-
PDF
- Split View
-
Views
-
Cite
Cite
Chunfeng Song, Yaron Shargall, Hui Li, Bo Tian, Shuo Chen, Jinbai Miao, Yili Fu, Bin You, Bin Hu, Prevalence of venous thromboembolism after lung surgery in China: a single-centre, prospective cohort study involving patients undergoing lung resections without perioperative venous thromboembolism prophylaxis, European Journal of Cardio-Thoracic Surgery, Volume 55, Issue 3, March 2019, Pages 455–460, https://doi.org/10.1093/ejcts/ezy323
Close - Share Icon Share
Abstract
Venous thromboembolism (VTE) is a common postoperative complication. Previous studies have shown that the incidence of VTE after major thoracic surgery ranges from 2.3% to 15%. However, there have been no such data from China so far. To evaluate the incidence of postoperative VTE, we conducted a single-centre, prospective cohort study.
Patients who underwent lung resections between July 2016 and March 2017 were enrolled in this study. None of the patients received any prophylaxis perioperatively. All patients were screened for deep venous thrombosis (DVT) using non-invasive duplex lower-extremity ultrasonography 30 days before surgery and within 30 days after surgery and before discharge. Chest tomography, pulmonary embolism protocol was carried out if patients had one of the following conditions: (i) typical symptoms of pulmonary embolism, (ii) high Caprini score (≥9 points) and (iii) newly diagnosed postoperative DVT.
Two hundred and sixty-two patients undergoing lung surgery were enrolled, including 115 benign and 147 malignant disease cases. The procedures included 84 sublobar lung resections, 161 lobectomies, 5 pneumonectomies and 12 mixed procedures. The overall postoperative incidence of VTE was 11.5% (30 of 262). Twenty-four patients were diagnosed with DVT (80.0%) and 6 with DVT + pulmonary embolism (20.0%). None of the patients diagnosed with VTE had obvious symptoms of VTE. The median time for VTE detection was 5 days postoperatively. The incidence of VTE was 7.0% in patients with benign lung diseases and 15.0% in those with malignant lung diseases (P < 0.05). Using the Caprini risk assessment model, 63 cases were scored as low risk, 179 as moderate risk and 20 as high risk, and each group had an incidence of postoperative VTE of 0%, 12.3% (22 of 179) and 40.0% (8 of 20), respectively (P < 0.05). In patients with lung cancer, 98% were moderate or high risk, and only 3 patients were scored in the low risk category. The incidence of VTE in patients at moderate risk and high risk was 12.0% and 36.8%, respectively, while it was 0 in low-risk patients.
The following conclusions were drawn: (i) the overall incidence of postoperative VTE after lung surgery without VTE prophylaxis is substantial; (ii) lower-extremity ultrasonography was helpful in detecting asymptomatic DVT in symptomatic or high-risk patients; and (iii) VTE prophylaxis should be considered as a mandatory part of perioperative care.
ChiCTR-EOC-17010577.
INTRODUCTION
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), is a common postoperative complication with substantial morbidity, mortality and resource utilization [1]. Recent evidence shows that the overall VTE rate after thoracic surgery is 2.3% and that 60-day VTE incidence following lung cancer resections is 5.2% [2]. More contemporary results indicate that patients undergoing thoracic surgery are in one of the highest risk groups for developing VTE [3] and that the incidence of VTE after lung resection varies from 5% to 15.2% [4–6]. While perioperative VTE prophylaxis is considered the standard of care by all major organizations and guidelines [7–9], the majority of thoracic surgeons in China do not endorse the significance of postoperative VTE. In addition, currently there are no consensus guidelines for VTE screening or prevention in patients undergoing thoracic surgery worldwide [10]. As a result, many centres in China do not routinely prescribe perioperative VTE prophylaxis, neither mechanical nor chemical, for patients undergoing thoracic surgical resections.
Perioperative or postoperative VTE can be asymptomatic, which may be the reason for the low incidence of postoperative VTE reported in patients undergoing thoracic surgery [11, 12]. Fatal or near-fatal PE can be the first clinical manifestation of postoperative VTE in many patients, which may cause severe clinical consequences. It is, therefore, important to predict those patients who are more likely to develop VTE and to detect those who have already developed VTE. The Caprini score is a widely used VTE risk assessment model (RAM) in a number of surgical specialties, which is also recommended by the American College of Chest Physicians as a decision tool for identifying patients with a high risk of VTE and determining the appropriate ways of prophylaxis amongst patients with abdominal and pelvic cancer [13]. Studies using a modified Caprini score for VTE risk assessment among patients after lung cancer resection showed that with a high-risk score cut-off of 9, the sensitivity, specificity and accuracy were 83.3%, 60.5% and 61.6%, respectively. Scores in the low-, moderate- and high-risk groups were associated with a VTE incidence of 0%, 1.7% and 10.3%, respectively; that is, patients with a higher Caprini score are more likely to develop postoperative VTE [2].
Unfortunately, there are no screening or prevention methods for perioperative VTE in patients undergoing lung surgery in China. No prospective studies have been conducted in China so far. As a result, the incidence of perioperative VTE in Chinese patients undergoing thoracic surgery remains unknown, and the usage of perioperative VTE prophylaxis, while broadly adopted and recommended worldwide, is not a common practice in China. We, therefore, conducted a single-centre, prospective, observational cohort study that aimed at detecting perioperative VTE events that occurred before discharge in patients undergoing lung resections at our centre and which were not treated with VTE prophylaxis and at identifying those patients who were at high risk of developing VTE.
PATIENTS AND METHODS
This prospective study was approved by the Beijing Chao-Yang Hospital Institutional Review Board. Patients who underwent lung surgery between July 2016 and March 2017 were enrolled in the study. The follow-up period ended upon patients’ discharge from the hospital.
The Caprini score was calculated for all the patients based on the modified scoring system used by the Boston Medical Center [10, 14]. Patients were divided into 3 categories according to their score: low-risk group (0–4 points), moderate-risk group (5–8 points) and high-risk group (9 or more points). All patients undergoing lung surgery were included in this study. With the exception of routine preoperative assessment (chest and upper abdomen computed tomography examination, brain magnetic resonance imaging, bone nuclear scan, cardiac echocardiogram and pulmonary function tests), all the patients were screened for DVT using non-invasive duplex lower-extremity ultrasonography 30 days before surgery and within 30 days after surgery before discharge. Computed tomographic pulmonary angiography (CTPA) was carried out if patients had one of the following conditions: (i) typical symptoms of PE (chest pain, haemoptysis, dyspnoea or persistent hypoxaemia), (ii) high Caprini score (≥9 points) and (iii) new diagnosis of postoperative DVT.
Patients were excluded based on the presence of any of the following criteria: missing records, not screened for DVT after surgery or before discharge from hospital, anticoagulation before the surgery and the need for anticoagulation due to other reasons such as arrhythmias.
The definition of a VTE event was any PE or DVT identified by CTPA or duplex proximal lower limbs venous ultrasonography. Patients who were discharged without postoperative image inspection were excluded from this analysis. Other details of the patients’ medical history, including general information [age, gender, body mass index (BMI), hospitalization time and diagnosis]; comorbidities (hypertension, diabetes mellitus and coronary atherosclerotic heart disease); and information about the surgical procedure (surgical approach, length of operation and volume of blood loss) were also recorded.
The Statistical Package for Social Science software (version 18.0, SPSS Inc., Chicago, IL, USA) was used for all analyses. Continuous variables were assessed by the underlying assumption of normality. Normally distributed variables were expressed as mean and standard deviation and other variables as the median and associated interquartile range. The Student’s t-test was used to compare the mean of the continuous variables between the 2 groups, and the Pearson χ2 test or the Fisher’s exact test (the minimum expected cell size is 5) was performed for categorical variables. P-value ≤0.05 indicated statistical significance. Multivariable logistic regression was further performed among the variables (P < 0.1) in univariable analysis.
RESULTS
Incidence of postoperative venous thromboembolism
A total of 262 patients were enrolled for analysis after exclusion of 23 patients because of anticoagulation administered prior to surgery (Table 1). There were no cases of death or adverse bleeding events after surgery and before discharge among the patients enrolled in the study. Postoperative discharge days ranged from 3 to 54 days. The mean time and median length of stay were 7.32 and 6 days, respectively. Two (0.7%) patients were diagnosed with pulmonary infection after surgery, 6 (2.2%) transient atrial fibrillation and 25 (9.5%) prolonged air leak (leakage time longer than 5 days after minimally invasive surgery (MIS) or longer than 7 days after open surgery). One patient required a second operation because of pulmonary infection and empyema following the first operation.
| Variables . | Number (%) or mean ± SD . |
|---|---|
| Age (years) | 54.73 ± 14.46 |
| Gender | |
| Male | 149 (56.9) |
| Female | 113 (43.1) |
| Disease type | |
| Benign | 115 (43.9) |
| Malignancy | 147 (56.1) |
| Comorbidities | |
| Hypertension | 63 (24.0) |
| CHD | 17 (6.5) |
| Diabetes | 35 (13.4) |
| BMI >25 (kg/m2) | 75 (28.6) |
| Hospitalization time (days) | 12.79 ± 6.58 |
| Surgical approach | |
| Minimally invasive surgery | 203 (77.5) |
| Open surgery | 59 (22.5) |
| Length of operation (min) | 163.46 ± 72.73 |
| Volume of blood loss (ml) | 181.32 |
| Serious acute lung disease (<1 month)a | 22 (8.4) |
| Positive duplex ultrasound | 60 (22.9) |
| Hospitalization time | 12.79 ± 6.58 |
| Variables . | Number (%) or mean ± SD . |
|---|---|
| Age (years) | 54.73 ± 14.46 |
| Gender | |
| Male | 149 (56.9) |
| Female | 113 (43.1) |
| Disease type | |
| Benign | 115 (43.9) |
| Malignancy | 147 (56.1) |
| Comorbidities | |
| Hypertension | 63 (24.0) |
| CHD | 17 (6.5) |
| Diabetes | 35 (13.4) |
| BMI >25 (kg/m2) | 75 (28.6) |
| Hospitalization time (days) | 12.79 ± 6.58 |
| Surgical approach | |
| Minimally invasive surgery | 203 (77.5) |
| Open surgery | 59 (22.5) |
| Length of operation (min) | 163.46 ± 72.73 |
| Volume of blood loss (ml) | 181.32 |
| Serious acute lung disease (<1 month)a | 22 (8.4) |
| Positive duplex ultrasound | 60 (22.9) |
| Hospitalization time | 12.79 ± 6.58 |
Serious acute lung diseases: combined lung diseases like pulmonary infection in lung cancer cases; positive duplex ultrasound: abnormal results of US examination before operation, such as intravenous expansion or blood stasis.
BMI: body mass index; CHD: congenital heart disease; SD: standard deviation.
| Variables . | Number (%) or mean ± SD . |
|---|---|
| Age (years) | 54.73 ± 14.46 |
| Gender | |
| Male | 149 (56.9) |
| Female | 113 (43.1) |
| Disease type | |
| Benign | 115 (43.9) |
| Malignancy | 147 (56.1) |
| Comorbidities | |
| Hypertension | 63 (24.0) |
| CHD | 17 (6.5) |
| Diabetes | 35 (13.4) |
| BMI >25 (kg/m2) | 75 (28.6) |
| Hospitalization time (days) | 12.79 ± 6.58 |
| Surgical approach | |
| Minimally invasive surgery | 203 (77.5) |
| Open surgery | 59 (22.5) |
| Length of operation (min) | 163.46 ± 72.73 |
| Volume of blood loss (ml) | 181.32 |
| Serious acute lung disease (<1 month)a | 22 (8.4) |
| Positive duplex ultrasound | 60 (22.9) |
| Hospitalization time | 12.79 ± 6.58 |
| Variables . | Number (%) or mean ± SD . |
|---|---|
| Age (years) | 54.73 ± 14.46 |
| Gender | |
| Male | 149 (56.9) |
| Female | 113 (43.1) |
| Disease type | |
| Benign | 115 (43.9) |
| Malignancy | 147 (56.1) |
| Comorbidities | |
| Hypertension | 63 (24.0) |
| CHD | 17 (6.5) |
| Diabetes | 35 (13.4) |
| BMI >25 (kg/m2) | 75 (28.6) |
| Hospitalization time (days) | 12.79 ± 6.58 |
| Surgical approach | |
| Minimally invasive surgery | 203 (77.5) |
| Open surgery | 59 (22.5) |
| Length of operation (min) | 163.46 ± 72.73 |
| Volume of blood loss (ml) | 181.32 |
| Serious acute lung disease (<1 month)a | 22 (8.4) |
| Positive duplex ultrasound | 60 (22.9) |
| Hospitalization time | 12.79 ± 6.58 |
Serious acute lung diseases: combined lung diseases like pulmonary infection in lung cancer cases; positive duplex ultrasound: abnormal results of US examination before operation, such as intravenous expansion or blood stasis.
BMI: body mass index; CHD: congenital heart disease; SD: standard deviation.
Among the 262 patients enrolled, there were 115 with benign diagnosis (Table 2) and 147 with malignancies. Lung malignancies included 93 (63.3%) adenocarcinoma, 34 (23.1%) squamous cell carcinoma, 8 (5.4%) metastatic diseases, 8 (5.4%) neuroendocrine tumours, 2 (1.4%) sarcomatoid carcinomas, 1 (0.7%) adenosquamous carcinoma and 1 (0.7%) synovial sarcoma. The surgical procedures included 73 (27.9%) lung wedge resection, 11 (4.2%) pulmonary segmentectomy, 161 (61.5%) lobectomy, 5 (1.9%) pneumonectomy and 12 (4.6%) mixed procedures (7 cases of both lobectomy and lung wedge resection, 4 cases of both lobectomy and segmentectomy and 1 case of both lung wedge resection and segmentectomy).
| . | Number . |
|---|---|
| Benign lung tumours | 21 |
| Pulmonary vesicles, emphysema | 32 |
| Bronchiectasis | 27 |
| Tuberculosis | 7 |
| Pulmonary mycosis | 5 |
| Cryptogenic organizing pneumonia | 10 |
| Interstitial pneumonia | 3 |
| Pulmonary abscess | 4 |
| Pulmonary atelectasis | 3 |
| Pulmonary cyst | 2 |
| Pulmonary arterio-venous fistula | 1 |
| . | Number . |
|---|---|
| Benign lung tumours | 21 |
| Pulmonary vesicles, emphysema | 32 |
| Bronchiectasis | 27 |
| Tuberculosis | 7 |
| Pulmonary mycosis | 5 |
| Cryptogenic organizing pneumonia | 10 |
| Interstitial pneumonia | 3 |
| Pulmonary abscess | 4 |
| Pulmonary atelectasis | 3 |
| Pulmonary cyst | 2 |
| Pulmonary arterio-venous fistula | 1 |
| . | Number . |
|---|---|
| Benign lung tumours | 21 |
| Pulmonary vesicles, emphysema | 32 |
| Bronchiectasis | 27 |
| Tuberculosis | 7 |
| Pulmonary mycosis | 5 |
| Cryptogenic organizing pneumonia | 10 |
| Interstitial pneumonia | 3 |
| Pulmonary abscess | 4 |
| Pulmonary atelectasis | 3 |
| Pulmonary cyst | 2 |
| Pulmonary arterio-venous fistula | 1 |
| . | Number . |
|---|---|
| Benign lung tumours | 21 |
| Pulmonary vesicles, emphysema | 32 |
| Bronchiectasis | 27 |
| Tuberculosis | 7 |
| Pulmonary mycosis | 5 |
| Cryptogenic organizing pneumonia | 10 |
| Interstitial pneumonia | 3 |
| Pulmonary abscess | 4 |
| Pulmonary atelectasis | 3 |
| Pulmonary cyst | 2 |
| Pulmonary arterio-venous fistula | 1 |
A total of 30 patients were detected with VTE, for an overall incidence of VTE after thoracic surgeries of 11.5% (30 of 262), of which 80.0% (24 of 30) were DVT and 20.0% (6 of 30) were both DVT and PE. Importantly, none of the patients diagnosed with VTE had obvious symptoms of VTE (acute severe chest pain, dyspnoea or swollen legs). Among the 30 VTE cases, 93.3% (28/30) of the VTE cases were diagnosed within 1 week after surgery. The mean time of postoperative VTE diagnosed was 4.97 postoperative days (ranged from 2 to 23 days), and the median time was 5 days (with the first quartile being 3 days and the third quartile being 6 days). The incidence of VTE was 7.0% (8 of 115) in patients with benign lung diseases and 15.0% (22 of 147) in patients who were operated on for malignant diseases (P < 0.05) (Fig. 1).
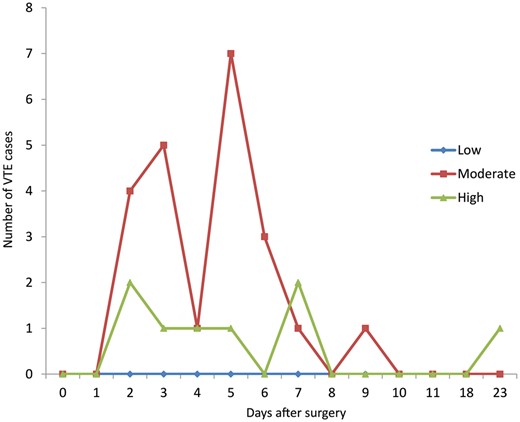
Incidence of VTE over time postoperatively stratified by Caprini RAM. VTE: venous thromboembolism.
Caprini score assessment
Among 262 patients in total, 63 patients were found to be at low risk (0–4 points), 179 at moderate risk (5–8 points) and 20 patients at high risk (9 points and more). Scores in the low-, moderate- and high-risk groups were associated with a VTE incidence of 0%, 12.3% (22 of 179) and 40.0% (8 of 20), respectively (P < 0.05). In patients diagnosed with lung cancer, only 3 patients scored low, 125 patients scored moderately (5–8 points) and 19 patients scored high (9 points and more). The corresponding VTE incidence for these patients was 0% (0 out of 3), 12.0% (15 of 125) and 36.8% (7 of 19) (P < 0.05).
Patients who experienced VTE were older than those who did not have it (mean age 65.10 vs 53.38 years, P < 0.05) (Table 3). Patients with malignant diseases were more likely to develop VTE (P < 0.05). As for comorbidities, hypertension was associated with a higher incidence of VTE (P < 0.05), while coronary heart diseases and diabetes mellitus were not associated with a higher risk for VTE occurrence. Longer surgeries (≥45 min) were associated with a higher incidence of VTE (P < 0.05). However, the surgical approach (MIS versus open resection), type of procedure (lobectomy, wedge and pneumonectomy), blood loss volumes and serious acute lung diseases (combined lung diseases such as pulmonary infection in lung cancer cases) were not associated with the perioperative development of VTE.
| Characteristics . | Non-VTE (n = 232) . | VTE (n = 30) . | P-value . |
|---|---|---|---|
| Age (years), mean (SD) | 53.38 (14.60) | 65.10 (7.59) | 0.004 |
| Gender, n (%) | 0.052 | ||
| Male | 137 (59.1) | 12 (40.0) | |
| Female | 95 (40.9) | 18 (60.0) | |
| Disease type, n (%) | 0.032 | ||
| Benign | 107 (46.1) | 8 (26.7) | |
| Malignant | 125 (53.9) | 22 (73.3) | |
| Comorbidities, n (%) | |||
| Hypertension | 51 (22.0) | 12 (40.0) | 0.040 |
| CHD | 14 (6.0) | 3 (10.0) | 0.424 |
| Diabetes | 34 (14.7) | 1 (3.3) | 0.148 |
| BMI >25 (kg/m2) | 62 (26.7) | 13 (43.3) | 0.058 |
| Surgical approach, n (%) | 0.062 | ||
| Minimally invasive surgery | 184 (79.3) | 19 (63.3) | |
| Open surgery | 48 (20.7) | 11 (36.7) | |
| Surgical procedure, n (%) | 0.301 | ||
| Lung wedge resection | 69 (29.7) | 4 (13.3) | |
| Segmentectomy | 10 (4.3) | 1 (3.3) | |
| Lobectomy | 138 (59.5) | 23 (76.7) | |
| Pneumonectomy | 5 (2.2) | 0 (0) | |
| Mixed procedure | 10 (4.3) | 2 (6.7) | |
| Duration of operation (min) | 159.87 (72.53) | 191.27 (69.32) | 0.026 |
| Volume of blood loss (ml) | 171.92 | 254.00 | 0.354 |
| Caprini score, n (%) | <0.001 | ||
| Low risk (0–4) | 63 (27.2) | 0 (0) | |
| Moderate risk (5–8) | 157 (24.6) | 22 (73.3) | |
| High risk (≥9) | 12 (5.2) | 8 (26.7) | |
| Serious acute lung disease (<1 month), n (%) | 19 (8.2) | 3 (10.0) | 0.474 |
| Positive duplex ultrasound, n (%) | 47 (20.3) | 13 (43.3) | 0.005 |
| Characteristics . | Non-VTE (n = 232) . | VTE (n = 30) . | P-value . |
|---|---|---|---|
| Age (years), mean (SD) | 53.38 (14.60) | 65.10 (7.59) | 0.004 |
| Gender, n (%) | 0.052 | ||
| Male | 137 (59.1) | 12 (40.0) | |
| Female | 95 (40.9) | 18 (60.0) | |
| Disease type, n (%) | 0.032 | ||
| Benign | 107 (46.1) | 8 (26.7) | |
| Malignant | 125 (53.9) | 22 (73.3) | |
| Comorbidities, n (%) | |||
| Hypertension | 51 (22.0) | 12 (40.0) | 0.040 |
| CHD | 14 (6.0) | 3 (10.0) | 0.424 |
| Diabetes | 34 (14.7) | 1 (3.3) | 0.148 |
| BMI >25 (kg/m2) | 62 (26.7) | 13 (43.3) | 0.058 |
| Surgical approach, n (%) | 0.062 | ||
| Minimally invasive surgery | 184 (79.3) | 19 (63.3) | |
| Open surgery | 48 (20.7) | 11 (36.7) | |
| Surgical procedure, n (%) | 0.301 | ||
| Lung wedge resection | 69 (29.7) | 4 (13.3) | |
| Segmentectomy | 10 (4.3) | 1 (3.3) | |
| Lobectomy | 138 (59.5) | 23 (76.7) | |
| Pneumonectomy | 5 (2.2) | 0 (0) | |
| Mixed procedure | 10 (4.3) | 2 (6.7) | |
| Duration of operation (min) | 159.87 (72.53) | 191.27 (69.32) | 0.026 |
| Volume of blood loss (ml) | 171.92 | 254.00 | 0.354 |
| Caprini score, n (%) | <0.001 | ||
| Low risk (0–4) | 63 (27.2) | 0 (0) | |
| Moderate risk (5–8) | 157 (24.6) | 22 (73.3) | |
| High risk (≥9) | 12 (5.2) | 8 (26.7) | |
| Serious acute lung disease (<1 month), n (%) | 19 (8.2) | 3 (10.0) | 0.474 |
| Positive duplex ultrasound, n (%) | 47 (20.3) | 13 (43.3) | 0.005 |
BMI: body mass index; CHD: congenital heart disease; SD: standard deviation; VTE: venous thromboembolism.
| Characteristics . | Non-VTE (n = 232) . | VTE (n = 30) . | P-value . |
|---|---|---|---|
| Age (years), mean (SD) | 53.38 (14.60) | 65.10 (7.59) | 0.004 |
| Gender, n (%) | 0.052 | ||
| Male | 137 (59.1) | 12 (40.0) | |
| Female | 95 (40.9) | 18 (60.0) | |
| Disease type, n (%) | 0.032 | ||
| Benign | 107 (46.1) | 8 (26.7) | |
| Malignant | 125 (53.9) | 22 (73.3) | |
| Comorbidities, n (%) | |||
| Hypertension | 51 (22.0) | 12 (40.0) | 0.040 |
| CHD | 14 (6.0) | 3 (10.0) | 0.424 |
| Diabetes | 34 (14.7) | 1 (3.3) | 0.148 |
| BMI >25 (kg/m2) | 62 (26.7) | 13 (43.3) | 0.058 |
| Surgical approach, n (%) | 0.062 | ||
| Minimally invasive surgery | 184 (79.3) | 19 (63.3) | |
| Open surgery | 48 (20.7) | 11 (36.7) | |
| Surgical procedure, n (%) | 0.301 | ||
| Lung wedge resection | 69 (29.7) | 4 (13.3) | |
| Segmentectomy | 10 (4.3) | 1 (3.3) | |
| Lobectomy | 138 (59.5) | 23 (76.7) | |
| Pneumonectomy | 5 (2.2) | 0 (0) | |
| Mixed procedure | 10 (4.3) | 2 (6.7) | |
| Duration of operation (min) | 159.87 (72.53) | 191.27 (69.32) | 0.026 |
| Volume of blood loss (ml) | 171.92 | 254.00 | 0.354 |
| Caprini score, n (%) | <0.001 | ||
| Low risk (0–4) | 63 (27.2) | 0 (0) | |
| Moderate risk (5–8) | 157 (24.6) | 22 (73.3) | |
| High risk (≥9) | 12 (5.2) | 8 (26.7) | |
| Serious acute lung disease (<1 month), n (%) | 19 (8.2) | 3 (10.0) | 0.474 |
| Positive duplex ultrasound, n (%) | 47 (20.3) | 13 (43.3) | 0.005 |
| Characteristics . | Non-VTE (n = 232) . | VTE (n = 30) . | P-value . |
|---|---|---|---|
| Age (years), mean (SD) | 53.38 (14.60) | 65.10 (7.59) | 0.004 |
| Gender, n (%) | 0.052 | ||
| Male | 137 (59.1) | 12 (40.0) | |
| Female | 95 (40.9) | 18 (60.0) | |
| Disease type, n (%) | 0.032 | ||
| Benign | 107 (46.1) | 8 (26.7) | |
| Malignant | 125 (53.9) | 22 (73.3) | |
| Comorbidities, n (%) | |||
| Hypertension | 51 (22.0) | 12 (40.0) | 0.040 |
| CHD | 14 (6.0) | 3 (10.0) | 0.424 |
| Diabetes | 34 (14.7) | 1 (3.3) | 0.148 |
| BMI >25 (kg/m2) | 62 (26.7) | 13 (43.3) | 0.058 |
| Surgical approach, n (%) | 0.062 | ||
| Minimally invasive surgery | 184 (79.3) | 19 (63.3) | |
| Open surgery | 48 (20.7) | 11 (36.7) | |
| Surgical procedure, n (%) | 0.301 | ||
| Lung wedge resection | 69 (29.7) | 4 (13.3) | |
| Segmentectomy | 10 (4.3) | 1 (3.3) | |
| Lobectomy | 138 (59.5) | 23 (76.7) | |
| Pneumonectomy | 5 (2.2) | 0 (0) | |
| Mixed procedure | 10 (4.3) | 2 (6.7) | |
| Duration of operation (min) | 159.87 (72.53) | 191.27 (69.32) | 0.026 |
| Volume of blood loss (ml) | 171.92 | 254.00 | 0.354 |
| Caprini score, n (%) | <0.001 | ||
| Low risk (0–4) | 63 (27.2) | 0 (0) | |
| Moderate risk (5–8) | 157 (24.6) | 22 (73.3) | |
| High risk (≥9) | 12 (5.2) | 8 (26.7) | |
| Serious acute lung disease (<1 month), n (%) | 19 (8.2) | 3 (10.0) | 0.474 |
| Positive duplex ultrasound, n (%) | 47 (20.3) | 13 (43.3) | 0.005 |
BMI: body mass index; CHD: congenital heart disease; SD: standard deviation; VTE: venous thromboembolism.
Using logistic regression, we found that age, gender and BMI >25 (kg/m2) are independent risk factors of VTE (P < 0.05) (Table 4).
| . | B . | S.E. . | Wals . | P-value . | OR . | 95% CI . | |
|---|---|---|---|---|---|---|---|
| Lower . | Upper . | ||||||
| Age (years) | 0.096 | 0.026 | 14.193 | 0.000 | 1.101 | 1.047 | 1.158 |
| Disease type | −0.799 | 0.476 | 2.815 | 0.093 | 0.450 | 0.177 | 1.144 |
| Hypertension | 0.158 | 0.749 | 0.044 | 0.833 | 1.171 | 0.270 | 5.084 |
| Duration of operation | 0.003 | 0.003 | 0.823 | 0.364 | 1.003 | 0.996 | 1.010 |
| Positive duplex ultrasound | −0.444 | 0.454 | 0.957 | 0.328 | 0.642 | 0.264 | 1.561 |
| Gender | 1.051 | 0.448 | 5.502 | 0.019 | 2.860 | 1.189 | 6.884 |
| BMI >25 (kg/m2) | −0.950 | 0.453 | 4.399 | 0.036 | 0.387 | 0.159 | 0.940 |
| Constant | −7.637 | 2.117 | 13.016 | 0.000 | 0.000 | ||
| . | B . | S.E. . | Wals . | P-value . | OR . | 95% CI . | |
|---|---|---|---|---|---|---|---|
| Lower . | Upper . | ||||||
| Age (years) | 0.096 | 0.026 | 14.193 | 0.000 | 1.101 | 1.047 | 1.158 |
| Disease type | −0.799 | 0.476 | 2.815 | 0.093 | 0.450 | 0.177 | 1.144 |
| Hypertension | 0.158 | 0.749 | 0.044 | 0.833 | 1.171 | 0.270 | 5.084 |
| Duration of operation | 0.003 | 0.003 | 0.823 | 0.364 | 1.003 | 0.996 | 1.010 |
| Positive duplex ultrasound | −0.444 | 0.454 | 0.957 | 0.328 | 0.642 | 0.264 | 1.561 |
| Gender | 1.051 | 0.448 | 5.502 | 0.019 | 2.860 | 1.189 | 6.884 |
| BMI >25 (kg/m2) | −0.950 | 0.453 | 4.399 | 0.036 | 0.387 | 0.159 | 0.940 |
| Constant | −7.637 | 2.117 | 13.016 | 0.000 | 0.000 | ||
BMI: body mass index; CI: confidence interval; OR: odds ratio.
| . | B . | S.E. . | Wals . | P-value . | OR . | 95% CI . | |
|---|---|---|---|---|---|---|---|
| Lower . | Upper . | ||||||
| Age (years) | 0.096 | 0.026 | 14.193 | 0.000 | 1.101 | 1.047 | 1.158 |
| Disease type | −0.799 | 0.476 | 2.815 | 0.093 | 0.450 | 0.177 | 1.144 |
| Hypertension | 0.158 | 0.749 | 0.044 | 0.833 | 1.171 | 0.270 | 5.084 |
| Duration of operation | 0.003 | 0.003 | 0.823 | 0.364 | 1.003 | 0.996 | 1.010 |
| Positive duplex ultrasound | −0.444 | 0.454 | 0.957 | 0.328 | 0.642 | 0.264 | 1.561 |
| Gender | 1.051 | 0.448 | 5.502 | 0.019 | 2.860 | 1.189 | 6.884 |
| BMI >25 (kg/m2) | −0.950 | 0.453 | 4.399 | 0.036 | 0.387 | 0.159 | 0.940 |
| Constant | −7.637 | 2.117 | 13.016 | 0.000 | 0.000 | ||
| . | B . | S.E. . | Wals . | P-value . | OR . | 95% CI . | |
|---|---|---|---|---|---|---|---|
| Lower . | Upper . | ||||||
| Age (years) | 0.096 | 0.026 | 14.193 | 0.000 | 1.101 | 1.047 | 1.158 |
| Disease type | −0.799 | 0.476 | 2.815 | 0.093 | 0.450 | 0.177 | 1.144 |
| Hypertension | 0.158 | 0.749 | 0.044 | 0.833 | 1.171 | 0.270 | 5.084 |
| Duration of operation | 0.003 | 0.003 | 0.823 | 0.364 | 1.003 | 0.996 | 1.010 |
| Positive duplex ultrasound | −0.444 | 0.454 | 0.957 | 0.328 | 0.642 | 0.264 | 1.561 |
| Gender | 1.051 | 0.448 | 5.502 | 0.019 | 2.860 | 1.189 | 6.884 |
| BMI >25 (kg/m2) | −0.950 | 0.453 | 4.399 | 0.036 | 0.387 | 0.159 | 0.940 |
| Constant | −7.637 | 2.117 | 13.016 | 0.000 | 0.000 | ||
BMI: body mass index; CI: confidence interval; OR: odds ratio.
DISCUSSION
Unlike North America, Europe and some Asian countries, postoperative VTE in patients undergoing thoracic surgeries in China has so far been neglected. Screening and prophylactic anticoagulation for patients with a high risk of developing VTE have become clinical best practices, and a considerable amount of studies and papers have been published [14–24]. In fact, some Chinese surgeons, especially orthopaedics, obstetrics and gynaecology-oncology physicians have started paying attention to perioperative VTE prevention in recent years, and some related consensus and guidance articles have been published and adopted [21, 25]. Yet, preventive treatment for VTE in patients undergoing thoracic surgery is almost absent in clinical practice in China.
In 2016, China participated in an international questionnaire survey on postoperative VTE prevention among thoracic surgeons, which was launched by the ESTS–AATS Joint Task Force on VTE Prophylaxis. The results indicated that almost half (46.9%) of the Chinese physicians think that there is no VTE prevention strategy in their working hospital [26]. There are several reasons that may explain this situation in China. First, the incidence of VTE after major thoracic surgeries has long been undervalued. Almost all the previous published papers are cases of patients diagnosed with severe PE, and so there is no complete epidemiological information in China, let alone the results of asymptomatic DVT screening or high-risk patients screening for PE. Secondly, there is no consensus on VTE prevention in China. Therefore, the real data on the incidence of postoperative VTE in patients undergoing thoracic surgery in China have clinical significance. Moreover, as many centres do not use VTE prophylaxis after thoracic surgery, understanding the real magnitude of VTE is of paramount importance. Therefore, we conducted this single-centre prospective cohort study. To the best of our knowledge, this is the first study of its kind on thoracic surgery in China.
In this study, all patients were screened for DVT using non-invasive duplex lower-extremity ultrasonography after surgery, and CTPA was carried out only if patients had typical symptoms of PE, high Caprini score (>9 points) or newly diagnosed postoperative DVT. We found that the overall incidence of VTE after lung surgery was 11.5%, which was significantly higher than that reported in previous studies [6, 10, 12]. On further analysis, the incidence of VTE after malignant lung diseases (including lung cancer and metastatic lung cancer) was found to be 14.97%, which was more than twice as much as the incidence of VTE after surgery for benign lung diseases (7.0%). This conclusion corresponded with the results published earlier, where malignancy was a key risk factor for VTE [19].
Importantly, none of the patients enrolled had typical symptoms of PE or DVT. The potential reasons for this phenomenon may be because most of the VTE cases were patients diagnosed with DVT who had scarcely any subjective symptoms and could be easily ignored after the surgery. In this study, 24 (80.0%) patients were diagnosed with DVT and 6 (20.0%) patients were diagnosed with both DVT and PE, which indicated that lower-extremity ultrasonography is an efficient way to detect most VTE events after thoracic resections [20, 27]. It is worth mentioning that the median time of postoperative VTE was 5 postoperative days (ranging from 2 to 23 days). Hence, 93.3% of the VTE cases could be detected within a week after the surgery. As such, lower-extremity ultrasonography is likely necessary and highly recommended before discharge for screening potential DVT cases, especially in a clinical setting where VTE prevention and prophylaxis is absent [27, 28].
The Caprini RAM has been widely applied for screening patients who are at a high risk of VTE after surgery, and this was also used in this study [29]. Our results showed that the incidence of VTE in low-, moderate- and high-risk groups for VTE was 0%, 12.3% and 40.0%, respectively, which clearly highlights the predictive effectiveness of the Caprini RAM. On further analysis, among patients who underwent lung cancer resection, the incidence of VTE in the moderate-risk group was found to be 12.0% while that of high-risk group was up to 36.8%, which suggests that more attention should be given to lung cancer patients who are at a high risk of VTE. Additionally, we also noticed that advanced age, long duration of operation and positive duplex ultrasound results were associated with the occurrence of VTE. However, body mass index, surgical approach and volume of blood loss hardly had an effect on VTE [16, 17].
The most important fact is that none of the patients in this study received prophylaxis. As such, the results of this study are a good contemporary reflection of the true incidence of VTE in natural state and are amongst the very first publications for such a cohort in thoracic surgery. In an older prospective study, Ziomek et al. [30] analysed 77 patients who underwent lung surgery for pulmonary diseases. They found that 15 patients (25%) developed thromboembolism after surgery without perioperative pharmacological prophylaxis. However, VTE prophylaxis was not the best practice standard of care at those times. The incidence of VTE without any perioperative chemoprophylaxis has not been investigated in a thoracic surgical population since prophylaxis is routinely applied in most countries. However, unlike Europe and North America, a preventive treatment strategy for VTE and prophylactic anticoagulation have not been widely applied in China. Even in an era of minimally invasive surgery and advanced perioperative care, patients are still at a high risk for developing VTE after lung resection, as is clearly shown in our study. The fact that the incidence of VTE in this study was significantly higher than that reported in most of the previously published thoracic surgery literature further highlights the extreme importance of the need for a VTE prophylaxis policy among the thoracic surgical patient population in China [10, 12, 17].
Limitations
There are several limitations to our study. First, there was no prolonged follow-up of patients after discharge. Additionally, because of the high expense of CTPA, not every patient can afford the detection of PE, which might have led to some undiscovered cases of PE amongst the enrolled patients. Finally, this study is a single-centre study, which may cause bias. Multicentric, prospective studies are currently underway among the thoracic surgical patient population in China.
CONCLUSION
In conclusion, in this single-centre prospective cohort study, the overall incidence of VTE after thoracic surgery without perioperative VTE prophylaxis was substantial and was significantly higher than the incidence previously reported in the thoracic surgery literature. We believe that this is a reflection of the fact that the study cohort did not receive perioperative chemoprophylaxis. Malignancy was found to be a key risk factor of VTE occurrence in patients after lung resection, and the Caprini RAM correlated well in identifying patients at high risk for VTE. Lower-extremity ultrasonography for all patients after thoracic surgery and CTPA for high-risk patients can clearly increase the detection rate of VTE. Chemoprophylaxis is necessary because of the high incidence especially in high-risk patients.
Conflict of interest: none declared.
Footnotes
†Presented at the 26th European Conference on General Thoracic Surgery, Ljubljana, Slovenia, 27–30 May 2018.
REFERENCES
National Institute for Health and Care Excellence. Venous Thromboembolism: Reducing the Risk for Patients in Hospital, Clinical Guideline CG 92.
National Clinical Guideline Centre—Acute and Chronic Conditions (UK).
Author notes
Chunfeng Song and Yaron Shargall authors contributed equally to this article.





