-
PDF
- Split View
-
Views
-
Cite
Cite
Yosuke Inoue, Hitoshi Matsuda, Atsushi Omura, Yoshimasa Seike, Kyokun Uehara, Hiroaki Sasaki, Junjiro Kobayashi, What is the optimal surgical strategy for Stanford Type A acute aortic dissection in patients with a patent false lumen at the descending aorta?, European Journal of Cardio-Thoracic Surgery, Volume 54, Issue 5, November 2018, Pages 933–939, https://doi.org/10.1093/ejcts/ezy125
Close - Share Icon Share
Abstract
Aggressive total arch replacement (TAR) to obtain thrombosis of the distal false lumen (FL) in patients with Stanford Type A acute aortic dissection, particularly with a patent FL at the descending aorta, is discussed. The aim of this study was to examine the efficacy of our strategy.
In the last 20 years, we retrospectively reviewed the records of 518 patients with Type A acute aortic dissection who underwent an emergent surgery. Among them, 290 patients with a preoperative patent FL at the descending aorta were enrolled in this study. Patients were divided in 2 groups: the non-TAR group (n = 124; 68 ± 14 years) and the TAR group (n = 166; 61 ± 13 years).
In-hospital mortality was 11% (32/290) without significant difference between the 2 groups (the non-TAR group 13% vs the TAR group 10%, P = 0.45). The rates of FL thrombosis of the entire descending aorta were detected at 32% in the non-TAR group and 41% in the TAR group (P = 0.16). Freedom from distal aortic dilatation ≥50 mm was significantly higher in the TAR group (P = 0.03) than in the non-TAR group. Independent predictors of distal aortic dilatation >50 mm were patients in the non-TAR group (P = 0.01; hazard ratio 3.1, 95% confidence interval 1.28–8.05) and unachieved primary entry tear resection (P = 0.002; hazard ratio 6.2, 95% confidence interval 1.38–8.66).
Our surgical strategy with an aggressive entry resection with higher rate of TAR was acceptable. In patients with a patent FL at the descending aorta, TAR should be considered to prevent the future growth of the distal aorta.
INTRODUCTION
With the improvement in the surgical results of Type A acute aortic dissection (AAAD), problems encountered during long-term follow-up such as distal aortic dilatation and rupture have been focused. A patent false lumen (FL) at the distal aorta after the initial surgery for AAAD has been reported as the strong predictor for distal reoperation [1, 2]. Recently, extended arch repair with the frozen elephant trunk (FET) procedure has been utilized in AAAD to promote distal FL thrombosis. Conversely, some authors have questioned the application of more aggressive surgical procedures such as total arch replacement (TAR) with or without elephant trunk (ET) that extends to the descending aorta in AAAD [3, 4].
An optimal strategy to achieve FL thrombosis is particularly necessary in patients with a preoperative patent FL at the descending aorta. Our surgical policy has been based on tear-oriented surgery. In addition, we preferred TAR for younger patients (<50 years) with severely dissected supra-aortic branches and a patent FL at the descending aorta, existence of connective tissue disease and enlarged distal aortic arch (≥40 mm). We performed TAR without hesitation even though in octogenarians with primary entry at aortic arch. Non-TAR was preferred in frail patients or thermodynamically unstable patients who needed cardiopulmonary resuscitation. This study discussed the efficacy of our strategy with aggressive indication of TAR and installation of the conventional ET in patients with a patent FL at the descending aorta.
MATERIALS AND METHODS
Patients
From January 1997 to February 2017, 518 patients had undergone an emergent surgery for AAAD at the National Cerebral Cardiovascular Center. Among them, 290 patients with a preoperative patent FL at the descending aorta, which was only of classical double-barrel type, detected using early-phase contrast computed tomographic (CT) angiography, were enrolled in this study. In this study, 228 patients were excluded because of the following reasons: DeBakey Type II AAAD (n = 81), thrombosis of an FL at the descending aorta including very limited patent FL near the entry (n = 113) and insufficient preoperative evaluation using CT angiography (n = 9).
Patients were divided into 2 groups: the non-TAR group (n = 124) and the TAR group (n = 166). Table 1 shows the characteristics of all patients according to their group. In-hospital mortality was evaluated in all patients enrolled in this study. In 263 patients (the non-TAR group: n = 115, the TAR group: n = 148), postoperative enhanced CT angiography was performed, and FL thrombosis was evaluated. Long-term changes in the diameter at the distal aorta was evaluated in 225 hospital survivors (the non-TAR group: n = 94, the TAR group: n = 131). The mean follow-up period was 62 ± 56 (range 1–244) months (Fig. 1). This retrospective observational study was approved by the institutional review board (M22-052-8), and individual oral and written informed consent were obtained from each patient.
| Variables . | Overall (%) (n = 290) . | Non-TAR (%) (n = 124) . | TAR (%) (n = 166) . | P-value . |
|---|---|---|---|---|
| Age (years), mean ± SD | 67 ± 13 | 68 ± 13 | 61 ± 12 | <0.001 |
| Male gender, n (%) | 150 (52) | 47 (38) | 103 (62) | <0.001 |
| Preoperative status, n (%) | ||||
| Shock | 74 (26) | 39 (31) | 35 (21) | 0.056 |
| Cardiopulmonary resuscitation | 27 (9) | 18 (15) | 8 (4.8) | 0.003 |
| Organ malperfusion, n (%) | 106 (37) | 44 (35) | 62 (37) | 0.81 |
| Neck vessels | 54 (19) | 25 (20) | 29 (17) | 0.64 |
| Coronary | 27 (9) | 13 (10) | 14 (8.4) | 0.55 |
| Visceral | 16 (6) | 6 (4.8) | 10 (6.0) | 0.80 |
| Extremities | 32 (11) | 10 (8.0) | 22 (13) | 0.19 |
| Connective tissue disease, n (%) | 25 (9) | 6 (4.8) | 19 (11) | 0.055 |
| Octogenarians, n (%) | 46 (16) | 33 (27) | 15 (9) | <0.001 |
| AR: moderate or more, n (%) | 36 (12) | 16 (13) | 20 (12) | 0.86 |
| Serum creatinine ≥1.5, n (%) | 14 (4.8) | 10 (8.1) | 4 (2.5) | 0.049 |
| Dissection classification, n (%) | ||||
| DeBakey Type I | 248 (86) | 111 (90) | 137 (83) | 0.13 |
| Distal extent of aortic dissection, n (%) | ||||
| Descending aorta | 69 (24) | 32 (26) | 37 (22) | |
| Abdominal aorta | 68 (24) | 36 (29) | 32 (20) | |
| Iliac or beyond iliac | 153 (52) | 56 (45) | 97 (58) | 0.06 |
| Variables . | Overall (%) (n = 290) . | Non-TAR (%) (n = 124) . | TAR (%) (n = 166) . | P-value . |
|---|---|---|---|---|
| Age (years), mean ± SD | 67 ± 13 | 68 ± 13 | 61 ± 12 | <0.001 |
| Male gender, n (%) | 150 (52) | 47 (38) | 103 (62) | <0.001 |
| Preoperative status, n (%) | ||||
| Shock | 74 (26) | 39 (31) | 35 (21) | 0.056 |
| Cardiopulmonary resuscitation | 27 (9) | 18 (15) | 8 (4.8) | 0.003 |
| Organ malperfusion, n (%) | 106 (37) | 44 (35) | 62 (37) | 0.81 |
| Neck vessels | 54 (19) | 25 (20) | 29 (17) | 0.64 |
| Coronary | 27 (9) | 13 (10) | 14 (8.4) | 0.55 |
| Visceral | 16 (6) | 6 (4.8) | 10 (6.0) | 0.80 |
| Extremities | 32 (11) | 10 (8.0) | 22 (13) | 0.19 |
| Connective tissue disease, n (%) | 25 (9) | 6 (4.8) | 19 (11) | 0.055 |
| Octogenarians, n (%) | 46 (16) | 33 (27) | 15 (9) | <0.001 |
| AR: moderate or more, n (%) | 36 (12) | 16 (13) | 20 (12) | 0.86 |
| Serum creatinine ≥1.5, n (%) | 14 (4.8) | 10 (8.1) | 4 (2.5) | 0.049 |
| Dissection classification, n (%) | ||||
| DeBakey Type I | 248 (86) | 111 (90) | 137 (83) | 0.13 |
| Distal extent of aortic dissection, n (%) | ||||
| Descending aorta | 69 (24) | 32 (26) | 37 (22) | |
| Abdominal aorta | 68 (24) | 36 (29) | 32 (20) | |
| Iliac or beyond iliac | 153 (52) | 56 (45) | 97 (58) | 0.06 |
AR: aortic valve regurgitation; SD: standard deviation; TAR: total arch replacement.
| Variables . | Overall (%) (n = 290) . | Non-TAR (%) (n = 124) . | TAR (%) (n = 166) . | P-value . |
|---|---|---|---|---|
| Age (years), mean ± SD | 67 ± 13 | 68 ± 13 | 61 ± 12 | <0.001 |
| Male gender, n (%) | 150 (52) | 47 (38) | 103 (62) | <0.001 |
| Preoperative status, n (%) | ||||
| Shock | 74 (26) | 39 (31) | 35 (21) | 0.056 |
| Cardiopulmonary resuscitation | 27 (9) | 18 (15) | 8 (4.8) | 0.003 |
| Organ malperfusion, n (%) | 106 (37) | 44 (35) | 62 (37) | 0.81 |
| Neck vessels | 54 (19) | 25 (20) | 29 (17) | 0.64 |
| Coronary | 27 (9) | 13 (10) | 14 (8.4) | 0.55 |
| Visceral | 16 (6) | 6 (4.8) | 10 (6.0) | 0.80 |
| Extremities | 32 (11) | 10 (8.0) | 22 (13) | 0.19 |
| Connective tissue disease, n (%) | 25 (9) | 6 (4.8) | 19 (11) | 0.055 |
| Octogenarians, n (%) | 46 (16) | 33 (27) | 15 (9) | <0.001 |
| AR: moderate or more, n (%) | 36 (12) | 16 (13) | 20 (12) | 0.86 |
| Serum creatinine ≥1.5, n (%) | 14 (4.8) | 10 (8.1) | 4 (2.5) | 0.049 |
| Dissection classification, n (%) | ||||
| DeBakey Type I | 248 (86) | 111 (90) | 137 (83) | 0.13 |
| Distal extent of aortic dissection, n (%) | ||||
| Descending aorta | 69 (24) | 32 (26) | 37 (22) | |
| Abdominal aorta | 68 (24) | 36 (29) | 32 (20) | |
| Iliac or beyond iliac | 153 (52) | 56 (45) | 97 (58) | 0.06 |
| Variables . | Overall (%) (n = 290) . | Non-TAR (%) (n = 124) . | TAR (%) (n = 166) . | P-value . |
|---|---|---|---|---|
| Age (years), mean ± SD | 67 ± 13 | 68 ± 13 | 61 ± 12 | <0.001 |
| Male gender, n (%) | 150 (52) | 47 (38) | 103 (62) | <0.001 |
| Preoperative status, n (%) | ||||
| Shock | 74 (26) | 39 (31) | 35 (21) | 0.056 |
| Cardiopulmonary resuscitation | 27 (9) | 18 (15) | 8 (4.8) | 0.003 |
| Organ malperfusion, n (%) | 106 (37) | 44 (35) | 62 (37) | 0.81 |
| Neck vessels | 54 (19) | 25 (20) | 29 (17) | 0.64 |
| Coronary | 27 (9) | 13 (10) | 14 (8.4) | 0.55 |
| Visceral | 16 (6) | 6 (4.8) | 10 (6.0) | 0.80 |
| Extremities | 32 (11) | 10 (8.0) | 22 (13) | 0.19 |
| Connective tissue disease, n (%) | 25 (9) | 6 (4.8) | 19 (11) | 0.055 |
| Octogenarians, n (%) | 46 (16) | 33 (27) | 15 (9) | <0.001 |
| AR: moderate or more, n (%) | 36 (12) | 16 (13) | 20 (12) | 0.86 |
| Serum creatinine ≥1.5, n (%) | 14 (4.8) | 10 (8.1) | 4 (2.5) | 0.049 |
| Dissection classification, n (%) | ||||
| DeBakey Type I | 248 (86) | 111 (90) | 137 (83) | 0.13 |
| Distal extent of aortic dissection, n (%) | ||||
| Descending aorta | 69 (24) | 32 (26) | 37 (22) | |
| Abdominal aorta | 68 (24) | 36 (29) | 32 (20) | |
| Iliac or beyond iliac | 153 (52) | 56 (45) | 97 (58) | 0.06 |
AR: aortic valve regurgitation; SD: standard deviation; TAR: total arch replacement.
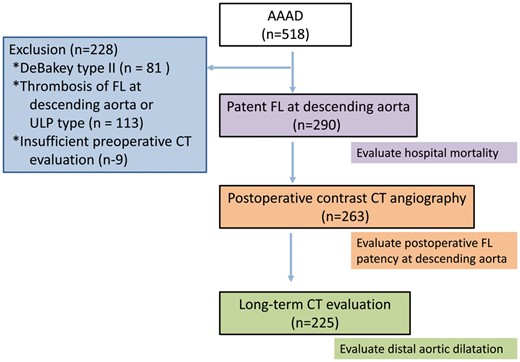
A flowchart of the study population. Based on the listed exclusion criteria, 290 patients were enrolled in this study. AAAD: Type A acute aortic dissection; CT: computed tomography; FL: false lumen; ULP: ulcer like projection.
Surgical technique
All operative manoeuvres were performed through median sternotomy. Arterial cannulation was achieved primarily through the right axillary and left common femoral arteries [5, 6]. In patients in whom the dissection extended up to the right axillary artery or who required cardiopulmonary resuscitation, single femoral artery cannulation was selected to establish cardiopulmonary bypass. Within the last 3 years, direct cannulation into the true lumen of the ascending aorta using the Seldinger technique was occasionally utilized depending on aortic morphology and the surgeon’s preference.
The first priority in the surgery for AAAD is entry resection, and TAR is chosen with certainty when the primary entry at the proximal descending aorta can be resected through median sternotomy. In addition, TAR is also preferred in case of enlarged aortic arch, severe dissection with circumferential dissection and/or involving supra-aortic branches, patients aged <50 years and patients with connective tissue disorder.
In all patients, open distal anastomosis was performed under hypothermic circulatory arrest at 20–28°C and antegrade selective cerebral perfusion. No aortic clamp was applied to avoid embolic event, malperfusion syndrome or formation of new intimal tear.
In patients undergoing TAR (166/290, 57%), distal anastomosis was performed using a conventional ET and not the FET procedure. In TAR, rewarming was started after the distal anastomosis and the reconstruction of the left subclavian artery under hypothermia for approximately 90 min. Then, the proximal anastomosis was carried out, and other supra-aortic branches were reconstructed after the aortic declamp under normothermic condition. Selective cerebral perfusion was sustained after the reconstruction of all supra-aortic branches.
When non-TAR replacement was performed (124/290, 43%), we routinely replaced the ascending aorta just below the innominate artery and beyond the top of the pericardium. Among non-TAR, hemiarch replacement was performed in 115 patients. For hemiarch replacement without neck vessel reconstruction, another non-TAR was performed with reconstruction of the brachiocephalic artery alone or brachiocephalic and left carotid arteries. Distal anastomosis in hemiarch replacement was reinforced using the felt sandwich technique (74/124, 60%) [7] or adventitial inversion technique (50/124, 40%) [8, 9].
For proximal anastomosis, direct anastomosis with a felt strip after the dissected wall fixed with glue had been performed. After 2013, we applied the ‘proximal stepwise anastomosis technique’ to suture the reversed short graft inside and felt outside after the fixation with glue and end-to-end anastomosis of the un-reversed short and distal main grafts, which enables a secure and easy anastomosis [10]. Gelatin–resorcin–formalin glue, fibrin sealant or BioGlue was applied according to their availability and the surgeon’s preference for the obliteration of the FL and the reinforcement of the proximal stump of the aorta.
Concomitant procedures included coronary bypass grafting in 22 patients, aortic valve replacement in 6 patients, aortic root surgery in 33 patients and limb bypass surgery in 13 patients. Intraoperative parameters were different between the 2 groups, except for the lowest core temperature (Table 2).
| Variables . | Overall (%) (n = 290) . | Non-TAR (%) (n = 124) . | TAR (%) (n = 166) . | P-value . |
|---|---|---|---|---|
| Operation time, mean ± SD | 496 ± 213 | 446 ± 184 | 533 ± 227 | <0.001 |
| CPB time, mean ± SD | 269 ± 130 | 224 ± 100 | 302 ± 139 | <0.001 |
| Cardiac ischaemic time, mean ± SD | 149 ± 63 | 130 ± 56 | 163 ± 64 | <0.001 |
| Antegrade cerebral perfusion, mean ± SD | 126 ± 79 | 60 ± 46 | 176 ± 60 | <0.001 |
| Lower body circulatory arrest time, mean ± SD | 61 ± 26 | 52 ± 26 | 68 ± 23 | <0.001 |
| Lowest core temperature, mean ± SD | 24.5 ± 2.5 | 24.6 ± 2.8 | 24.3 ± 2.1 | 0.51 |
| Location of primary entry tear, n (%) | ||||
| Ascending aorta | 183 (63) | 113 (91) | 70 (42) | <0.001 |
| Aortic arch ∼ distal arch | 92 (32) | 7 (5.8) | 85 (51) | <0.001 |
| Descending aorta/unknown | 15 (5) | 4 (3.2) | 11 (6.6) | 0.01 |
| Concomitant surgery, n (%) | ||||
| Root reimplantation | 10 (3.4) | 5 (4.0) | 5 (3.0) | 0.75 |
| Root replacement | 23 (7.9) | 12 (9.7) | 11 (6.6) | 0.38 |
| Aortic valve replacement | 6 (2.1) | 3 (2.4) | 3 (1.8) | 0.70 |
| CABG | 22 (7.5) | 10 (8.1) | 12 (7.2) | 0.83 |
| Limb bypass | 13 (4.5) | 5 (4.0) | 8 (4.8) | 1 |
| Early results, n (%) | ||||
| 30-Day mortality | 20 (6.9) | 8 (6.5) | 12 (7.2) | 1 |
| In-hospital mortality | 32 (11.0) | 16 (12.9) | 16 (9.6) | 0.45 |
| TND | 40 (13.7) | 20 (16) | 20 (12) | 0.30 |
| New-onset PND | 31 (10.7) | 15 (12) | 16 (9.6) | 0.56 |
| New-introduction HD | 11 (7.2) | 7 (5.6) | 4 (2.4) | 0.21 |
| Tracheostomy | 31 (10.7) | 15 (12) | 16 (9.6) | 0.44 |
| Re-exploration for bleeding | 44 (15) | 16 (13) | 28 (17) | 0.41 |
| Deep sternal infection | 10 (3.4) | 2 (1.6) | 8 (4.8) | 0.19 |
| Median ICU stay (days), median (IQR) | 5 (0–81) | 5 (0–81) | 5 (0–59) | 0.71 |
| Median hospital stay (days) | 30 (0–217) | 30 (0–217) | 30 (0–176) | 0.51 |
| Variables . | Overall (%) (n = 290) . | Non-TAR (%) (n = 124) . | TAR (%) (n = 166) . | P-value . |
|---|---|---|---|---|
| Operation time, mean ± SD | 496 ± 213 | 446 ± 184 | 533 ± 227 | <0.001 |
| CPB time, mean ± SD | 269 ± 130 | 224 ± 100 | 302 ± 139 | <0.001 |
| Cardiac ischaemic time, mean ± SD | 149 ± 63 | 130 ± 56 | 163 ± 64 | <0.001 |
| Antegrade cerebral perfusion, mean ± SD | 126 ± 79 | 60 ± 46 | 176 ± 60 | <0.001 |
| Lower body circulatory arrest time, mean ± SD | 61 ± 26 | 52 ± 26 | 68 ± 23 | <0.001 |
| Lowest core temperature, mean ± SD | 24.5 ± 2.5 | 24.6 ± 2.8 | 24.3 ± 2.1 | 0.51 |
| Location of primary entry tear, n (%) | ||||
| Ascending aorta | 183 (63) | 113 (91) | 70 (42) | <0.001 |
| Aortic arch ∼ distal arch | 92 (32) | 7 (5.8) | 85 (51) | <0.001 |
| Descending aorta/unknown | 15 (5) | 4 (3.2) | 11 (6.6) | 0.01 |
| Concomitant surgery, n (%) | ||||
| Root reimplantation | 10 (3.4) | 5 (4.0) | 5 (3.0) | 0.75 |
| Root replacement | 23 (7.9) | 12 (9.7) | 11 (6.6) | 0.38 |
| Aortic valve replacement | 6 (2.1) | 3 (2.4) | 3 (1.8) | 0.70 |
| CABG | 22 (7.5) | 10 (8.1) | 12 (7.2) | 0.83 |
| Limb bypass | 13 (4.5) | 5 (4.0) | 8 (4.8) | 1 |
| Early results, n (%) | ||||
| 30-Day mortality | 20 (6.9) | 8 (6.5) | 12 (7.2) | 1 |
| In-hospital mortality | 32 (11.0) | 16 (12.9) | 16 (9.6) | 0.45 |
| TND | 40 (13.7) | 20 (16) | 20 (12) | 0.30 |
| New-onset PND | 31 (10.7) | 15 (12) | 16 (9.6) | 0.56 |
| New-introduction HD | 11 (7.2) | 7 (5.6) | 4 (2.4) | 0.21 |
| Tracheostomy | 31 (10.7) | 15 (12) | 16 (9.6) | 0.44 |
| Re-exploration for bleeding | 44 (15) | 16 (13) | 28 (17) | 0.41 |
| Deep sternal infection | 10 (3.4) | 2 (1.6) | 8 (4.8) | 0.19 |
| Median ICU stay (days), median (IQR) | 5 (0–81) | 5 (0–81) | 5 (0–59) | 0.71 |
| Median hospital stay (days) | 30 (0–217) | 30 (0–217) | 30 (0–176) | 0.51 |
CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; HD: haemodialysis; ICU: Intensive care unit; IQR: interquartile range; PND: permanent neurological deficit; SD: standard deviation; TAR: total arch replacement; TND: transient neurological deficit.
| Variables . | Overall (%) (n = 290) . | Non-TAR (%) (n = 124) . | TAR (%) (n = 166) . | P-value . |
|---|---|---|---|---|
| Operation time, mean ± SD | 496 ± 213 | 446 ± 184 | 533 ± 227 | <0.001 |
| CPB time, mean ± SD | 269 ± 130 | 224 ± 100 | 302 ± 139 | <0.001 |
| Cardiac ischaemic time, mean ± SD | 149 ± 63 | 130 ± 56 | 163 ± 64 | <0.001 |
| Antegrade cerebral perfusion, mean ± SD | 126 ± 79 | 60 ± 46 | 176 ± 60 | <0.001 |
| Lower body circulatory arrest time, mean ± SD | 61 ± 26 | 52 ± 26 | 68 ± 23 | <0.001 |
| Lowest core temperature, mean ± SD | 24.5 ± 2.5 | 24.6 ± 2.8 | 24.3 ± 2.1 | 0.51 |
| Location of primary entry tear, n (%) | ||||
| Ascending aorta | 183 (63) | 113 (91) | 70 (42) | <0.001 |
| Aortic arch ∼ distal arch | 92 (32) | 7 (5.8) | 85 (51) | <0.001 |
| Descending aorta/unknown | 15 (5) | 4 (3.2) | 11 (6.6) | 0.01 |
| Concomitant surgery, n (%) | ||||
| Root reimplantation | 10 (3.4) | 5 (4.0) | 5 (3.0) | 0.75 |
| Root replacement | 23 (7.9) | 12 (9.7) | 11 (6.6) | 0.38 |
| Aortic valve replacement | 6 (2.1) | 3 (2.4) | 3 (1.8) | 0.70 |
| CABG | 22 (7.5) | 10 (8.1) | 12 (7.2) | 0.83 |
| Limb bypass | 13 (4.5) | 5 (4.0) | 8 (4.8) | 1 |
| Early results, n (%) | ||||
| 30-Day mortality | 20 (6.9) | 8 (6.5) | 12 (7.2) | 1 |
| In-hospital mortality | 32 (11.0) | 16 (12.9) | 16 (9.6) | 0.45 |
| TND | 40 (13.7) | 20 (16) | 20 (12) | 0.30 |
| New-onset PND | 31 (10.7) | 15 (12) | 16 (9.6) | 0.56 |
| New-introduction HD | 11 (7.2) | 7 (5.6) | 4 (2.4) | 0.21 |
| Tracheostomy | 31 (10.7) | 15 (12) | 16 (9.6) | 0.44 |
| Re-exploration for bleeding | 44 (15) | 16 (13) | 28 (17) | 0.41 |
| Deep sternal infection | 10 (3.4) | 2 (1.6) | 8 (4.8) | 0.19 |
| Median ICU stay (days), median (IQR) | 5 (0–81) | 5 (0–81) | 5 (0–59) | 0.71 |
| Median hospital stay (days) | 30 (0–217) | 30 (0–217) | 30 (0–176) | 0.51 |
| Variables . | Overall (%) (n = 290) . | Non-TAR (%) (n = 124) . | TAR (%) (n = 166) . | P-value . |
|---|---|---|---|---|
| Operation time, mean ± SD | 496 ± 213 | 446 ± 184 | 533 ± 227 | <0.001 |
| CPB time, mean ± SD | 269 ± 130 | 224 ± 100 | 302 ± 139 | <0.001 |
| Cardiac ischaemic time, mean ± SD | 149 ± 63 | 130 ± 56 | 163 ± 64 | <0.001 |
| Antegrade cerebral perfusion, mean ± SD | 126 ± 79 | 60 ± 46 | 176 ± 60 | <0.001 |
| Lower body circulatory arrest time, mean ± SD | 61 ± 26 | 52 ± 26 | 68 ± 23 | <0.001 |
| Lowest core temperature, mean ± SD | 24.5 ± 2.5 | 24.6 ± 2.8 | 24.3 ± 2.1 | 0.51 |
| Location of primary entry tear, n (%) | ||||
| Ascending aorta | 183 (63) | 113 (91) | 70 (42) | <0.001 |
| Aortic arch ∼ distal arch | 92 (32) | 7 (5.8) | 85 (51) | <0.001 |
| Descending aorta/unknown | 15 (5) | 4 (3.2) | 11 (6.6) | 0.01 |
| Concomitant surgery, n (%) | ||||
| Root reimplantation | 10 (3.4) | 5 (4.0) | 5 (3.0) | 0.75 |
| Root replacement | 23 (7.9) | 12 (9.7) | 11 (6.6) | 0.38 |
| Aortic valve replacement | 6 (2.1) | 3 (2.4) | 3 (1.8) | 0.70 |
| CABG | 22 (7.5) | 10 (8.1) | 12 (7.2) | 0.83 |
| Limb bypass | 13 (4.5) | 5 (4.0) | 8 (4.8) | 1 |
| Early results, n (%) | ||||
| 30-Day mortality | 20 (6.9) | 8 (6.5) | 12 (7.2) | 1 |
| In-hospital mortality | 32 (11.0) | 16 (12.9) | 16 (9.6) | 0.45 |
| TND | 40 (13.7) | 20 (16) | 20 (12) | 0.30 |
| New-onset PND | 31 (10.7) | 15 (12) | 16 (9.6) | 0.56 |
| New-introduction HD | 11 (7.2) | 7 (5.6) | 4 (2.4) | 0.21 |
| Tracheostomy | 31 (10.7) | 15 (12) | 16 (9.6) | 0.44 |
| Re-exploration for bleeding | 44 (15) | 16 (13) | 28 (17) | 0.41 |
| Deep sternal infection | 10 (3.4) | 2 (1.6) | 8 (4.8) | 0.19 |
| Median ICU stay (days), median (IQR) | 5 (0–81) | 5 (0–81) | 5 (0–59) | 0.71 |
| Median hospital stay (days) | 30 (0–217) | 30 (0–217) | 30 (0–176) | 0.51 |
CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; HD: haemodialysis; ICU: Intensive care unit; IQR: interquartile range; PND: permanent neurological deficit; SD: standard deviation; TAR: total arch replacement; TND: transient neurological deficit.
Follow-up data collection
All patients were assessed using contrast CT evaluation within 1 month after the surgery if the renal function of the patients was not impaired. The diameter of the aorta was measured, and the FL was examined. Patients were usually followed up using CT at our outpatient clinic or at any of the several neighbouring hospitals for 6–12 months after hospital discharge and annually thereafter.
Definitions
‘Complete FL thrombosis’ was defined as the thrombosis of the FL as far as the diaphragm level detected using postoperative contrast CT. ‘Distal aortic reoperation’ was defined as surgery for the dissected descending or thoraco-abdominal aorta, except for isolated surgery for infrarenal abdominal aorta.
Statistical analysis
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [11]. Nominal variables were evaluated using the Fisher’s exact analysis, whereas continuous variables were analysed using the t-test or Mann–Whitney U-test. Long-term survival and freedom from reoperation were estimated by the Kaplan–Meier method and log-rank test, as well as logistic regression model. The Cox proportional hazards regression model was applied for the multivariable analysis of the freedom from reoperation. P-value <0.05 was considered as statistically significant.
RESULTS
Preoperative and intraoperative data
Table 1 shows that age was significantly more advanced and preoperative shock state, particularly with cardiopulmonary resuscitation, was more prevalent in the non-TAR group than those in the TAR group. We tended to perform extended arch replacement in patients with connective tissue disease.
Early outcomes
Table 2 shows intraoperative data and surgical outcomes. Intraoperative lowest core temperature was not significantly different between the 2 groups. Percentages of concomitant procedures were not different between the 2 groups. Thirty-day mortality was not different (the non-TAR group: 8/124, 6.5% vs the TAR group: 12/166, 7.2%; P = 1), and in-hospital mortality was similar in the 2 groups (the non-TAR group: 16/124, 13% vs the TAR group: 16/166, 10%; P = 0.45). There were no significant differences in the incidence of permanent neurological deficit (P = 0.45) and haemodialysis (P = 0.21). Spinal cord ischaemia was not encountered in the 2 groups.
In 263 patients with sufficient postoperative contrast CT evaluation, the aortic diameter at the level of the distal arch and middle descending aorta was not statistically different (P = 0.69) between the 2 groups. Complete FL thrombosis was obtained in 32% (37/115) in the non-TAR group and 41% (61/148) in the TAR group (P = 0.16). Percentages of unachieved primary entry tear resection, which means retrograde dissection with primary entry located at unreachable descending aorta, were not statistically different between the 2 groups (Table 3).
| Variables . | Overall (%) (n = 263) . | Non-TAR (%) (n = 115) . | TAR (%) (n = 148) . | P-value . |
|---|---|---|---|---|
| Aortic diameter, mean ± SD | ||||
| At distal arch | 37.7 ± 6.1 | 37.5 ± 5.7 | 37.9 ± 6.2 | 0.69 |
| At the middle descending aorta | 35.2 ± 6.0 | 34.9 ± 6.1 | 35.4 ± 6.0 | 0.95 |
| Complete FL Thrombosis | 98 (37) | 37 (32) | 61 (41) | 0.16 |
| Unachieved primary entry resection, n (%) | 17 (6.5) | 5 (4.3) | 12 (8.1) | 0.31 |
| Variables . | Overall (%) (n = 263) . | Non-TAR (%) (n = 115) . | TAR (%) (n = 148) . | P-value . |
|---|---|---|---|---|
| Aortic diameter, mean ± SD | ||||
| At distal arch | 37.7 ± 6.1 | 37.5 ± 5.7 | 37.9 ± 6.2 | 0.69 |
| At the middle descending aorta | 35.2 ± 6.0 | 34.9 ± 6.1 | 35.4 ± 6.0 | 0.95 |
| Complete FL Thrombosis | 98 (37) | 37 (32) | 61 (41) | 0.16 |
| Unachieved primary entry resection, n (%) | 17 (6.5) | 5 (4.3) | 12 (8.1) | 0.31 |
FL: false lumen; TAR: total arch replacement.
| Variables . | Overall (%) (n = 263) . | Non-TAR (%) (n = 115) . | TAR (%) (n = 148) . | P-value . |
|---|---|---|---|---|
| Aortic diameter, mean ± SD | ||||
| At distal arch | 37.7 ± 6.1 | 37.5 ± 5.7 | 37.9 ± 6.2 | 0.69 |
| At the middle descending aorta | 35.2 ± 6.0 | 34.9 ± 6.1 | 35.4 ± 6.0 | 0.95 |
| Complete FL Thrombosis | 98 (37) | 37 (32) | 61 (41) | 0.16 |
| Unachieved primary entry resection, n (%) | 17 (6.5) | 5 (4.3) | 12 (8.1) | 0.31 |
| Variables . | Overall (%) (n = 263) . | Non-TAR (%) (n = 115) . | TAR (%) (n = 148) . | P-value . |
|---|---|---|---|---|
| Aortic diameter, mean ± SD | ||||
| At distal arch | 37.7 ± 6.1 | 37.5 ± 5.7 | 37.9 ± 6.2 | 0.69 |
| At the middle descending aorta | 35.2 ± 6.0 | 34.9 ± 6.1 | 35.4 ± 6.0 | 0.95 |
| Complete FL Thrombosis | 98 (37) | 37 (32) | 61 (41) | 0.16 |
| Unachieved primary entry resection, n (%) | 17 (6.5) | 5 (4.3) | 12 (8.1) | 0.31 |
FL: false lumen; TAR: total arch replacement.
The univariable analysis revealed that the significant risk factors for in-hospital mortality were shock, preoperative cardiopulmonary resuscitation and persistent malperfusion of the brain, coronary artery and visceral organs (Table 4). TAR was not a significant factor for in-hospital mortality [P = 0.45; odds ratio 0.72; 95% confidence interval (CI) 0.32–1.61].
| Variables . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| P-value . | OR (95% CI) . | P-value . | OR (95% CI) . | |
| Age ≥80 years | 0.07 | 2.2 (0.83–5.38) | 0.09 | 2.54 (0.87–7.37) |
| Female gender | 0.85 | 0.93 (0.41–2.07) | ||
| Shock | <0.01 | 9.48 (3.92–24.9) | 0.16 | 2.26 (0.72–7.15) |
| Preoperative CPR | <0.01 | 25.5 (9.19–75.4) | <0.001 | 9.1 (2.25–36.6) |
| Continuous coma | <0.01 | 11.1 (4.61–27.6) | 0.07 | 2.94 (0.91–9.44) |
| Malperfusion (overall) | <0.01 | 3.32 (1.47–7.82) | 0.7 | 1.26 (0.36–4.48) |
| Neck vessel | <0.63 | 1.26 (0.43–3.22) | ||
| Coronary | <0.01 | 5.17 (1.83–13.9) | 0.7 | 0.78 (0.16–3.90) |
| Visceral | 0.08 | 2.91 (0.64–10.5) | 0.17 | 3.24 (0.61–17.3) |
| Lower limb | 1 | 0.82 (0.15–2.90) | ||
| Serum creatinine ≥1.5 | <0.18 | 2.38 (0.40–9.76) | 0.3 | 2.49 (0.42–14.7) |
| TAR | 0.45 | 0.72 (0.32–1.61) | ||
| Connective tissue disease | 1 | 0.76 (0.08–3.38) | ||
| Variables . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| P-value . | OR (95% CI) . | P-value . | OR (95% CI) . | |
| Age ≥80 years | 0.07 | 2.2 (0.83–5.38) | 0.09 | 2.54 (0.87–7.37) |
| Female gender | 0.85 | 0.93 (0.41–2.07) | ||
| Shock | <0.01 | 9.48 (3.92–24.9) | 0.16 | 2.26 (0.72–7.15) |
| Preoperative CPR | <0.01 | 25.5 (9.19–75.4) | <0.001 | 9.1 (2.25–36.6) |
| Continuous coma | <0.01 | 11.1 (4.61–27.6) | 0.07 | 2.94 (0.91–9.44) |
| Malperfusion (overall) | <0.01 | 3.32 (1.47–7.82) | 0.7 | 1.26 (0.36–4.48) |
| Neck vessel | <0.63 | 1.26 (0.43–3.22) | ||
| Coronary | <0.01 | 5.17 (1.83–13.9) | 0.7 | 0.78 (0.16–3.90) |
| Visceral | 0.08 | 2.91 (0.64–10.5) | 0.17 | 3.24 (0.61–17.3) |
| Lower limb | 1 | 0.82 (0.15–2.90) | ||
| Serum creatinine ≥1.5 | <0.18 | 2.38 (0.40–9.76) | 0.3 | 2.49 (0.42–14.7) |
| TAR | 0.45 | 0.72 (0.32–1.61) | ||
| Connective tissue disease | 1 | 0.76 (0.08–3.38) | ||
CI: confidence interval; CPR: cardiopulmonary resuscitation; OR: odds ratio; TAR: total arch replacement.
| Variables . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| P-value . | OR (95% CI) . | P-value . | OR (95% CI) . | |
| Age ≥80 years | 0.07 | 2.2 (0.83–5.38) | 0.09 | 2.54 (0.87–7.37) |
| Female gender | 0.85 | 0.93 (0.41–2.07) | ||
| Shock | <0.01 | 9.48 (3.92–24.9) | 0.16 | 2.26 (0.72–7.15) |
| Preoperative CPR | <0.01 | 25.5 (9.19–75.4) | <0.001 | 9.1 (2.25–36.6) |
| Continuous coma | <0.01 | 11.1 (4.61–27.6) | 0.07 | 2.94 (0.91–9.44) |
| Malperfusion (overall) | <0.01 | 3.32 (1.47–7.82) | 0.7 | 1.26 (0.36–4.48) |
| Neck vessel | <0.63 | 1.26 (0.43–3.22) | ||
| Coronary | <0.01 | 5.17 (1.83–13.9) | 0.7 | 0.78 (0.16–3.90) |
| Visceral | 0.08 | 2.91 (0.64–10.5) | 0.17 | 3.24 (0.61–17.3) |
| Lower limb | 1 | 0.82 (0.15–2.90) | ||
| Serum creatinine ≥1.5 | <0.18 | 2.38 (0.40–9.76) | 0.3 | 2.49 (0.42–14.7) |
| TAR | 0.45 | 0.72 (0.32–1.61) | ||
| Connective tissue disease | 1 | 0.76 (0.08–3.38) | ||
| Variables . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| P-value . | OR (95% CI) . | P-value . | OR (95% CI) . | |
| Age ≥80 years | 0.07 | 2.2 (0.83–5.38) | 0.09 | 2.54 (0.87–7.37) |
| Female gender | 0.85 | 0.93 (0.41–2.07) | ||
| Shock | <0.01 | 9.48 (3.92–24.9) | 0.16 | 2.26 (0.72–7.15) |
| Preoperative CPR | <0.01 | 25.5 (9.19–75.4) | <0.001 | 9.1 (2.25–36.6) |
| Continuous coma | <0.01 | 11.1 (4.61–27.6) | 0.07 | 2.94 (0.91–9.44) |
| Malperfusion (overall) | <0.01 | 3.32 (1.47–7.82) | 0.7 | 1.26 (0.36–4.48) |
| Neck vessel | <0.63 | 1.26 (0.43–3.22) | ||
| Coronary | <0.01 | 5.17 (1.83–13.9) | 0.7 | 0.78 (0.16–3.90) |
| Visceral | 0.08 | 2.91 (0.64–10.5) | 0.17 | 3.24 (0.61–17.3) |
| Lower limb | 1 | 0.82 (0.15–2.90) | ||
| Serum creatinine ≥1.5 | <0.18 | 2.38 (0.40–9.76) | 0.3 | 2.49 (0.42–14.7) |
| TAR | 0.45 | 0.72 (0.32–1.61) | ||
| Connective tissue disease | 1 | 0.76 (0.08–3.38) | ||
CI: confidence interval; CPR: cardiopulmonary resuscitation; OR: odds ratio; TAR: total arch replacement.
The multivariable analysis revealed that a significant risk factor for hospital mortality was the requirement for preoperative cardiopulmonary resuscitation (P < 0.001; odds ratio 9.1, 95% CI 2.25–36.6).
Late outcomes
After postoperative contrast CT evaluation, 5 patients died during hospitalization. Among 258 hospital survivors, 7 patients have been lost from follow-up. Overall late death was encountered in 31 patients: the non-TAR group = 12 of 105 patients and the TAR group = 19 of 146 patients. The causes of death in the non-TAR group were due to cerebrovascular (n = 3), pulmonary (n = 2), renal (n = 1), malignancy (n = 1) and aorta-related death including sudden death (n = 5) and were due to cerebrovascular (n = 3), pulmonary (n = 5), cardiac (n = 3), renal (n = 2), malignancy (n = 4) and aorta-related death including sudden death (n = 3) in the TAR group. The 5- and 10-year survival rates of hospital survivors were 87% and 85% in the non-TAR group and 85% and 81% in the TAR group, respectively (Fig. 2A). Long-term survival rate did not reveal significant difference between the 2 groups (P = 0.55). The 5- and 10-year freedom from aorta-related death of hospital survivors were 96% and 94% in the non-TAR group and 98% and 94% in the TAR group, respectively (Fig. 2B), which also did not significantly differ between the 2 groups (P = 0.36).
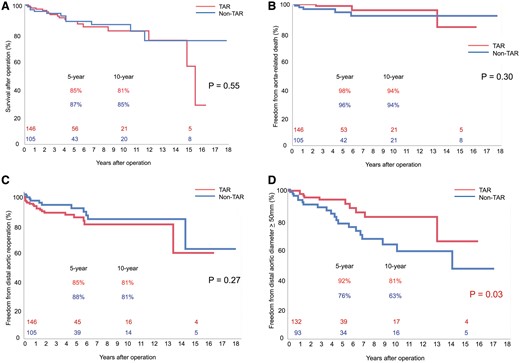
(A) Probability of cumulative overall survival about pursuable hospital survivors between the non-TAR and TAR groups. (B) Probability of freedom from aorta-related death between the non-TAR and TAR groups. (C) Probability of freedom from distal aortic reoperation between the non-TAR and TAR groups. (D) Probability of freedom from distal aortic dilation ≥50 mm between the non-TAR and TAR groups. TAR: total arch replacement.
Distal aortic reoperation and aortic diameter
Our reoperation criteria of the distal aorta were rapid expansion (≥5 mm within 6 months) or enlarge distal aortic diameter ≥55 mm.
Overall, 14 patients underwent distal aortic reoperation for distal aortic dilatation in the non-TAR group [12 replacements and 2 thoracic endovascular aortic repairs (TEVARs)]. Moreover, 23 patients underwent distal aortic reoperation in the TAR group (19 replacements and 4 TEVAR). Freedom from distal aortic reoperation at 5 and 10 years were 88% and 81% in the non-TAR group and 85% and 81% in the TAR group (Fig. 2C), with no significant differences between the 2 groups (P = 0.49).
The segmental aortic size changes in late phase of 225 patients with sufficient CT evaluation are shown in Table 5. Dilation of the proximal descending aorta and the middle descending aorta was significant in the non-TAR group. Freedom from distal aortic dilatation ≥ 50 mm was significantly higher in patients in the TAR group (P = 0.03) (Fig. 2D).
| Variables . | Overall (%) (n = 225) . | Non-TAR (%) (n = 94) . | TAR (%) (n = 131) . | P-value . |
|---|---|---|---|---|
| Aortic diameter, mean ± SD | ||||
| At aortic arch | 39 ± 6.2 | 39 ± 6.2 | NA | NA |
| At the proximal descending aorta | 40.8 ± 8.2 | 43.7 ± 8.1 | 38.4 ± 7.4 | <0.001 |
| At the middle descending aorta | 39.9 ± 8.2 | 41.9 ± 8.8 | 38.6 ± 7.7 | <0.001 |
| Variables . | Overall (%) (n = 225) . | Non-TAR (%) (n = 94) . | TAR (%) (n = 131) . | P-value . |
|---|---|---|---|---|
| Aortic diameter, mean ± SD | ||||
| At aortic arch | 39 ± 6.2 | 39 ± 6.2 | NA | NA |
| At the proximal descending aorta | 40.8 ± 8.2 | 43.7 ± 8.1 | 38.4 ± 7.4 | <0.001 |
| At the middle descending aorta | 39.9 ± 8.2 | 41.9 ± 8.8 | 38.6 ± 7.7 | <0.001 |
SD: standard deviation; TAR: total arch replacement.
| Variables . | Overall (%) (n = 225) . | Non-TAR (%) (n = 94) . | TAR (%) (n = 131) . | P-value . |
|---|---|---|---|---|
| Aortic diameter, mean ± SD | ||||
| At aortic arch | 39 ± 6.2 | 39 ± 6.2 | NA | NA |
| At the proximal descending aorta | 40.8 ± 8.2 | 43.7 ± 8.1 | 38.4 ± 7.4 | <0.001 |
| At the middle descending aorta | 39.9 ± 8.2 | 41.9 ± 8.8 | 38.6 ± 7.7 | <0.001 |
| Variables . | Overall (%) (n = 225) . | Non-TAR (%) (n = 94) . | TAR (%) (n = 131) . | P-value . |
|---|---|---|---|---|
| Aortic diameter, mean ± SD | ||||
| At aortic arch | 39 ± 6.2 | 39 ± 6.2 | NA | NA |
| At the proximal descending aorta | 40.8 ± 8.2 | 43.7 ± 8.1 | 38.4 ± 7.4 | <0.001 |
| At the middle descending aorta | 39.9 ± 8.2 | 41.9 ± 8.8 | 38.6 ± 7.7 | <0.001 |
SD: standard deviation; TAR: total arch replacement.
Table 6 shows the predictors of distal aortic dilatation ≥50 mm using the Cox proportional hazards model. The multivariable analysis revealed that non-TAR (P =0.01; hazard ratio 3.2, 95% CI 1.28–8.03) and unachieved primary entry resection (P =0.002; hazard ratio 6.2, 95% CI 1.38–8.66) were strong predictors of distal aortic dilatation.
Univariable and multivariable analyses of predictors of distal aortic dilatation ≥50 mm
| Variables . | Univariable . | Multivariable . | |
|---|---|---|---|
| P-value . | P-value . | HR (95% CI) . | |
| Age <50 years | 0.98 | ||
| Female gender | 0.38 | ||
| Connective tissue disease | 0.10 | 0.08 | 2.76 (0.87–8.70) |
| Re-entry located at | |||
| Celiac artery | 0.055 | 0.21 | 1.75 (0.72–4.26) |
| Superior mesenteric artery | 0.16 | ||
| Right renal artery | 0.89 | ||
| Left renal artery | 0.74 | ||
| Non-TAR | 0.03 | 0.01 | 3.21 (1.28–8.05) |
| Unachieved primary entry resection | <0.001 | 0.002 | 6.17 (1.38–8.66) |
| Patent FL status at descending aorta | <0.001 | 0.08 | 3.11 (0.86–11.04) |
| Variables . | Univariable . | Multivariable . | |
|---|---|---|---|
| P-value . | P-value . | HR (95% CI) . | |
| Age <50 years | 0.98 | ||
| Female gender | 0.38 | ||
| Connective tissue disease | 0.10 | 0.08 | 2.76 (0.87–8.70) |
| Re-entry located at | |||
| Celiac artery | 0.055 | 0.21 | 1.75 (0.72–4.26) |
| Superior mesenteric artery | 0.16 | ||
| Right renal artery | 0.89 | ||
| Left renal artery | 0.74 | ||
| Non-TAR | 0.03 | 0.01 | 3.21 (1.28–8.05) |
| Unachieved primary entry resection | <0.001 | 0.002 | 6.17 (1.38–8.66) |
| Patent FL status at descending aorta | <0.001 | 0.08 | 3.11 (0.86–11.04) |
CI: confidence interval; FL: false lumen; HR: hazard ratio; TAR: total arch replacement.
Univariable and multivariable analyses of predictors of distal aortic dilatation ≥50 mm
| Variables . | Univariable . | Multivariable . | |
|---|---|---|---|
| P-value . | P-value . | HR (95% CI) . | |
| Age <50 years | 0.98 | ||
| Female gender | 0.38 | ||
| Connective tissue disease | 0.10 | 0.08 | 2.76 (0.87–8.70) |
| Re-entry located at | |||
| Celiac artery | 0.055 | 0.21 | 1.75 (0.72–4.26) |
| Superior mesenteric artery | 0.16 | ||
| Right renal artery | 0.89 | ||
| Left renal artery | 0.74 | ||
| Non-TAR | 0.03 | 0.01 | 3.21 (1.28–8.05) |
| Unachieved primary entry resection | <0.001 | 0.002 | 6.17 (1.38–8.66) |
| Patent FL status at descending aorta | <0.001 | 0.08 | 3.11 (0.86–11.04) |
| Variables . | Univariable . | Multivariable . | |
|---|---|---|---|
| P-value . | P-value . | HR (95% CI) . | |
| Age <50 years | 0.98 | ||
| Female gender | 0.38 | ||
| Connective tissue disease | 0.10 | 0.08 | 2.76 (0.87–8.70) |
| Re-entry located at | |||
| Celiac artery | 0.055 | 0.21 | 1.75 (0.72–4.26) |
| Superior mesenteric artery | 0.16 | ||
| Right renal artery | 0.89 | ||
| Left renal artery | 0.74 | ||
| Non-TAR | 0.03 | 0.01 | 3.21 (1.28–8.05) |
| Unachieved primary entry resection | <0.001 | 0.002 | 6.17 (1.38–8.66) |
| Patent FL status at descending aorta | <0.001 | 0.08 | 3.11 (0.86–11.04) |
CI: confidence interval; FL: false lumen; HR: hazard ratio; TAR: total arch replacement.
DISCUSSION
With the improvement of early surgical results of AAAD, long-term problems including distal aortic dilatation and rupture have been focused on. Many authors have discussed the optimal extent of graft replacement for AAAD [3.4.6 12–15]; therefore, we investigated the influences of the extent of graft replacement, TAR vs non-TAR, on early and late outcomes in patients with a preoperative patent FL at the descending aorta.
The optimal surgical strategy in patients with AAAD focuses on 3 aspects: mortality, postoperative FL status and distal aortic reoperation.
Although the 30-day mortality rate after surgery for AAAD has been improved, it still remains at 10–20%, according to the recent analyses from facilities with high operative volume [16–18]. In this study, in-hospital mortality was 11% (32/290). This is a relatively higher rate than that reported by us previously, which revealed 7% in-hospital mortality [2]; this may reflect the more severe condition of patients with a patent FL at the descending aorta. These results led authors to question the indication of extensive surgical procedures.
Meanwhile, Omura et al. [12] reported that TAR did not have a negative influence on operative mortality in patients with DeBakey Type I acute aortic dissection. During the study period, when we detected a primary entry located at the proximal descending aorta, we usually indicated TAR as possible as we could. Our lower percentage of unachieved primary entry resection, 6.5% overall, is the result of this strategy. However, still the primary entry resection was confirmed as unresectable, and TAR or non-TAR was decided according to the patient condition. Our results also revealed that there was no significant difference in hospital mortality between the non-TAR and TAR groups, and only preoperative status needed cardiopulmonary resuscitation, which contributed for the operative death.
Several studies have reported that a patent FL at the distal aorta after the initial operation is the strong predictor for distal reoperation [1, 2]. To treat FL status and promote aortic remodelling, the exclusion of entry tear is one of the most important issues of surgical treatment of AAAD. In patients with preoperative complete thrombosis of an FL, postoperative FL status rarely becomes problematic. Non-TAR, hemiarch or partial arch replacement can be decided according to the tear-oriented strategy because the proportion of the primary entry tear at the aortic arch is as rare as 10% [19]. However, in patients with a preoperative patent FL in these patients, rates of complete thrombosis of an FL were not statistically different between the groups, but patients after TAR revealed a 10% increased rate of FL thrombosis at the descending aorta (32% in the non-TAR group vs 41% in the TAR group, P = 0.16). Because the overall rate of unachieved primary entry resection was as low as 4.3% in the non-TAR group and 8.1% in the TAR group, multiple factors may contribute to postoperative FL status.
In addition, the conventional ET was applied in these patients, and the FET procedure was not utilized [20]. The FET procedure has been reported to effectively promote FL thrombosis at 77–100% when compared with the conventional ET [21, 22]. Therefore, aggressive TAR with the FET procedure may be justified for relatively stable patient with a preoperative patent FL at the descending aorta.
The reported 5-year survival rate of the hospital survivors after treatment for AAAD was 70–80% [23]. Among mortalities after the initial operation, 10–20% were reportedly due to distal aortic rupture [23, 24], including TAR of as low as 10%. In our study, 5-year survival rates were 85–87% without significant differences between the non-TAR and TAR groups. TAR accounted for as high as 57% of patients, and annual follow-up rate of hospital survivors was 87%. Close observational follow-up using annual CT and aggressive indication of distal reoperation contributed to the equivalent overall survival outcomes.
Freedom from distal aortic reoperation was reportedly between 80% and 90% at 5–10 years after the initial surgery [1, 12]. In this study, freedom from distal aortic reoperation at 5 and 10 years were 88% and 81%, respectively, which agree with previous reports even with the higher rate of a preoperative patent FL.
In this study, predictors of distal aortic dilatation ≥50 mm were non-TAR and unachieved primary entry tear resection. Given that there was no significant difference in the distal aortic diameter immediately after the initial treatment and percentages of primary entry resection in each group, differences in the FL, such as pressure or flow pattern, would be expected. The number of intercostal arteries and their origin contributing as re-entry and celiac and superior mesenteric arteries, of which orifice might cause major re-entry, should be evaluated thereafter. Also, total event (n = 32) is relatively small for a multivariable analysis to investigate the significant predictor. Further study with a higher number of patients is mandatory.
The FET procedure would be useful not only for the closure of the remaining unresectable primary entry located from the middle to the distal descending aorta but also for avulsing the intercostal artery. TAR using the FET procedure for preoperatively stable patients with a patent FL at the descending aorta would improve long-term results. Furthermore, secondary TEVAR relatively at the early stage after the initial operation may contribute to improved long-term outcomes.
It would be expected that a higher percentage of FL thrombosis achieved by TAR would minimize the need for reoperation; however, there were no significant differences in this study. It might be caused by several factors. First, the age of the patient. Patient’s age was considered as one of the reasons why higher dilatation rate in non-TAR did not lead directly to higher distal reoperation rates. In this study, the average age of patients in the non-TAR group is approximately 7 years than that in the TAR group. Several factors, including patient’s frailty, complex surgical approach and larger distal aortic diameter as surgical indication for reoperation in the non-TAR group, may contribute to the hesitation of next operation. Second is unachieved primary entry resection rate. Not significant differences but differences about the unachieved primary entry resection rate (4.3% in the non-TAR group vs 8.1% in the TAR group) might contribute to the result, because secondary TEVAR is easier for patients with TAR and ET.
The mean follow-up period in this study was approximately 5 years, which was not long enough to follow this cohort because the life expectancy of a 67-year-old Japanese man, average age and majority gender of this cohort, is 17.99 years [25]. After the initial surgery for AAAD, patients would have been exposed to the continuing risk of aortic dilatation. It is obvious that arch replacement through repeated sternotomy or descending/thoraco-abdominal replacement through left thoracotomy with or without incision of the diaphragm and laparotomy are significant risks for the senescent patient with new comorbidities. Aortic dilatation involving aortic arch vessels after non-TAR requires complicated aortic surgery. In contrast, aortic dilatation after TAR does not require resternotomy, and the ET can be utilized for the second operation. TAR has considerable merit for future aortic surgery if it can be performed with acceptable mortality.
CONCLUSION
In conclusion, our aggressive strategy for patient with a preoperative patent FL at the descending aorta was acceptable. TAR with the FET procedure may be the first line or a good alternative treatment in the hope of obtaining FL thrombosis and preventing future growth of the distal aorta.
Footnotes
Presented at the 31st Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 7–11 October 2017.
ACKNOWLEDGEMENTS
Other than the author and the coauthors, 12 surgeons, including Yutaka Okita, Motomi Ando, Hitoshi Ogino and Kenji Minatoya, were involved as operating surgeons during the study period.
Conflict of interest: none declared.




