-
PDF
- Split View
-
Views
-
Cite
Cite
Luis F Tapias, Cameron D Wright, Michael Lanuti, Ashok Muniappan, Daniel Deschler, Douglas J Mathisen, Hyperbaric oxygen therapy in the prevention and management of tracheal and oesophageal anastomotic complications, European Journal of Cardio-Thoracic Surgery, Volume 57, Issue 6, June 2020, Pages 1203–1209, https://doi.org/10.1093/ejcts/ezz364
Close - Share Icon Share
Abstract
Failure of anastomotic healing is a morbid complication after airway or oesophageal surgery. Hyperbaric oxygen therapy (HBOT) has been used extensively in the management of complex wound-healing problems. We demonstrate the use of HBOT to rescue at-risk anastomoses or manage anastomotic failures in thoracic surgery.
Retrospective review of 25 patients who received HBOT as part of the management of tracheal or oesophageal anastomotic problems during 2007–2018. HBOT was delivered at 2 atm with 100% oxygen in 90-min sessions.
Twenty-three patients underwent airway resection and reconstruction while 2 patients underwent oesophagectomy. There were 16 (70%) laryngotracheal and 7 (30%) tracheal resections. Necrosis at the airway anastomosis was found in 13 (57%) patients, partial dehiscence in 2 (9%) patients and both in 6 (26%) patients. HBOT was prophylactic in 2 (9%) patients. Patients received a median of 9.5 HBOT sessions (interquartile range 5–19 sessions) over a median course of 8 days. The airway anastomosis healed in 20 of 23 (87%) patients. Overall, a satisfactory long-term airway outcome was achieved in 19 (83%) patients; 4 patients failed and required reoperation (2 tracheostomies and 1 T-tube). HBOT was used in 2 patients after oesophagectomy to manage focal necrosis or ischaemia at the anastomosis, with success in 1 patient. Complications from HBOT were infrequent and mild (e.g. ear discomfort).
HBOT should be considered as an adjunct in the management of anastomotic problems after airway surgery. It may also play a role after oesophagectomy. Possible mechanisms of action are rapid granulation, early re-epithelialization and angiogenesis.
INTRODUCTION
Tracheal and laryngotracheal resection and reconstruction are demanding procedures. To have a successful outcome requires strict attention to detail, careful patient evaluation, planning and intraoperative technique, as well as dedicated postoperative care [1]. A successful outcome can be achieved in over 90% of patients [2]. The most dreaded complications are those involving the anastomosis. These are observed in ∼9% of patients, with separation of the airway anastomosis occurring in 1–4% [2–4]. Certain patients are considered high risk for airway anastomotic complications [1, 3], and although infrequent, when anastomotic complications do occur, they are devastating. Strategies must be developed for the successful management of complications of the anastomosis that help mitigate permanent consequences such as the need for a tracheostomy or T-tube and voice impairment. Similarly, anastomotic complications after oesophagectomy occur in up to 3.9% of patients in our experience [5]. Approximately, one-third of these patients can be treated non-operatively, while two-thirds will go on to require some form of surgical intervention, which translates into increased morbidity, costs and decreased quality of life for these patients [5]. Effective strategies are needed to salvage at-risk anastomoses in thoracic surgery.
Systemic hyperbaric oxygen therapy (HBOT) consists of exposing patients to a pressurized environment, typically at 2–2.5 atmospheres, breathing 100% oxygen [6]. This results in substantial amounts of oxygen dissolved directly into the serum, reaching supraphysiological pO2 levels, which translates into a significant increase in total oxygen content, a more favourable oxygen diffusion gradient and increased oxygenation at the cellular level [6]. As such, HBOT is now an accepted treatment in the management of complex wound-healing problems where tissue hypoxia and ischaemia play a central role, like refractory chronic diabetic wounds, necrotizing soft tissue infections and radiation-induced necrosis and crush injuries [6]. Likewise, a large contributor to anastomotic failure is tissue ischaemia where HBOT may play a role to counteract its effects.
We have developed a strategy for very specific indications involving airway anastomotic complications that involves the use of HBOT. Since the institution of this treatment plan, success has been achieved in roughly 90% of patients with airway anastomotic problems. The main purpose of the present study is to present our experience with the use of HBOT in the prevention and management of anastomotic complications after tracheal surgery, while describing exploratory results of its application in the management of complications after oesophagectomy. This study expands on a previous report of our preliminary experience with HBOT [7]. Our results indicate that many patients can be salvaged from anastomotic complications by early institution of an aggressive non-operative treatment that includes HBOT.
MATERIALS AND METHODS
Patients, surgical treatment and postoperative care
This study included patients that received HBOT after tracheal or oesophageal surgery in the Division of Thoracic Surgery at Massachusetts General Hospital from 2007 until 2018. A prospectively maintained database was retrospectively reviewed for demographics, baseline characteristics, details on the operative procedures and anastomotic complications, delivery of HBOT and follow-up data, particularly, airway outcomes. Approval for this retrospective study was obtained from the Partners Human Research Committee Institutional Review Board (Protocol No. 2009P000111).
Tracheal or laryngotracheal resection and reconstruction in our institution are performed while strictly adhering to basic airway surgical principles that have been described in detail elsewhere [1]. In summary, these include careful consideration of the length of resection, limitation of the lateral dissection of the trachea, use of release manoeuvres and a meticulous anastomotic technique with multiple lubricated interrupted sutures using absorbable material. Coverage of the anastomosis with a local muscle flap is attempted in every case. Postoperatively, all patients routinely undergo surveillance bronchoscopy ∼5–7 days after surgery to evaluate the anastomosis allowing for the early identification of complications.
On the other hand, oesophagectomy is preferentially performed using a gastric conduit using the Ivor Lewis technique with an intrathoracic anastomosis. For proximal or middle third oesophageal cancers, we consider a modified McKeown approach with the anastomosis performed via the left neck. The oesophagogastric anastomosis is performed either with a circular stapler or handsewn and is not buttressed routinely. Postoperatively, patients are maintained nil per os with enteral nutrition provided via a jejunostomy tube, if applicable. All patients undergo a contrast swallow study ∼5–7 days after surgery to evaluate for anastomotic leaks and delayed gastric emptying.
Management of anastomotic complications
Airway anastomotic complications are managed following a few fundamental principles. First, airway stability needs to be determined. In the symptomatic patient with a concern for an unstable airway, the patient returns to the operating room emergently for bronchoscopic examination, possible re-exploration and stabilization with an airway appliance. Symptomatic patients with a stable airway proceed initially with bronchoscopy and computed tomography (CT) scan of the neck/chest. Asympto-matic patients who are found to have an anastomotic problem on routine surveillance bronchoscopy usually have stable airways. In general, patients with an anastomotic problem and a stable airway can be managed non-operatively, but should be treated aggressively with a combination of intravenous broad-spectrum antibiotics, inhaled nebulized tobramycin [8], nebulized saline treatments to help clear exudate from the affected area and heliox as indicated for symptomatic shortness of breath. Because of the risk of progression of the anastomotic problem, frequent bronchoscopic examinations every 2–3 days are highly recommended until it is determined that progress is being made. Finally, we also routinely consider patients with a documented stable airway for HBOT as an adjunct in the management of airway anastomotic complications based on our earlier preliminary experience [7].
Complications from the oesophagogastric anastomosis after oesophagectomy, namely leaks or ischaemia, are assessed by means of a contrast oesophagram and CT scan of the neck/chest. Depending on these findings and the general condition of the patient, we consider performing an oesophagogastroduo-denoscopy to assess the viability of the gastric conduit, the degree of disruption at the anastomosis and to potentially deliver a covered oesophageal stent [9]. Some patients with anastomotic leaks can be managed non-operatively with a combination of no oral intake, broad-spectrum intravenous antibiotics, percutaneous drainage of abscesses, nutritional support and frequent serial radiological or endoscopic examinations. We do not consider patients with leaks at the oesophagogastric anastomosis for candidacy for HBOT. However, we have explored its use in selected cases.
Hyperbaric oxygen therapy
It is important to emphasize that only patients with a documented stable airway can be considered for HBOT. HBOT was administered with 100% oxygen inside a pressurized chamber at 2 atmospheres in sessions of 90 min. Patients received HBOT once or twice per day depending on the appearance of the anastomosis and hospital logistics. We aimed to deliver a total of 20 sessions of HBOT at the start of treatment, but this could be shortened or extended. All patients were continuously monitored by a registered nurse with HBOT certification in a hospital-based HBOT centre and with HBOT-certified physician presence. Telemetry, oxygen saturation and vitals were monitored throughout the sessions. In addition to direct observation of the patient through the acrylic chamber, staff maintain periodic audio communication with the patient using the chamber speaker system. Patients were re-evaluated with bronchoscopy or oesophagogastroduodenoscopy at frequent intervals while receiving HBOT to monitor progression of the anastomosis.
Statistical analysis
Data extracted from the medical records of the patients were recorded in a database designed in Microsoft Office Excel 365 (Microsoft, Redmond, WA, USA). This database was exported to the statistical software Stata/SE 13 (StataCorp LP, College Station, TX, USA). Variables were analysed as proportions, means or medians according to their nature.
RESULTS
Baseline characteristics and airway surgical procedures
A total of 483 patients underwent tracheal or laryngotracheal resection during the study period for all indications. Of these, 23 (4.8%) patients received HBOT as part of the management of a complication of the airway anastomosis. Baseline patient characteristics and the surgical procedures performed are shown in Tables 1 and 2. Overall, a significant proportion of patients presented with known risk factors for anastomotic complications such as diabetes, previous tracheostomy (10 patients) and previous endoscopic interventions (8 patients) [1, 3]. Additionally, a significant number of patients underwent procedures with a higher risk for anastomotic complications such as laryngotracheal resections (16 patients) and reoperative procedures (6 patients) [1, 3]. The majority of patients received a local strap muscle flap (e.g. sternothyroid or sternohyoid muscle) to buttress the anastomosis.
Baseline characteristics of 23 patients receiving HBOT after airway surgery for high-risk or complicated tracheal anastomoses
| Variables . | Result . |
|---|---|
| Age (years), median (IQR) | 54.5 (47–64) |
| Gender, n (%) | |
| Male | 8 (34.8) |
| Female | 15 (65.22) |
| BMI (kg/m2), median (IQR) | 28.2 (23.6–34.4) |
| Smoking history, n (%) | 6 (26.1) |
| Comorbidities, n (%) | 16 (69.6) |
| Hypertension | 13 (56.5) |
| Obesity | 9 (39.1) |
| Diabetes | 9 (39.1) |
| Cardiac disease | 4 (17.4) |
| Cerebrovascular disease | 3 (13.0) |
| Renal insufficiency | 3 (13.0) |
| Chronic preoperative use of steroids, n (%) | 1 (4.4) |
| Diagnosis, n (%) | |
| PITS | 8 (34.8) |
| ILTS, primary | 7 (30.4) |
| ILTS, recurrent | 2 (8.7) |
| TOF | 3 (13.0) |
| Cancer | 3 (13.0) |
| Previous airway procedures, n (%) | |
| Tracheostomy | 10 (43.5) |
| Endoscopic intervention (e.g. dilatation, laser) | 8 (34.8) |
| Variables . | Result . |
|---|---|
| Age (years), median (IQR) | 54.5 (47–64) |
| Gender, n (%) | |
| Male | 8 (34.8) |
| Female | 15 (65.22) |
| BMI (kg/m2), median (IQR) | 28.2 (23.6–34.4) |
| Smoking history, n (%) | 6 (26.1) |
| Comorbidities, n (%) | 16 (69.6) |
| Hypertension | 13 (56.5) |
| Obesity | 9 (39.1) |
| Diabetes | 9 (39.1) |
| Cardiac disease | 4 (17.4) |
| Cerebrovascular disease | 3 (13.0) |
| Renal insufficiency | 3 (13.0) |
| Chronic preoperative use of steroids, n (%) | 1 (4.4) |
| Diagnosis, n (%) | |
| PITS | 8 (34.8) |
| ILTS, primary | 7 (30.4) |
| ILTS, recurrent | 2 (8.7) |
| TOF | 3 (13.0) |
| Cancer | 3 (13.0) |
| Previous airway procedures, n (%) | |
| Tracheostomy | 10 (43.5) |
| Endoscopic intervention (e.g. dilatation, laser) | 8 (34.8) |
BMI: body mass index; ILTS: idiopathic laryngotracheal stenosis; IQR: interquartile range; PITS: postintubation tracheal stenosis; TOF: tracheo-oesophageal fistula.
Baseline characteristics of 23 patients receiving HBOT after airway surgery for high-risk or complicated tracheal anastomoses
| Variables . | Result . |
|---|---|
| Age (years), median (IQR) | 54.5 (47–64) |
| Gender, n (%) | |
| Male | 8 (34.8) |
| Female | 15 (65.22) |
| BMI (kg/m2), median (IQR) | 28.2 (23.6–34.4) |
| Smoking history, n (%) | 6 (26.1) |
| Comorbidities, n (%) | 16 (69.6) |
| Hypertension | 13 (56.5) |
| Obesity | 9 (39.1) |
| Diabetes | 9 (39.1) |
| Cardiac disease | 4 (17.4) |
| Cerebrovascular disease | 3 (13.0) |
| Renal insufficiency | 3 (13.0) |
| Chronic preoperative use of steroids, n (%) | 1 (4.4) |
| Diagnosis, n (%) | |
| PITS | 8 (34.8) |
| ILTS, primary | 7 (30.4) |
| ILTS, recurrent | 2 (8.7) |
| TOF | 3 (13.0) |
| Cancer | 3 (13.0) |
| Previous airway procedures, n (%) | |
| Tracheostomy | 10 (43.5) |
| Endoscopic intervention (e.g. dilatation, laser) | 8 (34.8) |
| Variables . | Result . |
|---|---|
| Age (years), median (IQR) | 54.5 (47–64) |
| Gender, n (%) | |
| Male | 8 (34.8) |
| Female | 15 (65.22) |
| BMI (kg/m2), median (IQR) | 28.2 (23.6–34.4) |
| Smoking history, n (%) | 6 (26.1) |
| Comorbidities, n (%) | 16 (69.6) |
| Hypertension | 13 (56.5) |
| Obesity | 9 (39.1) |
| Diabetes | 9 (39.1) |
| Cardiac disease | 4 (17.4) |
| Cerebrovascular disease | 3 (13.0) |
| Renal insufficiency | 3 (13.0) |
| Chronic preoperative use of steroids, n (%) | 1 (4.4) |
| Diagnosis, n (%) | |
| PITS | 8 (34.8) |
| ILTS, primary | 7 (30.4) |
| ILTS, recurrent | 2 (8.7) |
| TOF | 3 (13.0) |
| Cancer | 3 (13.0) |
| Previous airway procedures, n (%) | |
| Tracheostomy | 10 (43.5) |
| Endoscopic intervention (e.g. dilatation, laser) | 8 (34.8) |
BMI: body mass index; ILTS: idiopathic laryngotracheal stenosis; IQR: interquartile range; PITS: postintubation tracheal stenosis; TOF: tracheo-oesophageal fistula.
| Variables . | Result, n (%) . |
|---|---|
| Type of resection | |
| Laryngotracheal | 16 (69.6) |
| Tracheal | 7 (30.4) |
| Nature of surgery | |
| First surgery | 17 (73.9) |
| Reoperation | 6 (26.1) |
| Release manoeuvres | 3 (13.0) |
| Tissue coverage of anastomosis | |
| Strap muscles | 17 (73.9) |
| Other tissue | 4 (14.4) |
| No coverage | 2 (8.7) |
| Variables . | Result, n (%) . |
|---|---|
| Type of resection | |
| Laryngotracheal | 16 (69.6) |
| Tracheal | 7 (30.4) |
| Nature of surgery | |
| First surgery | 17 (73.9) |
| Reoperation | 6 (26.1) |
| Release manoeuvres | 3 (13.0) |
| Tissue coverage of anastomosis | |
| Strap muscles | 17 (73.9) |
| Other tissue | 4 (14.4) |
| No coverage | 2 (8.7) |
| Variables . | Result, n (%) . |
|---|---|
| Type of resection | |
| Laryngotracheal | 16 (69.6) |
| Tracheal | 7 (30.4) |
| Nature of surgery | |
| First surgery | 17 (73.9) |
| Reoperation | 6 (26.1) |
| Release manoeuvres | 3 (13.0) |
| Tissue coverage of anastomosis | |
| Strap muscles | 17 (73.9) |
| Other tissue | 4 (14.4) |
| No coverage | 2 (8.7) |
| Variables . | Result, n (%) . |
|---|---|
| Type of resection | |
| Laryngotracheal | 16 (69.6) |
| Tracheal | 7 (30.4) |
| Nature of surgery | |
| First surgery | 17 (73.9) |
| Reoperation | 6 (26.1) |
| Release manoeuvres | 3 (13.0) |
| Tissue coverage of anastomosis | |
| Strap muscles | 17 (73.9) |
| Other tissue | 4 (14.4) |
| No coverage | 2 (8.7) |
Hyperbaric oxygen therapy after airway surgery
HBOT was used in 2 different ways after airway surgery. First, 2 patients received HBOT as a ‘preventive’ strategy. In these cases, HBOT was started early after surgery before a complication occurred in cases that were considered extremely high-risk for anastomotic complications. The first patient was a 50-year-old female with diabetes and diffuse large B-cell lymphoma of the thyroid who had received chemotherapy and radiation to the neck and presented with a recurrent 4-cm tracheo-oesophageal fistula after a previous failed repair without airway resection at another institution. She had a tracheostomy and had undergone previous multiple endoscopic procedures. She underwent primary repair of the oesophagus buttressed with a sternohyoid muscle flap and tracheal resection and reconstruction buttressed with a sternothyroid muscle flap. HBOT was started on postoperative day 2, and the patient received a total of 8 sessions over 8 days. The oesophageal repair and airway anastomosis healed without complications and she was discharged home on postoperative day 9. The second case of ‘preventive’ HBOT use involved a 57-year-old male who presented with a 5-cm adenoid cystic carcinoma of the middle and distal trachea involving only the membranous wall. Given the extent of the tumour, he was not a candidate for segmental airway resection with end-to-end anastomosis. To achieve local control, he underwent a right thoracotomy with resection of the membranous wall of the middle-distal trachea, and reconstruction with a cryopreserved aortic homograft with buttressing of the repair with intercostal and latissimus dorsi muscle flaps (Fig. 1A). HBOT started on postoperative day 3 for a total of 22 sessions over 12 days. Bronchoscopy revealed a satisfactorily healed graft in the posterior tracheal wall, and he was discharged home on postoperative day 15 (Fig. 1B). This case was previously reported as part of a series on the use of bioprosthetic materials for airway reconstruction [10].
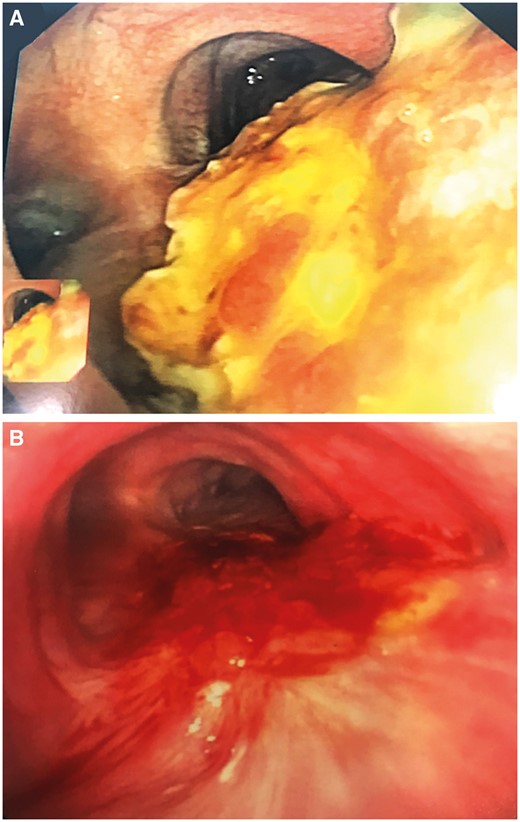
Prophylactic use of hyperbaric oxygen therapy in reconstruction of the membranous wall of the distal trachea with bioprosthetic material. (A) Early postoperative period. (B) Several weeks after surgery showing adequate incorporation and epithelialization of the bioprosthesis.
The remaining 21 of 23 patients received HBOT in a ‘reactive’ strategy after airway surgery. In all cases, a problem with anastomotic healing was identified during the postoperative period which led to a comprehensive management as detailed in the Methods section. Anastomotic problems included cartilage necrosis in 10 (47.6%) patients, mucosal necrosis in 3 (14.3%) patients, partial dehiscence of the airway anastomosis in 2 (9.5%) patients and both necrosis and partial dehiscence in 6 (28.6%) patients.
Table 3 shows the details of treatment with HBOT in all patients after airway surgery. Briefly, patients received a median of ∼10 HBOT sessions, over a median course of 8 days. All patients required additional bronchoscopies to evaluate the progression of anastomotic healing. A few cases required debridement of granulation tissue or dilatation of the airway anastomosis during bronchoscopy. The airway healed completely in 20 of 23 (87%) patients. Interestingly, none of these patients developed a clinically significant tracheal stenosis. The left panel of figure 2 shows the typical appearance of an anastomosis with a localized ischaemic and necrotic segment of cartilage that required the use of HBOT. The right panel shows the appearance of the anastomosis after HBOT, demonstrating rapid onset of granulation tissue and early re-epithelialization leading to a patent and non-inflamed airway. Figure 3 shows one of the most striking responses observed with HBOT. The left panel shows significant necrosis and partial anterior and posterior separation of the airway anastomosis in a 19-year-old patient who underwent third-time reoperation for a recurrent traumatic tracheo-oesophageal fistula after failed attempts in another country. The right panel shows the appearance of the airway at ∼20 weeks after surgery and after receiving 25 sessions of HBOT.
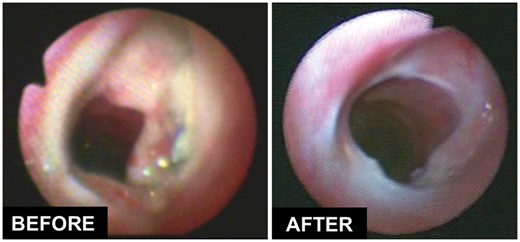
Effect of hyperbaric oxygen therapy on the healing of a tracheal anastomosis with cartilage necrosis but without dehiscence.
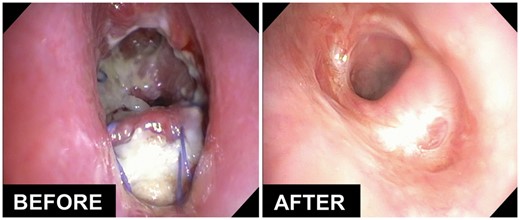
Effect of hyperbaric oxygen therapy on the healing of severe necrosis and partial dehiscence of the airway after a complex tracheo-oesophageal fistula repair.

Effect of HBOT on the management of gastric tip ischaemia after oesophagectomy. HBOT: hyperbaric oxygen therapy.
| Variables . | Result . |
|---|---|
| HBOT use, n (%) | |
| Preventive in high-risk anastomosis | 2 (8.7) |
| Reactive to anastomotic problem | 21 (91.3) |
| Anastomotic complications, n (%) | |
| Necrosis | 13 (61.9) |
| Anastomotic separation | 2 (9.5) |
| Necrosis + separation | 6 (28.6) |
| Number of HBOT sessions, median (IQR) | 9.5 (5–19) |
| Frequency of HBOT sessions, n (%) | |
| Daily | 10 (43.5) |
| Twice daily | 13 (56.5) |
| HBOT duration (days), median (IQR) | 8 (5–12) |
| Number of surveillance bronchoscopies, median (IQR) | 2.5 (1–4) |
| Need for bronchoscopic procedures, n (%) | |
| Mechanical debridement | 7 (30.4) |
| Laser debridement | 3 (13.0) |
| Dilatation | 1 (4.4) |
| Defect healed with HBOT, n (%) | 20 (87.0) |
| Days until defect healed, median (IQR) | 8 (5–14) |
| Overall airway result, n (%) | |
| Successful | 19 (82.6) |
| Unsuccessful | 4 (17.4) |
| HBOT complications, n (%) | |
| Ear discomfort | 2 (8.7) |
| Tympanostomy tubes | 1 (4.3) |
| Claustrophobia | 1 (4.3) |
| Variables . | Result . |
|---|---|
| HBOT use, n (%) | |
| Preventive in high-risk anastomosis | 2 (8.7) |
| Reactive to anastomotic problem | 21 (91.3) |
| Anastomotic complications, n (%) | |
| Necrosis | 13 (61.9) |
| Anastomotic separation | 2 (9.5) |
| Necrosis + separation | 6 (28.6) |
| Number of HBOT sessions, median (IQR) | 9.5 (5–19) |
| Frequency of HBOT sessions, n (%) | |
| Daily | 10 (43.5) |
| Twice daily | 13 (56.5) |
| HBOT duration (days), median (IQR) | 8 (5–12) |
| Number of surveillance bronchoscopies, median (IQR) | 2.5 (1–4) |
| Need for bronchoscopic procedures, n (%) | |
| Mechanical debridement | 7 (30.4) |
| Laser debridement | 3 (13.0) |
| Dilatation | 1 (4.4) |
| Defect healed with HBOT, n (%) | 20 (87.0) |
| Days until defect healed, median (IQR) | 8 (5–14) |
| Overall airway result, n (%) | |
| Successful | 19 (82.6) |
| Unsuccessful | 4 (17.4) |
| HBOT complications, n (%) | |
| Ear discomfort | 2 (8.7) |
| Tympanostomy tubes | 1 (4.3) |
| Claustrophobia | 1 (4.3) |
HBOT: hyperbaric oxygen therapy; IQR: interquartile range.
| Variables . | Result . |
|---|---|
| HBOT use, n (%) | |
| Preventive in high-risk anastomosis | 2 (8.7) |
| Reactive to anastomotic problem | 21 (91.3) |
| Anastomotic complications, n (%) | |
| Necrosis | 13 (61.9) |
| Anastomotic separation | 2 (9.5) |
| Necrosis + separation | 6 (28.6) |
| Number of HBOT sessions, median (IQR) | 9.5 (5–19) |
| Frequency of HBOT sessions, n (%) | |
| Daily | 10 (43.5) |
| Twice daily | 13 (56.5) |
| HBOT duration (days), median (IQR) | 8 (5–12) |
| Number of surveillance bronchoscopies, median (IQR) | 2.5 (1–4) |
| Need for bronchoscopic procedures, n (%) | |
| Mechanical debridement | 7 (30.4) |
| Laser debridement | 3 (13.0) |
| Dilatation | 1 (4.4) |
| Defect healed with HBOT, n (%) | 20 (87.0) |
| Days until defect healed, median (IQR) | 8 (5–14) |
| Overall airway result, n (%) | |
| Successful | 19 (82.6) |
| Unsuccessful | 4 (17.4) |
| HBOT complications, n (%) | |
| Ear discomfort | 2 (8.7) |
| Tympanostomy tubes | 1 (4.3) |
| Claustrophobia | 1 (4.3) |
| Variables . | Result . |
|---|---|
| HBOT use, n (%) | |
| Preventive in high-risk anastomosis | 2 (8.7) |
| Reactive to anastomotic problem | 21 (91.3) |
| Anastomotic complications, n (%) | |
| Necrosis | 13 (61.9) |
| Anastomotic separation | 2 (9.5) |
| Necrosis + separation | 6 (28.6) |
| Number of HBOT sessions, median (IQR) | 9.5 (5–19) |
| Frequency of HBOT sessions, n (%) | |
| Daily | 10 (43.5) |
| Twice daily | 13 (56.5) |
| HBOT duration (days), median (IQR) | 8 (5–12) |
| Number of surveillance bronchoscopies, median (IQR) | 2.5 (1–4) |
| Need for bronchoscopic procedures, n (%) | |
| Mechanical debridement | 7 (30.4) |
| Laser debridement | 3 (13.0) |
| Dilatation | 1 (4.4) |
| Defect healed with HBOT, n (%) | 20 (87.0) |
| Days until defect healed, median (IQR) | 8 (5–14) |
| Overall airway result, n (%) | |
| Successful | 19 (82.6) |
| Unsuccessful | 4 (17.4) |
| HBOT complications, n (%) | |
| Ear discomfort | 2 (8.7) |
| Tympanostomy tubes | 1 (4.3) |
| Claustrophobia | 1 (4.3) |
HBOT: hyperbaric oxygen therapy; IQR: interquartile range.
Complications directly related to HBOT were infrequent and minor. The most frequent complication was ear discomfort in 2 patients. Five patients were lost to long-term follow-up. Many of them were from different regions in the USA, who travelled to our centre to be evaluated and have surgery. Given the lack of complications, patients were instructed to continue follow-up locally with clear instructions to report back any issues. None of these 5 patients called again. In the remaining 18 patients, the mean follow-up duration was 12.8 ± 18.3 months. Overall, a satisfactory airway outcome was achieved in 19 of 23 (82.6%) patients during follow-up, with 4 patients failing non-operative management with HBOT. Looking at specific indications for HBOT, success was achieved in 2/2 (100%) cases of preventive use, 11/13 (84.6%) cases with necrosis only, 2/2 (100%) with partial dehiscence only and in 4/6 (66.7%) with both necrosis and partial dehiscence. We observed no differences in obtaining a satisfactory airway outcome between patients receiving once daily versus twice daily treatments (80% vs 84.6%, respectively; P = 1.000). Of the 4 failures, 3 patients required neck re-exploration. All had a laryngotracheal resection. One patient had a complex reoperation with evidence of calcified cartilage, and another was a debilitated patient on haemodialysis with postintubation subglottic stenosis from a previous prolonged course in the intensive care unit. Two patients developed anterior partial dehiscence of the anastomosis (size, 0.5–1 cm) that failed to improve with HBOT, while the third had cartilage necrosis, but no separation. Failure was determined by a persistent air leak from a neck drain in 1 patient, worsening stridor from severe glottic oedema in the setting of a fluid collection in the neck in another patient, and subcutaneous emphysema with rising leucocytosis in the third patient. All underwent re-exploration of the neck with drainage and debridement of necrotic and infected tissue. The patients with an anterior defect received an airway appliance through the defect (1 T-tube and 1 tracheostomy). The third patient did not have frank evidence of separation of the anastomosis upon re-exploration, but had a tracheostomy placed distally for protection of the airway. Finally, the fourth failure represented a patient with a recurrent tracheo-oesophageal fistula that required placement of a covered oesophageal stent.
Hyperbaric oxygen therapy after oesophageal surgery
Based on our experience after airway surgery, we explored the use of HBOT as an adjunct to support at-risk oesophagogastric anastomoses in 2 patients. The first patient was a 75-year-old man with recurrent hypopharyngeal squamous cell carcinoma after treatment with radiation therapy. He underwent a cervical exenteration with laryngectomy, pharyngectomy, transhiatal oesophagectomy with reconstruction with a long gastric conduit anastomosed to the hypopharynx (12 cm from the incisors) and pectoralis muscle flap coverage. At the end of the procedure, the tip of the gastric conduit with the anastomosis appeared mildly discoloured and under tension. Because of this, he underwent endoscopy on postoperative day 2 for an early assessment of the viability of the gastric conduit tip. This demonstrated no frank necrosis, but definite ischaemic changes of the gastric mucosa for a segment of 3 cm involving the anastomosis (Fig. 4). He was managed non-operatively with HBOT for a total of 15 sessions over 16 days. As shown in Fig. 4, HBOT was able to support the tissues through the period of ischaemia, and the patient never developed an anastomotic leak. Endoscopic evaluation 3 weeks after the end of HBOT showed healthy-appearing gastric mucosa. Unfortunately, despite clear margins of resection and negative lymph node involvement on final pathological examination of the surgical specimen, the patient experienced an aggressive early regional recurrence of his cancer and passed away ∼2 months after surgery while receiving hospice care.
The second case was a 56-year-old male patient with oesophageal adenocarcinoma who received neoadjuvant chemoradiation and underwent an open Ivor Lewis oesophagectomy; the anastomosis was not buttressed. On postoperative day 4, he experienced fever and was found to have bacteraemia with Gram-negative rods. A contrast swallow study on postoperative day 5 did not reveal an anastomotic leak. He was found to have cholangitis and underwent successful treatment with biliary drainage by endoscopic retrograde cholangiopancreatography. As part of the work-up for bacteraemia, he also underwent oesophagogastroduodenoscopy on postoperative day 6 that revealed a sharply demarcated non-circumferential area of ischaemia on the gastric side of the anastomosis measuring 1 cm without evidence of separation. He started HBOT in an attempt to salvage the anastomosis (11 sessions over 8 days). At that point, a frank leak was not identified; therefore, he did not undergo drainage or placement of an oesophageal stent initially. Unfortunately, the patient developed a broncho-neoesophageal fistula ∼3 weeks after surgery. The patient went on to require a prolonged hospitalization with multiple endoscopic interventions to manage this fistula. Therefore, HBOT was determined not successful in this case.
DISCUSSION
Tracheal surgery remains a demanding procedure. Careful attention to detail can lead to success in >90% of patients [2]. Those who have an anastomotic problem present a great challenge. It has been determined in our experience that risk factors for anastomotic complications include paediatric patients, those with prior radiation therapy to the neck, diabetes, undergoing laryngotracheal resections or revisional surgery, requiring long resections (i.e. >4 cm), or patients with previous tracheostomy or having required previous endoscopic interventions [1–3]. This study presents our experience with the use of HBOT as an adjunct in our institutional protocol for the aggressive non-operative management of airway anastomotic complications. If an anastomotic problem develops in the postoperative phase, we have shown that in a highly selected group of patients, HBOT might salvage the anastomosis in the majority of cases without further need for surgical re-exploration. This retrospective study demonstrates the success of this type of approach, as in our historical experience, these patients almost certainly would have required a tracheostomy or a T-tube to manage these complications.
One cannot underestimate the importance of careful selection of patients for HBOT. The underlying principle in all of these patients is the certainty of a stable anastomosis allowing patients to enter the hyperbaric therapy chamber, as it can take a few minutes (2–3 min in our institution) for gradual decompression of the chamber to remove patients safely. Therefore, an airway emergency while inside the pressurized chamber may prove catastrophic.
Oxygen plays a central role in inflammation and wound healing, and HBOT has demonstrated its efficacy in the treatment of complex wound-healing problems [6]. Similarly, the use of HBOT has been explored in the healing of the upper and lower central airways. Animal studies have shown the beneficial effects of HBOT in the healing of tracheal anastomoses with improved epithelialization and earlier neovascularization [11], even after radiation therapy [12]. Moreover, experiments have shown that HBOT can assist in the healing of auricular cartilage grafts used to replace anterior tracheal defects [13], or to support allotransplantation with cryopreserved tracheal grafts in a small animal model [14]. Clinically, after our initial report on the use of HBOT on 5 patients with tracheal anastomotic complications [7], similar independent observations have been made as case reports on the successful use of HBOT in the management of ischaemia of the anastomosis after carinal resection [15] and in the management of extensive tracheal necrosis after pharyngolaryngectomy [16]. Likewise, HBOT has proven beneficial in the management of 18 patients with severe laryngeal radionecrosis [17]. In this study, most patients received over 20 HBOT sessions and 13 (72%) patients showed major improvement of their symptoms and avoided a laryngectomy, thus preserving their voice [17]. Furthermore, 4 of 8 patients who had received a tracheostomy were decannulated. The remaining 5 (28%) patients ended up receiving a total laryngectomy (lack of response to HBOT in 4 patients and recurrent cancer in 1 patient) [17]. This study further supports the role of HBOT assisting in the healing of ischaemic or injured airway cartilage.
We have identified the value of routine coverage of the airway anastomosis with a muscle flap. This provides buttressing and provides a structure to work with when there is partial separation of the anastomosis. It should be stressed that this should be done in the vast majority of patients undergoing tracheal or laryngotracheal resection and reconstruction. With this approach, we have identified rapid granulations as well as early re-epithelialization and angiogenesis when using HBOT in complicated tracheal anastomoses. Additionally, we institute treatments with inhaled tobramycin, which might also assist in airway healing [8]. Using this strategy, no significant strictures developed in those patients with a successful response to HBOT.
The results presented here and our previous studies identifying patients at high risk for anastomotic complications [3] may suggest a preventive treatment strategy where a high-risk anastomosis might benefit from prophylactic use of HBOT. Even though this was not a controlled study, we demonstrated such a strategy in 2 patients requiring complex airway surgeries with successful healing of the airway repairs, thus avoiding complications. The prophylactic use of HBOT after high-risk airway surgery needs to be studied in detail, ideally in the setting of a randomized clinical trial, to prove its efficacy and identify the specific subset of patients who might benefit the most from it.
Finally, we explored the use of HBOT in the management of complications after oesophageal surgery. We showed success avoiding an anastomotic leak and frank necrosis of the ischaemic tip of a gastric conduit. We believe that a satisfactory outcome was obtained in that case because HBOT was started early, avoiding the establishment of transmural gastric necrosis, and there was significant soft tissue coverage of the area with a pectoralis muscle flap, which provided a framework for HBOT to assist in wound healing. In contrast, the second case was unsuccessful with an anastomotic leak and formation of a broncho-neoesophageal fistula. In retrospect, we believe that this patient had an early leak of the anastomosis and HBOT was started after this had already occurred. Additionally, this anastomosis was not buttressed; therefore, there was no framework for granulation tissue to form with the help of HBOT. Animal studies looking at the effects of HBOT on the healing of oesophagojejunal anastomoses have not shown a significant effect [18]. The use of HBOT to manage complications after oesophageal surgery remains exploratory and investigational, and we do not recommend it routinely.
Limitations
Our study has limitations. First, this is a single-institution experience at a high-volume academic centre; therefore, our results may not be reproduced in a different setting. Second, this was not a controlled study. There is inherent selection bias and there is no comparison group of patients who were managed non-operatively without HBOT to prove the magnitude of the benefit provided by HBOT. However, historical experience from our institution indicates that HBOT is helpful in these situations. Before use of HBOT, all patients with necrosis or partial dehiscence of the tracheal anastomosis ended up with a T-tube, tracheostomy or revision of the anastomosis. Our current results show that we have avoided reoperations and airway appliances in most patients with the use of HBOT. Therefore, it is now a routine part of our treatment strategy when airway anastomotic complications develop.
CONCLUSION
In conclusion, HBOT is a useful adjunct in the management of complex anastomotic problems after tracheal surgery or to support high-risk airway anastomoses. It should only be used when there is a documented stable airway and it may work best when a muscle flap coverage of the anastomosis is performed to provide a substrate for wound healing. Therefore, HBOT should be considered routinely in the management of anastomotic complications after tracheal or laryngotracheal resection and reconstruction. HBOT may play a role in cases after oesophagectomy, but this remains investigational.
Conflict of interest: all authors declare no conflicts of interest.
Author contributions
Luis F. Tapias: Data curation; Formal analysis; Methodology; Project administration; Software; Supervision; Writing—original draft; Writing—review & editing. Cameron D. Wright: Conceptualization; Data curation; Investigation; Methodology. Michael Lanuti: Data curation; Formal analysis; Methodology. Ashok Muniappan: Data curation; Formal analysis. Daniel Deschler: Conceptualization; Data curation; Methodology; Resources. Douglas J. Mathisen: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Writing—original draft; Writing—review & editing.
Presented at the 27th European Conference on General Thoracic Surgery, Dublin, Ireland, 9–12 June 2019.





