-
PDF
- Split View
-
Views
-
Cite
Cite
Samina Park, Kwanyong Hyun, Hyun Joo Lee, In Kyu Park, Young Tae Kim, Chang Hyun Kang, A study of the learning curve for robotic oesophagectomy for oesophageal cancer, European Journal of Cardio-Thoracic Surgery, Volume 53, Issue 4, April 2018, Pages 862–870, https://doi.org/10.1093/ejcts/ezx440
Close - Share Icon Share
Abstract
Robot-assisted minimally invasive oesophagectomy (RAMIE) enables radical, meticulous dissection of the oesophagus and lymph nodes. Our goal was to identify the effect of the learning curve for RAMIE when performing radical upper mediastinal dissection in patients with oesophageal cancer.
We conducted a retrospective review of a prospectively maintained database of patients who underwent RAMIE for oesophageal cancer between May 2008 and July 2016. The gain in proficiency for each postoperative outcome measure was presented using observed–expected cumulative sum (O-E CUSUM) curves. The change points were defined at the maximal distance from the zero axis.
A total of 140 patients were included. Squamous cell carcinoma (n = 131, 93.6%) was the dominant type. Thirty-day and 90-day deaths occurred in 1 and 5 patients (0.7% and 3.6%, respectively). The change points of the risk-adjusted O-E CUSUM curves were similar to those of the unadjusted O-E CUSUM curves with the exception of those for thoracic procedure time and vocal cord palsy. The number of harvested lymph nodes increased from 25 to 45 before and after 30 cases. The vocal cord palsy rate decreased from 36% to 17% before and after 60 cases. The total operation time decreased from 496 min to 431 min; the length of the hospital stay decreased from 24 days to 14 days; and the anastomotic leakage rate decreased from 15% to 2% before and after 80 cases.
Our study demonstrated a temporal improvement in postoperative outcomes based on accumulated experience with RAMIE. The risk-adjusted O-E CUSUM curves were similar to the unadjusted O-E CUSUM curves, which represents the significant impact of the effect of a learning period on the postoperative outcomes of RAMIE in patients with oesophageal cancer.
INTRODUCTION
Oesophagectomy and lymphadenectomy are the cornerstones of treatment in oesophageal cancer. Minimally invasive oesophagectomy (MIE) results in lower rates of postoperative respiratory complications and a better quality of life compared with open oesophagectomy [1, 2]. Video-assisted thoracic surgery (VATS) oesophagectomy has been widely adopted as a minimally invasive technique for oesophageal cancer with adequate oncological outcomes [3]. As part of a minimally invasive approach, the robotic technique has been applied to oesophageal cancer surgery, allowing radical and meticulous dissection of the oesophagus and lymph nodes using magnified images with a high degree of freedom. Since robot-assisted MIE (RAMIE) has been implemented, its feasibility and comparable oncological outcomes have been published by high-volume centres [4–6].
Despite a remarkable decrease in the number of postoperative deaths and advances in surgical techniques and postoperative care in recent decades, oesophagectomy is still accompanied by a high mortality rate compared to other high-risk surgeries, with a morbidity rate reaching 60%, irrespective of the technical approach [7–9]. Therefore, the use of standardized definitions for specific complications in oesophagectomy is essential to evaluate the influence and application of new surgical techniques [10]. In particular, radical oesophagectomy with lymphadenectomy is in itself a complex procedure. In addition, implementation of a robotic system for oesophagectomy is technically demanding and requires repeated modification during the set-up period, each of which varies considerably by the surgeon and the facility. Subsequently, sufficient experience is required to obtain technical proficiency and stable outcomes by tailoring technical aspects to patient physiology, even for experienced surgeons and centres [11, 12].
Previous studies of the learning curve for RAMIE have been performed in patients with oesophageal adenocarcinoma located in the distal oesophagus [4, 13]. However, the surgical strategy for oesophageal squamous cell carcinoma (ESCC) is different from that for distal oesophageal cancer. Oncological radical dissection along the upper mediastinal organs and lymphadenectomy along the bilateral recurrent laryngeal nerve (RLN) are important in ESCC. In our institute, we have used a robotic system for radical upper mediastinal dissection for ESCC, which is generally believed to have a steep learning curve and high morbidity rates. In this study, we attempted to identify the effect of the learning curve on RAMIE for ESCC when applying radical upper mediastinal dissection. Furthermore, we wanted to identify the number of cases needed to attain surgical proficiency as shown in short-term outcomes in RAMIE based on cumulative sum analysis.
MATERIALS AND METHODS
Study population
We conducted a retrospective review of a prospectively recorded database of 140 consecutive patients who underwent RAMIE performed by C.H.K. for oesophageal cancer among 467 oesophageal resections from May 2008 to July 2016. At first, RAMIE was performed only for early oesophageal cancer. However, the indications for RAMIE were gradually expanded, and recently RAMIE has become a major surgical modality in our institute. The thoracic and abdominal robotic procedures in RAMIE were applied separately according to disease progression and patient status. The contraindications for thoracic robotic oesophagectomy were (i) severe pleural adhesions, (ii) previous major chest surgery, (iii) a large oesophageal tumour that could not be reduced after neoadjuvant treatment, (iv) suspicious major airway invasion, (v) intolerance to single-lung ventilation and (vi) salvage oesophagectomy after definitive chemoradiation therapy. The contraindications for abdominal robotic procedures were (i) a previous history of peritonitis, (ii) previous major abdominal surgery, (iii) abdominal lymph node metastasis, (iv) suspicious invasion to adjacent organs and (v) severe obesity, in which case an abdominal robotic procedure is expected to require too much time. This study was approved by the institutional review board in our institute (H-1610-006-795).
Surgical technique
The surgical techniques for RAMIE in our institute have been modified several times to facilitate more complex surgery because our indications for RAMIE included more advanced tumours over time. In the first step of RAMIE, we used a 3-arm technique and cervical anastomosis exclusively. For dissection of lymph nodes along the bilateral RLNs and stable retraction of the trachea and main bronchus, we started to use a 4-arm technique beginning with the 9th case. Because the location of the tumour was mostly in the upper and mid-thoracic oesophagus, cervical anastomosis was performed in most patients, and intrathoracic anastomosis was performed sporadically. We used a circular stapler for 5 cases, however; we mostly performed a linear stapler or a robotic sewing technique for the anastomosis. The operation for patients who received neoadjuvant chemoradiation was performed sporadically in the early period. Routine robotic oesophagectomy after neoadjuvant chemoradiation started after the 98th case. The detailed technical aspects of RAMIE in our institute have been reported previously [14].
Outcome measures and definition of complication
Short-term outcome measures including total operation time, thoracic procedure time, length of hospital stay, number of harvested lymph nodes and operation-related complications that occurred within 30 days of surgery or during the in-hospital stay after RAMIE were evaluated. The severity of 3 surgical complications including anastomotic leak, conduit necrosis and vocal cord injury/palsy were designated based on the Esophagectomy Complications Consensus Group recommendations [10]. Laryngoscopic examination of the vocal cords by an otolaryngologist was routinely performed in all patients on postoperative Day 3 at our institution.
Statistical analysis
To calculate the learning curve for variables, an observed–expected cumulative sum (O-E CUSUM) curve was constructed. The notable change points were identified at the point showing the largest peak in the CUSUM curve. The significance of the change point was identified by comparing the postoperative outcomes before and after the change point. The unadjusted O-E CUSUM curves were constructed by assuming that the risk of an event has a single value of probability for all cases. We set the probability according to the mean value in related outcomes. However, preoperative patient factors and types of surgical procedures may affect postoperative outcomes. Therefore, the unadjusted O-E CUSUM curves may present results that could make it appear that the surgeon was performing poorly because he or she operated on more and more high-risk patients using complex surgical procedures. Therefore, we used risk-adjusted O-E CUSUM curves to assess accurately the surgeon’s actual performance. The curves plot the cumulative difference between the observed and the expected event according to the risk-adjustment model [15]. O-E CUSUM curves were adjusted for the following potential variables: age, gender, anastomosis location, tumour location, performance status, neoadjuvant chemoradiation treatment, lymph node dissection fields and stages using logistic or linear regression analysis. Linear regression models with log-transformed data are presented in the Supplementary Material, Appendices S1–S7. SPSS 22.0 (IBM Corporation, Armonk, NY, USA) was used for the statistical analyses. Categorical variables were presented as numbers with percentages, and continuous variables were presented as median values with quartiles or means with standard deviations. Comparisons before and after the change point for categorical variables were made using the χ2 test or the Fisher’s exact test. Comparisons before and after the change point for continuous variables were made using the independent t-test or the Mann–Whitney test.
RESULTS
Preoperative patient characteristics are presented in Table 1. The mean age was 65 years, and the majority of patients were men (n = 125, 89.3%). Squamous cell carcinoma (n = 131, 93.6%) was the dominant type, and the most common tumour locations were the cervical, upper or mid-thoracic oesophagus (n = 106, 75.7%). Early oesophageal cancer was the major indication in our cohort, including patients with 60 cT1 (45.7%) and 89 cN0 (63.6%).
| Variables . | n = 140 . |
|---|---|
| Age (years) | 65.1 ± 9.0 |
| Male gender | 125 (89.3) |
| Height (cm) | 164.7 ± 6.8 |
| Weight (kg) | 62.8 ± 10.5 |
| BMI (kg/m2) | 23.0 ± 3.1 |
| Pulmonary function test | |
| FVC (predicted %) | 100.7 ± 14.7 |
| FEV1 (predicted %) | 100.7 ± 18.6 |
| Comorbidity | |
| Hypertension | 62 (44.3) |
| Chronic obstructive pulmonary disease | 55 (39.2) |
| Diabetes mellitus | 25 (17.9) |
| History of malignancy in other organs | 18 (12.9) |
| History of pulmonary tuberculosis | 13 (9.3) |
| Stroke | 12 (8.6) |
| Coronary artery disease | 11 (7.9) |
| Peripheral vascular disease | 8 (5.7) |
| Atrial fibrillation | 7 (5.0) |
| Liver cirrhosis | 5 (3.5) |
| ECOG performance status | |
| 0 | 86 (61.4) |
| 1 | 54 (38.6) |
| Smoking status | |
| Never smoker | 30 (21.4) |
| Ex-smoker | 54 (38.6) |
| Current smoker | 56 (40.0) |
| Histology | |
| Squamous cell carcinoma | 131 (93.6) |
| Others | 9 (6.4) |
| Neoadjuvant CCRT | 24 (17.1) |
| Tumour location | |
| Cervical | 2 (1.4) |
| Upper | 18 (12.9) |
| Middle | 86 (61.4) |
| Lower | 34 (24.3) |
| Clinical stage | |
| T category | |
| cT1 | 60 (45.7) |
| cT2 | 52 (37.1) |
| cT3 | 28 (20.0) |
| N category | |
| cN0 | 89 (63.6) |
| cN+ | 51 (36.4) |
| Variables . | n = 140 . |
|---|---|
| Age (years) | 65.1 ± 9.0 |
| Male gender | 125 (89.3) |
| Height (cm) | 164.7 ± 6.8 |
| Weight (kg) | 62.8 ± 10.5 |
| BMI (kg/m2) | 23.0 ± 3.1 |
| Pulmonary function test | |
| FVC (predicted %) | 100.7 ± 14.7 |
| FEV1 (predicted %) | 100.7 ± 18.6 |
| Comorbidity | |
| Hypertension | 62 (44.3) |
| Chronic obstructive pulmonary disease | 55 (39.2) |
| Diabetes mellitus | 25 (17.9) |
| History of malignancy in other organs | 18 (12.9) |
| History of pulmonary tuberculosis | 13 (9.3) |
| Stroke | 12 (8.6) |
| Coronary artery disease | 11 (7.9) |
| Peripheral vascular disease | 8 (5.7) |
| Atrial fibrillation | 7 (5.0) |
| Liver cirrhosis | 5 (3.5) |
| ECOG performance status | |
| 0 | 86 (61.4) |
| 1 | 54 (38.6) |
| Smoking status | |
| Never smoker | 30 (21.4) |
| Ex-smoker | 54 (38.6) |
| Current smoker | 56 (40.0) |
| Histology | |
| Squamous cell carcinoma | 131 (93.6) |
| Others | 9 (6.4) |
| Neoadjuvant CCRT | 24 (17.1) |
| Tumour location | |
| Cervical | 2 (1.4) |
| Upper | 18 (12.9) |
| Middle | 86 (61.4) |
| Lower | 34 (24.3) |
| Clinical stage | |
| T category | |
| cT1 | 60 (45.7) |
| cT2 | 52 (37.1) |
| cT3 | 28 (20.0) |
| N category | |
| cN0 | 89 (63.6) |
| cN+ | 51 (36.4) |
Categorical variables were represented as n (%) and continuous variables as mean ± standard deviation.
BMI: body mass index; CCRT: concurrent chemoradiation treatment; ECOG: Eastern Cooperative Oncology Group; FEV1: forced expiratory volume at 1 s; FVC: forced vital capacity.
| Variables . | n = 140 . |
|---|---|
| Age (years) | 65.1 ± 9.0 |
| Male gender | 125 (89.3) |
| Height (cm) | 164.7 ± 6.8 |
| Weight (kg) | 62.8 ± 10.5 |
| BMI (kg/m2) | 23.0 ± 3.1 |
| Pulmonary function test | |
| FVC (predicted %) | 100.7 ± 14.7 |
| FEV1 (predicted %) | 100.7 ± 18.6 |
| Comorbidity | |
| Hypertension | 62 (44.3) |
| Chronic obstructive pulmonary disease | 55 (39.2) |
| Diabetes mellitus | 25 (17.9) |
| History of malignancy in other organs | 18 (12.9) |
| History of pulmonary tuberculosis | 13 (9.3) |
| Stroke | 12 (8.6) |
| Coronary artery disease | 11 (7.9) |
| Peripheral vascular disease | 8 (5.7) |
| Atrial fibrillation | 7 (5.0) |
| Liver cirrhosis | 5 (3.5) |
| ECOG performance status | |
| 0 | 86 (61.4) |
| 1 | 54 (38.6) |
| Smoking status | |
| Never smoker | 30 (21.4) |
| Ex-smoker | 54 (38.6) |
| Current smoker | 56 (40.0) |
| Histology | |
| Squamous cell carcinoma | 131 (93.6) |
| Others | 9 (6.4) |
| Neoadjuvant CCRT | 24 (17.1) |
| Tumour location | |
| Cervical | 2 (1.4) |
| Upper | 18 (12.9) |
| Middle | 86 (61.4) |
| Lower | 34 (24.3) |
| Clinical stage | |
| T category | |
| cT1 | 60 (45.7) |
| cT2 | 52 (37.1) |
| cT3 | 28 (20.0) |
| N category | |
| cN0 | 89 (63.6) |
| cN+ | 51 (36.4) |
| Variables . | n = 140 . |
|---|---|
| Age (years) | 65.1 ± 9.0 |
| Male gender | 125 (89.3) |
| Height (cm) | 164.7 ± 6.8 |
| Weight (kg) | 62.8 ± 10.5 |
| BMI (kg/m2) | 23.0 ± 3.1 |
| Pulmonary function test | |
| FVC (predicted %) | 100.7 ± 14.7 |
| FEV1 (predicted %) | 100.7 ± 18.6 |
| Comorbidity | |
| Hypertension | 62 (44.3) |
| Chronic obstructive pulmonary disease | 55 (39.2) |
| Diabetes mellitus | 25 (17.9) |
| History of malignancy in other organs | 18 (12.9) |
| History of pulmonary tuberculosis | 13 (9.3) |
| Stroke | 12 (8.6) |
| Coronary artery disease | 11 (7.9) |
| Peripheral vascular disease | 8 (5.7) |
| Atrial fibrillation | 7 (5.0) |
| Liver cirrhosis | 5 (3.5) |
| ECOG performance status | |
| 0 | 86 (61.4) |
| 1 | 54 (38.6) |
| Smoking status | |
| Never smoker | 30 (21.4) |
| Ex-smoker | 54 (38.6) |
| Current smoker | 56 (40.0) |
| Histology | |
| Squamous cell carcinoma | 131 (93.6) |
| Others | 9 (6.4) |
| Neoadjuvant CCRT | 24 (17.1) |
| Tumour location | |
| Cervical | 2 (1.4) |
| Upper | 18 (12.9) |
| Middle | 86 (61.4) |
| Lower | 34 (24.3) |
| Clinical stage | |
| T category | |
| cT1 | 60 (45.7) |
| cT2 | 52 (37.1) |
| cT3 | 28 (20.0) |
| N category | |
| cN0 | 89 (63.6) |
| cN+ | 51 (36.4) |
Categorical variables were represented as n (%) and continuous variables as mean ± standard deviation.
BMI: body mass index; CCRT: concurrent chemoradiation treatment; ECOG: Eastern Cooperative Oncology Group; FEV1: forced expiratory volume at 1 s; FVC: forced vital capacity.
Operative data are presented in Table 2. The median total operation and thoracic procedure times were 455 and 180 min, respectively. Cervical and thoracic anastomoses were performed in 107 (76.4%) and 33 (23.6%) patients, respectively. R0 resection was achieved in 135 (96.4%) patients. Thoracic robotic procedures were performed in all patients, and a robotic abdominal procedure was applied in 83 (59.3%) patients. A scatter plot showing abdominal procedures is presented in Supplementary Material, Appendix S8. Upper mediastinal lymph node dissection along the RLN was performed in 120 (85.7%) patients, and 3-field lymph node dissection was performed in 17 (12.1%) patients. The mean number of harvested lymph nodes was 41 ± 18.
| Variables . | n = 140 . |
|---|---|
| Total operation time (min) | 468 ± 96 |
| Thoracic procedure time (min) | 186 ± 61 |
| Anastomosis | |
| Cervical | 107 (76.4) |
| Thoracic | 33 (23.6) |
| Abdominal approach | |
| Laparotomy | 57 (40.7) |
| Robotic | 83 (59.3) |
| Conduit | |
| Stomach | 136 (97.1) |
| Colon | 4 (2.9) |
| Lymph node dissection | |
| 2-field/3-field | 123 (87.9)/17 (12.1) |
| RLN lymph node dissection | 120 (85.7) |
| Number of harvested lymph nodes | 41 ± 18 |
| R0 resection | 135 (96.4) |
| Pathological stage | |
| Complete remission | 10 (7.1) |
| I | 64 (45.7) |
| II | 34 (24.3) |
| III | 32 (22.9) |
| Variables . | n = 140 . |
|---|---|
| Total operation time (min) | 468 ± 96 |
| Thoracic procedure time (min) | 186 ± 61 |
| Anastomosis | |
| Cervical | 107 (76.4) |
| Thoracic | 33 (23.6) |
| Abdominal approach | |
| Laparotomy | 57 (40.7) |
| Robotic | 83 (59.3) |
| Conduit | |
| Stomach | 136 (97.1) |
| Colon | 4 (2.9) |
| Lymph node dissection | |
| 2-field/3-field | 123 (87.9)/17 (12.1) |
| RLN lymph node dissection | 120 (85.7) |
| Number of harvested lymph nodes | 41 ± 18 |
| R0 resection | 135 (96.4) |
| Pathological stage | |
| Complete remission | 10 (7.1) |
| I | 64 (45.7) |
| II | 34 (24.3) |
| III | 32 (22.9) |
Categorical variables were represented as n (%) and continuous variables as mean ± standard deviation.
RLN: recurrent laryngeal nerve.
| Variables . | n = 140 . |
|---|---|
| Total operation time (min) | 468 ± 96 |
| Thoracic procedure time (min) | 186 ± 61 |
| Anastomosis | |
| Cervical | 107 (76.4) |
| Thoracic | 33 (23.6) |
| Abdominal approach | |
| Laparotomy | 57 (40.7) |
| Robotic | 83 (59.3) |
| Conduit | |
| Stomach | 136 (97.1) |
| Colon | 4 (2.9) |
| Lymph node dissection | |
| 2-field/3-field | 123 (87.9)/17 (12.1) |
| RLN lymph node dissection | 120 (85.7) |
| Number of harvested lymph nodes | 41 ± 18 |
| R0 resection | 135 (96.4) |
| Pathological stage | |
| Complete remission | 10 (7.1) |
| I | 64 (45.7) |
| II | 34 (24.3) |
| III | 32 (22.9) |
| Variables . | n = 140 . |
|---|---|
| Total operation time (min) | 468 ± 96 |
| Thoracic procedure time (min) | 186 ± 61 |
| Anastomosis | |
| Cervical | 107 (76.4) |
| Thoracic | 33 (23.6) |
| Abdominal approach | |
| Laparotomy | 57 (40.7) |
| Robotic | 83 (59.3) |
| Conduit | |
| Stomach | 136 (97.1) |
| Colon | 4 (2.9) |
| Lymph node dissection | |
| 2-field/3-field | 123 (87.9)/17 (12.1) |
| RLN lymph node dissection | 120 (85.7) |
| Number of harvested lymph nodes | 41 ± 18 |
| R0 resection | 135 (96.4) |
| Pathological stage | |
| Complete remission | 10 (7.1) |
| I | 64 (45.7) |
| II | 34 (24.3) |
| III | 32 (22.9) |
Categorical variables were represented as n (%) and continuous variables as mean ± standard deviation.
RLN: recurrent laryngeal nerve.
The postoperative outcomes are presented in Table 3. Thirty- and 90-day operation-related deaths occurred in 1 (0.7%) patient and 5 (3.6%) patients, respectively. The cause of death within 30 days was toxic colitis caused by Clostridium difficile on postoperative Day 8. The causes of death within 90 days were respiratory failure by acute respiratory distress syndrome in 1 patient and fungal sepsis after recovery from acute respiratory distress syndrome in 1 patient. Overall, 3 (2.1%) patients died during postoperative hospitalization, and 2 additional patients died after discharge due to a delayed trachea-conduit fistula. One patient died of disseminated metastatic disease leading to hypercalcaemia after an uneventful discharge within 90 days after the operation. The overall postoperative complication rate was 57.8%, and the major complication rate was 14.3%.
| Variables . | n = 140 . |
|---|---|
| Mortality | |
| 30-day (operation-related) | 1 (0.7) |
| 90-day (operation-related) | 5 (3.6) |
| 90-day (all-cause) | 6 (4.3) |
| Length of stay (days) | |
| Mean ± standard deviation | 20.1 ± 20.5 |
| Median (lower quartile, upper quartile) | 14 (10, 19) |
| Reoperation within 90 days | 5 (3.6) |
| Exploration or drainage for anastomotic leak | 3 (2.1) |
| Ischaemia of colon | 1 (0.7) |
| Delayed tracheoconduit fistula | 1 (0.7) |
| Bleeding | 0 (0.0) |
| Conduit takedown | 0 (0.0) |
| Overall morbidity | 81 (57.9) |
| Respiratory complication | 12 (8.8) |
| Vocal cord palsy | 35 (25.0) |
| Type I | 17 (12.1) |
| Type II | 15 (10.7) |
| Type III | 3 (2.1) |
| Anastomotic leak | 13 (9.3) |
| Type I | 7 (5.0) |
| Type II | 3 (2.1) |
| Type III | 3 (2.1) |
| Conduit necrosis | 0 (0.0) |
| Othersa | 40 (28.6) |
| Variables . | n = 140 . |
|---|---|
| Mortality | |
| 30-day (operation-related) | 1 (0.7) |
| 90-day (operation-related) | 5 (3.6) |
| 90-day (all-cause) | 6 (4.3) |
| Length of stay (days) | |
| Mean ± standard deviation | 20.1 ± 20.5 |
| Median (lower quartile, upper quartile) | 14 (10, 19) |
| Reoperation within 90 days | 5 (3.6) |
| Exploration or drainage for anastomotic leak | 3 (2.1) |
| Ischaemia of colon | 1 (0.7) |
| Delayed tracheoconduit fistula | 1 (0.7) |
| Bleeding | 0 (0.0) |
| Conduit takedown | 0 (0.0) |
| Overall morbidity | 81 (57.9) |
| Respiratory complication | 12 (8.8) |
| Vocal cord palsy | 35 (25.0) |
| Type I | 17 (12.1) |
| Type II | 15 (10.7) |
| Type III | 3 (2.1) |
| Anastomotic leak | 13 (9.3) |
| Type I | 7 (5.0) |
| Type II | 3 (2.1) |
| Type III | 3 (2.1) |
| Conduit necrosis | 0 (0.0) |
| Othersa | 40 (28.6) |
Categorical variables were represented as n (%) and continuous variables as mean ± standard deviation or median with range.
Other morbidities included chyle leak, atrial fibrillation, acute kidney injury, wound problem and delirium.
| Variables . | n = 140 . |
|---|---|
| Mortality | |
| 30-day (operation-related) | 1 (0.7) |
| 90-day (operation-related) | 5 (3.6) |
| 90-day (all-cause) | 6 (4.3) |
| Length of stay (days) | |
| Mean ± standard deviation | 20.1 ± 20.5 |
| Median (lower quartile, upper quartile) | 14 (10, 19) |
| Reoperation within 90 days | 5 (3.6) |
| Exploration or drainage for anastomotic leak | 3 (2.1) |
| Ischaemia of colon | 1 (0.7) |
| Delayed tracheoconduit fistula | 1 (0.7) |
| Bleeding | 0 (0.0) |
| Conduit takedown | 0 (0.0) |
| Overall morbidity | 81 (57.9) |
| Respiratory complication | 12 (8.8) |
| Vocal cord palsy | 35 (25.0) |
| Type I | 17 (12.1) |
| Type II | 15 (10.7) |
| Type III | 3 (2.1) |
| Anastomotic leak | 13 (9.3) |
| Type I | 7 (5.0) |
| Type II | 3 (2.1) |
| Type III | 3 (2.1) |
| Conduit necrosis | 0 (0.0) |
| Othersa | 40 (28.6) |
| Variables . | n = 140 . |
|---|---|
| Mortality | |
| 30-day (operation-related) | 1 (0.7) |
| 90-day (operation-related) | 5 (3.6) |
| 90-day (all-cause) | 6 (4.3) |
| Length of stay (days) | |
| Mean ± standard deviation | 20.1 ± 20.5 |
| Median (lower quartile, upper quartile) | 14 (10, 19) |
| Reoperation within 90 days | 5 (3.6) |
| Exploration or drainage for anastomotic leak | 3 (2.1) |
| Ischaemia of colon | 1 (0.7) |
| Delayed tracheoconduit fistula | 1 (0.7) |
| Bleeding | 0 (0.0) |
| Conduit takedown | 0 (0.0) |
| Overall morbidity | 81 (57.9) |
| Respiratory complication | 12 (8.8) |
| Vocal cord palsy | 35 (25.0) |
| Type I | 17 (12.1) |
| Type II | 15 (10.7) |
| Type III | 3 (2.1) |
| Anastomotic leak | 13 (9.3) |
| Type I | 7 (5.0) |
| Type II | 3 (2.1) |
| Type III | 3 (2.1) |
| Conduit necrosis | 0 (0.0) |
| Othersa | 40 (28.6) |
Categorical variables were represented as n (%) and continuous variables as mean ± standard deviation or median with range.
Other morbidities included chyle leak, atrial fibrillation, acute kidney injury, wound problem and delirium.
Efficacy outcomes and postoperative complications
The risk-adjusted O-E CUSUM curve demonstrated that the change point of the number of harvested lymph nodes was the 28th case. The mean number of harvested lymph nodes increased significantly from 25 to 45 (P < 0.001) after the 28th case (Fig. 1A). One other minor change point was identified on the O-E CUSUM curve. A temporary decrease was identified at the 106th case; it was related to active involvement of the patients who underwent neoadjuvant chemoradiation. The number of harvested lymph nodes increased consistently and did not reach a steady state for the whole study period.
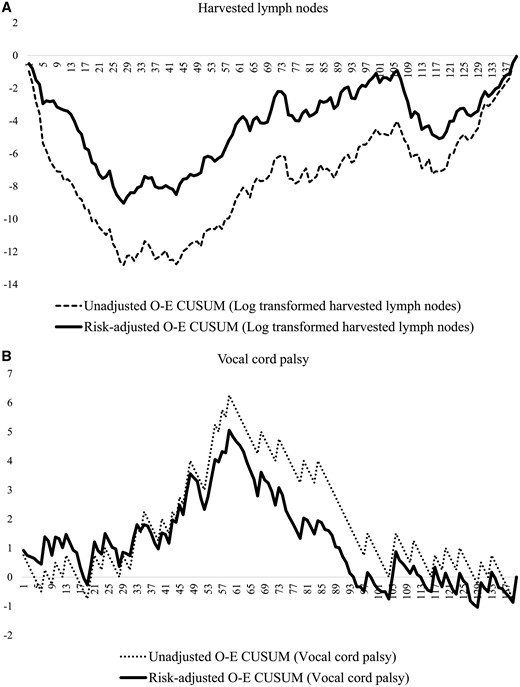
O-E CUSUM plots of the number of (A) harvested lymph nodes and (B) vocal cord palsy. O-E CUSUM: observed–expected cumulative sum.
The rate of vocal cord palsy decreased significantly from 36% to 17% (P < 0.001) around the 60th case (Fig. 1B). After an initial steady state, the rate of vocal cord palsy rate increased sharply around the 12th case, which is the point where we routinely started RLN lymph node dissection. Before the 60th case, the vocal cord palsy rate increased in proportion to the number of harvested lymph nodes. However, after the 60th case, the rate decreased dramatically.
Figure 2 demonstrates a notable change point in the risk-adjusted O-E CUSUM curves of total operative time and thoracic procedure time around the 80th case. Risk-adjusted O-E CUSUM curves showed patterns and change points that were similar to those of unadjusted O-E CUSUM curves, except thoracic procedure time. A significant reduction of the total operation time (496 vs 431 min, P < 0.001) was found at the 80th case (Fig. 2A); however, the thoracic procedure time (188 vs 182 min, P = 0.540) did not show a significant difference (Fig. 2B). The risk-adjusted O-E CUSUM curves for anastomotic leakage and length of hospital stay showed similar change points around the 85th case (Fig. 3), which were similar to those of the operative times. Significant reductions in the anastomotic leakage rate (15% vs 2%, P = 0.010) and length of hospital stay (24 days vs 14 days, P = 0.003) were identified at that point. However, respiratory complications developed consistently during the study period without a definite pattern (Fig. 4).
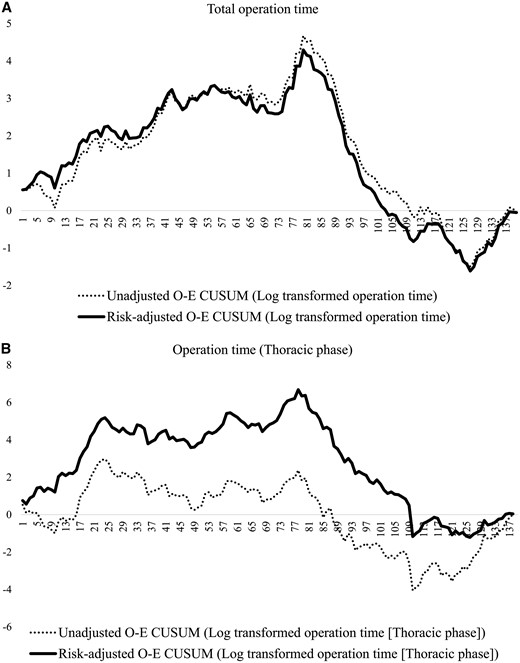
O-E CUSUN plots of total operation time (A) and thoracic operation time (B).
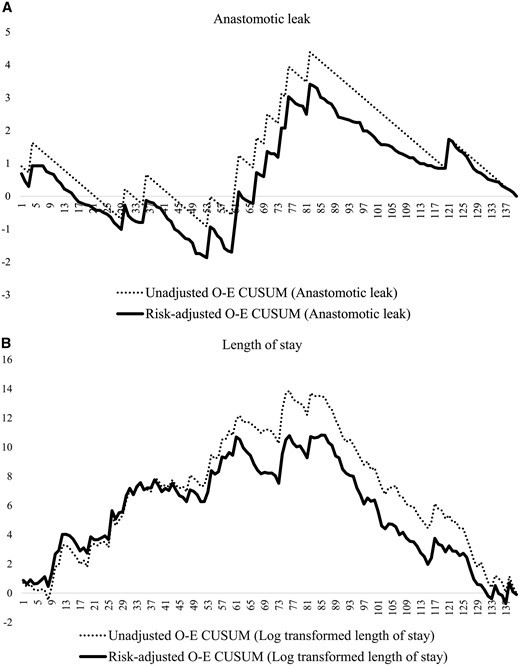
O-E CUSUM plots of (A) anastomotic leak and (B) length of hospital stay. O-E CUSUM: observed–expected cumulative sum.
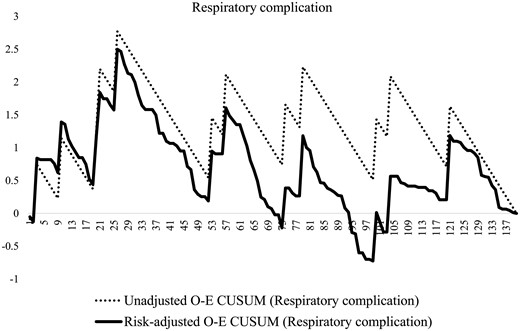
O-E CUSUM plots of respiratory complications. O-E CUSUM: observed–expected cumulative sum.
DISCUSSION
We present a learning curve study evaluating short-term outcome measures in RAMIE. Because the surgical indications for RAMIE changed gradually from low-risk patients with early-stage disease to high-risk patients with advanced stage disease, we used risk-adjusted O-E CUSUM curves to evaluate the proficiency gain of the surgeon by adjusting patient selection bias. The risk-adjusted O-E CUSUM curves showed patterns similar to those of the unadjusted O-E CUSUM curves, which represent a significant impact of the learning period on postoperative outcome improvement in RAMIE regardless of potential risk factors. We identified 3 notable change points; the first point was found around 30 cases for the number of harvested lymph nodes, the second point was found around 60 cases for vocal cord palsy and the third point was found around 80 cases for total operation time, anastomotic leakage rate and length of hospital stay (Table 4). Improvement of each outcome measure was achieved at different levels of experience; therefore, overall improvement of surgical outcomes is expected to require a significant learning period for RAMIE for treatment of oesophageal cancer.
| Variables . | Change pointsa . | Before vs after cut-off point case . | P-value . |
|---|---|---|---|
| Total operation time (min) | 80 | 496 vs 431 | <0.001 |
| Thoracic procedure time (min) | 78 | 188 vs 182 | 0.540 |
| Length of stay (days) | 85 | 24 vs 14 | 0.003 |
| Resected lymph node (number) | 28 | 25 vs 45 | <0.001 |
| Complications | |||
| Vocal cord palsy rate | 59 | 36% vs 17% | 0.013 |
| Anastomotic leakage rate | 82 | 15% vs 2% | 0.010 |
| Respiratory complication rate | 21 | 19% vs 7% | 0.083 |
| Variables . | Change pointsa . | Before vs after cut-off point case . | P-value . |
|---|---|---|---|
| Total operation time (min) | 80 | 496 vs 431 | <0.001 |
| Thoracic procedure time (min) | 78 | 188 vs 182 | 0.540 |
| Length of stay (days) | 85 | 24 vs 14 | 0.003 |
| Resected lymph node (number) | 28 | 25 vs 45 | <0.001 |
| Complications | |||
| Vocal cord palsy rate | 59 | 36% vs 17% | 0.013 |
| Anastomotic leakage rate | 82 | 15% vs 2% | 0.010 |
| Respiratory complication rate | 21 | 19% vs 7% | 0.083 |
Change points were identified in risk-adjusted observed–expected cumulative sum curves.
| Variables . | Change pointsa . | Before vs after cut-off point case . | P-value . |
|---|---|---|---|
| Total operation time (min) | 80 | 496 vs 431 | <0.001 |
| Thoracic procedure time (min) | 78 | 188 vs 182 | 0.540 |
| Length of stay (days) | 85 | 24 vs 14 | 0.003 |
| Resected lymph node (number) | 28 | 25 vs 45 | <0.001 |
| Complications | |||
| Vocal cord palsy rate | 59 | 36% vs 17% | 0.013 |
| Anastomotic leakage rate | 82 | 15% vs 2% | 0.010 |
| Respiratory complication rate | 21 | 19% vs 7% | 0.083 |
| Variables . | Change pointsa . | Before vs after cut-off point case . | P-value . |
|---|---|---|---|
| Total operation time (min) | 80 | 496 vs 431 | <0.001 |
| Thoracic procedure time (min) | 78 | 188 vs 182 | 0.540 |
| Length of stay (days) | 85 | 24 vs 14 | 0.003 |
| Resected lymph node (number) | 28 | 25 vs 45 | <0.001 |
| Complications | |||
| Vocal cord palsy rate | 59 | 36% vs 17% | 0.013 |
| Anastomotic leakage rate | 82 | 15% vs 2% | 0.010 |
| Respiratory complication rate | 21 | 19% vs 7% | 0.083 |
Change points were identified in risk-adjusted observed–expected cumulative sum curves.
The benefits of MIE over open oesophagectomy in oesophageal cancer have been widely reported with reduced pneumonia and a fair quality of life [1, 3]. Although not proven in a randomized controlled study, equivalent survival times between MIE and open oesophagectomy have been suggested [16]. MIE is now a major technique in oesophagectomy and is being implemented worldwide [9, 17, 18]. However, it is questionable whether one can demonstrate comparable outcomes with MIE using a large-scale multicentre database because of the short history of MIE. The heterogeneous outcomes from centres with different levels of experience may not be comparable to well-controlled outcomes from experienced centres. A longer operation time and higher reoperation rate of MIE compared to open oesophagectomy have been reported because of the learning curve [9, 17, 18]. Oesophagectomy, irrespective of a minimally invasive or open technique, requires a significant learning period and sufficient case volume to obtain competent short-term and long-term outcomes [19–22]. Moreover, surgeon volume has a more significant impact on postoperative outcomes than hospital volume [21, 23, 24]. Therefore, the experience of the surgeon is becoming more and more important, and the centralization issue has been raised by nationwide researchers [19, 25, 26].
We tried to identify the effect of the learning curve on each postoperative outcome by analysing the initial result of RAMIE in this study. We started RAMIE in 2008 and included all consecutive cases of RAMIE from the first case in this study. However, because we were already conducting VATS MIE with competent early outcomes at that time, a higher level goal was necessary to start a new surgical programme. We set a primary goal of RAMIE as a complete upper mediastinal lymph node dissection, especially along the bilateral RLNs, because dissection along the bilateral RLN by VATS is technically demanding, and incomplete dissection for fear of causing vocal cord palsy is common. The benefits of RAMIE include the ability to perform complex procedures with improved visualization and facilitation of meticulous dissection of the oesophagus and lymph nodes. In terms of oncological completeness, we achieved a high R0 resection rate and a sufficient number of lymph nodes from upper mediastinal lymphadenectomy, which is comparable to the results of VATS MIE [1, 12, 14]. However, the actual practice of RAMIE was not always the same as our expectation. In the first 8 cases, the surgeon were unable to perform the upper mediastinal lymph node dissection along the RLNs. We needed time to become accustomed to the robotic surgical system to understand the basic mechanism, optimize port sites and devices and train assistant surgeons. After we started upper mediastinal lymph node dissection, the number of dissected lymph nodes did not increase for a while; instead, the vocal cord palsy rate increased sharply from the 20th case. This result represents a learning period with adverse damage to the RLNs without improving the lymph node yield. The risk-adjusted O-E CUSUM curve demonstrated that the lymph node yield was improved from the 28th case and continued to improve. This phenomenon coincides well with other reports in the literature showing that the yield of lymph nodes increased continuously for hundreds of cases and that a significant volume of cases was required to achieve maximal lymph node yield [22].
Vocal cord palsy and subsequent aspiration pneumonia were the most concerning complications from RLN lymph node dissection. In our series, the incidence of vocal cord palsy increased in proportion to the number of harvested lymph nodes until the 63rd case, meaning that increased radical lymph node dissection was likely related to an increased risk of nerve damage. However, after the 63rd case, the vocal cord palsy rate decreased dramatically although the number of dissected lymph nodes increased persistently. We think that this was the point where proficiency for nerve protection was reached. Better visualization by the 4-arm technique, avoiding blind grasping of perineural tissue, minimizing electrothermal damage, avoiding completely skeletonizing the RLN and leaving some perineural tissue around the RLNs to preserve neural blood flow were the major technical modifications during this period. Suda et al. [27] also reported that the vocal cord palsy rate could be significantly reduced by the robotic technique compared with VATS MIE during RLN lymph node dissection. Our results also support the idea that improved lymph node yield with reduced RLN injury could be possible with RAMIE after sufficient experience was gained in robot-assisted RLN lymph node dissection. Interestingly, vocal cord palsy does not appear to be highly correlated with respiratory complications, suggesting that it may be mitigated by a postoperative management plan comprising active surveillance of vocal cord injury and aggressive intervention to prevent aspiration.
Previous studies of RAMIE reported a variable operative time from 6 to 8 h, similar to the results in this study [4, 28]. We reviewed the overall process of the operations and tried to minimize any unnecessary delays that would increase the operative time. We also started intrathoracic anastomosis for distal oesophageal cancer to avoid the need for additional cervical incision. All these changes contributed to the reduction of the operation time after the 80th case. After stabilizing the vocal cord palsy rate, the overall management protocol was modified to reduce anastomosis leakage beginning at the 80th case.
The reoperation rate in this study was 3.5%, which was much lower than that of previously reported large-scale studies (7.0–9.9%) derived from comparison studies between open and VATS MIE [9, 17, 18]. Furthermore, there was no conduit necrosis or postoperative bleeding requiring reoperation in our study. These findings suggest the ability of the robot system to safely handle the graft and control the bleeding focus meticulously. Our results were partly dependent on magnified 3D visualization and freely controllable devices of the robotic system.
Limitations
This study comprised a relatively small number of cases in a single centre, and we set the goal for oncological completeness first and then moved to improvement of early outcomes. Furthermore, we did not demonstrate oncological outcomes of survival and recurrence in this study. Therefore, the results should be taken cautiously in terms of generalization to other centres.
Our study suggested a significantly large case volume compared with that for open oesophagectomy [29]. In general, minimally invasive surgery required a long learning period compared with traditional open surgery. Furthermore, a decrease in postoperative deaths was achieved in fewer cases than were necessary to determine other oncological outcomes and quality measurements including operative times, lymph nodes yield, long-term survival and postoperative morbidities [4, 22].
To explore the learning effect on operative and postoperative outcome measures, we defined the change point of each outcome as the point showing the largest peak in the O-E CUSUM plot. O-E CUSUM plots could show intuitively valuable ways to recognize changes in performance [15]. However, the arbitrary use of the change point in O-E CUSUM has a weak statistical power, and the change points should be interpreted with caution [30].
The robotic platform is an extremely expensive system to be adopted worldwide, and its cost-effectiveness should be evaluated as well. However, considering that upper mediastinal lymph node metastasis is common in ESCC and that complete dissection of lymph nodes along the RLN is important for accurate staging and reducing regional recurrence, achieving complete upper mediastinal lymphadenectomy with RAMIE could be effective for ESCC.
CONCLUSIONS
To our knowledge, this is the first study demonstrating the volume of cases needed to attain stabilized short-term outcomes after RAMIE. We identified the effect of the learning curve for RAMIE in oesophageal cancer and the improvement of postoperative outcome measures achieved at different numbers of cases. In conclusion, RAMIE showed an acceptable rate of mortality and morbidity, and a significantly large number of cases was required for overall improvement of surgical outcomes in oesophageal cancer.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
ACKNOWLEDGEMENTS
The authors appreciated the statistical advice from the Medical Research Collaborating Center at the Seoul National University Hospital and the Seoul National University College of Medicine.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr I. Opitz(Zürich, Switzerland): Can you comment on this very high number of turnover point of the learning curve at 80 procedures for most of your variables? Is this comparable to open oesophageal surgery or other video thoracoscopic minimally invasive resections? Are there any data from the literature?
Dr S. Park(Seoul, Korea): The 80 cases is a cut-off value of the total operation time, so we thought that in case of other quality measures it was lower than that. According to the previous literature, mostly in open oesophagectomy, around 30–35 cases were necessary for learning period. We think that the number for learning period in robotic oesophagectomy was higher than other gastrointerstinal (GI) oncological surgery because of the difficulty to implement upper mediastinal lymph node dissection. We thought that larger number of cases is required because of the complexity of oesophagectomy and mediastinal lymph node dissection.
Dr Opitz: But this is a little bit in contrast, for example, for to robotic surgery for lung cancer, where the learning curve is actually flatter compared with open or minimal invasive procedure. How do you explain this?
Dr G. Rocco(Naples, Italy): I’m sure you will come up with an answer during the coffee break. Let’s move on.
Author notes
Presented at the 25th European Conference on General Thoracic Surgery, Innsbruck, Austria, 28-31 May 2017.




