-
PDF
- Split View
-
Views
-
Cite
Cite
Yaron Shargall, Alessandro Brunelli, Sudish Murthy, Laura Schneider, Fabrizio Minervini, Luca Bertolaccini, John Agzarian, Lori-Ann Linkins, Peter Kestenholz, Hui Li, Gaetano Rocco, Philippe Girard, Federico Venuta, Marc Samama, Marco Scarci, Masaki Anraku, Pierre-Emmanuel Falcoz, Alan Kirk, Piergiorgio Solli, Wayne Hofstetter, Meinoshin Okumura, James Douketis, Virginia Litle, Venous thromboembolism prophylaxis in thoracic surgery patients: an international survey, European Journal of Cardio-Thoracic Surgery, Volume 57, Issue 2, February 2020, Pages 331–337, https://doi.org/10.1093/ejcts/ezz191
Close - Share Icon Share
Abstract
Venous thromboembolic events (VTE) after thoracic surgery (TS) can be prevented with mechanical and chemical prophylaxis. Unlike other surgical specialties, TS lacks evidence-based guidelines. In the process of developing these guidelines, an understanding of the current prophylaxis methods practiced internationally is necessary and is described in this article.
A 26-item survey was distributed to members of the European Society of Thoracic Surgeons (ESTS), American Association of Thoracic Surgery (AATS), Japanese Association for Chest Surgery (JACS) and Chinese Society for Thoracic and Cardiovascular Surgery (CSTCS) electronically or in person. Participants were asked to report their current prophylaxis selection, timing of initiation and duration of prophylaxis, perceived risk factors and the presence and adherence to institutional VTE guidelines for patients undergoing TS for malignancies.
In total, 1613 surgeons anonymously completed the survey with an overall 36% response rate. Respondents were senior surgeons working in large academic hospitals (≥70%, respectively). More than 83.5% of ESTS, AATS and JACS respondents report formal TS thromboprophylaxis protocols in their institutions, but 53% of CSTCS members report not having such a protocol. The regions varied in the approaches utilized for VTE prophylaxis, the timing of initiation perioperatively and the use and type of extended prophylaxis. Respondents reported that multiple risk factors and sources of information impact their VTE prophylaxis decision-making processes, and these factors vastly diverge regionally.
There is little agreement internationally on the optimal approach to thromboprophylaxis in the TS population, and guidelines will be helpful and vastly welcomed.
INTRODUCTION
Venous thromboembolic events (VTE), consisting of deep vein thrombosis and pulmonary embolus are recognized complications following major thoracic surgery (TS) [1–5] with the potential to result in postoperative morbidity and mortality [6–10]. Prophylaxis measures are recommended to ameliorate the risk of these events, using mechanical and/or pharmaceutical interventions perioperatively based on individual risk profile [11–15]. The American College of Chest Physicians 9th Edition guidelines finds adequate (Grade 1B) evidence in the postoperative TS population to recommend the use of in-hospital routine VTE prophylaxis with either unfractionated heparin or low-molecular-weight heparin (LMWH) [13], but there is little to no evidence supporting the use of extended prophylaxis in any of the major international guideline organizations [11–15], despite the fact that more than at least 23% of VTE after TS occurs after hospital discharge [10]. The European Society of Anaesthesia (ESA) defined TS patients with malignancies as a high-risk population for VTE and recommended in-hospital pharmacological prophylaxis in addition to intermittent pneumatic compression (Grade 2B) [15]. In other high-risk surgical oncology specialties, such as major pelvic and abdominal cancer surgery, the American Society of Clinical Oncology (ASCO) guidelines [12] recommend that all major cancer surgeries receive prophylaxis starting before/around surgery and continuing for at least 7–10 days post-discharge, with further extended prophylaxis recommended for up to 4 weeks for major high-risk abdominal or pelvic surgery. The ACCP, European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) guidelines [11,13,14] concur, recommending extended LMWH prophylaxis for up to 4 weeks in the abdominal and pelvic cancer population.
In June 2016, a working group including members of the European Society of Thoracic Surgeons (ESTS), American Association for Thoracic Surgery (AATS), International Society for Thrombosis and Haemostasis (ISTH), Japanese Association for Chest Surgery (JACS) and Chinese Society for Thoracic and Cardiovascular Surgery (CSTCS) was established with the aim of investigating current VTE prophylaxis practices after thoracic cancer surgery worldwide and generating evidence-based specific guidelines to address the prophylaxis needs of this population. Out of this collaboration, we identified a need to survey the international TS community to understand current practices and risk factors, institutional guidelines in existence and adherence to current/future guidelines as baseline knowledge before developing and disseminating TS guidelines. The results of this survey are described in this article.
MATERIALS AND METHODS
Participants within each of the respective TS organizations (ESTS, AATS, JACS and CSTCS) were invited by their corresponding VTE working group member/s to complete a 26-item survey regarding VTE prophylaxis use in their TS patients.
The questionnaire was developed by an executive group of ESTS and AATS TS members with input from lead ISTH thrombosis experts. It was originally drafted in English for the ESTS/AATS membership, and translated by lead thoracic surgical investigators in Japan and China for their respective membership groups. There was no pilot testing or validation performed on this survey prior to distribution although internal consistency reliability testing using the Cronbach’s alpha measure was reported [16].
The survey was distributed electronically via LimeSurvey in October 2016 (ESTS and AATS) and via email (JACS) or WeChat (CSTCS) in November 2016 with anonymous response collection to all active members of each organization. All potential respondents received at least 2 reminder emails at 2–3-week intervals, with survey completion closed December 2016. Participants were asked to complete the survey once in the case of those with membership in multiple organizations to reduce the risk of duplication, which was estimated to be <3% of memberships.
All data were imported into a single database, with descriptive data summarized as frequencies with absolute number and percentages. Tests of statistical significance were not performed for individual questions, as the number of possible comparisons is too high to reveal meaningful conclusions.
RESULTS
A total of 1613 physicians completed the survey, 195 (12.1%) from ESTS, 139 (8.6%) from AATS, 1150 (71.3%) from CSTCS and 129 (8.0%) from JACS. Based on membership numbers at the time of survey distribution, the response rate is estimated to be roughly 25%, 30%, 35% and 55% of the memberships, respectively, for ESTS, AATS, CSTCS and JACS participation. Internal consistency was excellent with a Cronbach’s alpha of 0.84, implying excellent reliability of the questionnaire relating to the individual questions [17]. The survey report is summarized in Supplementary Material, Appendix 1.
Respondent characteristics and institutional volumes
Table 1 describes the respondent characteristics. Survey respondents represented 49 countries across 5 continents. Respondents were generally mid-career to senior surgeons with at least 11 years of experience (n = 1114, 69.1%), 78.9% (n = 1273) practicing at academic teaching hospitals. Respondents generally practiced in high-volume thoracic surgical centres, according to the Canadian TS standard for oncological resections definition, which defines high-volume centres as those with ≥150 anatomic lung resections and 20 oesophagectomies annually [18], with oesophagectomy volumes meeting this criterion for all regions but Japan. At least 50% of anatomical lung resections for cancer were performed using minimally invasive techniques. Regions diverged with regard to oesophagectomies, where CSTCS (92.4%) and AATS (76.3%) members regularly undertake such resections, but the majority of ESTS (69.2%) and JACS (98.4%) members do not.
| Characteristics . | Response . | ||||
|---|---|---|---|---|---|
| . | Overall . | ESTS . | AATS . | CSTCS . | JACS . |
| Years in practice (years), n (%) | |||||
| 1–5 | 219 (13.6) | 31 (15.9) | 24 (17.3) | 163 (14.2) | 1 (0.8) |
| 6–10 | 280 (17.4) | 51 (26.2) | 25 (18.0) | 201 (17.5) | 3 (2.3) |
| 11–20 | 493 (30.6) | 48 (24.6) | 45 (32.4) | 371 (32.3) | 29 (22.5) |
| 20+ | 621 (38.5) | 65 (33.3) | 45 (32.4) | 415 (36.1) | 96 (74.4) |
| Clinical practice location, n (%) | |||||
| Academic teaching hospital | 1273 (78.9) | 122 (62.6) | 106 (76.3) | 985 (85.7) | 60 (46.5) |
| Community teaching hospital | 206 (12.8) | 22 (11.3) | 16 (11.5) | 143 (12.4) | 25 (19.4) |
| Private practice | 53 (3.3) | 10 (5.1) | 11 (7.9) | 22 (1.9) | 10 (7.8) |
| Public hospital | 81 (5.0) | 41 (21.0) | 6 (4.3) | 0 (0) | 34 (26.4) |
| Institutional oncological lung resections (per year), mean ± SD | 262 ± 481 | 263 ± 388 | 622 ± 1093 | 132 ± 75 | |
| Institutional oncological lung resections (per year), median (range) | 150 (10–6000) | 175 (2–3000) | 200 (0–10 000) | 120 (15–500) | |
| Percentage of institutional minimally invasive oncological lung resections (per year), n (%) | |||||
| None | 67 (4.2) | 15 (7.7) | 2 (1.4) | 49 (4.3) | 1 (0.8) |
| <10 | 146 (9.1) | 18 (9.2) | 1 (0.7) | 125 (10.9) | 2 (1.6) |
| 10–25 | 139 (8.6) | 26 (13.3) | 3 (2.2) | 107 (9.3) | 3 (2.3) |
| 25–50 | 233 (14.4) | 48 (24.6) | 12 (8.6) | 154 (13.4) | 19 (14.7) |
| 50–75 | 469 (29.1) | 43 (22.1) | 43 (30.9) | 355 (30.9) | 28 (21.7) |
| 75–100 | 559 (34.7) | 45 (23.1) | 78 (56.1) | 360 (31.3) | 76 (58.9) |
| Oesophagectomy performed, n (%) | |||||
| Yes | 1231 (76.3) | 60 (30.8) | 106 (76.3) | 1063 (92.4) | 2 (1.6) |
| Institutional oesophagectomy (per year), mean ± SD | 34 ± 33 | 48 ± 50 | 234 ± 373 | 17 ± 26 | |
| Institutional oesophagectomy (per year), median (range) | 27 (0–140) | 30 (5–300) | 100 (2–5000) | 4 (0–60) | |
| Percentage of institutional minimally invasive/hybrid oesophagectomy (per year), n (%) | |||||
| None | 193 (15.5) | 28 (40.0) | 17 (15.9) | 144 (13.5) | 4 (57.1) |
| <10 | 263 (21.1) | 9 (12.9) | 12 (11.2) | 242 (22.7) | 0 (0) |
| 10–25 | 183 (14.7) | 6 (8.6) | 8 (7.5) | 169 (15.9) | 0 (0) |
| 25–50 | 247 (19.8) | 12 (17.1) | 22 (20.6) | 212 (19.9) | 1 (14.3) |
| 50–75 | 213 (17.1) | 10 (14.3) | 19 (17.8) | 183 (17.2) | 1 (14.3) |
| 75–100 | 148 (11.9) | 5 (7.1) | 29 (27.1) | 113 (10.6) | 1 (14.3) |
| Characteristics . | Response . | ||||
|---|---|---|---|---|---|
| . | Overall . | ESTS . | AATS . | CSTCS . | JACS . |
| Years in practice (years), n (%) | |||||
| 1–5 | 219 (13.6) | 31 (15.9) | 24 (17.3) | 163 (14.2) | 1 (0.8) |
| 6–10 | 280 (17.4) | 51 (26.2) | 25 (18.0) | 201 (17.5) | 3 (2.3) |
| 11–20 | 493 (30.6) | 48 (24.6) | 45 (32.4) | 371 (32.3) | 29 (22.5) |
| 20+ | 621 (38.5) | 65 (33.3) | 45 (32.4) | 415 (36.1) | 96 (74.4) |
| Clinical practice location, n (%) | |||||
| Academic teaching hospital | 1273 (78.9) | 122 (62.6) | 106 (76.3) | 985 (85.7) | 60 (46.5) |
| Community teaching hospital | 206 (12.8) | 22 (11.3) | 16 (11.5) | 143 (12.4) | 25 (19.4) |
| Private practice | 53 (3.3) | 10 (5.1) | 11 (7.9) | 22 (1.9) | 10 (7.8) |
| Public hospital | 81 (5.0) | 41 (21.0) | 6 (4.3) | 0 (0) | 34 (26.4) |
| Institutional oncological lung resections (per year), mean ± SD | 262 ± 481 | 263 ± 388 | 622 ± 1093 | 132 ± 75 | |
| Institutional oncological lung resections (per year), median (range) | 150 (10–6000) | 175 (2–3000) | 200 (0–10 000) | 120 (15–500) | |
| Percentage of institutional minimally invasive oncological lung resections (per year), n (%) | |||||
| None | 67 (4.2) | 15 (7.7) | 2 (1.4) | 49 (4.3) | 1 (0.8) |
| <10 | 146 (9.1) | 18 (9.2) | 1 (0.7) | 125 (10.9) | 2 (1.6) |
| 10–25 | 139 (8.6) | 26 (13.3) | 3 (2.2) | 107 (9.3) | 3 (2.3) |
| 25–50 | 233 (14.4) | 48 (24.6) | 12 (8.6) | 154 (13.4) | 19 (14.7) |
| 50–75 | 469 (29.1) | 43 (22.1) | 43 (30.9) | 355 (30.9) | 28 (21.7) |
| 75–100 | 559 (34.7) | 45 (23.1) | 78 (56.1) | 360 (31.3) | 76 (58.9) |
| Oesophagectomy performed, n (%) | |||||
| Yes | 1231 (76.3) | 60 (30.8) | 106 (76.3) | 1063 (92.4) | 2 (1.6) |
| Institutional oesophagectomy (per year), mean ± SD | 34 ± 33 | 48 ± 50 | 234 ± 373 | 17 ± 26 | |
| Institutional oesophagectomy (per year), median (range) | 27 (0–140) | 30 (5–300) | 100 (2–5000) | 4 (0–60) | |
| Percentage of institutional minimally invasive/hybrid oesophagectomy (per year), n (%) | |||||
| None | 193 (15.5) | 28 (40.0) | 17 (15.9) | 144 (13.5) | 4 (57.1) |
| <10 | 263 (21.1) | 9 (12.9) | 12 (11.2) | 242 (22.7) | 0 (0) |
| 10–25 | 183 (14.7) | 6 (8.6) | 8 (7.5) | 169 (15.9) | 0 (0) |
| 25–50 | 247 (19.8) | 12 (17.1) | 22 (20.6) | 212 (19.9) | 1 (14.3) |
| 50–75 | 213 (17.1) | 10 (14.3) | 19 (17.8) | 183 (17.2) | 1 (14.3) |
| 75–100 | 148 (11.9) | 5 (7.1) | 29 (27.1) | 113 (10.6) | 1 (14.3) |
| Characteristics . | Response . | ||||
|---|---|---|---|---|---|
| . | Overall . | ESTS . | AATS . | CSTCS . | JACS . |
| Years in practice (years), n (%) | |||||
| 1–5 | 219 (13.6) | 31 (15.9) | 24 (17.3) | 163 (14.2) | 1 (0.8) |
| 6–10 | 280 (17.4) | 51 (26.2) | 25 (18.0) | 201 (17.5) | 3 (2.3) |
| 11–20 | 493 (30.6) | 48 (24.6) | 45 (32.4) | 371 (32.3) | 29 (22.5) |
| 20+ | 621 (38.5) | 65 (33.3) | 45 (32.4) | 415 (36.1) | 96 (74.4) |
| Clinical practice location, n (%) | |||||
| Academic teaching hospital | 1273 (78.9) | 122 (62.6) | 106 (76.3) | 985 (85.7) | 60 (46.5) |
| Community teaching hospital | 206 (12.8) | 22 (11.3) | 16 (11.5) | 143 (12.4) | 25 (19.4) |
| Private practice | 53 (3.3) | 10 (5.1) | 11 (7.9) | 22 (1.9) | 10 (7.8) |
| Public hospital | 81 (5.0) | 41 (21.0) | 6 (4.3) | 0 (0) | 34 (26.4) |
| Institutional oncological lung resections (per year), mean ± SD | 262 ± 481 | 263 ± 388 | 622 ± 1093 | 132 ± 75 | |
| Institutional oncological lung resections (per year), median (range) | 150 (10–6000) | 175 (2–3000) | 200 (0–10 000) | 120 (15–500) | |
| Percentage of institutional minimally invasive oncological lung resections (per year), n (%) | |||||
| None | 67 (4.2) | 15 (7.7) | 2 (1.4) | 49 (4.3) | 1 (0.8) |
| <10 | 146 (9.1) | 18 (9.2) | 1 (0.7) | 125 (10.9) | 2 (1.6) |
| 10–25 | 139 (8.6) | 26 (13.3) | 3 (2.2) | 107 (9.3) | 3 (2.3) |
| 25–50 | 233 (14.4) | 48 (24.6) | 12 (8.6) | 154 (13.4) | 19 (14.7) |
| 50–75 | 469 (29.1) | 43 (22.1) | 43 (30.9) | 355 (30.9) | 28 (21.7) |
| 75–100 | 559 (34.7) | 45 (23.1) | 78 (56.1) | 360 (31.3) | 76 (58.9) |
| Oesophagectomy performed, n (%) | |||||
| Yes | 1231 (76.3) | 60 (30.8) | 106 (76.3) | 1063 (92.4) | 2 (1.6) |
| Institutional oesophagectomy (per year), mean ± SD | 34 ± 33 | 48 ± 50 | 234 ± 373 | 17 ± 26 | |
| Institutional oesophagectomy (per year), median (range) | 27 (0–140) | 30 (5–300) | 100 (2–5000) | 4 (0–60) | |
| Percentage of institutional minimally invasive/hybrid oesophagectomy (per year), n (%) | |||||
| None | 193 (15.5) | 28 (40.0) | 17 (15.9) | 144 (13.5) | 4 (57.1) |
| <10 | 263 (21.1) | 9 (12.9) | 12 (11.2) | 242 (22.7) | 0 (0) |
| 10–25 | 183 (14.7) | 6 (8.6) | 8 (7.5) | 169 (15.9) | 0 (0) |
| 25–50 | 247 (19.8) | 12 (17.1) | 22 (20.6) | 212 (19.9) | 1 (14.3) |
| 50–75 | 213 (17.1) | 10 (14.3) | 19 (17.8) | 183 (17.2) | 1 (14.3) |
| 75–100 | 148 (11.9) | 5 (7.1) | 29 (27.1) | 113 (10.6) | 1 (14.3) |
| Characteristics . | Response . | ||||
|---|---|---|---|---|---|
| . | Overall . | ESTS . | AATS . | CSTCS . | JACS . |
| Years in practice (years), n (%) | |||||
| 1–5 | 219 (13.6) | 31 (15.9) | 24 (17.3) | 163 (14.2) | 1 (0.8) |
| 6–10 | 280 (17.4) | 51 (26.2) | 25 (18.0) | 201 (17.5) | 3 (2.3) |
| 11–20 | 493 (30.6) | 48 (24.6) | 45 (32.4) | 371 (32.3) | 29 (22.5) |
| 20+ | 621 (38.5) | 65 (33.3) | 45 (32.4) | 415 (36.1) | 96 (74.4) |
| Clinical practice location, n (%) | |||||
| Academic teaching hospital | 1273 (78.9) | 122 (62.6) | 106 (76.3) | 985 (85.7) | 60 (46.5) |
| Community teaching hospital | 206 (12.8) | 22 (11.3) | 16 (11.5) | 143 (12.4) | 25 (19.4) |
| Private practice | 53 (3.3) | 10 (5.1) | 11 (7.9) | 22 (1.9) | 10 (7.8) |
| Public hospital | 81 (5.0) | 41 (21.0) | 6 (4.3) | 0 (0) | 34 (26.4) |
| Institutional oncological lung resections (per year), mean ± SD | 262 ± 481 | 263 ± 388 | 622 ± 1093 | 132 ± 75 | |
| Institutional oncological lung resections (per year), median (range) | 150 (10–6000) | 175 (2–3000) | 200 (0–10 000) | 120 (15–500) | |
| Percentage of institutional minimally invasive oncological lung resections (per year), n (%) | |||||
| None | 67 (4.2) | 15 (7.7) | 2 (1.4) | 49 (4.3) | 1 (0.8) |
| <10 | 146 (9.1) | 18 (9.2) | 1 (0.7) | 125 (10.9) | 2 (1.6) |
| 10–25 | 139 (8.6) | 26 (13.3) | 3 (2.2) | 107 (9.3) | 3 (2.3) |
| 25–50 | 233 (14.4) | 48 (24.6) | 12 (8.6) | 154 (13.4) | 19 (14.7) |
| 50–75 | 469 (29.1) | 43 (22.1) | 43 (30.9) | 355 (30.9) | 28 (21.7) |
| 75–100 | 559 (34.7) | 45 (23.1) | 78 (56.1) | 360 (31.3) | 76 (58.9) |
| Oesophagectomy performed, n (%) | |||||
| Yes | 1231 (76.3) | 60 (30.8) | 106 (76.3) | 1063 (92.4) | 2 (1.6) |
| Institutional oesophagectomy (per year), mean ± SD | 34 ± 33 | 48 ± 50 | 234 ± 373 | 17 ± 26 | |
| Institutional oesophagectomy (per year), median (range) | 27 (0–140) | 30 (5–300) | 100 (2–5000) | 4 (0–60) | |
| Percentage of institutional minimally invasive/hybrid oesophagectomy (per year), n (%) | |||||
| None | 193 (15.5) | 28 (40.0) | 17 (15.9) | 144 (13.5) | 4 (57.1) |
| <10 | 263 (21.1) | 9 (12.9) | 12 (11.2) | 242 (22.7) | 0 (0) |
| 10–25 | 183 (14.7) | 6 (8.6) | 8 (7.5) | 169 (15.9) | 0 (0) |
| 25–50 | 247 (19.8) | 12 (17.1) | 22 (20.6) | 212 (19.9) | 1 (14.3) |
| 50–75 | 213 (17.1) | 10 (14.3) | 19 (17.8) | 183 (17.2) | 1 (14.3) |
| 75–100 | 148 (11.9) | 5 (7.1) | 29 (27.1) | 113 (10.6) | 1 (14.3) |
Institutional venous thromboembolic events prophylaxis protocol use
There was regional variation in terms of the degree of surgeons’ involvement and availability/adoption of institutional thromboprophylaxis protocols. The majority of respondents make the decision for inpatient VTE prophylaxis without input from other services (Fig. 1), although CSTCS members generally (68.1%) consult with another service such as haematology/internal medicine before making the final decision.
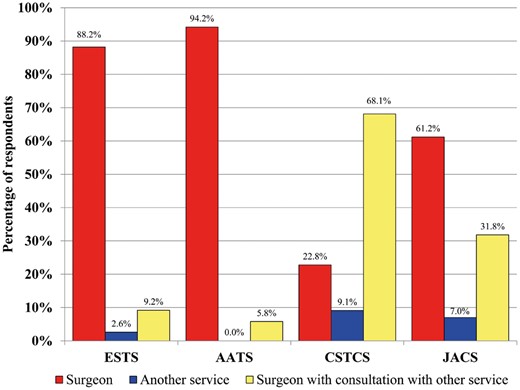
Surgeon involvement in decision-making surrounding inpatient venous thromboembolic events prophylaxis approaches for thoracic surgery patients. AATS: American Association of Thoracic Surgery; CSTCS: Chinese Society for Thoracic and Cardiovascular Surgery; ESTS: European Society of Thoracic Surgeons; JACS: Japanese Association for Chest Surgery.
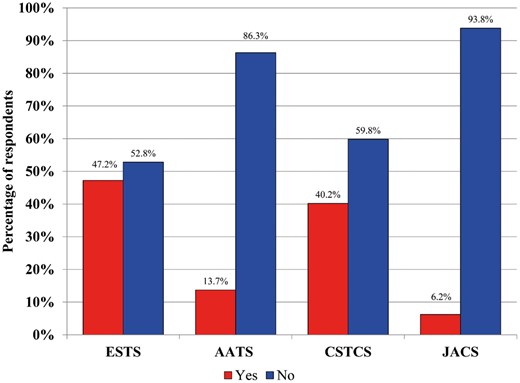
In terms of the availability of a formal institutional VTE prophylaxis protocol, JACS (n = 124, 96.1%), ESTS (n = 178, 91.3%) and AATS (n = 116, 83.5%) respondents overwhelmingly have such a protocol in place, while CSTCS respondents reported a smaller majority (n = 611, 53.1%). All respondents apply this protocol to the majority of their TS patients [JACS (n = 123, 95.3%), CSTCS (n = 577, 94.4%), ESTS (n = 182, 93.3%) and AATS (n = 126, 90.6%)]. The reasons for not using the institutional thromboprophylaxis protocol varied, but focused around: (i) lack of guidelines regarding VTE prophylaxis, (ii) attitudes that prophylaxis needs are patient-dependent, (iii) lack of consensus within the service, (iv) disagreement with the prescribed protocol or (v) protocol is not specific to the TS service. A total of 89% of respondents reported that they would be likely or very likely to use VTE thromboprophylaxis guidelines specific to the TS population, a finding that was universal across all regions (Fig. 2).
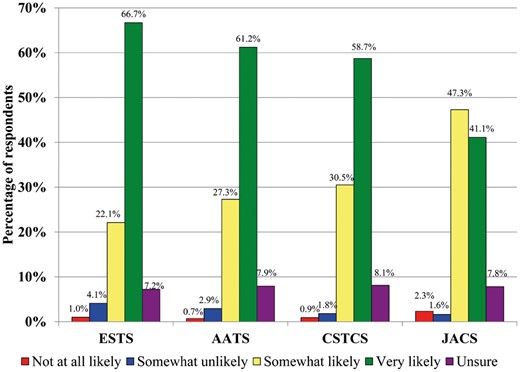
Likelihood of respondents to use venous thromboembolic thromboprophylaxis guidelines specific to thoracic surgery. AATS: American Association of Thoracic Surgery; CSTCS: Chinese Society for Thoracic and Cardiovascular Surgery; ESTS: European Society of Thoracic Surgeons; JACS: Japanese Association for Chest Surgery.
Perceptions of key risk factors influencing venous thromboembolic events prophylaxis selection in oncological lung resections
Participants were asked to consider the risk factors they consider when selecting in-hospital VTE prophylaxis. Overall, a personal history of VTE, patients with known thrombophilia but no VTE events, obesity (defined as body mass index >30 kg/m2) advanced age and duration of surgery longer than 4–6 h stood out as factors most likely to indicate a requirement for VTE prophylaxis (n = 1438, 1310, 1290, 1257 and 1067, respectively). The organizations agreed on the leading risk factors, with some variance. Respondents do not routinely utilize dedicated VTE risk scores such as the Caprini risk-stratification protocol for extended chemoprophylaxis in moderate to high-risk subjects (n = 373). Regarding the use of epidural pain control, respondents generally report that the use of such an approach does not influence their later choice in VTE prophylaxis (n = 475/622, 76.4%).
In-hospital venous thromboembolic events prophylaxis approaches in lung cancer resections
LMWH (n = 1058, 65.6%) and mechanical thromboprophylaxis consisting of either compression stockings (n = 597, 37.0%) or sequential compression devices (SCDs, n = 580, 36.0%), were the most common in-hospital thromboprophylaxis approaches after lung cancer surgery. As depicted in Fig. 3, prophylaxis selection was highly regional within the pharmaceutical and mechanical categories. CSTCS members uniquely utilize acetylsalicylic acid (ASA) as a prophylaxis option (9.3%), while the other three organizations use this modality <4% of the time, and report that no in-hospital prophylaxis is used 13.7% of the time, a finding that contrasts greatly with the other regions. ESTS, AATS and JACS participants are more likely to report starting treatment within 2–6 h of incision time (39.5%, 65.5% and 84.5%, respectively) in oncological lung resections, while the CSTCS membership reports a common practice of starting prophylaxis within 1 day postoperatively (67.0%, n = 770).
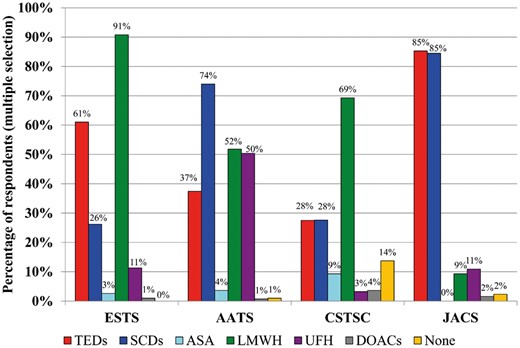
Proportion of respondents who routinely prescribe in-hospital thromboprophylaxis for the average patient undergoing oncological lung resection. AATS: American Association of Thoracic Surgery; ASA: acetylsalicylic acid; CSTCS: Chinese Society for Thoracic and Cardiovascular Surgery; DOACs: Direct-acting Oral AntiCoagulants; ESTS: European Society of Thoracic Surgeons; JACS: Japanese Association for Chest Surgery; LMWH: low-molecular-weight heparin; SCDs: sequential compression devices; TEDs: compression stockings; UFH: unfractionated heparin.
Clinical experience is the most important factor to thoracic surgeon respondents regarding the selection of type and duration of VTE prophylaxis, although this is not uniform across the responding organizations. Figure 4 describes the variable preferences across associations.
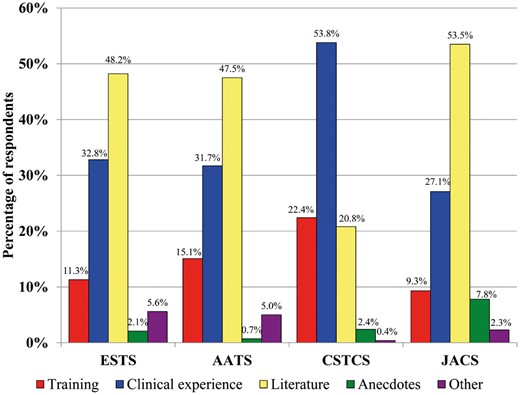
Factor that most influences decisions on the type and duration of venous thromboembolic prophylaxis for patients who are undergoing oncological lung resection. AATS: American Association of Thoracic Surgery; CSTCS: Chinese Society for Thoracic and Cardiovascular Surgery; ESTS: European Society of Thoracic Surgeons; JACS: Japanese Association for Chest Surgery.
Extended venous thromboembolic events prophylaxis approaches after lung cancer surgery
Respondents reported that extended thromboprophylaxis is not routinely prescribed after oncological lung resections (64.0%, n = 1032), although the proportion of use differs depending on the region (Fig. 5). LMWH is most commonly used for extended prophylaxis, but ASA and mechanical prophylaxis are regularly utilized (Fig. 6). Of those who prescribe out-of-hospital extended prophylaxis, LMWH is the most commonly prescribed modality for ESTS (62.4%, n = 133) and AATS (54.7%, n = 52) members while CSTCS members prefer ASA (26.1%, n = 373) and JACS respondents tend to utilize Direct-acting Oral AntiCoagulants (DOACs) primarily in this circumstance (43.9%, n = 18). Across every respondent region, extended prophylaxis is administered for up to 4 weeks postoperatively (44.0%, n = 437) after an oncological lung resection, when prescribed.
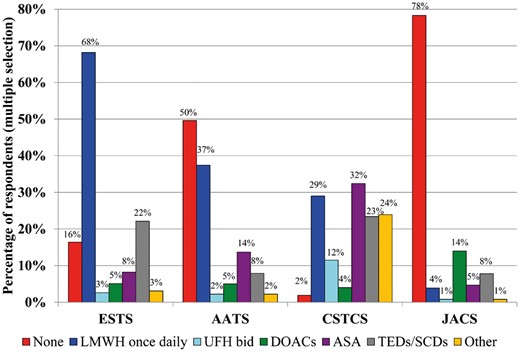
Proportion of respondents who routinely prescribe post-discharge thromboprophylaxis for the average patient undergoing an oncological lung resection. AATS: American Association of Thoracic Surgery; ASA: acetylsalicylic acid; CSTCS: Chinese Society for Thoracic and Cardiovascular Surgery; DOACs: Direct-acting Oral AntiCoagulants; ESTS: European Society of Thoracic Surgeons; JACS: Japanese Association for Chest Surgery; LMWH: low-molecular-weight heparin; SCDs: sequential compression devices; TEDs: compression stockings; UFH bid: unfractionated heparin twice daily.
Proportion of respondents who routinely recommend extended thromboprophylaxis for lung resections following hospital discharge. AATS: American Association of Thoracic Surgery; CSTCS: Chinese Society for Thoracic and Cardiovascular Surgery; ESTS: European Society of Thoracic Surgeons; JACS: Japanese Association for Chest Surgery.
The factors considered in choosing an extended thromboprophylaxis course in the lung population are similar to the leading factors for in-hospital prophylaxis selection. Respondents noted that patients with a personal history of VTE (n = 1262), thrombophilia but no personal history of VTE (n = 1208) and obesity (n = 920) were most likely to need extended prophylaxis. There is little agreement between regions regarding the next most important risk factors after these leading factors (Supplementary Material, Appendix 1, Question 22).
Extended venous thromboembolic events prophylaxis approaches after oesophagectomy
Standard practices for extended thromboprophylaxis after oesophagectomy see roughly a third of cases prescribed at least 1 prophylaxis approach (36.8%, n = 454), although ESTS respondents provide extended prophylaxis to this subpopulation 62.3% of the time, while AATS and CSTCS members prescribe extended prophylaxis less often (32.4% and 35.9%, respectively), and JACS respondents do not provide extended prophylaxis for these patients. LMWH once daily is most often prescribed (37.6%, n = 384), with mechanical prophylaxis at home in 27.0%. While DOACs are not favoured by CSTCS members for extended prophylaxis after lung resection, 22.4% of respondents report using these oral drugs in the extended timeframe after oesophagectomy. Amongst respondents who do prescribe out-of-hospital prophylaxis for oesophagectomy patients, treatments are most commonly administered for up to 4 weeks after surgery (43.7%, n = 271). For oesophagectomy cases, CSTCS surgeons are most likely to rely on clinical experience (52.5%), while AATS members are most likely to factor in the evidence in the literature when making thromboprophylaxis decisions in this population (49.5%), and ESTS members are about equally likely to consider clinical experience (40.0%) and literature (38.5%).
DISCUSSION
Overall, the current VTE thromboprophylaxis approaches are variable within and between international thoracic surgeon organizations. These results are in line with a recently published survey conducted amongst Canadian Thoracic Surgeons, Anaesthetists and Haematologists, which found considerable variability regarding the timing of initiation, duration of administration, and the agents used for VTE prophylaxis around TS across Canada [17]. Another largely North American survey by Zwischenberger et al. [19], focusing on postoesophagectomy VTE prophylaxis, echoed these findings, and particularly emphasized the under-treatment of this high-risk population. Both of these surveys had a similar response rate of roughly 35% of respondents in a single region but with small sample sizes, while our survey is the largest to date in the TS literature.
TS patients undergoing oncological resections are considered to be at high risk for VTE [1–5,15], which may be under-estimated due to non-specific symptoms and radiological findings that may be attributed to the surgery itself rather than VTE. Major abdominal oncological surgery is recognized as high-risk in VTE population [11], but the thoracic population ought to be considered at a particularly high-risk due to extensive surgical intervention directly involving manipulation of the pulmonary arteries and stapling/tying of arterial branches [20], dependent limb position [1] in the operating room and duration of the procedures. Treatment decision-making is based on a balance of cost-benefits, and part of this calculation involves adequate appraisal of the risks associated with failing to treat/prevent a given event, the risks associated with the treatment itself, and the likelihood of the event occurring for an individual.
The rates of VTE and VTE screening internationally was not examined in this survey, and it is difficult to ascertain an understanding of these rates in the literature due to disparate study designs, data collection techniques (national databases using readmitted patients versus screening-detected), and poor data reporting (varied follow-up duration and unreported data) [4,21]. The geographical distribution of VTE does appear to vary, with East Asian surgical oncology patients appearing to be at a slightly lower risk of VTE development [21] relative to European and North American populations, however, no firm conclusions can be made given the limitations of these systematic reviews.
This study indicates that there is a disagreement as to which factors are important in prophylaxis selection, so there is no consensus on which patients are at a particularly high risk of VTE. Major postoperative complications and advanced age are independent risk factors indicating a need for extended prophylaxis in surgical oncology patients in the literature [10,22,23], but survey respondents did not unequivocally prioritize these factors in their decision-making, nor did they report using validated VTE risk scores in any region. VTE risk scores, such as the Caprini score, put an emphasis on advanced age, a personal or family history of VTE, prior malignancy and long, extensive surgeries [24] in VTE prophylaxis selection, and have been associated with successfully identifying at-risk patients [25] who may need more intensive approaches.
This study observed that the rate of patients being discharged home with VTE prophylaxis is low, which is in keeping with evidence-based guidelines in TS that currently do not support the usage of extended thromboprophylaxis [11,12,15], however, a complementary Delphi process study (in review) evaluating current practice worldwide, has noted that the decision for discharging patients home with extended-duration VTE prophylaxis is solely based on individual surgeons’ decision-making and not influenced by any guidelines. While some respondents appear to make VTE thromboprophylaxis decisions that are concordant with evidence-based ACCP [11], ASCO [12] and ESA [15] recommendations, demonstrating a preference for LMWH/unfractionated heparin and mechanical prophylaxis where appropriate, there is clear deviation towards practices that are not currently supported by literature, such as initiating prophylaxis a day after surgery, not using prophylaxis at all, or use of ASA and DOACs for either in-hospital or extended prophylaxis. A large portion of survey respondents in Europe and Japan indicated that they refer to the literature to inform their decision-making. However, a Cochrane review of thromboprophylaxis safety and efficacy in thoracic patients found that the evidence was so poor that no population-wide recommendations could adequately be made [26]. Those surgeons who rely on the literature are faced with poor or conflicting evidence on which to inform their treatment decisions. There is a large minority across all regions, but weighted by a majority in the large Chinese cohort, that relies on clinical experience and knowledge gained during the training phase of a surgeon’s career, which can perpetuate non-evidence-based practices. Whether surgeons rely on inadequate literature or learned experience for their thromboprophylaxis decisions, these are challenges that lead to non-guideline concurrent treatments.
In TS VTE prophylaxis guidelines and literature, there is no evidence supporting extended out-of-hospital prophylaxis. Major pelvic and abdominal oncological surgery have generated sufficient quality evidence supporting the use of extended prophylaxis for up to 4 weeks after surgery with the ENOXACAN [27] and CANBESURE [28] placebo-controlled randomized controlled trials and an open-label randomized controlled trial comparison of 7-day vs 28-day dalteparin [29], leading to specific ACCP/ASCO guidelines [11,12]. Since many respondents noted that they rely on evidence in the literature to justify treatment decisions, this lack of supporting data may explain the widely varying adoption of extended prophylaxis approaches between thoracic surgeons seen in our study.
The vast majority of ESTS, AATS and JACS institutions possess a VTE prophylaxis protocol presumably designed with the best evidence to date, and respective members report to have adopted these institutional protocols in their practice. However, the variance in the survey findings indicates that ideal practice and reality are far apart. While reasons for guideline non-compliance were not fully explored in this study, respondents generally noted that a lack of literature supporting local recommendations, or recommendations that are non-specific to the thoracic oncology population were likely to lead to deviations from best practices. In some jurisdictions, it is also important to consider that some prophylaxis approaches are costly and/or less available, and may impact the selection of therapy accordingly.
Limitations
There are three primary limitations to this survey. The first is that the survey has a relatively low response rate of 36%. While the authors attempted to maximize responses with multiple reminders to the membership of each organization, this remains a limitation, although this study is the largest TS-specific thromboprophylaxis survey to date with feedback from 5 continents. A second potential limitation is the disproportionately larger number of respondents from CSTCS (71% of total respondents). This heavily weighted group could drastically impact the overall averages and first-glance findings, particularly when the Chinese respondents diverged from the other organizations. As an example, Fig. 1 clearly depicts this weighting, which sees 40% of surgeons primarily responsible for VTE prophylaxis selection if the Chinese respondents are included, yet when excluding the Chinese cohort, this mean number increases to 83%. Also, the availability of a unit/hospital prophylaxis protocol was present in 63.8% of institutions overall, yet this proportion increases to 90.3% without the Chinese weighting. This weighting issue was thought to potentially obscure overall trends, so individual region findings were reported to avoid losing signal. Finally, the respondents were mostly academically-based, so the survey findings may not be reflective of the experience in smaller centres or community hospitals. However, given that academic centres typically perform the majority of case volumes, the authors are confident that the practices presented in this study reflect the treatment of the vast majority of TS cases in the regions participating in the survey.
Moving forward, there is a need to gather consensus on the risk factors most important to thromboprophylaxis decision-making, and to generate adequate high-quality evidence to inform guidelines specific to this high-risk population for developing postoperative VTE complications. Many surgeons rely on the literature to provide evidence to inform treatment selections, but the current paucity of data is not helpful in providing the necessary evidence base.
Conflict of interest: none declared.
REFERENCES
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cancer-associated Venous Thromboembolic Disease; Version 2.2018.





