-
PDF
- Split View
-
Views
-
Cite
Cite
Patrick O Myers, Hitendu Dave, Oliver Kretschmar, Tornike Sologashvili, Raymond Pfister, René Prêtre, Cylinder mitral and tricuspid valve replacement in neonates and small children, European Journal of Cardio-Thoracic Surgery, Volume 58, Issue 5, November 2020, Pages 964–968, https://doi.org/10.1093/ejcts/ezaa196
Close - Share Icon Share
Abstract
Atrioventricular valve replacement in small children is associated with high morbidity and mortality. There are no prostheses available with a diameter ˂15 mm. This study reports our initial experience with a cylinder valve for mitral and tricuspid valve replacement in infants and small children.
Our cylinder valve was hand-made for patients requiring atrioventricuclar valve replacement with an annulus of <15 mm. A 12-mm Contegra valve was prepared and placed inside a 14-mm Gore-Tex tube graft and sutured on both extremities.
Eight patients were included, with a median age of 6.9 months (range 1 day to 38 months). Four had mitral and 4 had tricuspid valve replacement. All implants were technically successful, with no significant regurgitation, no stenosis and no left ventricular outflow tract obstruction. There were 3 early deaths from low cardiac output, in patients with significant associated lesions (severe neonatal Ebstein’s, pulmonary artery-intact ventricular septum, biventricular conversion from Norwood stage 1). Two patients required early reintervention: 1 for balloon dilatation for stenosis and 1 for reoperation for paravalvular leak. During follow-up, 2 patients had mitral valve replacement with a 16-mm mechanical valve at 9 and 20 months from the cylinder valve implantation. The remaining 2 patients are alive and well 2 years and 2 months after the procedure.
Cylinder valve replacement of atrioventricular valves was feasible without any technical issues. It was successful in getting out of a difficult situation and allows for somatic growth and implantation of a reasonably-sized mechanical prosthesis on the annulus.
INTRODUCTION
Mitral and tricuspid valve repair is always the first option in infants and small children, with improving outcomes [1–3]. Some valves remain unrepairable, and a suitable alternative for replacement is not always available: mechanical valves are only available down to a size 16 and they are associated with significant morbidity and mortality in small children, particularly with supra-annular implantation [4–7]. A hypoplastic annulus cannot always accept such a valve, despite techniques described to increase the mitral annulus [8]. The Melody valve (Medtronic, Minneapolis, MN, USA), a bovine jugular venous valve mounted inside a stent, has been used as an off-label and modified surgical mitral bioprosthetic valve [9–11].
We have used the Contegra (Medtronic, Minneapolis, MN, USA) bovine jugular venous valve tailor-mounted inside a Gore-Tex (Gore, Flagstaff, AZ, USA) tube graft for the replacement of the mitral valve in infants and small children whose native mitral annulus could not accept a mechanical valve. We describe our experience using this valve for mitral or tricuspid valve replacement in small children.
METHODS
Study design
This study is a retrospective analysis of all patients who underwent mitral or tricuspid valve replacement with a cylinder valve at our institution between 2005 and 2018. The primary end point was mortality; the secondary end points were reinterventions or reoperations on the replaced valve. All patients underwent follow-up to death or August 2019.
Ethical statement
The Ethics Committee (EC) approved the submission and publication of this study, and informed consent was waived by EC. Families consented to the off-label use of this tailored valve and alternative options were discussed in detail.
Surgical technique
The heart was accessed through a full median sternotomy, taking down adhesions to the chest wall from prior operations. Cardiopulmonary bypass was initiated with aortic and caval cannulation. The heart was arrested with cold blood cardioplegia. After assessment of the mitral valve and the decision that the valve was not repairable, the anterior leaflet was resected and the annulus sized. If it measured less than the smallest off-the-shelf mechanical valve (15 mm), our cylinder valve was prepared on the back table. A 12-mm Contegra graft was prepared on the back table. During this time, the aortic clamp was released and the heart was left beating. With the mitral annulus fully open, the risk of air embolism is eliminated. The Contegra graft was cut flush with the venous valve limits to shorten its height as much as possible. A 14-mm Gore-Tex tube graft was prepared and cut to the same length. The Contegra valve was placed inside the Gore-Tex tube graft and sutured on extremity in place using a locking continuous 6–0 polypropylene suture. This tube graft provides enough support of the venous valve commissural posts to avoid having to use a stent. Many separate tacking sutures were placed in the intercommissural triangles and in the sinuses, so as to obliterate any space for fluid to accumulate between the venous valve and the tube graft. The final result is illustrated in Fig. 1. In our initial trials, one sinus (the one placed under the aortic annulus) was resected to leave the smallest height possible and avoid left ventricular outflow tract obstruction. However, this did not provide any significant benefit and was abandoned, simplifying the procedure with circular running sutures at the top and bottom of the tube graft. A marking suture was placed at mid-level of the cylinder to facilitate implantation. The cylinder was positioned within the mitral or tricuspid annulus, and the valve was sutured (along the marking suture) using a running 5–0 polypropylene suture. The pliability of the Gore-Tex wall allows the use of a continuous suture, which has the advantage of insuring adapting the annulus on the graft with no distortion. It was not necessary to fix the distal valve to the papillary muscles or apex, as it has been described with Melody mitral valve replacement.
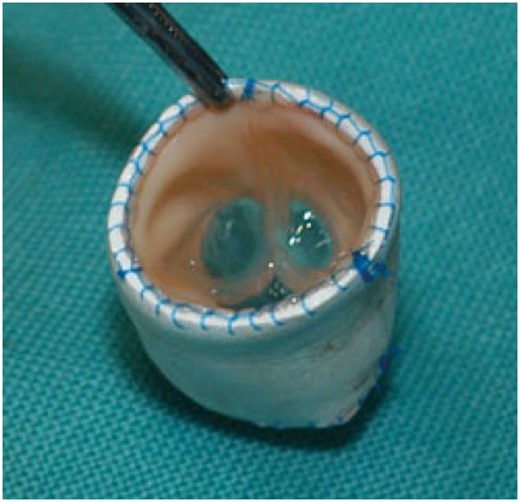
Cylinder valve before implantation. The 12-mm Contegra bovine internal jugular venous valve is trimmed and placed inside a 14-mm Gore-Tex tube graft.
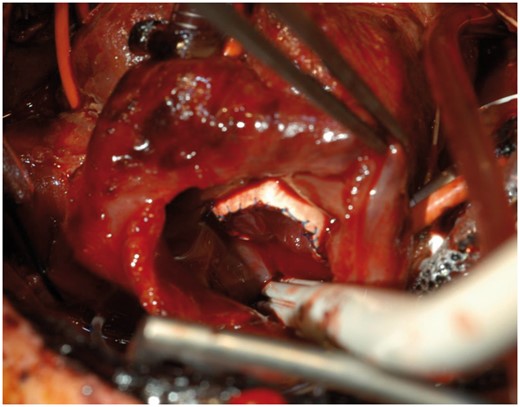
Cylinder valve implanted. View through the right atrium of the valve sutured in place.
Data are presented as mean ± standard deviation or median (range) where appropriate.
RESULTS
Eight patients were included during the study period, with a median age of 6.9 months. Baseline patient characteristics are summarized in Table 1. An implanted cylinder valve is illustrated in Figure 2. Four patients required mitral valve replacement, while 4 required tricuspid replacement. The main mechanism of disease was atrioventricular (AV) valve regurgitation in 4 patients (50%), followed by prosthetic valve dysfunction in 2 patients (25%). This was a difficult patient group, comprising several patients with significant non-cardiac congenital anomalies and all having had prior cardiac operations, including a single-ventricle palliation. One patient was on extracorporeal membrane oxygenation (ECMO) prior to the procedure and was successfully weaned, while 1 patient with biventricular conversion (including aortic valve replacement with a 12-mm Contegra, Damus–Kay–Stansel take-down, tricuspid valve repair, atrial septal defect repair) required ECMO after replacement. Operative procedures are summarized in Table 2. All patients received a 12-mm Contegra valve inside a 14-mm tube graft, with the exception of 2 patients. The first was a 9-month-old girl with severe Ebstein’s anomaly associated with a hypoplastic right ventricle and pulmonary stenosis, with a previously repaired and then replaced tricuspid valve. She presented with degeneration and stenosis of her tricuspid Mosaic bioprosthetic valve. This was replaced with a 22-mm Contegra valve inside a 24-mm tube graft. The second patient, a 17-month old with prior partial AV canal defect repair, who previously required left (mitral) AV replacement with an ATS 16-mm mechanical prosthesis, presented with stenosis of the mechanical prosthesis from pannus overgrowth and regurgitation of the right AV valve. The mitral valve prosthesis was replaced with a 16-mm Contegra inside an 18-mm graft.
| Variables . | Values . |
|---|---|
| Patients (N) | 8 |
| Age (months), median (range) | 6.9 (0–8) |
| Neonates, n (%) | 2 (25) |
| Infantsa, n (%) | 5 (62.5) |
| Small children, n (%) | 3 (37.5) |
| Male:female ratio | 1:1 |
| Valve needing replacement, n (%) | |
| Mitral | 4 (50) |
| Tricuspid | 4 (50) |
| Predominant AV valve disease, n (%) | |
| AV regurgitation | 4 (50) |
| AV stenosis | 1 (12.5) |
| Mixed disease | 1 (12.5) |
| Prosthetic valve dysfunction | 2 (25) |
| Associated anomalies, n (%) | |
| Aortic regurgitation | 1 (12.5) |
| Aortic stenosis | 1 (12.5) |
| Tricuspid regurgitation | 1 (12.5) |
| Atrial septal defect | 1 (12.5) |
| Partial AV canal defect | 2 (25) |
| Ventricular septal defect | 1 (12.5) |
| Ebstein’s anomaly | 3 (37.5) |
| Pulmonary atresia IVS | 1 (12.5) |
| Pulmonary stenosis and hypoplastic RV | 1 (12.5) |
| Non-cardiac anomalies, n (%) | |
| Hirschsprung’s disease | 1 (12.5) |
| Lung hypoplasia | 1 (12.5) |
| Prior interventions, n (%) | |
| Balloon aortic valvuloplasty | 1 (12.5) |
| Stage-1 single-ventricle palliation: Damus–Kay–Stansel, BT shunt, atrial septectomy | 1 (12.5) |
| Mitral valve repair | 1 (12.5) |
| Aortic valve repair | 1 (12.5) |
| AV canal defect repair | 2 (25) |
| Mitral valve replacement | 1 (12.5) |
| Tricuspid valve replacement | 1 (12.5) |
| ECMO | 1 (12.5) |
| Variables . | Values . |
|---|---|
| Patients (N) | 8 |
| Age (months), median (range) | 6.9 (0–8) |
| Neonates, n (%) | 2 (25) |
| Infantsa, n (%) | 5 (62.5) |
| Small children, n (%) | 3 (37.5) |
| Male:female ratio | 1:1 |
| Valve needing replacement, n (%) | |
| Mitral | 4 (50) |
| Tricuspid | 4 (50) |
| Predominant AV valve disease, n (%) | |
| AV regurgitation | 4 (50) |
| AV stenosis | 1 (12.5) |
| Mixed disease | 1 (12.5) |
| Prosthetic valve dysfunction | 2 (25) |
| Associated anomalies, n (%) | |
| Aortic regurgitation | 1 (12.5) |
| Aortic stenosis | 1 (12.5) |
| Tricuspid regurgitation | 1 (12.5) |
| Atrial septal defect | 1 (12.5) |
| Partial AV canal defect | 2 (25) |
| Ventricular septal defect | 1 (12.5) |
| Ebstein’s anomaly | 3 (37.5) |
| Pulmonary atresia IVS | 1 (12.5) |
| Pulmonary stenosis and hypoplastic RV | 1 (12.5) |
| Non-cardiac anomalies, n (%) | |
| Hirschsprung’s disease | 1 (12.5) |
| Lung hypoplasia | 1 (12.5) |
| Prior interventions, n (%) | |
| Balloon aortic valvuloplasty | 1 (12.5) |
| Stage-1 single-ventricle palliation: Damus–Kay–Stansel, BT shunt, atrial septectomy | 1 (12.5) |
| Mitral valve repair | 1 (12.5) |
| Aortic valve repair | 1 (12.5) |
| AV canal defect repair | 2 (25) |
| Mitral valve replacement | 1 (12.5) |
| Tricuspid valve replacement | 1 (12.5) |
| ECMO | 1 (12.5) |
Including neonates.
AV: atrioventricular valve (mitral or tricuspid); BT, Blalock-Taussig; ECMO: extracorporeal membrane oxygenation; IVS: intact ventricular septum; RV: right ventricle.
| Variables . | Values . |
|---|---|
| Patients (N) | 8 |
| Age (months), median (range) | 6.9 (0–8) |
| Neonates, n (%) | 2 (25) |
| Infantsa, n (%) | 5 (62.5) |
| Small children, n (%) | 3 (37.5) |
| Male:female ratio | 1:1 |
| Valve needing replacement, n (%) | |
| Mitral | 4 (50) |
| Tricuspid | 4 (50) |
| Predominant AV valve disease, n (%) | |
| AV regurgitation | 4 (50) |
| AV stenosis | 1 (12.5) |
| Mixed disease | 1 (12.5) |
| Prosthetic valve dysfunction | 2 (25) |
| Associated anomalies, n (%) | |
| Aortic regurgitation | 1 (12.5) |
| Aortic stenosis | 1 (12.5) |
| Tricuspid regurgitation | 1 (12.5) |
| Atrial septal defect | 1 (12.5) |
| Partial AV canal defect | 2 (25) |
| Ventricular septal defect | 1 (12.5) |
| Ebstein’s anomaly | 3 (37.5) |
| Pulmonary atresia IVS | 1 (12.5) |
| Pulmonary stenosis and hypoplastic RV | 1 (12.5) |
| Non-cardiac anomalies, n (%) | |
| Hirschsprung’s disease | 1 (12.5) |
| Lung hypoplasia | 1 (12.5) |
| Prior interventions, n (%) | |
| Balloon aortic valvuloplasty | 1 (12.5) |
| Stage-1 single-ventricle palliation: Damus–Kay–Stansel, BT shunt, atrial septectomy | 1 (12.5) |
| Mitral valve repair | 1 (12.5) |
| Aortic valve repair | 1 (12.5) |
| AV canal defect repair | 2 (25) |
| Mitral valve replacement | 1 (12.5) |
| Tricuspid valve replacement | 1 (12.5) |
| ECMO | 1 (12.5) |
| Variables . | Values . |
|---|---|
| Patients (N) | 8 |
| Age (months), median (range) | 6.9 (0–8) |
| Neonates, n (%) | 2 (25) |
| Infantsa, n (%) | 5 (62.5) |
| Small children, n (%) | 3 (37.5) |
| Male:female ratio | 1:1 |
| Valve needing replacement, n (%) | |
| Mitral | 4 (50) |
| Tricuspid | 4 (50) |
| Predominant AV valve disease, n (%) | |
| AV regurgitation | 4 (50) |
| AV stenosis | 1 (12.5) |
| Mixed disease | 1 (12.5) |
| Prosthetic valve dysfunction | 2 (25) |
| Associated anomalies, n (%) | |
| Aortic regurgitation | 1 (12.5) |
| Aortic stenosis | 1 (12.5) |
| Tricuspid regurgitation | 1 (12.5) |
| Atrial septal defect | 1 (12.5) |
| Partial AV canal defect | 2 (25) |
| Ventricular septal defect | 1 (12.5) |
| Ebstein’s anomaly | 3 (37.5) |
| Pulmonary atresia IVS | 1 (12.5) |
| Pulmonary stenosis and hypoplastic RV | 1 (12.5) |
| Non-cardiac anomalies, n (%) | |
| Hirschsprung’s disease | 1 (12.5) |
| Lung hypoplasia | 1 (12.5) |
| Prior interventions, n (%) | |
| Balloon aortic valvuloplasty | 1 (12.5) |
| Stage-1 single-ventricle palliation: Damus–Kay–Stansel, BT shunt, atrial septectomy | 1 (12.5) |
| Mitral valve repair | 1 (12.5) |
| Aortic valve repair | 1 (12.5) |
| AV canal defect repair | 2 (25) |
| Mitral valve replacement | 1 (12.5) |
| Tricuspid valve replacement | 1 (12.5) |
| ECMO | 1 (12.5) |
Including neonates.
AV: atrioventricular valve (mitral or tricuspid); BT, Blalock-Taussig; ECMO: extracorporeal membrane oxygenation; IVS: intact ventricular septum; RV: right ventricle.
| Characteristics . | Values, n (%) . |
|---|---|
| Concomitant procedures | |
| Biventricular conversion | 1 (12.5) |
| Tricuspid valve repair | 3 (37.5) |
| Fenestrated atrial septal defect repair | 3 (37.5) |
| Transannular monocusp pulmonary valvotomy | 1 (12.5) |
| Right atrial reduction plasty | 1 (12.5) |
| ECMO | 1 (12.5) |
| ECMO weaning and decannulation | 1 (12.5) |
| Valve size | |
| 12-mm Contegra inside 14-mm graft | 6 (75) |
| 16-mm Contegra inside 18-mm graft | 1 (12.5) |
| 22-mm Contegra inside 24-mm graft | 1 (12.5) |
| Characteristics . | Values, n (%) . |
|---|---|
| Concomitant procedures | |
| Biventricular conversion | 1 (12.5) |
| Tricuspid valve repair | 3 (37.5) |
| Fenestrated atrial septal defect repair | 3 (37.5) |
| Transannular monocusp pulmonary valvotomy | 1 (12.5) |
| Right atrial reduction plasty | 1 (12.5) |
| ECMO | 1 (12.5) |
| ECMO weaning and decannulation | 1 (12.5) |
| Valve size | |
| 12-mm Contegra inside 14-mm graft | 6 (75) |
| 16-mm Contegra inside 18-mm graft | 1 (12.5) |
| 22-mm Contegra inside 24-mm graft | 1 (12.5) |
ECMO: extracorporeal membrane oxygenation.
| Characteristics . | Values, n (%) . |
|---|---|
| Concomitant procedures | |
| Biventricular conversion | 1 (12.5) |
| Tricuspid valve repair | 3 (37.5) |
| Fenestrated atrial septal defect repair | 3 (37.5) |
| Transannular monocusp pulmonary valvotomy | 1 (12.5) |
| Right atrial reduction plasty | 1 (12.5) |
| ECMO | 1 (12.5) |
| ECMO weaning and decannulation | 1 (12.5) |
| Valve size | |
| 12-mm Contegra inside 14-mm graft | 6 (75) |
| 16-mm Contegra inside 18-mm graft | 1 (12.5) |
| 22-mm Contegra inside 24-mm graft | 1 (12.5) |
| Characteristics . | Values, n (%) . |
|---|---|
| Concomitant procedures | |
| Biventricular conversion | 1 (12.5) |
| Tricuspid valve repair | 3 (37.5) |
| Fenestrated atrial septal defect repair | 3 (37.5) |
| Transannular monocusp pulmonary valvotomy | 1 (12.5) |
| Right atrial reduction plasty | 1 (12.5) |
| ECMO | 1 (12.5) |
| ECMO weaning and decannulation | 1 (12.5) |
| Valve size | |
| 12-mm Contegra inside 14-mm graft | 6 (75) |
| 16-mm Contegra inside 18-mm graft | 1 (12.5) |
| 22-mm Contegra inside 24-mm graft | 1 (12.5) |
ECMO: extracorporeal membrane oxygenation.
All implants were technically successful, with no evidence of significant paravalvular or transvalvular regurgitation, no stenosis and no left ventricular outflow tract obstruction on post-bypass echocardiography.
There were 3 early deaths, 2 in patients with tricuspid valve replacement and 1 with mitral valve replacement. The first tricuspid patient was the one weaned from ECMO, who presented with persistent low cardiac output and was not a candidate for transplantation. The second was in a neonate with pulmonary atresia, with an intact ventricular septum, severe Ebstein’s anomaly, who underwent augmentation of the right ventricular outflow tract with a monocusp patch and tricuspid valve replacement and was considered a candidate for biventricular repair. The third was in the patient with biventricular conversion, who also presented low cardiac output and was not a transplant candidate. Two patients required an early reintervention on the mitral cylinder valve: one for dilatation with 8- and 12-mm balloons and the other for significant paravalvular leak, which required redoing the cylinder valve replacement with a new prosthesis. The patient with balloon dilatation presented with a mean gradient of 16 mmHg, confirmed by catheterization. The mechanism was stenosis of the distal construct, with fibrosis on the distal Gore-Tex tube. This was dilated with 8- and 12-mm Tyshak II and then 12-mm COEfficient balloons. The final result showed cracking of the distal tube graft fibrosis, with no recoil and reduction in the invasive mean gradient from 21 to 6 mmHg.
During late follow-up, 2 patients required a late reoperation on the mitral cylinder valve, both for mitral valve replacement with a 16-mm mechanical valve, at 9 and 20 months. These patients are thriving at late follow-up, at 9.75 and 9.9 months from their procedure. The remaining 3 patients are alive and well 2 months, 2 years and 10 years after the procedure.
Our protocol was to propose therapeutic anticoagulation for 6 weeks to 3 months (using either vitamin K antagonists or enoxaparin) followed by aspirin only. Among the patients with the longest follow-up, 1 received phenprocoumon for 3 months and then aspirin and 3 received enoxaparin (6 weeks in 1 patient, 3 months in 2 patients) and then aspirin. One of these patients never received aspirin despite this plan and required late replacement of his valve.
DISCUSSION
Mitral and tricuspid valve replacement in small children is challenging. The growth of the mitral annulus at this small age is exponential [12, 13], and currently approved prosthetic valves cannot grow with the child. In combination with borderline left or right heart structures, this makes for a difficult valve replacement, as evidenced by the poor results of mitral valve replacement in small children, with survival at 10 years of 33–75% (5–7) [4–6]. Furthermore, what should be done when the annulus is smaller than the smallest mechanical valve?
The present study reports our surgical experience after mitral or tricuspid valve replacement in infants and small children with a cylinder valve, a customized bovine internal jugular venous valve mounted inside a Gore-Tex tube graft for support. The acute haemodynamic performance of the valve was acceptable, although it was associated with significant early mortality, mainly associated with the high-risk patient population included. With the exception of 2 patients, all had an annulus that was too small to accommodate a mechanical prosthetic valve and they received a 12-mm valve inside a 14-mm tube graft. Among survivors, 2 patients underwent successful mechanical mitral valve replacement with a 16-mm within 2 years from the original replacement. These valves were placed in a modified supra-annular position, with sutures passing through the native annulus in a non-everting fashion and the valve brought down and resting on the atrial aspect of the annulus.
From a technical standpoint, the cylinder valve was easy to handle and position, with no issues of left ventricular outflow tract obstruction and good function of the venous valve. The valve takes time to prepare, and this can only be done after evaluating the mitral valve surgically. Nonetheless, once the native leaflets are removed, the clamp can be removed and preparing the valve can be done on the back table on a beating heart, as the risk of embolism once the native valve is removed is virtually non-existent and helps decrease ischaemic time. We have tested different techniques, at first scalloping out the sinuses to resemble a bioprosthetic valve and avoid outflow tract obstruction; however, this did not prove necessary and we have simplified it, leaving the sinuses intact. Contegra conduits are known for their porous nature, and we were concerned for leakage and fluid accumulation in the space between the valve and the tube graft. We have systematically placed tacking sutures in the intercommissural triangles to help support the venous valve. We have used tacking suture of the free wall to the sinus wall; however, this does not seem to add much and we tailor individually.
Other groups have reported using the Melody valve for mitral valve replacement [9, 11, 14–17]. It has been used in an off-label, modified form as a surgical mitral prosthetic valve [9, 11, 14–19]. The Melody stent takes a significant space within the left side of the heart. Modification in the Melody valve is of paramount importance [9, 20], and includes shortening the ventricular end of the valve [9] or folding over the distal stent struts to avoid disrupting the stent structure [21]. Several modifications in the implantation technique, including fixation to the inferior left ventricular wall [11] or implantation high in the left atrium and patch augmentation of the atrium [15], have also been proposed to avoid outflow tract obstruction. Contrary to the Melody valve, the cylinder valve takes much less space in a hypoplastic heart, and we have not had any issues with outflow tract or pulmonary venous obstruction. One advantage of the Melody valve, though, is that it is balloon expandable. Our cylinder valve is not expandable, although possible developments with radially stretchable Gore-Tex [22] could enable this.
Finally, the bovine jugular vein graft used in our cylinder valve is made of venous valve leaflets that are extremely light and fine (even ‘gossamer’ as described by Emani), requiring relatively small opening and closing forces [20]. These very low forces can be a significant advantage in a borderline hypoplastic heart or severe Ebstein’s anomaly with right ventricular dysfunction or hypoplasia, compared to the significantly larger opening and closing forces necessary to maintain adequate function of bioprosthetic and mechanical valves.
Limitations
This study has several limitations. First, this was a retrospective, non-interventional study designed to evaluate outcomes of an established clinical programme. All patients were managed as individuals and not according to a treatment protocol, which would have improved our ability to analyse outcomes. Our analyses were hampered by the limited patient sample. Nonetheless, this study is the first to look at the use of the cylinder valve in the mitral and tricuspid position in infants and small children.
CONCLUSIONS
Cylinder valve replacement of AV valves, in this initial experience, was feasible without any technical issues or difficulties of implantation. It was successful in getting us out of a difficult situation, without ventricular outflow or pulmonary venous obstruction, and allows for 2 years of somatic growth and implantation of a reasonably-sized mechanical prosthesis on the annulus. Expandable grafts may allow for a balloon-expandable prosthesis without the issues of the place taken by a large stent.
Presented at the 33rd Annual Meeting of the European Association for Cardio-Thoracic Surgery, Lisbon, Portugal, 3–5 October 2019.
ACKNOWLEDGEMENT
We thank Antonio Amodeo from Kinderspital Zurich for assisting in updating the data set.
Conflict of interest: none declared.
Author contributions
Patrick O. Myers: Conceptualization; Formal analysis; Methodology; Writing—original draft. Hitendu Dave: Conceptualization; Data curation; Writing—review & editing. Oliver Kretschmar: Validation; Writing—review & editing. Tornike Sologashvili: Writing—review & editing. Raymond Pfister: Supervision; Writing—review & editing. René Prêtre: Conceptualization; Resources; Validation; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Andrew J. Parry and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
Abbreviations
- left ventricular outflow obstruction
- mitral valve
- balloon dilatation
- tricuspid valve
- lung
- low cardiac output
- mitral valve replacement surgery
- child
- constriction, pathologic
- limb
- follow-up
- infant
- newborn
- repeat surgery
- sutures
- tissue transplants
- vomiting
- morbidity
- mortality
- interventricular septum
- atrioventricular valve
- study report
- replacement of tricuspid valve
- implants
- prostheses





