-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Chiappetta, Giovanni Leuzzi, Isabella Sperduti, Emilio Bria, Felice Mucilli, Filippo Lococo, Lorenzo Spaggiari, Giovanni Battista Ratto, Pier Luigi Filosso, Francesco Facciolo, Lymph-node ratio predicts survival among the different stages of non-small-cell lung cancer: a multicentre analysis, European Journal of Cardio-Thoracic Surgery, Volume 55, Issue 3, March 2019, Pages 405–412, https://doi.org/10.1093/ejcts/ezy311
Close - Share Icon Share
Abstract
The prognostic role of the number of resected and metastatic lymph nodes in non-small-cell lung cancer (NSCLC) is still being debated. The aim of this study was to evaluate the impact of lymphadenectomy in addition to the already validated variables in NSCLC survival.
From January 2002 to December 2012, data on 4858 patients with NSCLC undergoing anatomical lung resection and hilomediastinal lymphadenectomy in 6 institutions were analysed retrospectively. Established prognostic factors in addition to the number of resected lymph nodes and the ratio between the number of metastatic lymph nodes and the number of resected lymph nodes (NR) were correlated to overall survival (OS) and disease-free survival (DFS) using the multivariable Cox regression model. Harrell’s C-statistic with the 95% confidence interval (CI) was determined. Analysis by means of maximally selected log-rank statistics was performed to find optimal cut-off points in order to split patients into groups with different outcome probabilities.
The median numbers of resected lymph nodes and of metastatic lymph nodes were 17 (range 6–85) and 2 (1–36), respectively. Hilar (N1) and mediastinal (N2) metastases were identified in 21.3% and 20.0% of cases, respectively. Overall, the 5-year OS and DFS rates were 54.6% and 44.8%, respectively. At multivariable analysis, age, gender, pathological stage, R0 resection, type of surgery and NR correlated with longer OS rates; the same variables plus tumour grading were further related to DFS. C-statistics were 66.0 (95% CI 62.7–69.4) for DFS and 60.5 (95% CI 58.3–62.6) for OS. An NR <40% significantly correlated with a higher 5-year survival rate in the total sample (OS 57.6% vs 23.8%, P < 0.001; DFS 48.2% vs 11.4, P < 0.001) and in patients with N1 (OS 47.9% vs 36.1%, P = 0.03; DFS 39% vs 24.2%, P = 0.02) and N2 (OS 36.9% vs 21.8%, P < 0.001 DFS 23.9% vs 9.1%, P < 0.001).
Our study confirms that the number of resected lymph nodes is a strong prognostic indicator in NSCLC. In particular, an NR cut-off value of 40% may predict both OS and DFS.
INTRODUCTION
Lung cancer is the leading cause of death from tumours worldwide [1], with 5-year survival rates strictly related to the stage at diagnosis and decreasing from 54% for local, to 24% for regional and 4% for metastatic disease [2].
Lymph node assessment is one of the most important prognostic factors in non-small-cell lung cancer (NSCLC), with a worse prognosis for patients with hilar or mediastinal involvement.
In the current tumour, node and metastasis (TNM) NSCLC staging system [3], the node factor is still determined by the metastatic lymph node stations using the modified Naruke’s [4] or the International Association for the Study of Lung Cancer classification, which was developed to reconcile discrepancies in the Naruke and the Mountain-Dressler maps [5], both of which were based on topographic ‘zones’ independently from the metastatic pattern.
A number of researchers have analysed the different presentations of the metastatic nodes, focusing especially on involvement of the mediastinal nodes. These studies showed that several factors may affect prognosis, including the station involved, the number of metastatic stations [6], the type of metastasis (micro- or macrometastases) [7], lobe-specific metastasis [8] or the presence of skip metastases [9]. However, these data arise from single-centre experiences comprising only a few hundred cases.
Similar to the situation with other malignancies (e.g. breast, colon, bladder and gastric cancers) [10–14], the number of metastatic lymph nodes beyond the TNM classification has been investigated as a reliable prognostic factor also in NSCLC, by analysing the role of the number of resected lymph nodes or the ratio between the metastatic lymph nodes and the resected lymph nodes [i.e. lymph node ratio (NR)] [6, 15].
Despite encouraging findings from these studies in lung and other cancers, the TNM Classification of Malignant Tumours, 8th Edition addresses the lymphatic factor with no changes from the previous edition [3, 16].
The aim of our study was to investigate the role of the NR in terms of overall survival (OS) and disease-free survival (DFS) using a large multicentre database of 4858 patients and to assess if the NR may be effective in NSCLC prognosis, the goal being to integrate it into the TNM staging system.
METHODS
Approval for the study was obtained from the ethics committee of our institution as the proposer centre.
From January 2002 to December 2012, data on 4858 patients with NSCLC undergoing anatomical lung resection and hilomediastinal lymphadenectomy in 6 Italian institutions were analysed retrospectively. Indications for surgery were in accordance with the TNM 7th edition, but pathological results were reviewed according to the 8th edition.
Only patients with available data on surgery, pathological stage and follow-up were eligible for the analysis.
Surgery was performed by experienced thoracic surgeons and included patients undergoing anatomical resection (lobectomy, bilobectomy or pneumonectomy) associated with lymphadenectomy (systematic lymph node sampling or mediastinal lymph node dissection) [16] of hilar and mediastinal lymph nodes using a Naruke modified dissection [17]. Lymph node resection was conducted en bloc with the surrounding fat whenever possible, and in case of fragmentation, the lymph node parts were added to the corresponding node station for the histological analysis. If one or more lymph node fragments were detected, each fragment was counted as another lymph node.
For pathological evaluation and analysis of the lymph nodes, a gross section using haematoxylin-eosin stain was performed to determinate the presence of metastases; immunohistochemical analysis was used to confirm the diagnosis if additional investigations were needed [17]. The presence of micrometastases and infiltration of the capsule, if present, were considered lymph node metastases.
Surgical approaches were either thoracotomy (anterolateral or posterior) or video-assisted thoracic surgery, based on the preference of the surgeon and the centre.
Follow-up comprised an objective examination, blood tests and radiological examinations (computed tomography and positron emission tomography when available and indicated) every 3 to 6 months after surgery for the first 2 years postoperatively and then every 6 months for 5 years and 1 year thereafter.
OS was calculated using the interval between surgery and the last follow-up or death of all causes; DFS was calculated from the time of surgery to the occurrence of local relapse or distant metastases.
Patients were classified free from disease when objective examination and follow-up examinations ruled out relapses or metastases.
Clinical and pathological factors (age, sex, type of surgery, histological analysis, lymph node involvement, pathological stage, tumour grade and induction and adjuvant chemotherapy) plus the number of surgically removed lymph nodes, the metastatic lymph nodes and the ratio between the number of positive and removed nodes (NR), were investigated as predictors of OS and DFS. In particular, the analysis of the OS and DFS among the different TNM stages was conducted in R0 patients with at least 6 lymph nodes removed [18].
Statistical analysis
The associations between variables were tested using the Pearson χ2 test or the Fisher’s exact test, when appropriate. DFS and OS analyses were performed using Cox regression models (univariable and multivariable). The proportional hazards assumption of the Cox regression model was confirmed by analytical (Schoenfeld residuals test) and graphical methods. A multivariable model was developed using stepwise regression (forwards selection) by selecting significant variables upon univariable analysis. Enter limit and remove limit were P = 0.10 and P = 0.15, respectively. For the univariable and multivariable regression model, covariates included the following baseline characteristics: age, sex, type of surgery, histological assessment, lymph node involvement, pathological stage, radical resection, tumour grade, induction, adjuvant chemotherapy, numbers of resected and metastatic lymph nodes and the NR.
Survival curves were calculated by the Kaplan–Meier method and the log-rank test was used to assess differences between subgroups. Significance was defined by a P-value ≤0.05. The maximally selected log-rank statistical analysis was applied to the continuous variable in order to estimate the most appropriate cut-off values for splitting patients into groups with different DFS/OS probabilities. To evaluate the discriminative ability of the model, Harrell’s C-statistic with the 95% confidence interval (CI) was determined [19]. SPSS software (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.) and R software (version 3.5.1) were used for all analyses.
RESULTS
Demographics characteristics and surgical outcomes are reported in Table 1.
| Age (years), mean ± SD | 66.7 ± 17.7 |
| Sex, n (%) | |
| Male | 3405 (70.1) |
| Female | 1453 (29.9) |
| Surgery, n (%) | |
| Lobectomy | 3853 (79.4) |
| Right upper | 1477 (30.4) |
| Middle | 191 (4) |
| Right lower | 592 (12.2) |
| Left upper | 1040 (21.4) |
| Left lower | 552 (11.4) |
| Bilobectomy | 274 (5.6) |
| Upper | 126 (2.6) |
| Lower | 148 (3) |
| Pneumonectomy | 731 (15) |
| Right | 314 (6.5) |
| Left | 427 (7.5) |
| Histological diagnosis, n (%) | |
| Adenocarcinoma | 2872 (58.1) |
| Squamous cell | 1183 (23.9) |
| Carcinoids | 281 (4.9) |
| Other | 504 (11.1) |
| Grading | |
| 1 | 951 (19.6) |
| 2 | 1342 (27.6) |
| 3 | 1655 (34) |
| Missing data | 917 (18.8) |
| Lymphadenectomy | |
| Number of resected nodes, median (range) | 17 (6–85) |
| Number of metastatic nodes, median (range) | 2 (1–36) |
| Number of N1, n (%) | 841 (21.3) |
| Number of N2, n (%) | 780 (20) |
| pT, n (%) | |
| I | 1548 (32.3) |
| II | 2221 (47.1) |
| III | 654 (13.2) |
| IV | 283 (5.7) |
| Missing data | 152 (0.9) |
| Radicality, n (%) | |
| R0 | 4721 (97.3%) |
| R1 | 105 (2.2%) |
| R2 | 32 (0.5%) |
| Integrated treatments, n (%) | |
| Induction therapy | 858 (17.7%) |
| Adjuvant therapy | 1300 (26.8%) |
| Stage, n (%) | |
| I | 2117 (46.5) |
| II | 1174 (24.1) |
| III | 1337 (27.8) |
| IV | 78 (1.6) |
| Missing data | 152 (0.9) |
| Age (years), mean ± SD | 66.7 ± 17.7 |
| Sex, n (%) | |
| Male | 3405 (70.1) |
| Female | 1453 (29.9) |
| Surgery, n (%) | |
| Lobectomy | 3853 (79.4) |
| Right upper | 1477 (30.4) |
| Middle | 191 (4) |
| Right lower | 592 (12.2) |
| Left upper | 1040 (21.4) |
| Left lower | 552 (11.4) |
| Bilobectomy | 274 (5.6) |
| Upper | 126 (2.6) |
| Lower | 148 (3) |
| Pneumonectomy | 731 (15) |
| Right | 314 (6.5) |
| Left | 427 (7.5) |
| Histological diagnosis, n (%) | |
| Adenocarcinoma | 2872 (58.1) |
| Squamous cell | 1183 (23.9) |
| Carcinoids | 281 (4.9) |
| Other | 504 (11.1) |
| Grading | |
| 1 | 951 (19.6) |
| 2 | 1342 (27.6) |
| 3 | 1655 (34) |
| Missing data | 917 (18.8) |
| Lymphadenectomy | |
| Number of resected nodes, median (range) | 17 (6–85) |
| Number of metastatic nodes, median (range) | 2 (1–36) |
| Number of N1, n (%) | 841 (21.3) |
| Number of N2, n (%) | 780 (20) |
| pT, n (%) | |
| I | 1548 (32.3) |
| II | 2221 (47.1) |
| III | 654 (13.2) |
| IV | 283 (5.7) |
| Missing data | 152 (0.9) |
| Radicality, n (%) | |
| R0 | 4721 (97.3%) |
| R1 | 105 (2.2%) |
| R2 | 32 (0.5%) |
| Integrated treatments, n (%) | |
| Induction therapy | 858 (17.7%) |
| Adjuvant therapy | 1300 (26.8%) |
| Stage, n (%) | |
| I | 2117 (46.5) |
| II | 1174 (24.1) |
| III | 1337 (27.8) |
| IV | 78 (1.6) |
| Missing data | 152 (0.9) |
pT: pathological T; SD: standard deviation.
| Age (years), mean ± SD | 66.7 ± 17.7 |
| Sex, n (%) | |
| Male | 3405 (70.1) |
| Female | 1453 (29.9) |
| Surgery, n (%) | |
| Lobectomy | 3853 (79.4) |
| Right upper | 1477 (30.4) |
| Middle | 191 (4) |
| Right lower | 592 (12.2) |
| Left upper | 1040 (21.4) |
| Left lower | 552 (11.4) |
| Bilobectomy | 274 (5.6) |
| Upper | 126 (2.6) |
| Lower | 148 (3) |
| Pneumonectomy | 731 (15) |
| Right | 314 (6.5) |
| Left | 427 (7.5) |
| Histological diagnosis, n (%) | |
| Adenocarcinoma | 2872 (58.1) |
| Squamous cell | 1183 (23.9) |
| Carcinoids | 281 (4.9) |
| Other | 504 (11.1) |
| Grading | |
| 1 | 951 (19.6) |
| 2 | 1342 (27.6) |
| 3 | 1655 (34) |
| Missing data | 917 (18.8) |
| Lymphadenectomy | |
| Number of resected nodes, median (range) | 17 (6–85) |
| Number of metastatic nodes, median (range) | 2 (1–36) |
| Number of N1, n (%) | 841 (21.3) |
| Number of N2, n (%) | 780 (20) |
| pT, n (%) | |
| I | 1548 (32.3) |
| II | 2221 (47.1) |
| III | 654 (13.2) |
| IV | 283 (5.7) |
| Missing data | 152 (0.9) |
| Radicality, n (%) | |
| R0 | 4721 (97.3%) |
| R1 | 105 (2.2%) |
| R2 | 32 (0.5%) |
| Integrated treatments, n (%) | |
| Induction therapy | 858 (17.7%) |
| Adjuvant therapy | 1300 (26.8%) |
| Stage, n (%) | |
| I | 2117 (46.5) |
| II | 1174 (24.1) |
| III | 1337 (27.8) |
| IV | 78 (1.6) |
| Missing data | 152 (0.9) |
| Age (years), mean ± SD | 66.7 ± 17.7 |
| Sex, n (%) | |
| Male | 3405 (70.1) |
| Female | 1453 (29.9) |
| Surgery, n (%) | |
| Lobectomy | 3853 (79.4) |
| Right upper | 1477 (30.4) |
| Middle | 191 (4) |
| Right lower | 592 (12.2) |
| Left upper | 1040 (21.4) |
| Left lower | 552 (11.4) |
| Bilobectomy | 274 (5.6) |
| Upper | 126 (2.6) |
| Lower | 148 (3) |
| Pneumonectomy | 731 (15) |
| Right | 314 (6.5) |
| Left | 427 (7.5) |
| Histological diagnosis, n (%) | |
| Adenocarcinoma | 2872 (58.1) |
| Squamous cell | 1183 (23.9) |
| Carcinoids | 281 (4.9) |
| Other | 504 (11.1) |
| Grading | |
| 1 | 951 (19.6) |
| 2 | 1342 (27.6) |
| 3 | 1655 (34) |
| Missing data | 917 (18.8) |
| Lymphadenectomy | |
| Number of resected nodes, median (range) | 17 (6–85) |
| Number of metastatic nodes, median (range) | 2 (1–36) |
| Number of N1, n (%) | 841 (21.3) |
| Number of N2, n (%) | 780 (20) |
| pT, n (%) | |
| I | 1548 (32.3) |
| II | 2221 (47.1) |
| III | 654 (13.2) |
| IV | 283 (5.7) |
| Missing data | 152 (0.9) |
| Radicality, n (%) | |
| R0 | 4721 (97.3%) |
| R1 | 105 (2.2%) |
| R2 | 32 (0.5%) |
| Integrated treatments, n (%) | |
| Induction therapy | 858 (17.7%) |
| Adjuvant therapy | 1300 (26.8%) |
| Stage, n (%) | |
| I | 2117 (46.5) |
| II | 1174 (24.1) |
| III | 1337 (27.8) |
| IV | 78 (1.6) |
| Missing data | 152 (0.9) |
pT: pathological T; SD: standard deviation.
The median follow-up calculated with the Kaplan–Meier reverse method was 50.3 months (95% CI 48.3–52.4).
The median numbers of resected and metastatic lymph nodes were 17 (range 6–85) and 2 (range 1–36), respectively; the median number of resected lymph node stations was 5 and the pathological TNM classification was stage I in 46.5%, stage II in 24.1%, stage III in 27.8% and stage IV in 1.6% cases. In particular, hilar (N1) and mediastinal (N2) node metastases were identified in 21.3% and 20.0% of cases, respectively, whereas histological analysis showed a predominance of adenocarcinomas (58.1%).
Five-year OS and DFS rates were 54.6% and 44.8%, respectively. At multivariable analysis, age, gender, pathological stage, R0 resection, type of surgery and NR related with a longer OS; the same variables associated with tumour grading were further associated with DFS (Table 2). The C-statistic was 66.0 (95% CI 62.7–69.4) for DFS and 60.5 (95% CI 58.3–62.6) for OS.
| Variables . | Multivariable DFS . | Multivariable OS . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |
| Gender | ||||
| Male vs female | 1.18 (1.03–1.35) | 0.02 | 1.41 (1.21–1.63) | <0.001 |
| Age (years) | ||||
| >66 vs ≤66 years | 1.52 (1.35–1.73) | <0.001 | 1.74 (1.53–1.99) | <0.001 |
| Lymph node ratio | ||||
| >40% vs ≤40% | 1.90 (1.53–2.36) | <0.001 | 1.87 (1.51–2.32) | <0.001 |
| R0 resection | ||||
| No vs yes | 1.66 (1.30–2.10) | <0.001 | 1.70 (1.32–2.18) | <0.001 |
| Grading | ||||
| 3 vs ½ | 1.28 (1.12–1.47) | <0.001 | 0.258 | |
| Pathological stage | <0.001 | <0.001 | ||
| II vs I | 1.83 (1.56–2.14) | <0.001 | 1.72 (1.45–2.04) | <0.001 |
| III/IV vs I | 2.60 (2.22–3.04) | <0.001 | 2.53 (2.15 (2.98) | <0.001 |
| Surgery | ||||
| Pneumonectomy vs lobectomy | 1.32 (1.09–1.59) | 0.004 | 1.38 (1.13–1.68) | 0.001 |
| *C-statistic 66.0 (95% CI 62.7–69.4) | *C-statistic 60.0 (95% CI 58.3–62.6) | |||
| Variables . | Multivariable DFS . | Multivariable OS . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |
| Gender | ||||
| Male vs female | 1.18 (1.03–1.35) | 0.02 | 1.41 (1.21–1.63) | <0.001 |
| Age (years) | ||||
| >66 vs ≤66 years | 1.52 (1.35–1.73) | <0.001 | 1.74 (1.53–1.99) | <0.001 |
| Lymph node ratio | ||||
| >40% vs ≤40% | 1.90 (1.53–2.36) | <0.001 | 1.87 (1.51–2.32) | <0.001 |
| R0 resection | ||||
| No vs yes | 1.66 (1.30–2.10) | <0.001 | 1.70 (1.32–2.18) | <0.001 |
| Grading | ||||
| 3 vs ½ | 1.28 (1.12–1.47) | <0.001 | 0.258 | |
| Pathological stage | <0.001 | <0.001 | ||
| II vs I | 1.83 (1.56–2.14) | <0.001 | 1.72 (1.45–2.04) | <0.001 |
| III/IV vs I | 2.60 (2.22–3.04) | <0.001 | 2.53 (2.15 (2.98) | <0.001 |
| Surgery | ||||
| Pneumonectomy vs lobectomy | 1.32 (1.09–1.59) | 0.004 | 1.38 (1.13–1.68) | 0.001 |
| *C-statistic 66.0 (95% CI 62.7–69.4) | *C-statistic 60.0 (95% CI 58.3–62.6) | |||
CI: confidence interval; DFS: disease-free survival; HR: hazard ratio; OS: overall survival.
| Variables . | Multivariable DFS . | Multivariable OS . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |
| Gender | ||||
| Male vs female | 1.18 (1.03–1.35) | 0.02 | 1.41 (1.21–1.63) | <0.001 |
| Age (years) | ||||
| >66 vs ≤66 years | 1.52 (1.35–1.73) | <0.001 | 1.74 (1.53–1.99) | <0.001 |
| Lymph node ratio | ||||
| >40% vs ≤40% | 1.90 (1.53–2.36) | <0.001 | 1.87 (1.51–2.32) | <0.001 |
| R0 resection | ||||
| No vs yes | 1.66 (1.30–2.10) | <0.001 | 1.70 (1.32–2.18) | <0.001 |
| Grading | ||||
| 3 vs ½ | 1.28 (1.12–1.47) | <0.001 | 0.258 | |
| Pathological stage | <0.001 | <0.001 | ||
| II vs I | 1.83 (1.56–2.14) | <0.001 | 1.72 (1.45–2.04) | <0.001 |
| III/IV vs I | 2.60 (2.22–3.04) | <0.001 | 2.53 (2.15 (2.98) | <0.001 |
| Surgery | ||||
| Pneumonectomy vs lobectomy | 1.32 (1.09–1.59) | 0.004 | 1.38 (1.13–1.68) | 0.001 |
| *C-statistic 66.0 (95% CI 62.7–69.4) | *C-statistic 60.0 (95% CI 58.3–62.6) | |||
| Variables . | Multivariable DFS . | Multivariable OS . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |
| Gender | ||||
| Male vs female | 1.18 (1.03–1.35) | 0.02 | 1.41 (1.21–1.63) | <0.001 |
| Age (years) | ||||
| >66 vs ≤66 years | 1.52 (1.35–1.73) | <0.001 | 1.74 (1.53–1.99) | <0.001 |
| Lymph node ratio | ||||
| >40% vs ≤40% | 1.90 (1.53–2.36) | <0.001 | 1.87 (1.51–2.32) | <0.001 |
| R0 resection | ||||
| No vs yes | 1.66 (1.30–2.10) | <0.001 | 1.70 (1.32–2.18) | <0.001 |
| Grading | ||||
| 3 vs ½ | 1.28 (1.12–1.47) | <0.001 | 0.258 | |
| Pathological stage | <0.001 | <0.001 | ||
| II vs I | 1.83 (1.56–2.14) | <0.001 | 1.72 (1.45–2.04) | <0.001 |
| III/IV vs I | 2.60 (2.22–3.04) | <0.001 | 2.53 (2.15 (2.98) | <0.001 |
| Surgery | ||||
| Pneumonectomy vs lobectomy | 1.32 (1.09–1.59) | 0.004 | 1.38 (1.13–1.68) | 0.001 |
| *C-statistic 66.0 (95% CI 62.7–69.4) | *C-statistic 60.0 (95% CI 58.3–62.6) | |||
CI: confidence interval; DFS: disease-free survival; HR: hazard ratio; OS: overall survival.
As previously mentioned, the pathological stage significantly related with prognosis, with a 5-year OS of 70.4% for stage I, 50.1% for stage II and 34.3 for stage III/IV (P < 0.001) (Fig. 1).
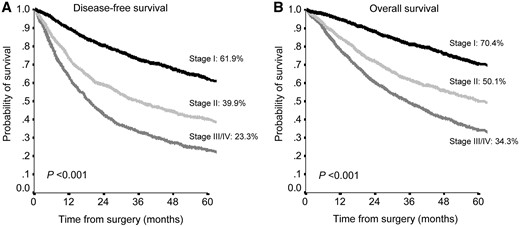
Disease-free survival (A) and overall survival (B) according to tumour, node and metastasis staging system in the entire population.
A NR <40% was significantly related with a higher 5-year survival rate in the total sample with an OS of 57.6% vs 23.8% (P < 0.001) and a DFS of 48.2% vs 11.4 (P < 0.001) (Fig. 2).
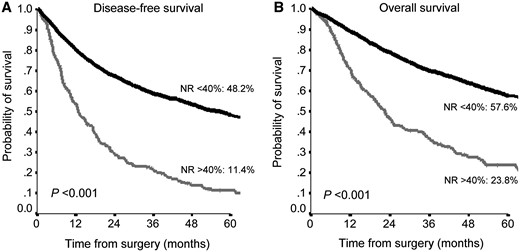
Disease-free survival (A) and overall survival (B) according to NR in the entire population. NR: node ratio.
When stratifying the patients by type of nodal involvement, an NR <40% strongly predicted a 5-year OS and a 5-year DFS both in patients with N1 (OS 47.9% vs 36.1%, P = 0.03; DFS 39% vs 24.2%, P = 0.02) and with N2 (OS 36.9% vs 21.8%, P < 0.001; DFS 23.9% vs 9.1%, P < 0.001) (Fig. 3).
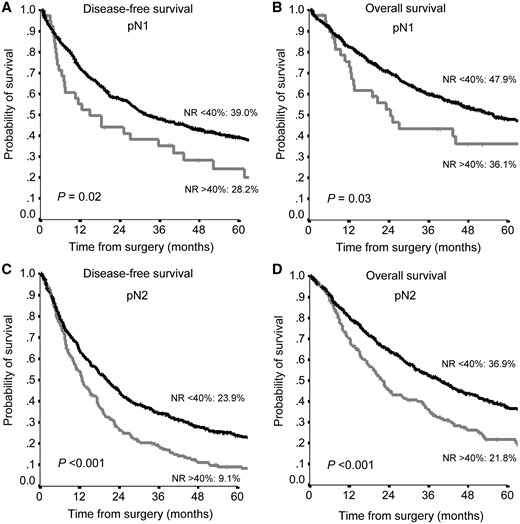
Disease-free survival (A, C) and overall survival (B, D) according to NR in pN1 patients (A, B) and pN2 (C, D) patients. NR: node ratio.
The prognostic role of NR <40% was also confirmed by its impact on the TNM stage. In particular in stage II, the 5-year OS was 50.6% vs 36.8% (P = 0.04) and the 5-year DFS was 40.1% vs 27.6% (P = 0.05), whereas in stage III, the NR showed a stronger predictive power than in stage II with a 5-year OS of 38.5% vs 21.5% (P < 0.001) and a 5-year DFS of 27.6% vs 9.5% (P < 0.001) (Fig. 4).
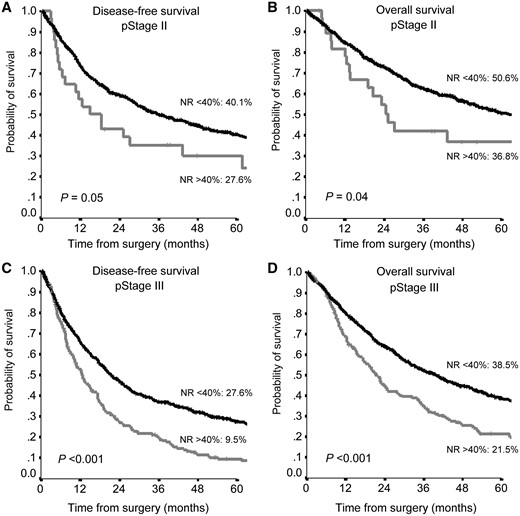
Disease-free survival (A, C) and overall survival (B, D) according to NR in pStage II (A, B) and pStage III (C, D). NR: node ratio.
DISCUSSION
Despite the fact that the current [3] and the past editions [16] of the NSCLC TNM Staging System classify the N factor in terms of topographical nodal stations, it seems fairly clear that other factors may also influence the prognosis. In fact, a significant number of studies reported longer survival rates in patients with lobe-specific node metastases [8] or skip [9] metastases, whereas others underlined the prognostic role of the number of metastatic lymph nodes [20, 21]. All these findings explain the high variability in terms of prognosis in these patients, suggesting that other parameters beyond the TNM classification may integrate and make the clinical scenario clearer.
Based on our experience, we focused our attention on the NR in patients with NSCLC, with the challenge of identifying different subgroups of patients using parameters that were easy to obtain and with an immediate impact: the number of resected lymph nodes, the number of metastatic lymph nodes and the NR. We speculated that such parameters should be used in all patients undergoing anatomical resection plus lymphadenectomy, allowing stratification in different subgroups on the basis of different cut-offs of NR.
In particular, we found that NR <40% was the optimal cut-off, similar to the value that emerged from other studies, which ranged between 35% and 50% [22–24].
We further confirmed NR as an independent prognostic factor by stratifying patients by stage and nodal involvement. Indeed, the cut-off of NR <40% is also effective in the different stages (II and III) and in the case of N1 or N2 involvement.
NR stratification is particularly important in patients with N1 disease or at stage II where schedules and indications for adjuvant therapies are still being debated [25]. This approach would allow more aggressive adjuvant therapies plus require strict follow-up in patients with a high NR compared to those with a low NR. Indeed, analysis of patients who were diagnosed as pathological stage II showed a greater 5-year OS rate than in those with an NR <40% [50.6% vs 36.8% in patients with NR >40% (P = 0.04)]. These data agree with those from other studies [22, 26] also describing the validity of the NR ratio in this stage of disease, with significant benefit on the survival of patients with a low NR.
Moreover, the NR seems to have an important role in stage III with N2 involvement. Our results agree with those of Urban [24], who reported the benefits of postoperative radiotherapy only in the subgroup of patients with an NR >50%. Therefore, in this stage, the NR may also have a new role for tailoring treatment, with the possibility of changing the type of adjuvant treatment schedule and combinations on the basis of the lymph NR.
Another point of interest is that the NR may be a useful and effective parameter in both lymph node sampling and radical dissection. In fact, the optimal strategy for lymph node assessment during thoracic surgery is still a matter for debate, with some studies reporting a higher number of resected lymph nodes after radical mediastinal lymph node dissection compared to lymph node sampling, although advantages in terms of survival are not yet well defined [22, 25, 27, 28]. These are some of the main reasons we do not think that there are any differences on the basis of the kind of lymphadenectomy, in addition to the following considerations.
The first is that we were looking for a parameter that may be used in every situation. Indeed, different guidelines suggest different possibilities in the choice of sampling or radical dissection on the basis of the histological analysis, the location and the clinical staging of the NSCLC [17].
The second consideration is that, if lymphadenectomy is conducted correctly, it permits an exhaustive lymph node assessment, as we showed in this work with a high number of resected nodes (median of 17 resected nodes).
Indeed, the NR described the ratio of the spread of the tumour and may be validated independently using 1 of the following 2 strategies to possibly reduce the effects of confounding factors such as lymph node fragmentation or interindividual differences in the number of lymph nodes in the lymphatic chain. Indeed, the NR may also stay stable if both the number of metastatic lymph nodes and the number of resected lymph nodes increase if the lymph nodes are fragmented, decreasing the confounding effect of fragmentation. Of course, this point may be valid in the case of correct lymph node sampling or dissection when a minimum number of resected nodes are partially fragmented, whereas it loses any validity if only few of the resected lymph nodes are totally fragmented.
Finally, this study started from a need that was also declared by the 8th TNM staging system [16]: the possibility of subclassifying patients and individuating other prognostic factors in cases of lymph-node metastases. In fact, as previously mentioned, the stratification of patients in the same stage on the basis of the lymph node pattern is requested by surgeons, oncologists and physicians, and this question has also been addressed by the International Association for the Study of Lung Cancer [29]. In particular, the committee tested a subclassification by considering the number and the kind of lymph node involvement, but this solution presented some limitations and some overlap between the different stages. Conversely, we proposed an approach that would be valid for all stages, easy to calculate and obtain, and that permits identification of the characteristics of each stage and determination of the prognosis in a clear way, by dividing the stage with a number based on the corresponding lymph NR. Indeed, we propose that the main role of the NR is to integrate the information about the different stages of the TNM, using a parameter that is obtained quickly and is easy to use to stratify prognosis and to plan further therapeutic approaches.
Limitations
This study has some limitations: first, it is retrospective in nature. Indeed, because the data were collected from different centres, the variability of the surgical approaches and the extent of the lymphadenectomy should be considered when interpreting the results, although radical lymphadenectomy was routinely performed at all involved institutions. In particular, we tried to limit confounding factors in many ways. Firstly, the high median number of resected nodes and resected stations confirmed the effectiveness of the procedure in different centres; secondly, for NR analysis among different stages, we considered patients with at least 6 lymph nodes resected. Finally, on the basis of our data, we think that the technique adopted for lymphadenectomy was not fundamentally useful for NR analysis because of the extremely accurate method of data collection and the median number of resected nodes (17 in our paper), that permits a strong and valid statistical analysis. On the other hand, data from different centres may validate our analysis and confirm that our findings may be extended to different cohorts of patients and whether our cut-off should be validated using a larger database and prospective trials.
CONCLUSIONS
We confirmed the role of the NR as an independent prognostic factor for OS and DFS in patients undergoing curative resection for NSCLC. In particular, we showed that a cut-off of 40% was able to predict the prognosis and stratify patients on the basis of N1/N2 involvement or pathological stage. Indeed, the NR allowed us to determine the prognosis for those in pathological stages II and III, suggesting that it should be integrated into the actual staging system. It provides the opportunity to stratify patients within the different stages of the disease and may represent an innovative instrument for surveillance planning and adjuvant therapies.
Footnotes
†Presented at the 25th European Conference on General Thoracic Surgery, Innsbruck, Austria, 28–31 May 2017.
ACKNOWLEDGEMENTS
A special thanks to Elizabeth Katherine Anna Triumbari and Ugo Grossi for language and text supervision.
Conflict of interest: none declared.
REFERENCES
The Japan Lung Cancer Society. Classification of Lung Cancer, 1st English edn. Tokyo: Kanehara & Co,





