-
PDF
- Split View
-
Views
-
Cite
Cite
Findra Setianingrum, Riina Rautemaa-Richardson, Rajesh Shah, David W Denning, Clinical outcomes of patients with chronic pulmonary aspergillosis managed surgically, European Journal of Cardio-Thoracic Surgery, Volume 58, Issue 5, November 2020, Pages 997–1003, https://doi.org/10.1093/ejcts/ezaa137
Close - Share Icon Share
Abstract
Surgical resection is one treatment modality for chronic pulmonary aspergillosis (CPA), and sometimes a preoperative presumption of lung cancer turns out to be CPA. We have audited our surgical experience with regard to risk factors for relapse, and the value of postoperative monitoring of Aspergillus-immunogolubulin G (IgG) titres.
All patients with CPA surgically treated at National Aspergillosis Centre (NAC), Manchester, UK (2007–2018), were retrospectively evaluated. Surgical procedures, underlying disorders, Aspergillus-IgG titres (ImmunoCap) and antifungal therapy were evaluated for symptom control, operative complications, CPA relapse and mortality.
A total of 61 patients with CPA (28 males, 33 females) were operated on primarily for antifungal therapy failure (51%, n = 31) and presumed lung malignancies (38%, n = 23). Procedures included lobectomy (64%, n = 39), wedge resection (28%, n = 17), segmentectomy (n = 3), pneumonectomy (n = 3) and decortication (n = 2). Overall, 25 (41%) patients relapsed, 26 months (standard deviation: 24.8 months) after surgery. Antifungal therapy before surgery (P = 0.002) or both before and after surgery (P = 0.005) were protective for relapse. The relapse rate within 3 years after surgery (33%, n = 20) was higher than the 3–10 years after surgery (8%, n = 5). At the end of follow-up, the median Aspergillus-IgG titre was lower than at relapse in 12 patients (67 vs 126 mg/l) (P = 0.016).
Surgery in these selected patients with CPA resulted in favourable outcomes. Relapse is common after surgical treatment of CPA but can be minimized with antifungal therapy, emphasizing the importance of an accurate diagnosis prior to surgery.
INTRODUCTION
Aspergillosis can present as chronic infection of the lung, called chronic pulmonary aspergillosis (CPA) [1]. CPA is thought to affect more than 3 million people around the world, perhaps 40% as a late complication of pulmonary tuberculosis [2]. The estimated annual burden of CPA exceeds 3 million cases and has a particularly high annual mortality rate (15%) with more than 450 000 deaths per year [3]. The most common antecedent conditions include chronic obstructive pulmonary disease (COPD), pulmonary tuberculosis (TB), prior pneumothorax, sarcoidosis and non-tuberculous mycobacterial infections [4, 5]. There is a growing body of literature on CPA, a tribute to awareness and advanced diagnostic techniques [6–9].
Surgery is one of the treatment options for CPA, especially for single aspergilloma, for those with recurrent haemoptysis despite bronchial artery embolization or with azole-resistant disease [10–13]. Debate continues about the best strategies for the surgical management of CPA in view of the prevention of CPA relapse and reduces the postoperative morbidity [8, 11, 14]. A previous study from our centre reported a high relapse rate (26%) for CPA cases after surgery [11]. Postoperative morbidity ranges from 23% to 57% in published literature [11, 13, 15–18]. The efficacy of antifungal therapy is still questionable in terms of regimens, the duration and timescale of surgery [14, 19, 20], despite being recommended [6, 11].
To evaluate the clinical outcome of CPA surgery, we performed a retrospective audit of patients with CPA who underwent surgery focusing on risk factor for relapse, serology monitoring and antifungal treatment. We also evaluated the postoperative morbidity and mortality of the patients.
MATERIALS AND METHODS
This was a retrospective cohort study evaluating 61 patients with CPA who were treated at the National Aspergillosis Centre (NAC), Manchester, UK, over an 11-year period (2007–2018). Being a retrospective study, ethics review and patients’ informed consent were waived. Data were anonymized prior to analysis and were collected as part of a clinical audit. The diagnosis of CPA for every patient was confirmed by the European Society of Clinical Microbiology and Infectious Diseases and European Respiratory Society (ESCMID/ERS) guidelines [6]. The diagnosis of CPA was established if the following were present: (i) one or more cavities on chest radiology; (ii) positive laboratory results of Aspergillus from microscopy, culture, biopsy or positive Aspergillus immunogolubulin G (IgG); and (iii) persistent symptoms (≥3 months) including cough, fatigue, haemoptysis, dyspnoea and weight loss. Patients were categorized into 3 types of CPA: single aspergillosis, chronic cavitary and nodules [6].
For the analyses, the patients were classified into relapse and non-relapse groups of CPA. Relapse was defined as deterioration in 2 of the following parameters: clinical, radiological, serology or sputum microbiological markers of CPA activity. Clinical deterioration was any deterioration in systemic or respiratory symptoms, as reported by the patient and documented in the patient’s notes. Radiological deterioration was defined as a worsening of respiratory imaging, either in chest X-ray or chest computed tomography scan, according to the reporting radiologist. Clinical and radiological deterioration should not have been attributable to other disease processes, notably concurrent pulmonary infection (e.g. bacterial super-infection or non-tuberculous mycobacterial infection), overt deterioration in underlying disease (e.g. sarcoidosis, rheumatoid arthritis or lung cancer) or toxicity of non-antifungal drugs. Serological deterioration was defined as any increase in Aspergillus IgG.
Microbiological deterioration was defined as a new positive culture of Aspergillus spp. from respiratory secretions or a positive Aspergillus polymerase chain reaction (PCR), deemed in the opinion of the treating physician to represent a significant result. The clinical decision of the treating physician to re-institute antifungal therapy was used as a surrogate marker of relapse.
We reviewed the clinical features, laboratory results (culture, Aspergillus IgG, PCR) and radiological appearances of the patients with CPA 3–6 months before (before time point) and 6–8 months after surgery (after time point). Surgical indication, histological findings and history of therapy were also documented from medical records. Assessment of the patients included the observation of relapse or non-relapse of CPA after surgery. The association of serum Aspergillus-IgG conversion with CPA relapse or CPA non-relapse was analysed in the 37 patients whose Aspergillus-specific IgG records were complete during follow-up.
Statistical analysis
Demographic data collected included age, type and number of other pulmonary conditions and history of antifungal therapy. The difference between continuous variables was analysed using Student’s t-test. Aspergillus-IgG titres are shown as median and interquartile range. The Friedman’s test and the Wilcoxon signed-rank test were used to compare Aspergillus-IgG titres at different time points. Fisher’s exact test or χ2 test was used for categorical variables. McNemar’s test was used to compare the categorical variables before and after surgery. A P-value of <0.05 was considered as statistically significant. Statistical analysis was performed with the use of IBM SPSS 25 statistic software.
A multivariable logistic regression model was performed to determine factors associated with the clinical outcome of CPA. Furthermore, a backwards selection procedure with 0.157 as critical level for the P-value was used [21]. All the variables were included in analysis: gender, age, category of CPA, pulmonary conditions (prior TB, asthma, pneumothorax, bronchiectasis, COPD, recurrent chest infection, lung malignancy, previous lung surgery, fungal asthma, pneumonia, lung abscess and number of pulmonary condition) and extrapulmonary conditions (mannose-binding lectin deficient, gamma-interferon deficient, diabetes mellitus, coronary heart disease, hypertension, autoimmune disorder, rheumatoid disorder, non-pulmonary malignancy, antifungal any time before surgery, perioperative antifungal and antifungal after surgery). Odds ratio (OR) along with 95% confidence interval (CI) are presented.
Survival analysis was performed to investigate the impact of antifungal therapy before surgery on the relapse and/or death. The Kaplan–Meier plot and the log-rank test were applied to investigate whether relapse and/or death were influenced by antifungal therapy before surgery.
RESULTS
A total of 61 patients with CPA (28 males, 33 females) were included in this study with a mean age of 55.5 years (range 26–84 years) (Table 1). The most common underlying pulmonary condition was COPD (31%, n = 19). Thirty-two (52%) patients had more than 1 pulmonary condition as possible risk factors for CPA. No pre-existing pulmonary condition could be identified in 4 (7%) patients. Ten patients had immunosuppressive therapy before surgery, 13 patients were mannose-binding lectin deficient and 3 patients were gamma-interferon deficient, but most were not tested.
| . | All (n = 61) . | Relapse (n = 25) . | Non-relapse (n = 36) . | P-value . |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 28 | 14 (50) | 14 (50) | 0.12 |
| Female | 33 | 11 (33) | 22 (67) | |
| Age (years), mean (range) | 55.5 (26–84) | 58 (34–77) | 53.8 (26–84) | 0.22 |
| Category of CPA, n (%) | ||||
| Simple aspergilloma | 28 | 12 (43) | 16 (57) | 0.78 |
| CCPA | 17 | 4 (24) | 13 (76) | 0.085 |
| Aspergillus nodules | 22 | 10 (40) | 12 (33) | 0.59 |
| Pulmonary conditionsa | ||||
| Prior tuberculosis, n (%) | 10 | 1 (10) | 9 (90) | 0.037 |
| Asthma, n (%) | 18 | 5 (28) | 13 (72) | 0.18 |
| Pneumothorax, n (%) | 13 | 7 (54) | 6 (46) | 0.29 |
| Bronchiectasis, n (%) | 14 | 5 (36) | 9 (64) | 0.65 |
| COPD, n (%) | 19 | 12 (63) | 7 (37) | 0.018 |
| Recurrent chest infections, n (%) | 12 | 4 (33) | 8 (67) | 0.75 |
| Lung malignancy, n (%) | 2 | 0 | 2 (100) | 0.51 |
| Previous lung surgery, n (%) | 9 | 5 (56) | 4 (44) | 0.47 |
| Fungal asthma, n (%) | 5 | 1 (20) | 4 (80) | 0.64 |
| Pneumonia, n (%) | 9 | 0 | 9 (100) | 0.006 |
| Lung abscess, n (%) | 5 | 2 (40) | 3 (60) | 1 |
| Number of pulmonary conditions, mean (SD) | 1.9 (1.2) | 1.7 (1) | 2 (1.3) | 0.24 |
| Extrapulmonary conditions, n (%) | ||||
| Mannose binding lectin deficient | 13 | 6 (46) | 7 (54) | 0.70 |
| Gamma interferon deficient | 3 | 0 | 3 (100) | 0.26 |
| Diabetes mellitus | 3 | 1 (33) | 2 (67) | 1 |
| Coronary heart disease | 8 | 3 (38) | 5 (62) | 1 |
| Hypertension | 10 | 4 (40) | 6 (60) | 1 |
| Autoimmune disorder | 2 | 1 (50) | 1 (50) | 1 |
| Rheumatoid arthritis | 4 | 2 (50) | 2 (50) | 1 |
| Non-pulmonary malignancy | 8 | 2 (25) | 6 (75) | 0.45 |
| . | All (n = 61) . | Relapse (n = 25) . | Non-relapse (n = 36) . | P-value . |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 28 | 14 (50) | 14 (50) | 0.12 |
| Female | 33 | 11 (33) | 22 (67) | |
| Age (years), mean (range) | 55.5 (26–84) | 58 (34–77) | 53.8 (26–84) | 0.22 |
| Category of CPA, n (%) | ||||
| Simple aspergilloma | 28 | 12 (43) | 16 (57) | 0.78 |
| CCPA | 17 | 4 (24) | 13 (76) | 0.085 |
| Aspergillus nodules | 22 | 10 (40) | 12 (33) | 0.59 |
| Pulmonary conditionsa | ||||
| Prior tuberculosis, n (%) | 10 | 1 (10) | 9 (90) | 0.037 |
| Asthma, n (%) | 18 | 5 (28) | 13 (72) | 0.18 |
| Pneumothorax, n (%) | 13 | 7 (54) | 6 (46) | 0.29 |
| Bronchiectasis, n (%) | 14 | 5 (36) | 9 (64) | 0.65 |
| COPD, n (%) | 19 | 12 (63) | 7 (37) | 0.018 |
| Recurrent chest infections, n (%) | 12 | 4 (33) | 8 (67) | 0.75 |
| Lung malignancy, n (%) | 2 | 0 | 2 (100) | 0.51 |
| Previous lung surgery, n (%) | 9 | 5 (56) | 4 (44) | 0.47 |
| Fungal asthma, n (%) | 5 | 1 (20) | 4 (80) | 0.64 |
| Pneumonia, n (%) | 9 | 0 | 9 (100) | 0.006 |
| Lung abscess, n (%) | 5 | 2 (40) | 3 (60) | 1 |
| Number of pulmonary conditions, mean (SD) | 1.9 (1.2) | 1.7 (1) | 2 (1.3) | 0.24 |
| Extrapulmonary conditions, n (%) | ||||
| Mannose binding lectin deficient | 13 | 6 (46) | 7 (54) | 0.70 |
| Gamma interferon deficient | 3 | 0 | 3 (100) | 0.26 |
| Diabetes mellitus | 3 | 1 (33) | 2 (67) | 1 |
| Coronary heart disease | 8 | 3 (38) | 5 (62) | 1 |
| Hypertension | 10 | 4 (40) | 6 (60) | 1 |
| Autoimmune disorder | 2 | 1 (50) | 1 (50) | 1 |
| Rheumatoid arthritis | 4 | 2 (50) | 2 (50) | 1 |
| Non-pulmonary malignancy | 8 | 2 (25) | 6 (75) | 0.45 |
One patient in relapse group had sarcoidosis. History of non-tuberculous mycobacterium infection and chest trauma was found in 1 patient in non-relapse group, respectively.
CCPA: chronic cavitary pulmonary aspergillosis; COPD: chronic obstructive pulmonary disease; CPA: chronic pulmonary aspergillosis; SD: standard deviation.
| . | All (n = 61) . | Relapse (n = 25) . | Non-relapse (n = 36) . | P-value . |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 28 | 14 (50) | 14 (50) | 0.12 |
| Female | 33 | 11 (33) | 22 (67) | |
| Age (years), mean (range) | 55.5 (26–84) | 58 (34–77) | 53.8 (26–84) | 0.22 |
| Category of CPA, n (%) | ||||
| Simple aspergilloma | 28 | 12 (43) | 16 (57) | 0.78 |
| CCPA | 17 | 4 (24) | 13 (76) | 0.085 |
| Aspergillus nodules | 22 | 10 (40) | 12 (33) | 0.59 |
| Pulmonary conditionsa | ||||
| Prior tuberculosis, n (%) | 10 | 1 (10) | 9 (90) | 0.037 |
| Asthma, n (%) | 18 | 5 (28) | 13 (72) | 0.18 |
| Pneumothorax, n (%) | 13 | 7 (54) | 6 (46) | 0.29 |
| Bronchiectasis, n (%) | 14 | 5 (36) | 9 (64) | 0.65 |
| COPD, n (%) | 19 | 12 (63) | 7 (37) | 0.018 |
| Recurrent chest infections, n (%) | 12 | 4 (33) | 8 (67) | 0.75 |
| Lung malignancy, n (%) | 2 | 0 | 2 (100) | 0.51 |
| Previous lung surgery, n (%) | 9 | 5 (56) | 4 (44) | 0.47 |
| Fungal asthma, n (%) | 5 | 1 (20) | 4 (80) | 0.64 |
| Pneumonia, n (%) | 9 | 0 | 9 (100) | 0.006 |
| Lung abscess, n (%) | 5 | 2 (40) | 3 (60) | 1 |
| Number of pulmonary conditions, mean (SD) | 1.9 (1.2) | 1.7 (1) | 2 (1.3) | 0.24 |
| Extrapulmonary conditions, n (%) | ||||
| Mannose binding lectin deficient | 13 | 6 (46) | 7 (54) | 0.70 |
| Gamma interferon deficient | 3 | 0 | 3 (100) | 0.26 |
| Diabetes mellitus | 3 | 1 (33) | 2 (67) | 1 |
| Coronary heart disease | 8 | 3 (38) | 5 (62) | 1 |
| Hypertension | 10 | 4 (40) | 6 (60) | 1 |
| Autoimmune disorder | 2 | 1 (50) | 1 (50) | 1 |
| Rheumatoid arthritis | 4 | 2 (50) | 2 (50) | 1 |
| Non-pulmonary malignancy | 8 | 2 (25) | 6 (75) | 0.45 |
| . | All (n = 61) . | Relapse (n = 25) . | Non-relapse (n = 36) . | P-value . |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 28 | 14 (50) | 14 (50) | 0.12 |
| Female | 33 | 11 (33) | 22 (67) | |
| Age (years), mean (range) | 55.5 (26–84) | 58 (34–77) | 53.8 (26–84) | 0.22 |
| Category of CPA, n (%) | ||||
| Simple aspergilloma | 28 | 12 (43) | 16 (57) | 0.78 |
| CCPA | 17 | 4 (24) | 13 (76) | 0.085 |
| Aspergillus nodules | 22 | 10 (40) | 12 (33) | 0.59 |
| Pulmonary conditionsa | ||||
| Prior tuberculosis, n (%) | 10 | 1 (10) | 9 (90) | 0.037 |
| Asthma, n (%) | 18 | 5 (28) | 13 (72) | 0.18 |
| Pneumothorax, n (%) | 13 | 7 (54) | 6 (46) | 0.29 |
| Bronchiectasis, n (%) | 14 | 5 (36) | 9 (64) | 0.65 |
| COPD, n (%) | 19 | 12 (63) | 7 (37) | 0.018 |
| Recurrent chest infections, n (%) | 12 | 4 (33) | 8 (67) | 0.75 |
| Lung malignancy, n (%) | 2 | 0 | 2 (100) | 0.51 |
| Previous lung surgery, n (%) | 9 | 5 (56) | 4 (44) | 0.47 |
| Fungal asthma, n (%) | 5 | 1 (20) | 4 (80) | 0.64 |
| Pneumonia, n (%) | 9 | 0 | 9 (100) | 0.006 |
| Lung abscess, n (%) | 5 | 2 (40) | 3 (60) | 1 |
| Number of pulmonary conditions, mean (SD) | 1.9 (1.2) | 1.7 (1) | 2 (1.3) | 0.24 |
| Extrapulmonary conditions, n (%) | ||||
| Mannose binding lectin deficient | 13 | 6 (46) | 7 (54) | 0.70 |
| Gamma interferon deficient | 3 | 0 | 3 (100) | 0.26 |
| Diabetes mellitus | 3 | 1 (33) | 2 (67) | 1 |
| Coronary heart disease | 8 | 3 (38) | 5 (62) | 1 |
| Hypertension | 10 | 4 (40) | 6 (60) | 1 |
| Autoimmune disorder | 2 | 1 (50) | 1 (50) | 1 |
| Rheumatoid arthritis | 4 | 2 (50) | 2 (50) | 1 |
| Non-pulmonary malignancy | 8 | 2 (25) | 6 (75) | 0.45 |
One patient in relapse group had sarcoidosis. History of non-tuberculous mycobacterium infection and chest trauma was found in 1 patient in non-relapse group, respectively.
CCPA: chronic cavitary pulmonary aspergillosis; COPD: chronic obstructive pulmonary disease; CPA: chronic pulmonary aspergillosis; SD: standard deviation.
The main indications for surgery included the failure of antifungal therapy (51%, n = 31) and the suspicion of lung malignancy (38%, n = 23) (Table 2). Patients who failed antifungal therapy reported recurrent haemoptysis (61%, n = 19) and adverse effects of therapy (45%, n = 14) or carried a resistant isolate of Aspergillus fumigatus (26%, n = 8) and had radiological deterioration of the lung lesion(s) (10%, n = 3). None of the patients with suspected malignancy had received antifungal therapy prior to surgery. Of these, 13% (n = 3) reported haemoptysis.
| Indications . | All (n = 61) . | Relapse (n = 25), n (%) . | Non-relapsea (n = 36), n (%) . | P-value . |
|---|---|---|---|---|
| Localized disease | 4 | 2 (50) | 2 (50) | 1 |
| Long-term immunosuppression | 4 | 2 (50) | 2 (50) | 1 |
| Failed therapy | 31 | 7 (23) | 24 (77) | 0.003 |
| Resistant isolates | 8 | 0 | 8 (100) | 0.017 |
| Recurrent haemoptysis | 19 | 4 (21) | 15 (79) | 0.033 |
| Adverse reactions | 14 | 2 (14) | 12 (86) | 0.021 |
| Increase size of the lesion | 3 | 2 (67) | 1 (33) | 0.56 |
| Presumed malignancy | 23 | 15 (65) | 8 (35) | 0.003 |
| Indications . | All (n = 61) . | Relapse (n = 25), n (%) . | Non-relapsea (n = 36), n (%) . | P-value . |
|---|---|---|---|---|
| Localized disease | 4 | 2 (50) | 2 (50) | 1 |
| Long-term immunosuppression | 4 | 2 (50) | 2 (50) | 1 |
| Failed therapy | 31 | 7 (23) | 24 (77) | 0.003 |
| Resistant isolates | 8 | 0 | 8 (100) | 0.017 |
| Recurrent haemoptysis | 19 | 4 (21) | 15 (79) | 0.033 |
| Adverse reactions | 14 | 2 (14) | 12 (86) | 0.021 |
| Increase size of the lesion | 3 | 2 (67) | 1 (33) | 0.56 |
| Presumed malignancy | 23 | 15 (65) | 8 (35) | 0.003 |
One case of simple aspergilloma from non-relapse group was found during surgery for pneumothorax management.
| Indications . | All (n = 61) . | Relapse (n = 25), n (%) . | Non-relapsea (n = 36), n (%) . | P-value . |
|---|---|---|---|---|
| Localized disease | 4 | 2 (50) | 2 (50) | 1 |
| Long-term immunosuppression | 4 | 2 (50) | 2 (50) | 1 |
| Failed therapy | 31 | 7 (23) | 24 (77) | 0.003 |
| Resistant isolates | 8 | 0 | 8 (100) | 0.017 |
| Recurrent haemoptysis | 19 | 4 (21) | 15 (79) | 0.033 |
| Adverse reactions | 14 | 2 (14) | 12 (86) | 0.021 |
| Increase size of the lesion | 3 | 2 (67) | 1 (33) | 0.56 |
| Presumed malignancy | 23 | 15 (65) | 8 (35) | 0.003 |
| Indications . | All (n = 61) . | Relapse (n = 25), n (%) . | Non-relapsea (n = 36), n (%) . | P-value . |
|---|---|---|---|---|
| Localized disease | 4 | 2 (50) | 2 (50) | 1 |
| Long-term immunosuppression | 4 | 2 (50) | 2 (50) | 1 |
| Failed therapy | 31 | 7 (23) | 24 (77) | 0.003 |
| Resistant isolates | 8 | 0 | 8 (100) | 0.017 |
| Recurrent haemoptysis | 19 | 4 (21) | 15 (79) | 0.033 |
| Adverse reactions | 14 | 2 (14) | 12 (86) | 0.021 |
| Increase size of the lesion | 3 | 2 (67) | 1 (33) | 0.56 |
| Presumed malignancy | 23 | 15 (65) | 8 (35) | 0.003 |
One case of simple aspergilloma from non-relapse group was found during surgery for pneumothorax management.
The final diagnoses of the 61 patients set after the surgery were simple aspergilloma (46%, n = 28), chronic cavitary pulmonary aspergillosis (CCPA; 28%, n = 17) and Aspergillus nodules (36%, n = 22). Aspergillus hyphae were seen in lung tissue using Grocott stain in 56 (92%) patients. The histopathological analyses of the samples from patients with suspected malignancies showed septate fungal hyphae suggestive of Aspergillus spp. complex in all cases.
Prior to surgery, cough (67%, n = 41), haemoptysis (39%, n = 24) and dyspnoea (38%, n = 23) were the most common symptoms (Table 3). At 6–8 months after surgery, 23 (38%) patients experienced cough, 18 (30%) patients had dyspnoea and 6 (10%) patients had haemoptysis.
| . | Before surgery, n (%) . | After surgery, n (%) . | P-value . |
|---|---|---|---|
| Non-relapse (n = 36) | |||
| Haemoptysis | 16 (44) | 4 (11) | <0.001 |
| Cough | 27 (75) | 13 (36) | 0.01 |
| Dyspnoea | 16 (44) | 8 (22) | 0.057 |
| Chest pain | 4 (11) | 5 (14) | 1 |
| Asymptomatic | 2 (6) | 13 (36) | 0.003 |
| Relapse (n = 25) | |||
| Haemoptysis | 8 (32) | 2 (8) | 0.031 |
| Cough | 14 (56) | 10 (40) | 0.29 |
| Dyspnoea | 7 (28) | 10 (40) | 0.45 |
| Chest pain | 3 (12) | 3 (12) | 1 |
| Asymptomatic | 3 (12) | 8 (32) | 0.13 |
| . | Before surgery, n (%) . | After surgery, n (%) . | P-value . |
|---|---|---|---|
| Non-relapse (n = 36) | |||
| Haemoptysis | 16 (44) | 4 (11) | <0.001 |
| Cough | 27 (75) | 13 (36) | 0.01 |
| Dyspnoea | 16 (44) | 8 (22) | 0.057 |
| Chest pain | 4 (11) | 5 (14) | 1 |
| Asymptomatic | 2 (6) | 13 (36) | 0.003 |
| Relapse (n = 25) | |||
| Haemoptysis | 8 (32) | 2 (8) | 0.031 |
| Cough | 14 (56) | 10 (40) | 0.29 |
| Dyspnoea | 7 (28) | 10 (40) | 0.45 |
| Chest pain | 3 (12) | 3 (12) | 1 |
| Asymptomatic | 3 (12) | 8 (32) | 0.13 |
| . | Before surgery, n (%) . | After surgery, n (%) . | P-value . |
|---|---|---|---|
| Non-relapse (n = 36) | |||
| Haemoptysis | 16 (44) | 4 (11) | <0.001 |
| Cough | 27 (75) | 13 (36) | 0.01 |
| Dyspnoea | 16 (44) | 8 (22) | 0.057 |
| Chest pain | 4 (11) | 5 (14) | 1 |
| Asymptomatic | 2 (6) | 13 (36) | 0.003 |
| Relapse (n = 25) | |||
| Haemoptysis | 8 (32) | 2 (8) | 0.031 |
| Cough | 14 (56) | 10 (40) | 0.29 |
| Dyspnoea | 7 (28) | 10 (40) | 0.45 |
| Chest pain | 3 (12) | 3 (12) | 1 |
| Asymptomatic | 3 (12) | 8 (32) | 0.13 |
| . | Before surgery, n (%) . | After surgery, n (%) . | P-value . |
|---|---|---|---|
| Non-relapse (n = 36) | |||
| Haemoptysis | 16 (44) | 4 (11) | <0.001 |
| Cough | 27 (75) | 13 (36) | 0.01 |
| Dyspnoea | 16 (44) | 8 (22) | 0.057 |
| Chest pain | 4 (11) | 5 (14) | 1 |
| Asymptomatic | 2 (6) | 13 (36) | 0.003 |
| Relapse (n = 25) | |||
| Haemoptysis | 8 (32) | 2 (8) | 0.031 |
| Cough | 14 (56) | 10 (40) | 0.29 |
| Dyspnoea | 7 (28) | 10 (40) | 0.45 |
| Chest pain | 3 (12) | 3 (12) | 1 |
| Asymptomatic | 3 (12) | 8 (32) | 0.13 |
The details of the surgical procedures are shown in Table 4. Seventeen complications developed in 15 patients (25%). One patient with CCPA suffered from both pleural infection and bronchopleural fistula and subsequently relapsed. Pulmonary embolism and prolonged air leak were observed in 1 patient with pulmonary nodules in the non-relapse group. The most common complication of surgery was pleural infection (12%, n = 7). The most common surgical techniques performed in patients with complications were lobectomy (15%, n = 9) compared to wedge resection (7%, n = 4), decortications (2%, n = 1) and pneumonectomy (2%, n = 1). CCPA was the most common CPA syndrome observed in patients with surgical complications (15%, n = 9) including 1 patient with a mixed type of CCPA and nodule lesion on radiography.
| Surgical procedures . | All (n = 61), n (%) . | Relapse (n = 25), n (%) . | Remission (n = 36), n (%) . | P-value . |
|---|---|---|---|---|
| Wedge resection | 17 (28) | 9 (36) | 8 (22) | 0.24 |
| Lobectomy | 39 (64) | 14 (56) | 25 (69) | 0.28 |
| Segmentectomy | 3 (5) | 2 (8) | 1 (3) | 0.56 |
| Pneumonectomy | 3 (5) | 0 | 3 (8) | 0.26 |
| Decortication | 2 (3) | 0 | 2 (6) | 0.51 |
| Surgical procedures . | All (n = 61), n (%) . | Relapse (n = 25), n (%) . | Remission (n = 36), n (%) . | P-value . |
|---|---|---|---|---|
| Wedge resection | 17 (28) | 9 (36) | 8 (22) | 0.24 |
| Lobectomy | 39 (64) | 14 (56) | 25 (69) | 0.28 |
| Segmentectomy | 3 (5) | 2 (8) | 1 (3) | 0.56 |
| Pneumonectomy | 3 (5) | 0 | 3 (8) | 0.26 |
| Decortication | 2 (3) | 0 | 2 (6) | 0.51 |
CPA: chronic pulmonary aspergillosis.
| Surgical procedures . | All (n = 61), n (%) . | Relapse (n = 25), n (%) . | Remission (n = 36), n (%) . | P-value . |
|---|---|---|---|---|
| Wedge resection | 17 (28) | 9 (36) | 8 (22) | 0.24 |
| Lobectomy | 39 (64) | 14 (56) | 25 (69) | 0.28 |
| Segmentectomy | 3 (5) | 2 (8) | 1 (3) | 0.56 |
| Pneumonectomy | 3 (5) | 0 | 3 (8) | 0.26 |
| Decortication | 2 (3) | 0 | 2 (6) | 0.51 |
| Surgical procedures . | All (n = 61), n (%) . | Relapse (n = 25), n (%) . | Remission (n = 36), n (%) . | P-value . |
|---|---|---|---|---|
| Wedge resection | 17 (28) | 9 (36) | 8 (22) | 0.24 |
| Lobectomy | 39 (64) | 14 (56) | 25 (69) | 0.28 |
| Segmentectomy | 3 (5) | 2 (8) | 1 (3) | 0.56 |
| Pneumonectomy | 3 (5) | 0 | 3 (8) | 0.26 |
| Decortication | 2 (3) | 0 | 2 (6) | 0.51 |
CPA: chronic pulmonary aspergillosis.
Intraoperative spillage of the fungal material occurred in 3 patients. Two of these patients developed complications after surgery (pleural infection and persistent pneumothorax). These patients received intravenous amphotericin B or intravenous micafungin subsequently after surgery and recovered well.
Overall, 25 patients relapsed after surgery giving a recurrence rate of 41%. The mean time to relapse was 49.1 months (95% CI 27–71.1 months) after surgery. Risk factor analysis (univariable) revealed that COPD was a risk factor for relapse, whereas TB sequelae and pneumonia were protective. The multivariable logistic model showed that pneumonia (OR 0.033, 95% CI 0.002–0.496; P = 0.014) and antifungal therapy any time before surgery (OR 0.072, 95% CI 0.011–0.465; P = 0.006) were significant, but COPD and TB sequelae were not. Surgical complications in the relapse and non-relapse groups occurred in 8 patients and 7 patients, respectively.
Antifungal therapy
A total of 40 (66%) patients received antifungal therapy before, perioperatively and/or after surgery. Of them, 4 patients received antifungal only within 6 months before surgery, 16 patients received antifungal therapy continuously (within 6 months before surgery, perioperatively and after surgery), 11 patients received antifungal therapy only after surgery, 8 patients received antifungal therapy perioperatively and after surgery and 1 patient received antifungal therapy for 6 months before and after surgery. The duration of antifungal therapy was very variable; 4 patients received an antifungal therapy for <6 months after surgery; and the longest duration was 22 months before surgery, with itraconazole. There were significant differences between the relapse and non-relapse groups with regard to antifungal therapy given within the 6 months before surgery (minimal duration of 3 months or intravenous antifungal therapy of any duration) (P = 0.048), perioperatively (P = 0.010) and both before and after surgery (P = 0.005). We defined the term ‘antifungal therapy any point before surgery’ as antifungal therapy 6 months before surgery and/or perioperatively. Overall, antifungal therapy at any point before surgery was highly protective of relapse (P = 0.002), in contrast to a lack of significant benefit if only given after surgery (P = 0.15) (Table 5).
| Time . | All (n = 61), n (%) . | Relapse (n = 25), n (%) . | Non-relapse (n = 36), n (%) . | P-value* . |
|---|---|---|---|---|
| Any point before surgery | 29 (48) | 6 (24) | 23 (64) | 0.002 |
| Within 6 months | 21 (34) | 5 (20) | 16 (44) | 0.048 |
| Perioperative | 24 (39) | 5 (20) | 19 (53) | 0.010 |
| After surgery | 36 (59) | 12 (48) | 24 (67) | 0.15 |
| Before and after surgery | 25 (59) | 5 (48) | 20 (67) | 0.005 |
| Any point related to surgery | 40 (66) | 13 (52) | 27 (75) | 0.063 |
| Time . | All (n = 61), n (%) . | Relapse (n = 25), n (%) . | Non-relapse (n = 36), n (%) . | P-value* . |
|---|---|---|---|---|
| Any point before surgery | 29 (48) | 6 (24) | 23 (64) | 0.002 |
| Within 6 months | 21 (34) | 5 (20) | 16 (44) | 0.048 |
| Perioperative | 24 (39) | 5 (20) | 19 (53) | 0.010 |
| After surgery | 36 (59) | 12 (48) | 24 (67) | 0.15 |
| Before and after surgery | 25 (59) | 5 (48) | 20 (67) | 0.005 |
| Any point related to surgery | 40 (66) | 13 (52) | 27 (75) | 0.063 |
P-values <0.0083 were considered statistically significant.
| Time . | All (n = 61), n (%) . | Relapse (n = 25), n (%) . | Non-relapse (n = 36), n (%) . | P-value* . |
|---|---|---|---|---|
| Any point before surgery | 29 (48) | 6 (24) | 23 (64) | 0.002 |
| Within 6 months | 21 (34) | 5 (20) | 16 (44) | 0.048 |
| Perioperative | 24 (39) | 5 (20) | 19 (53) | 0.010 |
| After surgery | 36 (59) | 12 (48) | 24 (67) | 0.15 |
| Before and after surgery | 25 (59) | 5 (48) | 20 (67) | 0.005 |
| Any point related to surgery | 40 (66) | 13 (52) | 27 (75) | 0.063 |
| Time . | All (n = 61), n (%) . | Relapse (n = 25), n (%) . | Non-relapse (n = 36), n (%) . | P-value* . |
|---|---|---|---|---|
| Any point before surgery | 29 (48) | 6 (24) | 23 (64) | 0.002 |
| Within 6 months | 21 (34) | 5 (20) | 16 (44) | 0.048 |
| Perioperative | 24 (39) | 5 (20) | 19 (53) | 0.010 |
| After surgery | 36 (59) | 12 (48) | 24 (67) | 0.15 |
| Before and after surgery | 25 (59) | 5 (48) | 20 (67) | 0.005 |
| Any point related to surgery | 40 (66) | 13 (52) | 27 (75) | 0.063 |
P-values <0.0083 were considered statistically significant.
Perioperatively, antifungal therapy was administered to 5 patients in the relapse group and to 19 patients in the non-relapse group (P = 0.010). Thirty-six patients received antifungal therapy after surgery: 12 (33%) patients who subsequently relapsed and 24 (67%) patients who did not (P > 0.05). Among the relapse group, the fastest relapse occurred 2 months after the patient stopped antifungal therapy and the longest was 5.5 years after stopping antifungal therapy.
Aspergillus-IgG monitoring
We investigated the association of relapse risk with Aspergillus-IgG titre in 37 patients for whom we had data (Table 6). Among these patients, 12 (32%) patients experienced a CPA relapse and 25 (68%) patients did not. Wilcoxon signed-rank tests were carried out for the 3 pair comparisons of the IgG titres in the relapse groups. At the end of the follow-up, the median Aspergillus-IgG titre was significantly lower than at the time of relapse (67 vs 126 mg/l, P = 0.016). However, the median Aspergillus-IgG titre at the end of follow-up in the non-relapse CPA group was significantly lower compared with before surgery (52 vs 75 mg/l, P = 0.003).
| Group . | Median (IQR) (mg/l) . | P-value* . |
|---|---|---|
| Relapse (n = 12) | ||
| 6 months before relapse | 78 (33–91) | 0.22 |
| Relapse time | 126 (79.8–198) | |
| End of follow-up | 67 (44–117.3) | |
| Two times comparison for relapse | ||
| 6 months before relapse versus relapse time | 0.18 | |
| Relapse time versus end of follow-up | 0.016 | |
| 6 months before relapse versus end of follow-up | 0.92 | |
| Non-relapse (n = 25) | ||
| Before surgery | 75 (55.3–156) | 0.003 |
| End of follow-up | 52 (34–75) | |
| Group . | Median (IQR) (mg/l) . | P-value* . |
|---|---|---|
| Relapse (n = 12) | ||
| 6 months before relapse | 78 (33–91) | 0.22 |
| Relapse time | 126 (79.8–198) | |
| End of follow-up | 67 (44–117.3) | |
| Two times comparison for relapse | ||
| 6 months before relapse versus relapse time | 0.18 | |
| Relapse time versus end of follow-up | 0.016 | |
| 6 months before relapse versus end of follow-up | 0.92 | |
| Non-relapse (n = 25) | ||
| Before surgery | 75 (55.3–156) | 0.003 |
| End of follow-up | 52 (34–75) | |
P-values <0.017 were considered statistically significant for ‘two times comparison for relapse’.
IQR: interquartile range.
| Group . | Median (IQR) (mg/l) . | P-value* . |
|---|---|---|
| Relapse (n = 12) | ||
| 6 months before relapse | 78 (33–91) | 0.22 |
| Relapse time | 126 (79.8–198) | |
| End of follow-up | 67 (44–117.3) | |
| Two times comparison for relapse | ||
| 6 months before relapse versus relapse time | 0.18 | |
| Relapse time versus end of follow-up | 0.016 | |
| 6 months before relapse versus end of follow-up | 0.92 | |
| Non-relapse (n = 25) | ||
| Before surgery | 75 (55.3–156) | 0.003 |
| End of follow-up | 52 (34–75) | |
| Group . | Median (IQR) (mg/l) . | P-value* . |
|---|---|---|
| Relapse (n = 12) | ||
| 6 months before relapse | 78 (33–91) | 0.22 |
| Relapse time | 126 (79.8–198) | |
| End of follow-up | 67 (44–117.3) | |
| Two times comparison for relapse | ||
| 6 months before relapse versus relapse time | 0.18 | |
| Relapse time versus end of follow-up | 0.016 | |
| 6 months before relapse versus end of follow-up | 0.92 | |
| Non-relapse (n = 25) | ||
| Before surgery | 75 (55.3–156) | 0.003 |
| End of follow-up | 52 (34–75) | |
P-values <0.017 were considered statistically significant for ‘two times comparison for relapse’.
IQR: interquartile range.
Time of relapse-free survival after surgery
We analysed relapse time or death as an event using the Kaplan–Meier analysis comparing patients who received antifungal therapy at any point before surgery (n = 29, Table 5) and those who had no antifungal therapy before surgery (n = 32) (Fig. 1). The median relapse-free survival time after surgery in patients who received antifungal therapy and those who did not was 88.4 months (95% CI 55.7–121.6 months) and 30.4 months (95% CI 19.7–41 months), respectively (P = 0.003). The relapse rate in the first 3 years of observation after surgery (33%, n = 20) was higher than in the period 3–10 years after surgery (8%, n = 5).
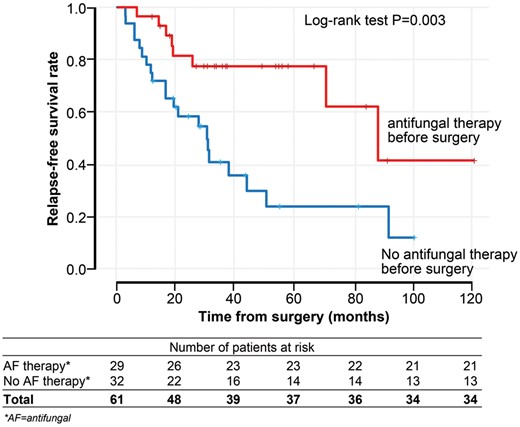
The relapse-free survival rate according to administration of AF therapy before surgery. AF: antifungal.
The 1-, 3-, 5- and 10-year observed overall survival rates were 100%, 93%, 93% and 92%, respectively. There were no intraoperative deaths during the study period. The mortality rate after surgery was 8% (5 patients). CPA relapse contributed to death in 1 patient at 17 months after surgery. Cause of death was unclear in 4 patients since these patients showed no evidence of CPA relapse at the last observation, one of them having been discharged from clinic 54 months after surgery. Of these 4 patients, 1 patient died at 14 months, 1 patient died at 18 months, another patient died at 19 months and 1 patient died at 88 months after surgery.
DISCUSSION
CPA is the result of a slowly destructive process of the lung parenchyma, mostly occurring in patients with a pre-existing cavity as a complication of other lung diseases [22]. Prior tuberculosis, non-tuberculous mycobacterial infection or pneumothorax or concurrent COPD are several of the most common underlying lung diseases related to CPA [22, 23]. Two main factors to consider in the management of CPA are the clinical presentation of CPA and the applicability of the surgical option [6, 11]. A recent study reported bilateral CPA as a risk factor of relapse following cessation of antifungal therapy [24]. Patients who experienced failed therapy as indications for CPA surgery relapsed less often (P = 0.003) in our study. In particular, none of the 8 patients with azole resistance documented prior to surgery relapsed. Presumed malignancy was a more common reason for surgery in those who relapsed (60%, n = 15) compared to those who did not (22%, n = 8) (P = 0.003) as shown in Table 2.
COPD was found to be the most common underlying disease in this study (31%). Previous series have noted COPD as the frequent underlying disease in patients with CPA [11, 12, 25]. Pneumonia and antifungal therapy any time before surgery appear to be protective of relapse whereas COPD and other underlying diseases were neutral. Our data are most consistent for a protective role of antifungal therapy at any point before surgery or a combination of before and after CPA surgery.
Most of the current literature regarding CPA focuses on the clinical presentation of the patients before surgery [10, 16, 20, 25, 26]. There is no study published comparing signs and symptoms of patients before and after surgery. As others have found, haemoptysis and cough were 2 main complaints of patients [11, 17]. Overall, 61 patients showed a significant decrease in haemoptysis and cough and more were asymptomatic at 6–8 months after surgery. The decrease in the number of patients complaining of cough and haemoptysis is significant between before and after surgery in all 61 patients and in the non-relapse group (n = 36) (P < 0.05). Haemoptysis decreased significantly in the relapse group after surgery (Table 3). These data support the value of CPA surgery in improving the quality of life by decreasing pulmonary symptoms. We found that the signs and symptoms of CPA at 6–8 months after surgery are present more often in those who relapse. Patients with persistent symptoms after 6–8 months should be evaluated for relapse on an ongoing basis.
While most studies in the literature divide CPA surgery into simple and complex pulmonary aspergilloma [11–13, 16, 25], we focused on the long-term monitoring of patients after surgery, including monitoring of Aspergillus-IgG titre. This is the first study reporting the Aspergillus-IgG titre monitoring in CPA surgery patients. Six months before relapse, the median Aspergillus-IgG titre was lower than at relapse time (78 vs 126 mg/l), but this difference is not significant (P = 0.18). In those who did not relapse, the Aspergillus-IgG titres decreased significantly compared to baseline titre before surgery. Aspergillus-IgG titre may be useful as a marker for monitoring CPA progression, but our conclusions are limited by the small sample size (n = 37).
The relapse rate in this study (41%) is higher than in our prior series (26%) [11]. Several factors could explain this observation. One possible explanation for this might be that more than one-third of the population were initially misdiagnosed with lung cancer and received no antifungal therapy before surgery. Overall, 60% of patients with presumed malignancy experienced CPA relapse. It can therefore be assumed that antifungal therapy before surgery is beneficial to prevent CPA relapse. Another factor contributing to the high relapse rate is a longer follow-up period—the mean duration of our series is 36 months (standard deviation: 3–121 months). A previous study with 30 months of median follow-up reported no CPA relapse after surgery [25]. Another possible explanation for these results may be that more advanced and more complex CPA cases are referred to our centre as we are the national referral centre for the UK.
There is no consensus about antifungal therapy duration for CPA surgery cases. Every centre has their own protocol, and it probably varies according to each patient’s condition. This is the first study that analysed 3 different time points of antifungal therapy (before, perioperatively and after surgery) correlated with the clinical outcome of patients. Various studies have reviewed the use of antifungal therapy; most of them showed no positive findings regarding antifungal therapy and surgery outcome [19, 27, 28]. However, new, more active regimens such as voriconazole were not included in these studies. One study found a benefit of antifungal therapy given 2 weeks before surgery and 3 months after surgery, but the number of patients was limited (7 patients) [29]. A recent study found a positive effect of perioperative antifungal in 55 patients but most of those who progressed or died without antifungal therapy had subacute invasive aspergillosis, not CPA [20]. Compared to other series [12, 20, 29], we have better evidence supporting a positive effect of antifungal therapy with a larger number of patients and more detail on the timing of antifungal administration. Antifungal therapy at any point before surgery may provide 88.4 months relapse free after surgery. We recommend administering antifungal therapy at least before surgery (at least 3 months of oral therapy or intravenous with any duration, or during the perioperative period) or continuously both before and after surgery to optimize the outcome of CPA surgery.
CONCLUSION
In summary, this study has identified a good outcome of CPA surgery in terms of clinical symptoms and the decrease of Aspergillus-IgG level, especially in those who do not relapse. The high relapse rate possibly reflects the complexities of CPA cases managed at the NAC and early detection of relapse. The research has also shown the possible beneficial use of antifungal therapy before or during surgery. The accuracy of preoperative diagnosis is crucial to optimize the early management of CPA. Intense monitoring of patients within 3 years after CPA surgery is important due to the high relapse rate in this time period.
Presented at the 9th Trends in Medical Mycology, Nice, France, 12 October 2019.
ACKNOWLEDGEMENTS
The authors are grateful to Chris Harris and all the staffs at the National Aspergillosis Centre for their help in collecting the data. They also would like to thank John Belcher of the Department of Medical Statistics and Lembaga Pengelola Dana Pendidikan (LPDP), Republik Indonesia, for awarding a scholarship to support the studies of the first author.
FUNDING
The scholarship to support the studies of the first author (FS) was provided by Lembaga Pengelola Dana Pendidikan/Indonesia Endowment Fund for Education (20160222045506). DWD and RR-R are partly supported by the NIHR Manchester Biomedical Research Centre.
Conflict of interest: DWD and family hold Founder shares in F2G Ltd., a University of Manchester spin-out antifungal discovery company.He acts or has recently acted as a consultant to Astellas, Sigma Tau, Basilea, Scynexis, Cidara, Biosergen, Quintiles, Pulmatrix, Pulmocide, and Zambon. In the last 3 years, he has been paid for talks on behalf of Astellas, Dynamiker, Gilead, Merck, and Pfizer. He is a longstanding member of the Infectious Disease Society of America Aspergillosis Guidelines group, the European Society for Clinical Microbiology and Infectious Diseases Aspergillosis Guidelines group, and the British Society for Medical Mycology Standards of Care committee. RR-R reports personal fees from Gilead Sciences and Astellas. FS and RS declare no conflicts of interest.
Author contributions
Findra Setianingrum: Data curation; Formal analysis; Investigation; Methodology; Writing—original draft. Riina Rautemaa-Richardson: Conceptualization; Methodology; Supervision; Writing—review & editing. Rajesh Shah: Data curation; Methodology; Validation; Writing—review & editing. David W. Denning: Conceptualization; Methodology; Supervision; Writing—review & editing.
REFERENCES
Global Action Fund for Fungal Infections. Improving Outcomes for Patients with Fungal Infections Across the World. Geneva: GAFFI,
Abbreviations
- CCPA
Chronic cavitary pulmonary aspergillosis
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- CPA
Chronic pulmonary aspergillosis
- OR
Odds ratio





