hospital stay was significantly shorter for MIMVS patients (7.2 ± 4.6 vs 5.0 ± 2.7 days; P < 0.001). In the propensity score-matched analysis, 30-day mortality was low in both the sternotomy group (n = 2; 0.8%) and the MIMVS group (n = 1; 0.4%).
| . | Overall cohort . | Propensity score-matched cohort . | ||||
|---|---|---|---|---|---|---|
| Sternotomy (n = 294) . | Minimally invasive (n = 311) . | P-value . | Sternotomy (n = 251) . | Minimally invasive (n = 25)1 . | P-value . | |
| Reoperation for bleeding, n (%) | 20 (6.8) | 8 (2.6) | 0.013 | 18 (7.2) | 6 (2.4) | 0.012 |
| Stroke, n (%) | 3 (1.0) | 4 (1.3) | 0.76 | 2 (0.8) | 3 (1.2) | 0.65 |
| Perioperative myocardial infarction, n (%) | 5 (1.7) | 8 (2.6) | 0.46 | 4 (1.6) | 8 (3.2) | 0.24 |
| Postoperative peak creatinine kinase-MB (µg/l), mean ± SD | 49 ± 41 | 53 ± 57 | 0.32 | 49 ± 42 | 54 ± 61 | 0.24 |
| New-onset dialysis, n (%) | 12 (4.1) | 4 (1.3) | 0.032 | 11 (4.4) | 4 (1.6) | 0.067 |
| Postoperative peak creatinine (µmol/l), mean ± SD | 118 ± 69 | 105 ± 68 | 0.017 | 116 ± 70 | 105 ± 72 | 0.11 |
| Mechanical ventilation >48 h, n (%) | 10 (3.4) | 4 (1.3) | 0.084 | 8 (3.2) | 4 (1.6) | 0.24 |
| Extracorporeal membrane oxygenation, n (%) | 4 (1.4) | 1 (0.3) | 0.16 | 3 (1.2) | 1 (0.4) | 0.32 |
| Pacemaker implantation, n (%) | 20 (6.8) | 10 (3.2) | 0.042 | 18 (7.2) | 7 (2.8) | 0.024 |
| New-onset atrial fibrillation, n (%) | 72 (24.5) | 56 (18.0) | 0.051 | 61 (24.3) | 44 (17.5) | 0.062 |
| ≥1 unit red blood cells transfused, n (%) | 99 (33.7) | 56 (18.0) | <0.001 | 77 (30.7) | 48 (19.1) | 0.003 |
| Pericardiocentesis, n (%) | 4 (1.4) | 0 (0) | 0.039 | 4 (1.6) | 0 (0) | 0.045 |
| Pleurocentesis, n (%) | 11 (3.7) | 9 (2.9) | 0.56 | 8 (3.2) | 7 (2.8) | 0.79 |
| Reoperation for sternal insufficiency or mediastinitis, n (%) | 1 (0.3) | na | na | 1 (0.4) | na | na |
| Chest wound infection treated with antibiotics, n (%) | 2 (0.7) | 0 (0) | 0.15 | 2 (0.8) | 0 (0) | 0.16 |
| Pneumonia treated with antibiotics, n (%) | 5 (1.7) | 9 (2.9) | 0.33 | 4 (1.6) | 7 (2.8) | 0.36 |
| Post-repair mitral regurgitation >grade 1/4, n (%) | 16 (7.2) | 8 (3.2) | 0.052 | 11 (5.8) | 8 (3.9) | 0.37 |
| Reoperation for mitral regurgitation during hospital stay, n (%) | 7 (2.4) | 0 (0) | 0.006 | 6 (2.4) | 0 (0) | 0.014 |
| Intensive care unit stay (days), median (Q1–Q3) | 1 (1–1) | 1 (1–1) | 0.20 | 1 (1–1) | 1 (1–1) | 0.76 |
| In-hospital stay (days), mean ± SD | 7.3 ± 4.4 | 5.0 ± 2.6 | <0.001 | 7.2 ± 4.6 | 5.0 ± 2.7 | <0.001 |
| 30-Day mortality, n (%) | 2 (0.7) | 1 (0.3) | 0.53 | 2 (0.8) | 1 (0.4) | 0.56 |
| . | Overall cohort . | Propensity score-matched cohort . | ||||
|---|---|---|---|---|---|---|
| Sternotomy (n = 294) . | Minimally invasive (n = 311) . | P-value . | Sternotomy (n = 251) . | Minimally invasive (n = 25)1 . | P-value . | |
| Reoperation for bleeding, n (%) | 20 (6.8) | 8 (2.6) | 0.013 | 18 (7.2) | 6 (2.4) | 0.012 |
| Stroke, n (%) | 3 (1.0) | 4 (1.3) | 0.76 | 2 (0.8) | 3 (1.2) | 0.65 |
| Perioperative myocardial infarction, n (%) | 5 (1.7) | 8 (2.6) | 0.46 | 4 (1.6) | 8 (3.2) | 0.24 |
| Postoperative peak creatinine kinase-MB (µg/l), mean ± SD | 49 ± 41 | 53 ± 57 | 0.32 | 49 ± 42 | 54 ± 61 | 0.24 |
| New-onset dialysis, n (%) | 12 (4.1) | 4 (1.3) | 0.032 | 11 (4.4) | 4 (1.6) | 0.067 |
| Postoperative peak creatinine (µmol/l), mean ± SD | 118 ± 69 | 105 ± 68 | 0.017 | 116 ± 70 | 105 ± 72 | 0.11 |
| Mechanical ventilation >48 h, n (%) | 10 (3.4) | 4 (1.3) | 0.084 | 8 (3.2) | 4 (1.6) | 0.24 |
| Extracorporeal membrane oxygenation, n (%) | 4 (1.4) | 1 (0.3) | 0.16 | 3 (1.2) | 1 (0.4) | 0.32 |
| Pacemaker implantation, n (%) | 20 (6.8) | 10 (3.2) | 0.042 | 18 (7.2) | 7 (2.8) | 0.024 |
| New-onset atrial fibrillation, n (%) | 72 (24.5) | 56 (18.0) | 0.051 | 61 (24.3) | 44 (17.5) | 0.062 |
| ≥1 unit red blood cells transfused, n (%) | 99 (33.7) | 56 (18.0) | <0.001 | 77 (30.7) | 48 (19.1) | 0.003 |
| Pericardiocentesis, n (%) | 4 (1.4) | 0 (0) | 0.039 | 4 (1.6) | 0 (0) | 0.045 |
| Pleurocentesis, n (%) | 11 (3.7) | 9 (2.9) | 0.56 | 8 (3.2) | 7 (2.8) | 0.79 |
| Reoperation for sternal insufficiency or mediastinitis, n (%) | 1 (0.3) | na | na | 1 (0.4) | na | na |
| Chest wound infection treated with antibiotics, n (%) | 2 (0.7) | 0 (0) | 0.15 | 2 (0.8) | 0 (0) | 0.16 |
| Pneumonia treated with antibiotics, n (%) | 5 (1.7) | 9 (2.9) | 0.33 | 4 (1.6) | 7 (2.8) | 0.36 |
| Post-repair mitral regurgitation >grade 1/4, n (%) | 16 (7.2) | 8 (3.2) | 0.052 | 11 (5.8) | 8 (3.9) | 0.37 |
| Reoperation for mitral regurgitation during hospital stay, n (%) | 7 (2.4) | 0 (0) | 0.006 | 6 (2.4) | 0 (0) | 0.014 |
| Intensive care unit stay (days), median (Q1–Q3) | 1 (1–1) | 1 (1–1) | 0.20 | 1 (1–1) | 1 (1–1) | 0.76 |
| In-hospital stay (days), mean ± SD | 7.3 ± 4.4 | 5.0 ± 2.6 | <0.001 | 7.2 ± 4.6 | 5.0 ± 2.7 | <0.001 |
| 30-Day mortality, n (%) | 2 (0.7) | 1 (0.3) | 0.53 | 2 (0.8) | 1 (0.4) | 0.56 |
MB: myocardial band; na: not applicable; Q: quartile; SD: standard deviation.
| . | Overall cohort . | Propensity score-matched cohort . | ||||
|---|---|---|---|---|---|---|
| Sternotomy (n = 294) . | Minimally invasive (n = 311) . | P-value . | Sternotomy (n = 251) . | Minimally invasive (n = 25)1 . | P-value . | |
| Reoperation for bleeding, n (%) | 20 (6.8) | 8 (2.6) | 0.013 | 18 (7.2) | 6 (2.4) | 0.012 |
| Stroke, n (%) | 3 (1.0) | 4 (1.3) | 0.76 | 2 (0.8) | 3 (1.2) | 0.65 |
| Perioperative myocardial infarction, n (%) | 5 (1.7) | 8 (2.6) | 0.46 | 4 (1.6) | 8 (3.2) | 0.24 |
| Postoperative peak creatinine kinase-MB (µg/l), mean ± SD | 49 ± 41 | 53 ± 57 | 0.32 | 49 ± 42 | 54 ± 61 | 0.24 |
| New-onset dialysis, n (%) | 12 (4.1) | 4 (1.3) | 0.032 | 11 (4.4) | 4 (1.6) | 0.067 |
| Postoperative peak creatinine (µmol/l), mean ± SD | 118 ± 69 | 105 ± 68 | 0.017 | 116 ± 70 | 105 ± 72 | 0.11 |
| Mechanical ventilation >48 h, n (%) | 10 (3.4) | 4 (1.3) | 0.084 | 8 (3.2) | 4 (1.6) | 0.24 |
| Extracorporeal membrane oxygenation, n (%) | 4 (1.4) | 1 (0.3) | 0.16 | 3 (1.2) | 1 (0.4) | 0.32 |
| Pacemaker implantation, n (%) | 20 (6.8) | 10 (3.2) | 0.042 | 18 (7.2) | 7 (2.8) | 0.024 |
| New-onset atrial fibrillation, n (%) | 72 (24.5) | 56 (18.0) | 0.051 | 61 (24.3) | 44 (17.5) | 0.062 |
| ≥1 unit red blood cells transfused, n (%) | 99 (33.7) | 56 (18.0) | <0.001 | 77 (30.7) | 48 (19.1) | 0.003 |
| Pericardiocentesis, n (%) | 4 (1.4) | 0 (0) | 0.039 | 4 (1.6) | 0 (0) | 0.045 |
| Pleurocentesis, n (%) | 11 (3.7) | 9 (2.9) | 0.56 | 8 (3.2) | 7 (2.8) | 0.79 |
| Reoperation for sternal insufficiency or mediastinitis, n (%) | 1 (0.3) | na | na | 1 (0.4) | na | na |
| Chest wound infection treated with antibiotics, n (%) | 2 (0.7) | 0 (0) | 0.15 | 2 (0.8) | 0 (0) | 0.16 |
| Pneumonia treated with antibiotics, n (%) | 5 (1.7) | 9 (2.9) | 0.33 | 4 (1.6) | 7 (2.8) | 0.36 |
| Post-repair mitral regurgitation >grade 1/4, n (%) | 16 (7.2) | 8 (3.2) | 0.052 | 11 (5.8) | 8 (3.9) | 0.37 |
| Reoperation for mitral regurgitation during hospital stay, n (%) | 7 (2.4) | 0 (0) | 0.006 | 6 (2.4) | 0 (0) | 0.014 |
| Intensive care unit stay (days), median (Q1–Q3) | 1 (1–1) | 1 (1–1) | 0.20 | 1 (1–1) | 1 (1–1) | 0.76 |
| In-hospital stay (days), mean ± SD | 7.3 ± 4.4 | 5.0 ± 2.6 | <0.001 | 7.2 ± 4.6 | 5.0 ± 2.7 | <0.001 |
| 30-Day mortality, n (%) | 2 (0.7) | 1 (0.3) | 0.53 | 2 (0.8) | 1 (0.4) | 0.56 |
| . | Overall cohort . | Propensity score-matched cohort . | ||||
|---|---|---|---|---|---|---|
| Sternotomy (n = 294) . | Minimally invasive (n = 311) . | P-value . | Sternotomy (n = 251) . | Minimally invasive (n = 25)1 . | P-value . | |
| Reoperation for bleeding, n (%) | 20 (6.8) | 8 (2.6) | 0.013 | 18 (7.2) | 6 (2.4) | 0.012 |
| Stroke, n (%) | 3 (1.0) | 4 (1.3) | 0.76 | 2 (0.8) | 3 (1.2) | 0.65 |
| Perioperative myocardial infarction, n (%) | 5 (1.7) | 8 (2.6) | 0.46 | 4 (1.6) | 8 (3.2) | 0.24 |
| Postoperative peak creatinine kinase-MB (µg/l), mean ± SD | 49 ± 41 | 53 ± 57 | 0.32 | 49 ± 42 | 54 ± 61 | 0.24 |
| New-onset dialysis, n (%) | 12 (4.1) | 4 (1.3) | 0.032 | 11 (4.4) | 4 (1.6) | 0.067 |
| Postoperative peak creatinine (µmol/l), mean ± SD | 118 ± 69 | 105 ± 68 | 0.017 | 116 ± 70 | 105 ± 72 | 0.11 |
| Mechanical ventilation >48 h, n (%) | 10 (3.4) | 4 (1.3) | 0.084 | 8 (3.2) | 4 (1.6) | 0.24 |
| Extracorporeal membrane oxygenation, n (%) | 4 (1.4) | 1 (0.3) | 0.16 | 3 (1.2) | 1 (0.4) | 0.32 |
| Pacemaker implantation, n (%) | 20 (6.8) | 10 (3.2) | 0.042 | 18 (7.2) | 7 (2.8) | 0.024 |
| New-onset atrial fibrillation, n (%) | 72 (24.5) | 56 (18.0) | 0.051 | 61 (24.3) | 44 (17.5) | 0.062 |
| ≥1 unit red blood cells transfused, n (%) | 99 (33.7) | 56 (18.0) | <0.001 | 77 (30.7) | 48 (19.1) | 0.003 |
| Pericardiocentesis, n (%) | 4 (1.4) | 0 (0) | 0.039 | 4 (1.6) | 0 (0) | 0.045 |
| Pleurocentesis, n (%) | 11 (3.7) | 9 (2.9) | 0.56 | 8 (3.2) | 7 (2.8) | 0.79 |
| Reoperation for sternal insufficiency or mediastinitis, n (%) | 1 (0.3) | na | na | 1 (0.4) | na | na |
| Chest wound infection treated with antibiotics, n (%) | 2 (0.7) | 0 (0) | 0.15 | 2 (0.8) | 0 (0) | 0.16 |
| Pneumonia treated with antibiotics, n (%) | 5 (1.7) | 9 (2.9) | 0.33 | 4 (1.6) | 7 (2.8) | 0.36 |
| Post-repair mitral regurgitation >grade 1/4, n (%) | 16 (7.2) | 8 (3.2) | 0.052 | 11 (5.8) | 8 (3.9) | 0.37 |
| Reoperation for mitral regurgitation during hospital stay, n (%) | 7 (2.4) | 0 (0) | 0.006 | 6 (2.4) | 0 (0) | 0.014 |
| Intensive care unit stay (days), median (Q1–Q3) | 1 (1–1) | 1 (1–1) | 0.20 | 1 (1–1) | 1 (1–1) | 0.76 |
| In-hospital stay (days), mean ± SD | 7.3 ± 4.4 | 5.0 ± 2.6 | <0.001 | 7.2 ± 4.6 | 5.0 ± 2.7 | <0.001 |
| 30-Day mortality, n (%) | 2 (0.7) | 1 (0.3) | 0.53 | 2 (0.8) | 1 (0.4) | 0.56 |
MB: myocardial band; na: not applicable; Q: quartile; SD: standard deviation.
DISCUSSION
This study showed that MIMVS was associated with short-term outcomes comparable to those with mitral valve surgery performed through a sternotomy. The initiation of an MIMVS programme did not confer an increased risk for adverse events.
Previous studies have reported substantial differences in the baseline characteristics of patients selected for sternotomy mitral valve surgery versus MIMVS. The choice of a minimally invasive versus a sternotomy approach is often surgeon-specific, which results in a high risk for selection bias with high-risk patients more likely to undergo sternotomy mitral valve surgery [3, 8, 14]. To limit the risk for selection bias, we compared patients undergoing MIMVS to a cohort who underwent sternotomy mitral valve surgery during the period immediately prior to the introduction of an MIMVS programme. Selection bias should be reduced because the procedures were performed with 1 strategy during each period and the decision to use a minimally invasive procedure after the introduction of the MIMVS programme was not surgeon-specific but determined by general institutional directives. As demonstrated by our results, the 2 groups had similar baseline characteristics before matching. It is also noteworthy that all differences detected in postoperative outcomes in the propensity score-matched cohort also could be seen in the overall cohort, increasing the generalizability of our findings. In this study, MIMVS was associated with a lower incidence of reoperation for bleeding, lower need for transfusions and shorter in-hospital stay compared with sternotomy procedures. These results are in line with those of previous studies showing a reduced need for red blood cell transfusions [8, 10, 15] as well as shorter in-hospital stays [10, 16, 17].
MIMVS has been associated with a considerable learning curve with reports of the risk of high complication rates initially [12]. Studies have approximated an early learning curve of >75 operations per surgeon for satisfactory postoperative results and >50 operations per year and per surgeon to maintain these results [12, 13]. High surgeon volume correlates with higher repair rates and increased survival and freedom from reoperation [12, 18]. The results presented in this study relate the complete experience of starting a medium-volume MIMVS programme. The results include consecutive data from the introduction of the programme and are therefore subjected to a learning curve effect. In our opinion, the favourable outcomes seen in this series were achieved through well-established routines that included a limited number of surgeons performing all minimally invasive mitral valve procedures. Several different surgical techniques have been described for MIMVS but to standardize treatment, all procedures in this study were performed through a right-sided minithoracotomy, with video assistance allowing for smaller incisions and with peripheral femoral cannulation for extracorporeal circulation. No endoaortic balloon occlusion was used because the technique has been shown to be associated with a potentially increased risk of iatrogenic aortic dissection and stroke [9, 19].
Although it was not statistically significant, it is notable that the mitral valve repair rate was higher in the MIMVS group compared to that in the sternotomy group in both the overall series and in the matched analysis. With a right-sided minithoracotomy, the mitral valve is accessed through a left atrial incision anterior to the right-sided pulmonary veins, which allows an oblique view of the mitral valve with the heart in its natural position. With a sternotomy approach, the heart is rotated, which could in theory make assessment and repair of the mitral valve more difficult. Mitral valve repair with neochords is a general trend in mitral valve surgery, but it also appears easier in a minimally invasive setting, and we see a significantly greater use of artificial chordae in the MIMVS group. In line with previous studies, our study shows that MIMVS was associated with increased duration of extracorporeal circulation compared to sternotomy. In patients who have MIMVS, extracorporeal circulation is initiated before entry into the chest in order to enable collapse of the right lung, consequently prolonging the duration of extracorporeal circulation. Interestingly, aortic cross-clamp times were significantly reduced in MIMVS, which could be an additional indication that mitral valve repair was facilitated with a minimally invasive approach.
The minimally invasive group had significantly fewer concomitant procedures such as tricuspid annuloplasty, left atrial appendage closure and ablation. The latter could in part be explained by a relatively lower incidence of atrial fibrillation in the minimally invasive group. However, we note that the difference in concomitant procedures could lead to a lower risk of immediate postoperative complications in the minimally invasive group. The long-term effects of the procedural differences remain unclear and will be studied when data are available, but they are not within the scope of this study.
Limitations
This study has limitations. The fact that minimally invasive and sternotomy mitral valve surgical procedures were performed during 2 different time periods can be regarded as a limitation because other factors not related to the surgical technique might have changed between the periods. Also, the number of surgeons performing mitral valve surgery before and after the introduction of the minimally invasive programme differed, with fewer surgeons performing the minimally invasive mitral valve procedures compared with the number performing sternotomy procedures. Furthermore, this was a single-centre study, which limits its generalizability.
CONCLUSION
We concluded that MIMVS was associated with short-term outcomes comparable to those of mitral valve surgery performed through a sternotomy. Initiating a minimally invasive mitral valve programme with a limited number of surgeons and a well-designed institutional selection strategy did not confer an increased risk for adverse events. Minimally invasive surgery was associated with a lower risk for bleeding and shorter in-hospital stays compared with sternotomy procedures.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Funding
This study was supported by the Mats Kleberg Foundation and a donation from Fredrik Lundberg. M.D. was financially supported by a research grant from Karolinska Institutet [2018-02009].
Conflict of interest: none declared.
Author contributions
Mikael Kastengren: Conceptualization; Data curation; Investigation; Writing—original draft; Writing—review & editing. Peter Svenarud: Supervision; Writing—review & editing. Göran Källner: Supervision; Writing—review & editing. Anders Franco-Cereceda: Funding acquisition; Supervision; Writing—review & editing. Jan Liska: Supervision; Writing—review & editing. Isak Gran: Data curation. Magnus Dalén: Conceptualization; Formal analysis; Funding acquisition; Methodology; Supervision; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Evalsdas Girdauskas, Stefano Mastrobuoni and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- EuroSCORE
European System for Cardiac Operative Risk Evaluation
- MIMVS
Minimally invasive mitral valve surgery





