-
PDF
- Split View
-
Views
-
Cite
Cite
Katsuya Watanabe, Kentaro Sakamaki, Hiroyuki Ito, Tomoyuki Yokose, Kozo Yamada, Haruhiko Nakayama, Munetaka Masuda, Impact of the micropapillary component on the timing of recurrence in patients with resected lung adenocarcinoma, European Journal of Cardio-Thoracic Surgery, Volume 58, Issue 5, November 2020, Pages 1010–1018, https://doi.org/10.1093/ejcts/ezaa138
Close - Share Icon Share
Abstract
A micropapillary (MIP) component is reported to be associated with a poor prognosis in patients with completely resected lung adenocarcinoma. The purpose of this study was to investigate the impact of an MIP component on the timing of postoperative recurrence using hazard curves.
A total of 1289 patients with lung adenocarcinoma who underwent complete pulmonary resection from 2008 to 2015 were studied. Hazard curves representing the changes in hazard over time were evaluated.
The hazard curve displayed an initial wide, high peak within 1 year after surgery in patients with an MIP component, whereas some gentle peaks around the second year were noted in patients without an MIP component. The presence of an MIP component was associated with a worse recurrence-free survival and an early recurrence in stage I patients but not in advanced-stage patients. In multivariable Cox regression, the presence of an MIP component and lymph node metastasis, pleural invasion and gender were associated with a poor prognosis.
Patients with an MIP component retained a high risk of early recurrence after surgery, and the risk for recurrence persisted over the long term. Even after complete resection in stage I lung adenocarcinoma patients, an MIP component remains correlated with a poor prognosis.
INTRODUCTION
Lung cancer is the leading cause of cancer-related death worldwide, and adenocarcinoma is the most common histological type of non-small-cell lung cancer (NSCLC) [1]. Surgery remains the mainstay and most promising treatment for early-stage disease. However, despite curative-intent surgical resection, recurrence often develops. The recurrence risk after surgery for NSCLC changes throughout follow-up, and the pattern of recurrence differs according to the histological type and pathological stage [2, 3].
As most cases of adenocarcinoma have mixed subtypes rather than a single subtype, the outcomes after complete resection vary even among different subsets of patients with the same disease stage [4, 5]. A new histological classification by the International Association for the Study of Lung Cancer, American Thoracic Society and European Respiratory Society characterizes lung adenocarcinoma as a heterogeneous mixture of histological subtypes [6]. Previous studies have indicated that patients with lepidic-predominant subtype had the most favourable outcome, whereas those with solid (SOL)- and micropapillary (MIP)-predominant subtypes showed a worse prognosis [7]. An MIP component, which comprises small papillary (PAP) structures without fibrovascular cores, has been reported to be associated with a high degree of aggressiveness, advanced stage, high maximum standardized uptake value and distant metastasis, and it leads to a poor prognosis even if its pattern is not predominant [8–11].
Few studies have evaluated the relationship between histological subtypes and the changes in recurrence risk over time. In addition, studies focusing on the correlation between MIP components and postoperative recurrence are limited and the number of patients included in previous studies has been relatively small [12, 13]. It would be beneficial to understand the changes in the postoperative recurrence risk over time and how tumour-related factors affect these changes to identify patients at a high risk for early recurrence who would clinically benefit from intensive follow-up.
We therefore investigated the influence of histological subtypes and that of an MIP component on the timing of recurrence using hazard curves.
MATERIALS AND METHODS
Study design
A prospectively maintained surgical database at Kanagawa Cancer Center Hospital was reviewed retrospectively. The Institutional Review Board approved this retrospective study (IRB number: 2019-es-150) and waived the requirement for informed consent from individual patients. Between January 2008 and December 2015, 1809 consecutive patients who underwent complete pulmonary resection were enrolled in this study. Patients who died in the immediate postoperative period (within 30 days after surgery or during the initial hospitalization) were excluded. Ultimately, a total of 1289 patients (605 men, 684 women) with resected lung invasive adenocarcinoma were studied.
Preoperative staging investigations were routinely performed using high-resolution computed tomography (CT) scans of the chest and abdomen, and tumours were staged according to the tumour, lymph node and metastatic Classification of Malignant Tumours, seventh edition [14]. A single primary tumour was diagnosed in all patients, and no patients had a history of lung cancer (excluding those with multicentric cancers) or induction therapy. Sublobar resection was allowed for patients with a ground-glass opacity-dominant tumour on high-resolution CT or those who would otherwise tolerate conventional lobectomy. All other patients underwent lobectomy with lymph node dissection.
Histopathological investigation
Surgically resected specimens were routinely formalin fixed and stained using haematoxylin–eosin stain. According to the IASLC/ATS/ERS classification, each histological subtype and the ratio of histological components in 5% increments were recorded. The predominant subtypes in tumours were classified as lepidic, acinar, PAP, MIP and SOL. These 5 major histological subtypes were further divided into 3 groups based on the prognosis, as follows: low grade (lepidic), intermediate grade (acinar/PAP) and high grade (MIP/SOL).
We defined an MIP component as cells growing in PAP tufts without fibrovascular cores that appeared detached or connected to alveolar walls [6, 8], and the presence of an MIP component was considered when it occupied ≥5% of the entire tumour.
Patient follow-up
A physical examination, chest radiography and CT of the chest and abdomen were performed during the follow-up period. In general, chest radiography was performed every 3–6 months for the first 2 years and then at 6-month intervals or annually thereafter. CT was performed every 6 months in the first 2 years after surgery and annually thereafter. In patients with any signs or symptoms of recurrence, CT of the chest and abdomen, brain magnetic resonance imaging, bone scintigraphy and positron emission tomography were performed as required.
Recurrence was diagnosed based on the results of a physical examination and diagnostic imaging and was confirmed by the pathological examination of biopsy specimens if necessary. Second primary lung cancers (diagnosed when a new lung tumour with different histological features was detected on standard histological and immunological studies or when the clinical scenario was considered more compatible with a new primary tumour than local recurrence) were excluded.
Statistical analysis
Continuous variables were summarized as the mean and standard deviation and categorical variables as the frequencies and percentage. These variables were compared between MIP subgroups using both Student’s t-test and Fisher’s exact test.
The recurrence-free survival (RFS) was defined as the period from surgery until disease recurrence or death. Patients without an event were censored at the time of final follow-up. The RFS was analysed by survival curves and hazard curves. Survival curves were evaluated by the Kaplan–Meier method, and the differences by variables were compared by the log-rank test. To estimate hazard curves, we used the muhaz package for smoothing a hazard function for censored data proposed by Muller and Wang [15]. The period for estimating hazards was ∼1.5 months, which was calculated by default in the muhaz package. Multivariable Cox regression was conducted to evaluate the relationship of an MIP component and the RFS after adjusting for known prognostic factors, with a P-value of <0.1 in a univariable analysis. All analyses were conducted using the SAS 9.4 (SAS Institute, Cary, NC, USA) and R 3.6.2 (R Foundation for Computing, Vienna, Austria) software programmes. P-values <0.05 were considered to be statistically significant.
RESULTS
Clinicopathological features
The clinicopathological characteristics of 1289 patients with resected lung adenocarcinoma are summarized in Table 1; the median age was 69 years (range 23–93 years) and 281 (22.0%) patients had an MIP component of >5%. In brief, male gender, a smoking status, lobectomy or pneumonectomy, advanced-stage disease, intermediate or high malignancy grade and lymph node metastases were more frequent in patients with an MIP component than in those without such a component. The median follow-up was 47.4 months (range 1.1–115.6 months).
| . | Total . | MIP component . | P-value . | |
|---|---|---|---|---|
| Positive (n = 281), n (%) . | Negative (n = 1008), n (%) . | |||
| Age (years), range (median) | 23–93 | 0.821 | ||
| <70 | 684 | 151 (54) | 534 (53) | |
| ≥70 | 605 | 130 (46) | 474 (47) | |
| Gender | <0.001 | |||
| Male | 605 | 163 (58) | 442 (44) | |
| Female | 684 | 118 (42) | 566 (56) | |
| Smoking status | 0.002 | |||
| Former/current | 648 | 165 (59) | 483 (48) | |
| Never | 641 | 116 (41) | 525 (52) | |
| Surgical procedure | <0.001 | |||
| Wedge resection | 205 | 28 (10) | 177 (18) | |
| Segmentectomy | 159 | 17 (6) | 142 (14) | |
| Lobectomy | 916 | 232 (83) | 684 (68) | |
| Pneumonectomy | 9 | 4 (1) | 5 (1) | |
| Pathological stage | <0.001 | |||
| IA | 825 | 107 (38) | 718 (71) | |
| IB | 271 | 83 (30) | 188 (19) | |
| IIA | 83 | 37 (13) | 46 (5) | |
| IIB | 36 | 16 (6) | 20 (2) | |
| IIIA | 74 | 38 (14) | 36 (4) | |
| Malignancy grades | <0.001 | |||
| Low | 620 | 46 (16) | 572 (57) | |
| Intermediate | 526 | 179 (64) | 347 (34) | |
| High | 143 | 54 (19) | 89 (9) | |
| Lymph node metastasis | <0.001 | |||
| Yes | 128 | 69 (25) | 59 (6) | |
| No | 1161 | 212 (75) | 949 (94) | |
| Pleural invasion | <0.001 | |||
| Yes | 232 | 98 (35) | 134 (13) | |
| No | 1057 | 183 (65) | 874 (87) | |
| . | Total . | MIP component . | P-value . | |
|---|---|---|---|---|
| Positive (n = 281), n (%) . | Negative (n = 1008), n (%) . | |||
| Age (years), range (median) | 23–93 | 0.821 | ||
| <70 | 684 | 151 (54) | 534 (53) | |
| ≥70 | 605 | 130 (46) | 474 (47) | |
| Gender | <0.001 | |||
| Male | 605 | 163 (58) | 442 (44) | |
| Female | 684 | 118 (42) | 566 (56) | |
| Smoking status | 0.002 | |||
| Former/current | 648 | 165 (59) | 483 (48) | |
| Never | 641 | 116 (41) | 525 (52) | |
| Surgical procedure | <0.001 | |||
| Wedge resection | 205 | 28 (10) | 177 (18) | |
| Segmentectomy | 159 | 17 (6) | 142 (14) | |
| Lobectomy | 916 | 232 (83) | 684 (68) | |
| Pneumonectomy | 9 | 4 (1) | 5 (1) | |
| Pathological stage | <0.001 | |||
| IA | 825 | 107 (38) | 718 (71) | |
| IB | 271 | 83 (30) | 188 (19) | |
| IIA | 83 | 37 (13) | 46 (5) | |
| IIB | 36 | 16 (6) | 20 (2) | |
| IIIA | 74 | 38 (14) | 36 (4) | |
| Malignancy grades | <0.001 | |||
| Low | 620 | 46 (16) | 572 (57) | |
| Intermediate | 526 | 179 (64) | 347 (34) | |
| High | 143 | 54 (19) | 89 (9) | |
| Lymph node metastasis | <0.001 | |||
| Yes | 128 | 69 (25) | 59 (6) | |
| No | 1161 | 212 (75) | 949 (94) | |
| Pleural invasion | <0.001 | |||
| Yes | 232 | 98 (35) | 134 (13) | |
| No | 1057 | 183 (65) | 874 (87) | |
MIP: micropapillary.
| . | Total . | MIP component . | P-value . | |
|---|---|---|---|---|
| Positive (n = 281), n (%) . | Negative (n = 1008), n (%) . | |||
| Age (years), range (median) | 23–93 | 0.821 | ||
| <70 | 684 | 151 (54) | 534 (53) | |
| ≥70 | 605 | 130 (46) | 474 (47) | |
| Gender | <0.001 | |||
| Male | 605 | 163 (58) | 442 (44) | |
| Female | 684 | 118 (42) | 566 (56) | |
| Smoking status | 0.002 | |||
| Former/current | 648 | 165 (59) | 483 (48) | |
| Never | 641 | 116 (41) | 525 (52) | |
| Surgical procedure | <0.001 | |||
| Wedge resection | 205 | 28 (10) | 177 (18) | |
| Segmentectomy | 159 | 17 (6) | 142 (14) | |
| Lobectomy | 916 | 232 (83) | 684 (68) | |
| Pneumonectomy | 9 | 4 (1) | 5 (1) | |
| Pathological stage | <0.001 | |||
| IA | 825 | 107 (38) | 718 (71) | |
| IB | 271 | 83 (30) | 188 (19) | |
| IIA | 83 | 37 (13) | 46 (5) | |
| IIB | 36 | 16 (6) | 20 (2) | |
| IIIA | 74 | 38 (14) | 36 (4) | |
| Malignancy grades | <0.001 | |||
| Low | 620 | 46 (16) | 572 (57) | |
| Intermediate | 526 | 179 (64) | 347 (34) | |
| High | 143 | 54 (19) | 89 (9) | |
| Lymph node metastasis | <0.001 | |||
| Yes | 128 | 69 (25) | 59 (6) | |
| No | 1161 | 212 (75) | 949 (94) | |
| Pleural invasion | <0.001 | |||
| Yes | 232 | 98 (35) | 134 (13) | |
| No | 1057 | 183 (65) | 874 (87) | |
| . | Total . | MIP component . | P-value . | |
|---|---|---|---|---|
| Positive (n = 281), n (%) . | Negative (n = 1008), n (%) . | |||
| Age (years), range (median) | 23–93 | 0.821 | ||
| <70 | 684 | 151 (54) | 534 (53) | |
| ≥70 | 605 | 130 (46) | 474 (47) | |
| Gender | <0.001 | |||
| Male | 605 | 163 (58) | 442 (44) | |
| Female | 684 | 118 (42) | 566 (56) | |
| Smoking status | 0.002 | |||
| Former/current | 648 | 165 (59) | 483 (48) | |
| Never | 641 | 116 (41) | 525 (52) | |
| Surgical procedure | <0.001 | |||
| Wedge resection | 205 | 28 (10) | 177 (18) | |
| Segmentectomy | 159 | 17 (6) | 142 (14) | |
| Lobectomy | 916 | 232 (83) | 684 (68) | |
| Pneumonectomy | 9 | 4 (1) | 5 (1) | |
| Pathological stage | <0.001 | |||
| IA | 825 | 107 (38) | 718 (71) | |
| IB | 271 | 83 (30) | 188 (19) | |
| IIA | 83 | 37 (13) | 46 (5) | |
| IIB | 36 | 16 (6) | 20 (2) | |
| IIIA | 74 | 38 (14) | 36 (4) | |
| Malignancy grades | <0.001 | |||
| Low | 620 | 46 (16) | 572 (57) | |
| Intermediate | 526 | 179 (64) | 347 (34) | |
| High | 143 | 54 (19) | 89 (9) | |
| Lymph node metastasis | <0.001 | |||
| Yes | 128 | 69 (25) | 59 (6) | |
| No | 1161 | 212 (75) | 949 (94) | |
| Pleural invasion | <0.001 | |||
| Yes | 232 | 98 (35) | 134 (13) | |
| No | 1057 | 183 (65) | 874 (87) | |
MIP: micropapillary.
Survival analyses
A total of 152 (11.8%) of the 1289 patients experienced recurrence. Figure 1A shows the survival curves of recurrence after surgery by malignancy grade. Patients with high-grade tumours had a significantly worse RFS than those with intermediate- and low-grade tumours.
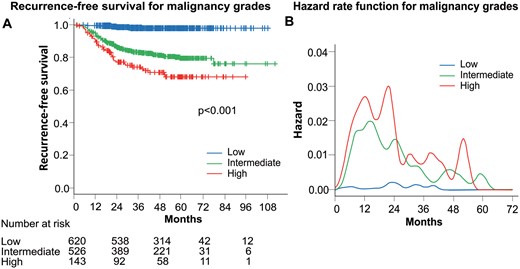
(A) Recurrence-free survival curves according to malignancy grades. (B) Smoothed hazard estimates for each malignancy grade.
Hazard curves by the malignancy grade revealed that the recurrence risk increased steeply towards the first peak around the first year after surgery for both high- and intermediate-grade tumours (Fig. 1B). There was no prominent peak for low-grade tumours during the follow-up period. The absolute magnitude of the maximum peak was highest for high-grade tumours, followed by intermediate- and low-grade tumours. As for the highest peak of recurrence, the hazard curve for intermediate-grade tumours displayed the highest peak ∼15 months after surgery. The maximum risk for high-grade tumours was observed ∼21 months after surgery, which was slightly later than the peak for intermediate-grade tumours.
When analysing the RFS by MIP subgroups, the patients with an MIP component had a significantly poorer RFS than those without an MIP component (P < 0.001) (Fig. 2A). Hazard curves by MIP components showed a sharp and high peak within 1 year after surgery in patients with an MIP component (Fig. 2B). In contrast, the hazard curve for patients without an MIP component showed some gentle peaks during the first 3 years after surgery and recurrence risk remained low through the rest of follow-up. Even though the recurrence risk for patients with an MIP component gradually decreased after the third year following surgery, it remained higher than that for patients without an MIP component for up to 5 years. The hazard curve for intermediate-grade showed that patients with an MIP component had a high risk and an early timing of recurrence (Fig. 2C). In contrast, the risk of recurrence for high-grade tumours with an MIP component peaked later than for those without an MIP component despite carrying a higher risk of recurrence (Fig. 2D).
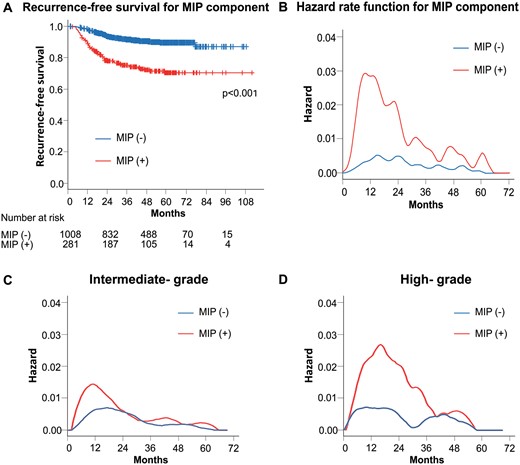
(A) Recurrence-free survival curves according to the presence of MIP component. (B) Smoothed hazard estimates for the presence of MIP component. (C) Smoothed hazard estimates of patients with intermediate-grade tumours according to the presence of an MIP component. (D) Smoothed hazard estimates of patients with high-grade tumours according to the presence of an MIP component. MIP: micropapillary.
The relationship of an MIP component with the RFS was analysed by the pathological stage. The RFS curves showed that the presence of an MIP component was associated with a poor RFS in stage I patients (P < 0.001) (Fig. 3A). However, no statistically significant differences were observed between patients with and without an MIP component among those with stages IIA–IIIA disease (P = 0.357) (Fig. 3B). The hazard curves in stages IA and IB patients showed that patients without an MIP component had a low risk of recurrence throughout the time interval. In contrast, the maximum peak of patients with an MIP component occurred around 1 year after surgery, which is indicative of early recurrence (Fig. 3C). In patients with stages IIA–IIIA, the hazard curves were quite similar between patients with and without an MIP component (Fig. 3D). A high risk of recurrence in patients with an MIP component was also observed in individual variables, such as gender, age, smoking status, surgical procedure, lymph node metastasis and pleural invasion (Supplementary Material, Figs S1–S6).
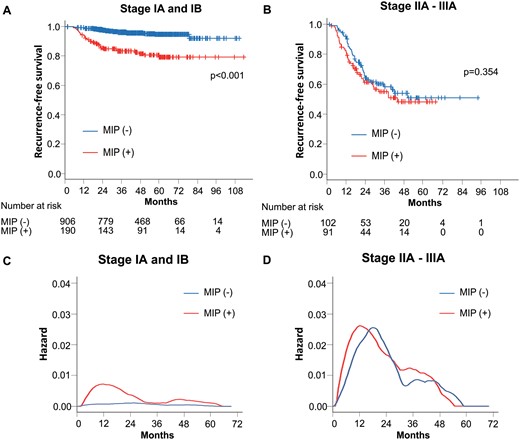
(A) Recurrence-free survival curves of patients with stage I according to the presence of MIP component. (B) Recurrence-free survival curves of patients with stages IIA–IIIA according to the presence of an MIP component. (C) Smoothed hazard estimates of patients with stage I according to the presence of an MIP component. (D) Smoothed hazard estimates of patients with stages IIA–IIIA according to the presence of an MIP component. MIP: micropapillary.
Regarding the surgical procedure in stage I patients, patients who underwent sublobar resection had a better RFS than those who received lobectomy or pneumonectomy (P = 0.004) (Fig. 4A). The RFS was worse for patients with an MIP component than those without an MIP component, regardless of the extent of resection (Fig. 4B and C).
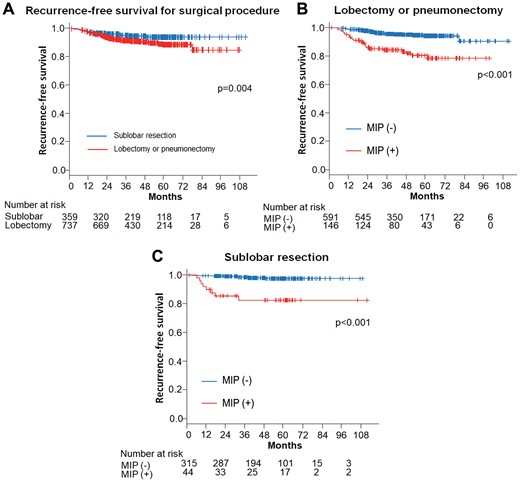
(A) Recurrence-free survival curves of patients with stage I according to the surgical procedure. (B) Recurrence-free survival curves of patients who underwent lobectomy or pneumonectomy according to the presence of MIP component. (C) Recurrence-free survival curves of patients who underwent sublobar resection according to the presence of MIP component. MIP: micropapillary.
The results of a multivariable analysis of the RFS are shown in Table 2. The hazard ratio of the presence of an MIP component was 1.898 (95% confidence interval 1.355–2.659; P < 0.001) in multivariable Cox regression, and an MIP component, as well as the sex, lymph node metastasis and pleural invasion, may be a prognostic factor.
| Variables . | Multivariables . | ||
|---|---|---|---|
| HR . | 95% CI . | P-value . | |
| Age (>70 years) | 1.010 | 0.732–1.393 | 0.954 |
| Gender (male) | 2.032 | 1.304–3.166 | 0.002 |
| Smoking status | 0.907 | 0.583–1.411 | 0.664 |
| Surgical procedure | 1.315 | 0.765–2.258 | 0.322 |
| Micropapillary component | 1.898 | 1.355–2.659 | <0.001 |
| Lymph node metastasis | 5.134 | 3.578–7.367 | <0.001 |
| Pleural invasion | 3.591 | 2.541–5.074 | <0.001 |
| Variables . | Multivariables . | ||
|---|---|---|---|
| HR . | 95% CI . | P-value . | |
| Age (>70 years) | 1.010 | 0.732–1.393 | 0.954 |
| Gender (male) | 2.032 | 1.304–3.166 | 0.002 |
| Smoking status | 0.907 | 0.583–1.411 | 0.664 |
| Surgical procedure | 1.315 | 0.765–2.258 | 0.322 |
| Micropapillary component | 1.898 | 1.355–2.659 | <0.001 |
| Lymph node metastasis | 5.134 | 3.578–7.367 | <0.001 |
| Pleural invasion | 3.591 | 2.541–5.074 | <0.001 |
CI: confidence interval; HR: hazard ratio; RFS: recurrence-free survival.
| Variables . | Multivariables . | ||
|---|---|---|---|
| HR . | 95% CI . | P-value . | |
| Age (>70 years) | 1.010 | 0.732–1.393 | 0.954 |
| Gender (male) | 2.032 | 1.304–3.166 | 0.002 |
| Smoking status | 0.907 | 0.583–1.411 | 0.664 |
| Surgical procedure | 1.315 | 0.765–2.258 | 0.322 |
| Micropapillary component | 1.898 | 1.355–2.659 | <0.001 |
| Lymph node metastasis | 5.134 | 3.578–7.367 | <0.001 |
| Pleural invasion | 3.591 | 2.541–5.074 | <0.001 |
| Variables . | Multivariables . | ||
|---|---|---|---|
| HR . | 95% CI . | P-value . | |
| Age (>70 years) | 1.010 | 0.732–1.393 | 0.954 |
| Gender (male) | 2.032 | 1.304–3.166 | 0.002 |
| Smoking status | 0.907 | 0.583–1.411 | 0.664 |
| Surgical procedure | 1.315 | 0.765–2.258 | 0.322 |
| Micropapillary component | 1.898 | 1.355–2.659 | <0.001 |
| Lymph node metastasis | 5.134 | 3.578–7.367 | <0.001 |
| Pleural invasion | 3.591 | 2.541–5.074 | <0.001 |
CI: confidence interval; HR: hazard ratio; RFS: recurrence-free survival.
DISCUSSION
In the present study, we confirmed that the hazard of postoperative recurrence peaked on multiple occasions after surgery and was not always constant. Despite similar hazard curves for malignancy grades, the peak timing of recurrence appeared earlier in intermediate-grade tumours than in high-grade tumours. This shorter recurrence-free interval in intermediate-grade tumours implies that entities with an aggressive growth nature existed even among those classified as intermediate-grade lesions. Concerning the presence of an MIP component, the hazard of recurrence for patients with an MIP component increased rapidly until ∼8 months after surgery and then decreased gradually thereafter, showing several delayed peaks. However, the recurrence risk remained higher than that for patients without an MIP component throughout the follow-up. Similarly, an early, large peak of recurrence in patients with an MIP component indicated the aggressiveness of adenocarcinoma with an MIP component. In addition, several delayed peaks of recurrence occurred at different times, suggesting the existence of slow-growing tumours with a longer recurrence-free interval as well.
Tumour recurrence after curative-intent surgery remains the primary cause of cancer-related death. One possible reason for this may be that clinically undetectable micrometastasis has already spread to distant sites in the body before surgery, suggesting that an underestimation of the true tumour stage might account for poor outcomes. Kamiya et al. [12] demonstrated that the presence of an MIP component (≥5% of the tumour) is likely to be associated with a high frequency of micrometastasis, as the cells in an MIP component have most likely acquired anoikis resistance (an ability to survive upon detachment) and facilitated anchorage-independent growth (a capacity to proliferate in the vasculature and lymphatic circulation). Zhao et al. [13] showed that the presence of an MIP component is associated with extensive lymph node metastasis and a poor prognosis, even if it is not predominant. Our present results and those of previous studies suggest the significance of focusing first on the predominant subtypes and then on the presence of an MIP component to predict the prognosis and timing of recurrence. In addition, it is necessary to know not only the total recurrence risk but also when recurrence is most likely to occur. Furthermore, it may be useful to identify patients at high risk for early recurrence within the same lung adenocarcinoma histological subtype.
Regarding the postoperative follow-up of NSCLC, at present, there is no clear-cut basis to recommend aggressive screening, and whether the early detection of recurrence contributes to an improved outcome remains unclear [16]. Recently, the development of new anticancer drugs and the advent of molecular-targeted therapy have prolonged the survival [17] and improved the quality of life [18] in patients with advanced, recurrent NSCLC. In addition, several recent investigations have indicated that EGFR mutations are frequently found (from 70.6% to 91.4%) in lung adenocarcinoma with an MIP component [5, 19, 20]. These findings suggest that even in biologically aggressive cases, if the presence of a certain biomarker is confirmed, patients with recurrent adenocarcinoma with an MIP component can be controlled with drug therapy. In Asia in particular, epidermal growth factor receptor (EGFR) mutations are frequently observed. Therefore, considering the effectiveness of treatment by EGFR-tyrosine kinase inhibitors, we may reasonably assume that the early detection and early treatment of recurrence may contribute to an improved quality of life and outcomes. Consequently, an analysis of the risk of recurrence after surgery based on histological subtypes is required for the detection of treatable recurrence while avoiding performing superfluous imaging studies.
To facilitate the early detection and early treatment of recurrence, the optimal postoperative follow-up protocol needs to be established. Our investigation provided more useful information concerning the postoperative follow-up strategy, allowing for the more specific identification of patients at high risk for early recurrence. First, patients with an MIP component had a markedly higher recurrence risk than those without an MIP component, especially during the first 2 years after surgery, and the recurrence risk remained high thereafter. This finding suggests that patients with an MIP component need more intensive surveillance, e.g. hospital visits for taking the patient’s history and performing a physical examination between the interval of CT examinations, throughout the entire postoperative period. Second, the hazard for recurrence in patients without an MIP component did not show an apparent peak during the first 2 years after surgery and remained relatively low. Therefore, a reduced surveillance intensity after 2 years of regular follow-up may be justified for patients without an MIP component. In accordance with currently recommended guidelines, not only CT-based surveillance but also a physical examination should be performed in cases with a high recurrence risk for such cohort of patients, considering the cost–benefit and patient satisfaction.
Another important finding in our study was that the presence of an MIP component was significantly associated with a worse RFS in stage I patients but not in stages IIA–IIIA patients. In addition, stage I patients with an MIP component, even when not a predominant pattern, had a poor prognosis, irrespective of the extent of surgery. We first assumed that the higher rate of advanced-stage patients was somewhat attributed to a worse RFS for patients with an MIP component than that without an MIP component. The difference in RFS among the same stage I patients may be the reason why tumours with an MIP component tend to have poor outcomes; however, a poor RFS in patients with an MIP component was not necessarily due to the high rate of advanced-stage disease in patients with an MIP component.
Concerning the surgical procedure, many studies have demonstrated that patients with a predominant ground-glass opacity component have excellent prognoses and are suitable candidates for sublobar resection [21, 22]. Patients who underwent sublobar resection in the present study had a better RFS than those who underwent lobectomy or pneumonectomy owing to the low percentage of adenocarcinoma with an MIP in patients with sublobar resection than in those with lobectomy or pneumonectomy (12.4% vs 25.5%, respectively). However, importantly, our result demonstrated that stage I patients with an MIP component showed a poor prognosis, regardless of whether complete pulmonary resection or sublobar resection was performed.
Several previous studies have reported that clinical stage I lung adenocarcinoma was pathologically upstaged more frequently in MIP-positive tumours than in MIP-negative tumours because of lymph node metastasis and lung micrometastasis [23, 24], and the presence of an MIP component was shown to be a risk factor for local recurrence in sublobar resection when the surgical margin was <1 cm [25]. These findings and our results imply that adenocarcinoma with an MIP component spreads through the lung tissue and that stage I patients with an MIP component, even after undergoing complete resection, may have a more advanced disease stage due to occult micrometastases not detected preoperatively. Therefore, it is appropriate to investigate the role of postoperative adjuvant chemotherapy for stage I lung adenocarcinoma patients with an MIP component.
Adjuvant chemotherapy is not currently recommended for stage IA patients, and the role of adjuvant chemotherapy in stage IB patients is limited and based on little evidence. Furthermore, the predictive effect of the IASLC/ATS/ERS classification on adjuvant chemotherapy for completely resected patients is still incompletely understood and merits further investigation. Recent studies have shown that patients with MIP/SOL-predominant tumours but not acinar/PAP-predominant tumours obtained a disease-free survival benefit from adjuvant chemotherapy [26–28], thus demonstrating the importance of accurately determining the histological subtype of stage IB lung adenocarcinoma for adjuvant chemotherapy. Finally, given the early peak of recurrence in hazard curves, it should be pointed out that stage I patients with an MIP component may be suitable candidates for adjuvant chemotherapy. Prospective, randomized multi-institutional trials will be needed to evaluate the prognostic and predictive value of histological subtypes for stage I patients benefitting from adjuvant chemotherapy.
Limitations
Several limitations associated with the present study warrant mention. As this study was a retrospective study conducted at a single institution, selection bias may exist. The sample size is also small, considering that only 152 events occurred among 1289 patients. Despite these limitations, however, our study provides preliminary insight into the postoperative follow-up strategy based on minor component subtypes as well as predominant histological subtypes for lung adenocarcinoma.
CONCLUSION
In conclusion, patients with an MIP component are at a high risk of early recurrence after surgery and the recurrence risk persisted over the long term. In addition, patients with stage I lung adenocarcinoma with an MIP component retain a high risk of early recurrence, regardless of the extent of surgery. These results are useful for determining the optimum postoperative follow-up strategy and the future design of clinical trials for aggressive adjuvant chemotherapy.
Supplementary material is available at EJCTS online.
Conflict of interest: none declared.
Author contributions
Katsuya Watanabe: Conceptualization; Investigation; Project administration; Writing—original draft. Kentaro Sakamaki: Formal analysis; Validation. Hiroyuki Ito: Data curation; Investigation; Writing—review & editing. Tomoyuki Yokose: Investigation; Validation. Kozo Yamada: Data curation. Haruhiko Nakayama: Data curation; Investigation; Writing—review & editing. Munetaka Masuda: Supervision.
Presented at the 32nd Annual Meeting of the European Association for Cardio-Thoracic Surgery, Milan, Italy, 18 October 2018.
REFERENCES
ABBREVIATIONS
- CT
Computed tomography
- MIP
Micropapillary
- NSCLC
Non-small-cell lung cancer
- PAP
Papillary
- RFS
Recurrence-free survival
- SOL
Solid





