-
PDF
- Split View
-
Views
-
Cite
Cite
Tommaso Hinna Danesi, Loris Salvador, Minimally invasive aortic valve replacement techniques using endoscopic surgery: ‘must dos’ and ‘preferences’, European Journal of Cardio-Thoracic Surgery, Volume 53, Issue suppl_2, May 2018, Pages ii27–ii28, https://doi.org/10.1093/ejcts/ezy087
Close - Share Icon Share
Abstract
Aortic valve replacement via a full sternotomy remains the gold standard for aortic stenosis treatment; however, minimally invasive techniques have grown in popularity and continue to evolve. A recent evolution of minimally invasive aortic valve replacement is endoscopic surgical aortic valve replacement: a miniaturized surgical approach under video guidance. To ensure a safe and reproducible procedure, we have developed ‘must dos’ and ‘preferences’ for endoscopic surgical aortic valve replacement. These include specific endoscopic surgical skills to avoid severe adverse events or an emergency conversion to a full sternotomy.
INTRODUCTION
Aortic valve replacement via a full sternotomy currently represents the gold standard for aortic stenosis treatment; however, minimally invasive techniques have grown in popularity and continue to evolve.
One minimally invasive aortic valve replacement procedure is endoscopic surgical aortic valve replacement (ESAVR): a video-guided operation using a miniaturized surgical approach to pass the prosthesis through the chest wall. Specific training and endoscopic surgical skills are required to perform ESAVR. We describe the ‘must dos’ and ‘preferences’ for ESAVR to ensure a safe and reproducible procedure.
Operating theatre set-up
Must do
The operating theatre set-up for ESAVR is similar to standard aortic valve replacement, except that a video column and specific long shaft instruments are needed [1].
Preference
For centres new to ESAVR, we recommend the use of 3D-video systems, which speed up the procedure and provide better depth feedback.
Patient positioning
Must do
The patient must be positioned with the right hemithorax elevated at an angle of 30°.
Surgical access
Must do
Usually, the right minithoracotomy (‘working port’) is done in the 2nd intercostal space (IS). The skin incision should be made just above the 3rd rib to easily reach both the 2nd or the 3rd IS, depending on the position of the ascending aorta. The small skin incision of 2–3 cm spares the right internal mammary artery.
A soft tissue retractor is required to elevate the working port. Two other 5-mm miniports are needed: 1 in the 2nd IS laterally to the midclavicular line to introduce the aortic clamp and 1 in the 3rd IS at the level of the anterior axillary line to introduce the ventricular vent line (Fig. 1).
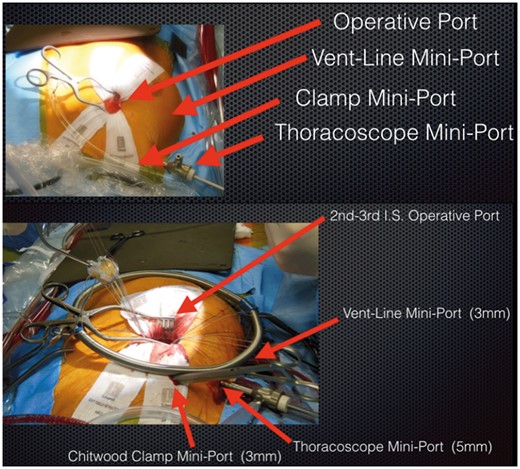
Preference
The working port can be positioned slightly laterally to the right margin sternal line to achieve a comfortable working angle.
Cardiopulmonary bypass
Must do
The working port does not enable direct aortic and right atrial cannulation; therefore, cardiopulmonary bypass is carried out through the femoral vessels, surgical exposure of the common femoral artery and percutaneous cannulation of the contralateral common femoral vein. Transoesophageal echocardiography is mandatory to avoid injuries during the positioning of the venous guide-wire and then the venous drainage cannula into the superior vena cava.
Vacuum-assisted venous drainage is needed. The vacuum should not exceed –60 mmHg in the venous reservoir to avoid red blood cell damage [2].
Preference
Using both femoral vessels of the groin means that the incision to expose the femoral artery is at least 2 cm.
Aortic cross-clamping, cardioplegia and ventricular venting
Must do
Aortic cross-clamping is achieved using a Chitwood clamp. Once cardiopulmonary bypass is in place, the transverse sinus must be dissected gently to avoid injuring the right branch of the pulmonary artery when positioning the aortic clamp. The first shot of cardioplegia is delivered into the ascending aorta and then into the coronary ostia. A retroplegia catheter cannot be positioned through the working port, so a vent line is placed through the superior right pulmonary vein and passed through the mini port into the 3rd IS.
Preferences
Cardioplegia and ventricular venting can be obtained by placing specific necklines (Propledge and Endovent, Edwards Lifesciences, Irvine, CA, USA) [3]. These lines keep the working port clear.
Surgical techniques
Must do
Aortotomy
Aortotomy should not extend toward the pulmonary artery. This zone is difficult to manage when bleeding occurs. The aortotomy should be high enough to allow sutureless or rapid-deployment prosthesis implantation, if needed.
Aortic valve replacement
The aortic valve is normally excised, and the annulus is decalcified.
Aortorrhaphy
After aortic declamping, bleeding from the aortotomy is difficult to control, so attention should be given when performing the medial part of the aortorrhaphy.
Deairing
Deairing during ESAVR is less efficient than with sternotomic access, but inflated carbon dioxide significantly reduces gas emboli.
Electrodes
Because the working port is small, a temporary electrode must be placed once the heart is empty, otherwise exposure to the right ventricle is difficult.
Drainages
Two small 24-Fr silastic drains will efficiently drain the chest. These can be passed through the mini port used to place the vent line and aortic clamp.
Preferences
Three retraction stitches placed at the nadir of the aortic valve commissures improve its exposure. In addition, 2 or 3 stitches will keep the aortic wall away from the thoracoscope field.
We suggest placing the right coronary sinus stitches first because this is the most difficult zone to handle with long-shaft instruments. When implanting a rapid-deployment prosthesis, it should be ensured that the valve is correctly positioned in the right coronary sinus as reaching it with the thoracoscope is difficult (Fig. 2).
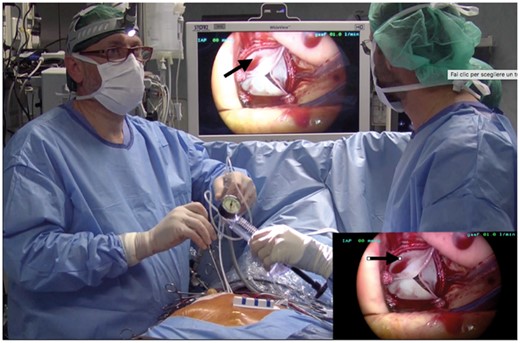
Right coronary sinus (black arrows) is surgically difficult to manage.
We close the aortotomy with hemicontinuous 4-0 polypropylene sutures.
CONCLUSION
The ‘must do’ and ‘preferences’ described will ensure a safe and reproducible procedure for ESAVR, which will benefit both experienced surgeons and those new to the minimally invasive approach.
Conflict of interest: none declared.




