-
PDF
- Split View
-
Views
-
Cite
Cite
Eilon Ram, Ilan Goldenberg, Yigal Kassif, Amit Segev, Jacob Lavee, Michal Einhorn-Cohen, Ehud Raanani, Real-life characteristics and outcomes of patients who undergo percutaneous coronary intervention versus coronary artery bypass grafting for left main coronary artery disease: data from the prospective Multi-vessel Coronary Artery Disease (MULTICAD) Israeli Registry, European Journal of Cardio-Thoracic Surgery, Volume 54, Issue 4, October 2018, Pages 717–723, https://doi.org/10.1093/ejcts/ezy115
Close - Share Icon Share
Abstract
Left main coronary artery involvement in patients with multivessel coronary artery disease provides a poor prognosis. Although the main strategy for revascularization is by coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI) is being used with increased frequency.
This prospective, 3-year follow-up study included 1063 consecutive patients with multivessel coronary artery disease enrolled between January and April 2013 from all 22 hospitals in Israel that perform coronary angiography and PCI.
Of the 1063 patients, 252 (24%) had left main coronary artery disease. Of them, 27% were treated by PCI and 73% by CABG. Factors associated with referral for PCI included older age [odds ratio (OR) 1.04; P = 0.021], renal impairment (OR 3.52; P = 0.006), prior PCI (OR 2.23; P = 0.041) and lower SYNTAX score (OR 1.05; P = 0.004). Kaplan–Meier survival analysis showed that after 3 years, all-cause mortality among left main coronary artery disease patients was significantly higher among those who underwent PCI versus CABG (26.9% vs 8.7%; P < 0.001). Multivariable analysis showed that PCI was associated with a >2-fold increased hazard for mortality compared with surgical revascularization (hazard ratio 2.13, 95% confidence interval 1.05–4.31; P = 0.036).
In real-life practice, clinical factors and a lower SYNTAX score affect the decision to perform PCI in left main coronary artery disease patients. Our findings suggest that CABG is associated with improved long-term survival compared to PCI in patients with left main coronary artery disease after adjustment for those factors.
INTRODUCTION
The left main (LM) coronary artery feeds blood to a large part of the myocardium, and therefore, patients with left main coronary artery disease (LMCAD) are at a higher morbidity and mortality risk. In patients with unprotected LMCAD, the superiority of coronary artery bypass grafting (CABG) over optimal medical treatment has been demonstrated in several studies [1] and is the standard of care and preferred strategy in the current guidelines [2]. Recently, as a result of technological refinements, such as a new generation of drug-eluting stents, intravascular imaging techniques and preferred revascularization strategies based on heart teams for decision-making, percutaneous coronary intervention (PCI) has become more popular in patients with unprotected LMCAD, despite inconsistent data from limited clinical trials and registries [3–6]. Notably, studies that failed to show the superiority of CABG over PCI in patients with LMCAD have few limitations regarding implementation on a real-life setting, such as careful patient selection with strict inclusion and exclusion criteria, use of a heart team involved in decision-making for every patient included in the study and a follow-up period that is not long enough to provide surgical revascularization, the advantage over PCI [5, 6].
Accordingly, the present study was carried out in a contemporary cohort of patients with multivessel CAD to identify factors associated with PCI versus CABG in patients with LMCAD and compare long-term survival between the 2 revascularization strategies in this high-risk population.
METHODS
Study design
The present study population comprised patients who were enrolled and prospectively followed-up in the Multi-vessel Coronary Artery Disease (MULTICAD) Israeli Registry, which included all 22 hospitals in Israel that perform coronary angiography. The aim of this Registry is to: (i) provide contemporary data on real-life management strategies of patients with multivessel CAD in Israel; (ii) provide information regarding the clinical course following CABG and multivessel PCI; (iii) identify risk factors for adverse events following revascularization; and (iv) compare the 2 revascularization approaches in risk subgroups defined by coronary anatomy and clinical factors.
From January through April 2013, 1112 consecutive patients were enrolled in the MULTICAD Registry. Inclusion criteria included all patients ≥18 years of age with multivessel CAD, defined as 2- or 3-vessel CAD involving the proximal or mid-left anterior descending (LAD) artery, and any unprotected left main CAD with >50% narrowing with or without additional CAD. Exclusion criteria included all patients who had undergone prior CABG and/or treated with primary PCI on this admission, those who had moderate or severe valvular disease and pregnant or nursing women. All patients were prospectively followed up for clinical events at 1 month and mortality over a 3-year period. Of the 1112 patients enrolled in the Registry, 49 patients were excluded from the present study [11 who had undergone a combined revascularization strategy with CABG and PCI and 38 patients who were treated conservatively (Fig. 1)]. Thus, the final cohort comprised 1063 patients.
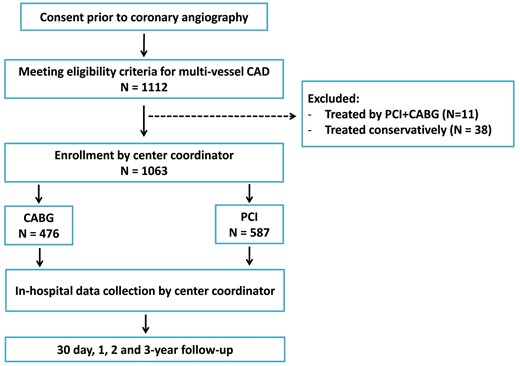
Flow chart summary from consent and eligibility, through a 3-year follow-up. CAD: coronary artery disease; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting.
Revascularization strategy was at the discretion of the treating team. The study was approved by the institutional review board of each of the participating centres, and all patients provided informed consent.
Data collection and follow-up
All data from the participating hospitals were collected and pooled into a designated database. All centres used standardized definitions to collect all data including demographic parameters, medical history, chronic and periprocedural medical treatment, echocardiography measurements, procedural information and outcome measures. All coronary catheterization compact discs were evaluated, and the coronary findings were adjudicated at a core laboratory (Rambam Medical Center). All patients were prospectively followed up for clinical events at 30 days and for mortality at 36 months. Mortality was ascertained from the Israeli Ministry of Interior Population Register through April 2016.
Statistical analysis
Data are presented as mean ± standard deviation, if normally distributed, or as median, if not normally distributed. Continuous variables were tested with the Kolmogorov–Smirnov test for normal distribution. Categorical variables are given as frequencies and percentages. A χ2 test was used for comparison of categorical variables between patients with and without left main CAD and between revascularization strategies (CABG and PCI), and a Student’s t-test was performed for comparison of continuous variables between the groups. The Kaplan–Meier survival analysis was performed to compare long-term mortality by the presence of LMCAD and by the revascularization strategy among patients with LMCAD, with statistical differences tested by the log-rank test.
To identify factors associated with referral to PCI among LMCAD patients, a multivariable logistic regression model was constructed. Candidate covariates are provided in Tables 1 and 2. Variables that were associated with referral to PCI (P < 0.1 in Table 2) were included in the regression model. In addition, we included prespecified clinically significant variables in the model. The variables included in the final model were age, gender, diabetes mellitus, hypertension, renal impairment, history of stroke, prior PCI, prior ischaemic heart disease and SYNTAX score (Fig. 2). Results are presented as odds ratio (OR), 95% confidence interval (CI) and P-value.
| . | With LMCAD (n = 252), n (%) . | Without LMCAD (n = 811), n (%) . | P-value . |
|---|---|---|---|
| Age (years), mean (SD) | 68 (11) | 64 (11) | <0.001 |
| Gender (male) | 200 (79) | 647 (80) | 0.958 |
| Hypertension | 195 (77) | 580 (72) | 0.106 |
| Current smoker | 61 (24) | 225 (28) | 0.306 |
| Hyperlipidaemia | 191 (77) | 588 (74) | 0.362 |
| Diabetes mellitus | 111 (44) | 364 (45) | 0.863 |
| Family history of CAD | 65 (31) | 212 (30) | 0.966 |
| Renal impairment | 29 (12) | 99 (12) | 0.874 |
| COPD | 22 (9) | 51 (6) | 0.233 |
| Prior IHD | 62 (25) | 237 (30) | 0.219 |
| Prior PCI | 81 (32) | 284 (35) | 0.439 |
| History of CVA/TIA | 23 (9) | 75 (9) | 1.000 |
| NYHA score III–IV | 3 (13) | 18 (26) | 0.290 |
| SYNTAX score, mean (SD) | 30 (11) | 20 (9) | <0.001 |
| EuroSCORE I, mean (SD) | 2.86 (2.47) | 2.41 (2.54) | 0.015 |
| Indication for angiography | 0.313 | ||
| Stable angina | 75 (30) | 197 (24) | |
| ACS | 148 (59) | 519 (64) | |
| CHF | 5 (2) | 22 (3) | |
| Other | 24 (10) | 73 (9) | |
| Medications on admission | |||
| Aspirin | 181 (73) | 544 (71) | 0.528 |
| Beta-blockers | 131 (53) | 409 (51) | 0.736 |
| ACE inhibitors | 124 (50) | 356 (45) | 0.185 |
| Statins | 177 (71) | 554 (69) | 0.578 |
| Antihyperglycaemic | 70 (31) | 222 (31) | 1.000 |
| Fusid | 22 (10) | 82 (12) | 0.623 |
| Coumadin | 9 (4) | 20 (3) | 0.459 |
| . | With LMCAD (n = 252), n (%) . | Without LMCAD (n = 811), n (%) . | P-value . |
|---|---|---|---|
| Age (years), mean (SD) | 68 (11) | 64 (11) | <0.001 |
| Gender (male) | 200 (79) | 647 (80) | 0.958 |
| Hypertension | 195 (77) | 580 (72) | 0.106 |
| Current smoker | 61 (24) | 225 (28) | 0.306 |
| Hyperlipidaemia | 191 (77) | 588 (74) | 0.362 |
| Diabetes mellitus | 111 (44) | 364 (45) | 0.863 |
| Family history of CAD | 65 (31) | 212 (30) | 0.966 |
| Renal impairment | 29 (12) | 99 (12) | 0.874 |
| COPD | 22 (9) | 51 (6) | 0.233 |
| Prior IHD | 62 (25) | 237 (30) | 0.219 |
| Prior PCI | 81 (32) | 284 (35) | 0.439 |
| History of CVA/TIA | 23 (9) | 75 (9) | 1.000 |
| NYHA score III–IV | 3 (13) | 18 (26) | 0.290 |
| SYNTAX score, mean (SD) | 30 (11) | 20 (9) | <0.001 |
| EuroSCORE I, mean (SD) | 2.86 (2.47) | 2.41 (2.54) | 0.015 |
| Indication for angiography | 0.313 | ||
| Stable angina | 75 (30) | 197 (24) | |
| ACS | 148 (59) | 519 (64) | |
| CHF | 5 (2) | 22 (3) | |
| Other | 24 (10) | 73 (9) | |
| Medications on admission | |||
| Aspirin | 181 (73) | 544 (71) | 0.528 |
| Beta-blockers | 131 (53) | 409 (51) | 0.736 |
| ACE inhibitors | 124 (50) | 356 (45) | 0.185 |
| Statins | 177 (71) | 554 (69) | 0.578 |
| Antihyperglycaemic | 70 (31) | 222 (31) | 1.000 |
| Fusid | 22 (10) | 82 (12) | 0.623 |
| Coumadin | 9 (4) | 20 (3) | 0.459 |
ACE: angiotensin-converting enzyme; ACS: acute coronary syndrome; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; CHF: congestive heart failure; CVA: cerebrovascular accident; IHD: ischaemic heart disease; LMCAD: left main coronary artery disease; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; SD: standard deviation; TIA: transient ischaemic attack.
| . | With LMCAD (n = 252), n (%) . | Without LMCAD (n = 811), n (%) . | P-value . |
|---|---|---|---|
| Age (years), mean (SD) | 68 (11) | 64 (11) | <0.001 |
| Gender (male) | 200 (79) | 647 (80) | 0.958 |
| Hypertension | 195 (77) | 580 (72) | 0.106 |
| Current smoker | 61 (24) | 225 (28) | 0.306 |
| Hyperlipidaemia | 191 (77) | 588 (74) | 0.362 |
| Diabetes mellitus | 111 (44) | 364 (45) | 0.863 |
| Family history of CAD | 65 (31) | 212 (30) | 0.966 |
| Renal impairment | 29 (12) | 99 (12) | 0.874 |
| COPD | 22 (9) | 51 (6) | 0.233 |
| Prior IHD | 62 (25) | 237 (30) | 0.219 |
| Prior PCI | 81 (32) | 284 (35) | 0.439 |
| History of CVA/TIA | 23 (9) | 75 (9) | 1.000 |
| NYHA score III–IV | 3 (13) | 18 (26) | 0.290 |
| SYNTAX score, mean (SD) | 30 (11) | 20 (9) | <0.001 |
| EuroSCORE I, mean (SD) | 2.86 (2.47) | 2.41 (2.54) | 0.015 |
| Indication for angiography | 0.313 | ||
| Stable angina | 75 (30) | 197 (24) | |
| ACS | 148 (59) | 519 (64) | |
| CHF | 5 (2) | 22 (3) | |
| Other | 24 (10) | 73 (9) | |
| Medications on admission | |||
| Aspirin | 181 (73) | 544 (71) | 0.528 |
| Beta-blockers | 131 (53) | 409 (51) | 0.736 |
| ACE inhibitors | 124 (50) | 356 (45) | 0.185 |
| Statins | 177 (71) | 554 (69) | 0.578 |
| Antihyperglycaemic | 70 (31) | 222 (31) | 1.000 |
| Fusid | 22 (10) | 82 (12) | 0.623 |
| Coumadin | 9 (4) | 20 (3) | 0.459 |
| . | With LMCAD (n = 252), n (%) . | Without LMCAD (n = 811), n (%) . | P-value . |
|---|---|---|---|
| Age (years), mean (SD) | 68 (11) | 64 (11) | <0.001 |
| Gender (male) | 200 (79) | 647 (80) | 0.958 |
| Hypertension | 195 (77) | 580 (72) | 0.106 |
| Current smoker | 61 (24) | 225 (28) | 0.306 |
| Hyperlipidaemia | 191 (77) | 588 (74) | 0.362 |
| Diabetes mellitus | 111 (44) | 364 (45) | 0.863 |
| Family history of CAD | 65 (31) | 212 (30) | 0.966 |
| Renal impairment | 29 (12) | 99 (12) | 0.874 |
| COPD | 22 (9) | 51 (6) | 0.233 |
| Prior IHD | 62 (25) | 237 (30) | 0.219 |
| Prior PCI | 81 (32) | 284 (35) | 0.439 |
| History of CVA/TIA | 23 (9) | 75 (9) | 1.000 |
| NYHA score III–IV | 3 (13) | 18 (26) | 0.290 |
| SYNTAX score, mean (SD) | 30 (11) | 20 (9) | <0.001 |
| EuroSCORE I, mean (SD) | 2.86 (2.47) | 2.41 (2.54) | 0.015 |
| Indication for angiography | 0.313 | ||
| Stable angina | 75 (30) | 197 (24) | |
| ACS | 148 (59) | 519 (64) | |
| CHF | 5 (2) | 22 (3) | |
| Other | 24 (10) | 73 (9) | |
| Medications on admission | |||
| Aspirin | 181 (73) | 544 (71) | 0.528 |
| Beta-blockers | 131 (53) | 409 (51) | 0.736 |
| ACE inhibitors | 124 (50) | 356 (45) | 0.185 |
| Statins | 177 (71) | 554 (69) | 0.578 |
| Antihyperglycaemic | 70 (31) | 222 (31) | 1.000 |
| Fusid | 22 (10) | 82 (12) | 0.623 |
| Coumadin | 9 (4) | 20 (3) | 0.459 |
ACE: angiotensin-converting enzyme; ACS: acute coronary syndrome; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; CHF: congestive heart failure; CVA: cerebrovascular accident; IHD: ischaemic heart disease; LMCAD: left main coronary artery disease; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; SD: standard deviation; TIA: transient ischaemic attack.
| . | CABG (n = 185), n (%) . | PCI (n = 67), n (%) . | P-value . |
|---|---|---|---|
| Age (years), mean (SD) | 66 (10) | 70 (12) | 0.029 |
| Gender (male) | 150 (81) | 50 (75) | 0.346 |
| Hypertension | 140 (76) | 55 (82) | 0.366 |
| Current smoker | 46 (25) | 15 (22) | 0.811 |
| Hyperlipidaemia | 137 (75) | 54 (82) | 0.362 |
| Diabetes mellitus | 82 (45) | 29 (43) | 0.970 |
| Family history of CAD | 44 (29) | 21 (36) | 0.364 |
| Renal impairment | 14 (8) | 15 (22) | 0.003 |
| COPD | 14 (8) | 8 (12) | 0.412 |
| Prior IHD | 39 (21) | 23 (36) | 0.035 |
| Prior PCI | 50 (27) | 31 (47) | 0.005 |
| History of CVA/TIA | 21 (11) | 2 (3) | 0.072 |
| SYNTAX score, mean (SD) | 31 (10) | 26 (11) | 0.006 |
| EuroSCORE I, mean (SD) | 2.64 (2.12) | 3.46 (3.17) | 0.020 |
| Indication for angiography | 0.920 | ||
| Stable angina | 109 (59) | 39 (58) | |
| ACS | 3 (2) | 2 (3) | |
| CHF | 18 (10) | 6 (9) | |
| Other | 55 (29) | 20 (30) | |
| Medications on admission | |||
| Aspirin | 134 (74) | 47 (70) | 0.605 |
| Beta-blockers | 92 (50) | 39 (58) | 0.352 |
| ACE inhibitors | 82 (45) | 42 (63) | 0.018 |
| Statins | 127 (70) | 50 (76) | 0.446 |
| Antihyperglycaemic | 52 (32) | 18 (29) | 0.821 |
| Fusid | 11 (7) | 11 (18) | 0.026 |
| Coumadin | 4 (2) | 5 (9) | 0.107 |
| . | CABG (n = 185), n (%) . | PCI (n = 67), n (%) . | P-value . |
|---|---|---|---|
| Age (years), mean (SD) | 66 (10) | 70 (12) | 0.029 |
| Gender (male) | 150 (81) | 50 (75) | 0.346 |
| Hypertension | 140 (76) | 55 (82) | 0.366 |
| Current smoker | 46 (25) | 15 (22) | 0.811 |
| Hyperlipidaemia | 137 (75) | 54 (82) | 0.362 |
| Diabetes mellitus | 82 (45) | 29 (43) | 0.970 |
| Family history of CAD | 44 (29) | 21 (36) | 0.364 |
| Renal impairment | 14 (8) | 15 (22) | 0.003 |
| COPD | 14 (8) | 8 (12) | 0.412 |
| Prior IHD | 39 (21) | 23 (36) | 0.035 |
| Prior PCI | 50 (27) | 31 (47) | 0.005 |
| History of CVA/TIA | 21 (11) | 2 (3) | 0.072 |
| SYNTAX score, mean (SD) | 31 (10) | 26 (11) | 0.006 |
| EuroSCORE I, mean (SD) | 2.64 (2.12) | 3.46 (3.17) | 0.020 |
| Indication for angiography | 0.920 | ||
| Stable angina | 109 (59) | 39 (58) | |
| ACS | 3 (2) | 2 (3) | |
| CHF | 18 (10) | 6 (9) | |
| Other | 55 (29) | 20 (30) | |
| Medications on admission | |||
| Aspirin | 134 (74) | 47 (70) | 0.605 |
| Beta-blockers | 92 (50) | 39 (58) | 0.352 |
| ACE inhibitors | 82 (45) | 42 (63) | 0.018 |
| Statins | 127 (70) | 50 (76) | 0.446 |
| Antihyperglycaemic | 52 (32) | 18 (29) | 0.821 |
| Fusid | 11 (7) | 11 (18) | 0.026 |
| Coumadin | 4 (2) | 5 (9) | 0.107 |
ACE: angiotensin-converting enzyme; ACS: acute coronary syndrome; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; CVA: cerebrovascular accident; IHD: ischaemic heart disease; LMCAD: left main coronary artery disease; PCI: percutaneous coronary intervention; SD: standard deviation; TIA: transient ischaemic attack.
| . | CABG (n = 185), n (%) . | PCI (n = 67), n (%) . | P-value . |
|---|---|---|---|
| Age (years), mean (SD) | 66 (10) | 70 (12) | 0.029 |
| Gender (male) | 150 (81) | 50 (75) | 0.346 |
| Hypertension | 140 (76) | 55 (82) | 0.366 |
| Current smoker | 46 (25) | 15 (22) | 0.811 |
| Hyperlipidaemia | 137 (75) | 54 (82) | 0.362 |
| Diabetes mellitus | 82 (45) | 29 (43) | 0.970 |
| Family history of CAD | 44 (29) | 21 (36) | 0.364 |
| Renal impairment | 14 (8) | 15 (22) | 0.003 |
| COPD | 14 (8) | 8 (12) | 0.412 |
| Prior IHD | 39 (21) | 23 (36) | 0.035 |
| Prior PCI | 50 (27) | 31 (47) | 0.005 |
| History of CVA/TIA | 21 (11) | 2 (3) | 0.072 |
| SYNTAX score, mean (SD) | 31 (10) | 26 (11) | 0.006 |
| EuroSCORE I, mean (SD) | 2.64 (2.12) | 3.46 (3.17) | 0.020 |
| Indication for angiography | 0.920 | ||
| Stable angina | 109 (59) | 39 (58) | |
| ACS | 3 (2) | 2 (3) | |
| CHF | 18 (10) | 6 (9) | |
| Other | 55 (29) | 20 (30) | |
| Medications on admission | |||
| Aspirin | 134 (74) | 47 (70) | 0.605 |
| Beta-blockers | 92 (50) | 39 (58) | 0.352 |
| ACE inhibitors | 82 (45) | 42 (63) | 0.018 |
| Statins | 127 (70) | 50 (76) | 0.446 |
| Antihyperglycaemic | 52 (32) | 18 (29) | 0.821 |
| Fusid | 11 (7) | 11 (18) | 0.026 |
| Coumadin | 4 (2) | 5 (9) | 0.107 |
| . | CABG (n = 185), n (%) . | PCI (n = 67), n (%) . | P-value . |
|---|---|---|---|
| Age (years), mean (SD) | 66 (10) | 70 (12) | 0.029 |
| Gender (male) | 150 (81) | 50 (75) | 0.346 |
| Hypertension | 140 (76) | 55 (82) | 0.366 |
| Current smoker | 46 (25) | 15 (22) | 0.811 |
| Hyperlipidaemia | 137 (75) | 54 (82) | 0.362 |
| Diabetes mellitus | 82 (45) | 29 (43) | 0.970 |
| Family history of CAD | 44 (29) | 21 (36) | 0.364 |
| Renal impairment | 14 (8) | 15 (22) | 0.003 |
| COPD | 14 (8) | 8 (12) | 0.412 |
| Prior IHD | 39 (21) | 23 (36) | 0.035 |
| Prior PCI | 50 (27) | 31 (47) | 0.005 |
| History of CVA/TIA | 21 (11) | 2 (3) | 0.072 |
| SYNTAX score, mean (SD) | 31 (10) | 26 (11) | 0.006 |
| EuroSCORE I, mean (SD) | 2.64 (2.12) | 3.46 (3.17) | 0.020 |
| Indication for angiography | 0.920 | ||
| Stable angina | 109 (59) | 39 (58) | |
| ACS | 3 (2) | 2 (3) | |
| CHF | 18 (10) | 6 (9) | |
| Other | 55 (29) | 20 (30) | |
| Medications on admission | |||
| Aspirin | 134 (74) | 47 (70) | 0.605 |
| Beta-blockers | 92 (50) | 39 (58) | 0.352 |
| ACE inhibitors | 82 (45) | 42 (63) | 0.018 |
| Statins | 127 (70) | 50 (76) | 0.446 |
| Antihyperglycaemic | 52 (32) | 18 (29) | 0.821 |
| Fusid | 11 (7) | 11 (18) | 0.026 |
| Coumadin | 4 (2) | 5 (9) | 0.107 |
ACE: angiotensin-converting enzyme; ACS: acute coronary syndrome; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; CVA: cerebrovascular accident; IHD: ischaemic heart disease; LMCAD: left main coronary artery disease; PCI: percutaneous coronary intervention; SD: standard deviation; TIA: transient ischaemic attack.
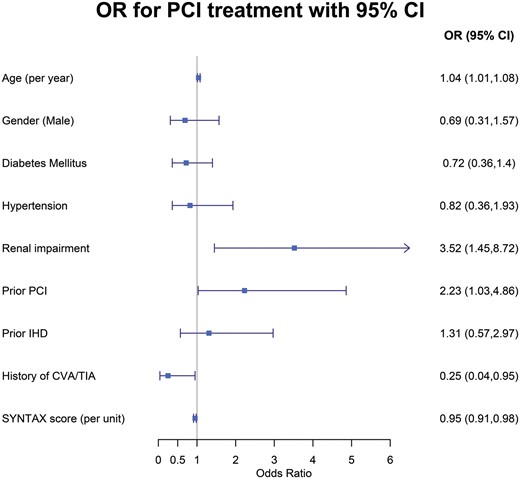
Predictors for referral to PCI vs CABG among patients with left main coronary artery disease. CABG: coronary artery bypass grafting; CI: confidence interval; CVA: cerebrovascular accident; IHD: ischaemic heart disease; OR: odds ratio; PCI: percutaneous coronary intervention; TIA: transient ischaemic attack.
A Cox proportional hazard model was performed in order to assess the association between the presence of LMCAD and all-cause 3-year mortality in the entire study population adjusted for potential confounders. Similar to the logistic regression models, candidate covariates are provided in Table 1. Variables that were associated with LMCAD (P < 0.1) were included in the Cox regression model. In addition, we included prespecified clinically significant variables in the model. The following variables were included in the model: age, gender, diabetes, hypertension, atrial fibrillation, congestive heart failure and SYNTAX score. In addition, we examined the interaction between treatment (PCI or CABG) and LMCAD (Supplementary Material, Table S1). Additional Cox proportional hazard model was constructed to assess the association between revascularization strategy (PCI or CABG) and long-term mortality in LMCAD patients adjusted to the following covariates: age, gender, diabetes, atrial fibrillation and SYNTAX score. Adjusted variables were selected as previously described. Results are presented as hazard ratio (HR), 95% CI and P-value. Proportionality of hazard assumption for both Cox models was tested using Schoenfelds’ residuals test. In cases where an assumption was violated, we performed analysis of interaction terms with treatment and time, with HR and P-values presented separately, with a P-value for interaction term (Fig. 3B).
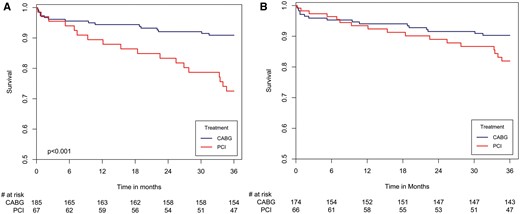
(A) Three-year survival curves in patients with left main coronary artery disease by revascularization strategy (CABG vs PCI). (B) Hazard plot for survival at three years in patients with left main coronary artery disease by revascularization strategy (PCI vs CABG), with propensity score adjustment. The covariates included in the model are age, gender, diabetes, hypertension, renal impairment, COPD, prior PCI, history of CVA/TIA, EuroSCORE and SYNTAX score. The figure depicts the hazard plots obtained from the propensity score-adjusted model; the number of patients at risk at each time point is derived from the Kaplan–Meier life table. Until 7 months, HR 0.407 (PCI vs CABG), 95% CI 0.097–1.718; P = 0.932. 7 months and thereafter, HR 3.361 (PCI vs CABG), 95% CI 1.329–8.5; P = 0.029. CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; CI: confidence interval; CVA: cerebrovascular accident; HR: hazard ratio; PCI: percutaneous coronary intervention; TIA: transient ischaemic attack.
Further analyses were performed to account for potential selection bias including: (i) propensity score regression adjustment in which a logistic regression model was applied to calculate conditional probability for being in the LMCAD group. The following variables were entered into the model: EuroSCORE, smoking, diabetes, hyperlipidaemia, hypertension, renal impairment, prior PCI or myocardial infarction, congestive heart failure, atrial fibrillation, revascularization strategy and SYNTAX score. We included the propensity score as a covariate in the Cox regression models (Supplementary Material, Fig. S2); (ii) propensity score regression adjustment in which a logistic regression model was applied to calculate conditional probability for revascularization by PCI among patients with LMCAD. The following variables were entered into the model: EuroSCORE, diabetes, hypertension, renal impairment, prior PCI and SYNTAX score. We included the propensity score as a covariate in the Cox regression models. Due to time-dependent association between treatment and outcomes, we used an interaction term in the model between time divided in 7 months and treatment (Fig. 3B); (iii) in order to adjust for differences between centre variation, we performed mixed-effects Cox model with an intercepts term as the random-effects component (i.e. the centre) normally distributed with a mean 0 and estimated standard deviation (Supplementary Material, Table S3).
Statistical significance was assumed when the null hypothesis could be rejected at a P-value <0.05. All P-values are results of 2-sided tests. Statistical analyses were conducted using R (version 3.4.1) [7] . The investigators initiated the study, had full access to and analysed all data and wrote the manuscript. All authors vouch for data and analysis.
RESULTS
Among the 1063 patients included in the study, 252 (24%) had multivessel CAD with involvement of the LM coronary artery and 811 (76%) had multivessel CAD without involvement of the LM coronary artery. The mean age of the entire cohort was 65 years, and 20% were women. The baseline clinical characteristics of the study patients by LMCAD involvement are presented in Table 1. Compared with patients without LM involvement, those with LM involvement were older, had a higher frequency of prior angina pectoris, a higher rate of atrial fibrillation and higher levels of nitrate usage. However, there were no statistically significant differences in other baseline clinical parameters between those with and without involvement of the LM coronary artery, such as gender, smoking, diabetes mellitus, hyperlipidaemia, family history of CAD, renal impairment, presence of chronic obstructive pulmonary disease, prior myocardial infarction, prior PCI, history of congestive heart failure, any prior cerebrovascular event and other chronic medical treatments (Table 1).
Factors associated with percutaneous coronary intervention vs coronary artery bypass grafting referral among patients with left main coronary artery disease
Among the 252 patients with LMCAD, 27% were treated by PCI and 73% by CABG. The clinical characteristics of patients with LMCAD by the revascularization strategy are presented in Table 2. Patients who underwent CABG were younger, with less renal impairment, with a lower rate of prior PCI and lower EuroSCORE but with a higher frequency of prior cerebrovascular events, more complex CAD, reflected by a significantly higher mean SYNTAX score (31 ± 10 vs 26 ± 11, P = 0.006) and with a higher rate of 3 or more vessel disease (83% vs. 69%, P = 0.019) (Table 2).
Multivariable logistic regression analysis showed that factors independently associated with referral for PCI vs CABG among patients with LMCAD (Fig. 2) included older age (4% increased odds for PCI vs CABG per 1-year increment in age; P = 0.021), the presence of renal impairment (OR 3.52; P = 0.006), a history of prior PCI (OR 2.23; P = 0.041), no history of stroke (OR 4.00; P = 0.076) and a lower SYNTAX score [5% reduced odds for PCI vs. CABG referral per 1-unit increment in the SYNTAX score (P = 0.004)].
Early and late outcomes by the presence of left main coronary artery disease
In-hospital complications occurred in a significantly greater proportion of study patients with, compared to those without, involvement of the LM coronary artery, including an experienced higher incidence of cardiogenic shock (2.3% vs 0.3%, P = 0.009), permanent pacemaker implantation due to any indication (1.4% vs 0%, P = 0.012), new-onset atrial fibrillation (14% vs 7%, P = 0.002) and sepsis (3.2% vs 0.5%, P = 0.004). Similarly, 30-day mortality rates were significantly higher among patients with, compared to those without, LMCAD (2.9% vs 0.7% respectively, P = 0.013).
The Kaplan–Meier survival analysis showed that mortality rates at 3 years of follow-up among patients with LMCAD were significantly higher (13.6%) compared with those who had multivessel CAD without LMCAD (6.6%; log-rank P-value = 0.001 for the overall difference during follow-up; Supplementary Material, Fig. S1). Consistent with the univariable findings, adjusted analysis, including a propensity score for PCI referral, using a propensity score for confounders, also demonstrated a significantly higher risk for 3-year mortality among patients with, compared to those without, LMCAD (Supplementary Material, Fig. S2 and Table 2). Additional independent predictors for long-term mortality among all study patients included older age, diabetes mellitus and atrial fibrillation (Supplementary Material, Table S2).
Early and late outcomes among patients with left main coronary artery disease by the revascularization strategy
All patients with LMCAD who underwent surgical revascularization received a graft to the LAD artery. Ninety-nine percent of the grafts to the LAD, 63% of the grafts to the left circumflex artery system and 29% of the grafts to the right coronary system were arterial grafts. In the PCI group, the type of stent used in the left main coronary artery was drug eluting in 81% and bare metal in 19%.
Among patients with LMCAD, in-hospital complications (such as cardiogenic shock, permanent pacemaker implantation, sepsis, myocardial infarction and cerebrovascular events) were not significantly different between those who underwent PCI versus CABG. Similarly, 30-day mortality was not significantly different between the 2 groups (PCI vs CABG 3% vs 2.9%, P = 1.000). However, Kaplan–Meier survival analysis (Fig. 3A) showed that at 3 years of follow-up, the rate of all-cause mortality among patients with LMCAD was significantly higher in those who underwent PCI (26.9%) vs CABG (8.7%; log-rank P-value <0.001 for the overall difference during follow-up). Consistent with the univariable Kaplan–Meier findings, multivariable analysis (Table 3) showed that PCI versus CABG referral was the most powerful predictor of long-term mortality among patients with LMCAD and was associated with a >2-fold mortality hazard increase. Additional predictors of mortality among patients with LMCAD included older age and diabetes mellitus (Table 3). Similar results were obtained after further adjustment for centre volume (Supplementary Material, Table S3).
Hazard ratio for mortality in the entire series of patients with LMCAD at 3 years
| . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| PCI vs CABG | 2.13 | 1.05–4.31 | 0.036 |
| Age | 1.07 | 1.03–1.11 | 0.001 |
| Male gender | 0.73 | 0.36–1.50 | 0.390 |
| SYNTAX score >32 | 0.66 | 0.30–1.48 | 0.314 |
| Diabetes mellitus | 2.12 | 1.05–4.27 | 0.036 |
| Atrial fibrillation | 2.10 | 0.91–4.86 | 0.084 |
| . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| PCI vs CABG | 2.13 | 1.05–4.31 | 0.036 |
| Age | 1.07 | 1.03–1.11 | 0.001 |
| Male gender | 0.73 | 0.36–1.50 | 0.390 |
| SYNTAX score >32 | 0.66 | 0.30–1.48 | 0.314 |
| Diabetes mellitus | 2.12 | 1.05–4.27 | 0.036 |
| Atrial fibrillation | 2.10 | 0.91–4.86 | 0.084 |
CABG: coronary artery bypass grafting; CI: confidence interval; HR: hazard ratio; LMCAD: left main coronary artery disease; PCI: percutaneous coronary intervention.
Hazard ratio for mortality in the entire series of patients with LMCAD at 3 years
| . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| PCI vs CABG | 2.13 | 1.05–4.31 | 0.036 |
| Age | 1.07 | 1.03–1.11 | 0.001 |
| Male gender | 0.73 | 0.36–1.50 | 0.390 |
| SYNTAX score >32 | 0.66 | 0.30–1.48 | 0.314 |
| Diabetes mellitus | 2.12 | 1.05–4.27 | 0.036 |
| Atrial fibrillation | 2.10 | 0.91–4.86 | 0.084 |
| . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| PCI vs CABG | 2.13 | 1.05–4.31 | 0.036 |
| Age | 1.07 | 1.03–1.11 | 0.001 |
| Male gender | 0.73 | 0.36–1.50 | 0.390 |
| SYNTAX score >32 | 0.66 | 0.30–1.48 | 0.314 |
| Diabetes mellitus | 2.12 | 1.05–4.27 | 0.036 |
| Atrial fibrillation | 2.10 | 0.91–4.86 | 0.084 |
CABG: coronary artery bypass grafting; CI: confidence interval; HR: hazard ratio; LMCAD: left main coronary artery disease; PCI: percutaneous coronary intervention.
Notably, propensity score modelling showed that the long-term mortality risk reduction associated with CABG versus PCI among patients with LMCAD was evident at 7 months following revascularization (Fig. 3B). Furthermore, a subgroup analysis showed that the lower mortality rates associated with CABG among patients with LMCAD were also evident among patients with a lower SYNTAX score (≤32; Fig. 4).
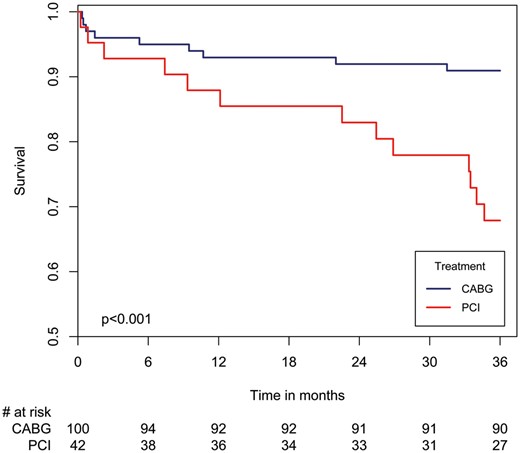
Three-year survival curves in patients with left main coronary artery disease and low-to-intermediate SYNTAX score (≤32) by revascularization strategy (CABG vs PCI). CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention.
DISCUSSION
The present study, carried out in a contemporary cohort of patients with multivessel CAD who were enrolled and prospectively followed up in a real-life setting, demonstrates several important implications regarding coronary revascularization among patients with LMCAD. We have shown that: (i) the presence of LMCAD is associated with a poor prognosis unrelated to the revascularization strategy; (ii) in a real-life setting, almost a third of stable patients without an indication for primary PCI but with significant LMCAD who demonstrate American College of Cardiology (ACC)/American Heart Association Class I indication for CABG are treated by PCI; (iii) revascularization by CABG in patients with multivessel CAD and involvement of the LM coronary artery is associated with improved 3-year survival compared with PCI after adjustment for confounding factors associated with a referral pattern, even in the subset of patients with a lower SYNTAX score and (iv) the survival benefit associated with CABG versus PCI referral among patients with LMCAD is evident after 7 months and continues thereafter.
The main goal of this study was to determine whether PCI achieves similar results as CABG in the treatment of unprotected LMCAD in an era of increasing use of PCI for patients with LMCAD. Previous publications of randomized studies (e.g. the EXCEL study [5]) have reported similar results between CABG and PCI in low-to-intermediate SYNTAX score (≤32) patients with LMCAD. One of the criticisms of these studies is that they do not reflect a real-life situation. All patients included in these randomized trials met strict inclusion and exclusion criteria, and all underwent discussion by a multidisciplinary heart team that decided that all patients were eligible for both CABG and PCI before being included in the study. In a real-life setting, only a minority of patients with LMCAD are discussed by a multidisciplinary heart team before the decision is made regarding which revascularization strategy to adopt, especially if they are referred for PCI eventually.
The present study included all 22 hospitals in Israel that perform angiography and PCI. Of them, 13 did not have an on-site cardiac surgery department or unit, and thus no heart team discussion occurred. In the 9 centres with on-site cardiac surgery services, a formal multidisciplinary heart team discussion was conducted per the discretion of the treating physician and not on a uniform basis. We believe that this reflects the situation more accurately in current practice worldwide.
Another criticism of the above-mentioned randomized studies is connected to their patient selection. While we included all patients from a national registry over a 4-month period, the EXCEL study [5] recruited patients from a total of 126 sites over a 4-year period; that is to say they recruited 3.7 patients per centre per year, which highly suggests that these were carefully selected patients, thereby raising the concern about implications on real-life practice. Moreover, this study followed up these patients for 3 years, while the advantage of CABG is known to be seen specifically in the long term, after 3–5 years and thereafter.
In the present study, among patients with LMCAD, there were some baseline differences between the 2 revascularization strategies. On the one hand, patients who underwent PCI were older, had more renal impairment and a higher rate of prior ischaemic heart disease treated by PCI; on the other hand, they had less history of cerebrovascular accidents and a less complicated coronary artery anatomy reflected by a lower mean SYNTAX score. Although we could not calculate the predicted patient risk due to lack of consistent data collection, we performed a multivariable analysis for confounders that included these baseline differences. The results suggested that in a real-life setting, CABG provides a better 3-year survival rate compared to PCI. Moreover, our results showed that in a subgroup of patients with low-to-intermediate SYNTAX score (≤32) and LMCAD, revascularization by CABG revealed a higher survival rate compared to PCI after 3 years of follow-up.
Many physicians and patients prefer PCI to CABG because of ageing, physical status, personal preference and other factors and therefore more patients with LMCAD are treated by PCI. During the last decade, the rate of isolated CABG has declined, and in clinical practice, there is a tendency to underuse surgical coronary revascularization in patients with LMCAD who are considered appropriate candidates [8]. Recent randomized studies that have shown that revascularization by PCI is not inferior to CABG regarding survival legitimizes this choice. We believe that PCI is a good choice to achieve revascularization in some groups of patients with LMCAD who are at high surgical risk or inoperable. However, patient selection is the key for success, and it should be highly acknowledged that all patients with LMCAD who are referred to PCI should be discussed by a multidisciplinary heart team and that the advantages and disadvantages of this strategy should be explained to the patient prior to the decision on revascularization strategy.
Limitations
We had no information regarding the main cause of death or the rate of cardiac events, such as recurrent revascularization, during the follow-up period. Analysis of cardiac events could reinforce the conclusion that CABG is favourable for the treatment of patients with LMCAD.
CONCLUSIONS AND CLINICAL IMPLICATIONS
We have shown that the presence of LMCAD among patients with multivessel CAD is a powerful risk factor for long-term mortality. Accordingly, a decision on the most appropriate revascularization strategy for each patient should be carefully considered by a multidisciplinary heart team. Our data indicate that in a contemporary real-life setting, approximately one-third of the patients with LMCAD involvement (even those with a lower SYNTAX score) are referred for PCI, despite increased long-term mortality rates associated with this revascularization strategy. These findings suggest that in eligible patients with LMCAD, CABG should be the preferred revascularization strategy when feasible.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 31st Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 7–10 October 2017.




