-
PDF
- Split View
-
Views
-
Cite
Cite
Isabelle Moneke, Jussuf T Kaifi, Raphael Kloeser, Patrick Samson, Benedikt Haager, Sebastian Wiesemann, Sven Diederichs, Bernward Passlick, Pulmonary metastasectomy for thyroid cancer as salvage therapy for radioactive iodine-refractory metastases, European Journal of Cardio-Thoracic Surgery, Volume 53, Issue 3, March 2018, Pages 625–630, https://doi.org/10.1093/ejcts/ezx367
Close - Share Icon Share
Abstract
Distant metastasis arising from thyroid cancer is rare but has been associated with significantly reduced long-term survival, especially when refractory to radioactive iodine ablation. We provide one of the largest studies worldwide reporting the outcome after salvage pulmonary metastasectomy for this entity, aiming to identify prognostic factors and to analyse surgical indication.
We retrospectively analysed the medical records of 43 patients who had undergone pulmonary metastasectomy for radioactive iodine-refractory thyroid cancer from 1985 to 2016.
The median follow-up period was 77 (95% confidence interval 41–113) months. Twenty-three (53%) patients were alive at the time of analysis. The majority of tumours were follicular thyroid cancer by histology, with 23% identified as Hurthle cell subtype. Five- and 10-year disease-specific (DS) survival was 84% and 59%, respectively. Thirty-one (72%) patients underwent R0-resection with a 5- and 10-year DS survival of 100% and 77%, respectively. This was significantly reduced to 62% and 22% (P = 0.013) in case of incomplete resection, respectively. Ten years after R0-metastasectomy, 17 (55%) patients were recurrence-free. Systematic mediastinal lymphadenectomy was performed in 16 (37%) patients and was associated with improved long-term DS survival (10 years 88% vs 46%, P = 0.034). Moreover, a reduction of > 80% in serum thyroglobulin levels post-metastasectomy correlates with better long-term DS survival (10 years 81% vs 36%, P = 0.007).
Pulmonary metastasectomy is associated with good survival for selected patients with radioactive iodine-refractory metastases of differentiated thyroid cancer, especially if R0-resection can be achieved. Moreover, it is worth considering whether a significant reduction of tumour load, as indicated by thyroglobulin serum levels, seems possible.
INTRODUCTION
Thyroid cancer is the most common endocrine malignancy worldwide, and its prevalence has been steadily increasing [1]. The primary treatment consists of surgery, selective postoperative radioiodine ablation therapy and thyroid-stimulating hormone-suppressive therapy [2]. The overall prognosis of differentiated thyroid cancer is very good with a 10-year survival of 85–93% [3]. Only 4–23% of patients develop distant metastases, with the lung being the most common site [4]. Distant metastasis, however, has been associated with significantly reduced long-term survival [3], accounting for a major part of cancer-specific deaths. Up-to-date radioactive iodine ablation is the mainstay treatment for metastatic-differentiated thyroid cancer [5]. Several factors such as tumour histopathology, age at diagnosis, site and size of metastases or visible radioactive iodine uptake on diagnostic whole-body scan have been demonstrated to play a critical role in its effectiveness [5]. It is, however, not always a curative treatment with more than half of the patients showing progressive disease over time [6]. For radioactive iodine ablation-refractory metastasis, the 10-year survival rate is only about 10–18% [5, 7]. Chemotherapy and external beam radiation therapy have yet to show promising results [7], and new molecular therapies are still being developed. Thus, if radioactive iodine ablation is not or no longer effective, surgical resection of pulmonary metastases can be offered to selected patients as a salvage option.
Back in 1947, Alexander and Haight [8, 9] were the first to report pulmonary metastasectomy as a treatment option and proposed the first selection criteria for surgery. They were modified by Thomford et al. [10] in 1965, and the development of new techniques steadily opens up new possibilities. Numerous retrospective studies have been conducted since then, including the hallmark study of the International Registry of Lung Metastases, which demonstrated pulmonary metastasectomy [9, 11] to be potentially curative.
The benefit of systematic mediastinal lymph node dissection in the context of pulmonary metastasectomy remains controversial throughout the literature, and the extent of it often varies between institutions [9, 12]. As shown in a survey conducted on behalf of the European Society of Thoracic Surgeons (ESTS) Pulmonary Metastasectomy Working Group in 2008, in most of the European institutions, only a lymph node sampling is done [13, 14]. Whether there lies an actual survival benefit in the removal of the nodes themselves or whether it serves a as purely diagnostic means to better plan adjuvant treatment for these patients remains to be further elucidated [9, 14]. Most authors mentioned in the studies above believe the latter. So far, little is known about the outcome after thoracic metastasectomy and systematic mediastinal lymphadenectomy in thyroid cancer.
The predictive value of serum thyroglobulin (TG) concentrations post-thyroidectomy for persistent disease, metastasis or tumour recurrence has been reported in multiple studies [15]. It is a very important diagnostic tool in the follow-up of patients with differentiated thyroid cancer [16].
To date, there are very few studies with small cohorts of patients reporting the outcome after pulmonary metastasectomy for differentiated thyroid cancer [17–19]. By histology, most of the reported cases were papillary, while at our institution, the majority of cases were follicular. The aim of our study was to analyse the overall outcome after salvage metastasectomy for radioactive iodine ablation-refractory pulmonary metastases with regard to patient profile, surgical extent and technique, disease-free survival and potential predictors for long-term survival.
PATIENTS AND METHODS
Patients
We performed a retrospective analysis of the database of medical records at the Department of Thoracic Surgery, Medical Center–University of Freiburg, a tertiary care cancer centre. The survival data were obtained from the above-mentioned hospital records and the cancer registry of the Comprehensive Cancer Center Freiburg (CCCF). We identified a total of 43 patients (19 men and 24 women) who had undergone pulmonary metastasectomy for differentiated thyroid cancer with curative intent from 1985 to 2016. Patients presenting with metastases to other organs without curative treatment option and patients with malignant pleural effusion or a known second malignancy at the time of metastasectomy were excluded.
According to the World Health Organization (WHO) classification, Hurthle cell thyroid cancer is considered as a subtype of follicular thyroid cancer [20]. In this study, there was no statistically significant difference between the overall outcomes of the 2 groups or any other of the analysed factors. They were therefore considered as one group.
All patients had undergone initial surgical therapy of the primary tumour followed by radioactive iodine ablation therapy. If metastases turned out to be fully or partly refractory to treatment, patients were considered for pulmonary metastasectomy to remove the remaining tumour. Pulmonary function was analysed to be compatible with the intended procedure. Anterolateral thoracotomy or video-assisted thoracoscopy was performed for resection of metastases by lung-sparing procedures, except in 2 cases where a lobectomy was performed. In 5 of the older cases with confirmed mediastinal masses, partial sternotomy was done. Staged thoracotomy was done for bilateral metastases in all cases but 2, where bilateral thoracotomy was performed during 1 session. Follow-up consisted of a regular clinical examination, blood markers (e.g. thyroid-stimulating hormone and TG), thyroid ultrasonography and whole-body scintigraphy. In case of elevated serum TG values with negative whole-body scintigraphy, a chest computed tomography scan or F-18-fluorodeoxyglucose–positron emission tomography/computed tomography scan was done to detect radioactive iodine-refractory metastases.
Definitions
The cause of death of the patients was analysed going through hospital records and the data provided by the cancer registry of the CCCF and divided into disease-specific (DS) death and death related to other causes, when clearly stated in the records. However carefully done, there is always a certain bias as the lines between disease-related death and death related to other causes are sometimes blurred. Survival was defined as the interval between pulmonary metastasectomy and the last follow-up or event. If the metastases could be completely removed, the resection status was defined as R0, in case of incomplete removal it was defined as R1, and in case the patient decided against contralateral surgery after 1 side had been completed it was categorized as R2.
In case of staged thoracotomy, systematic lymphadenectomy was only counted as such if performed on both sides. Metastases already present at the time of detection of the primary thyroid tumour were defined as synchronous, while metastases developing more than 3 months after the treatment of the primary tumour was completed were defined as metachronous. Complications arising within 30 days of surgery were defined as perioperative. Individual consent for the study was obtained from all patients, and the study was approved by the local ethics committee of the Medical Center–University of Freiburg. It is registered at the German Registry for Clinical Trials under the trial registration number 5100009230.
Statistical analysis
Survival was estimated using the Kaplan–Meier method [21]. The log-rank test was used for comparison of survival curves. After determining the prognostic factors with univariate analysis, multivariate analysis using the Cox proportional hazards regression model was carried out. The threshold for statistical significance was a P-value of <0.05. The threshold significance level in the univariate analysis for inclusion in the multivariate analysis was also a P-value of <0.05. All statistical analyses were conducted using GraphPadPrism (Version 7, GraphPad Software Inc., La Jolla, CA, USA) and SPSS software (Version 23, IBM Corporation, New York, NY, USA).
RESULTS
A total of 43 patients (19 men and 24 women) underwent pulmonary metastasectomy at our institution from January 1985 to January 2016 (Table 1). The median follow-up period was 77 (95% confidence interval 41–113) months. Twenty-three (53%) patients were alive at the time of analysis. The overall 5- and 10-year DS survival post-metastasectomy were 84% and 56%, respectively, with a median of 143 (95% confidence interval 107–179) months (Fig. 1). The median age at initial diagnosis with differentiated thyroid cancer was 57 (range 10–77) years, and the median age at the time of pulmonary metastasectomy was 66 (range 17–79) years. There was a median interval of 96 (range 0–381) months between thyroidectomy and metastasectomy. In terms of tumour histopathology, 35 (81%) patients were follicular, with 8 (23%) of them showing a Hurthle cell subtype. Eight (19%) patients were papillary.
| Variables . | n (%) . |
|---|---|
| Gender | |
| Male | 19 (44) |
| Female | 24 (56) |
| Histopathology | |
| Follicular−Hurthle cell | 35 (81)−8 (23) |
| Papillary | 8 (19) |
| TNM stage of primary tumour <45 years | |
| I (M0) | 8 (19) |
| II (M1) | 2 (5) |
| TNM stage of primary tumour >45 years | |
| I–III (M0) | 16 (37) |
| IV (M1) | 17 (40) |
| Median age at initial diagnosis (years) | 57 (range 10–77) |
| Median age at metastasectomy (years) | 66 (range 17–79) |
| Median interval until metastasectomy (months) | 96 (range 0–381) |
| Pulmonary metastases location | |
| Bilateral | 31 (72) |
| Positive lymph nodes | 9 (21) |
| Median number of metastases | 3 (range 1–60) |
| Median size of metastases in cm | 1 (range 0.3–10)a |
| Variables . | n (%) . |
|---|---|
| Gender | |
| Male | 19 (44) |
| Female | 24 (56) |
| Histopathology | |
| Follicular−Hurthle cell | 35 (81)−8 (23) |
| Papillary | 8 (19) |
| TNM stage of primary tumour <45 years | |
| I (M0) | 8 (19) |
| II (M1) | 2 (5) |
| TNM stage of primary tumour >45 years | |
| I–III (M0) | 16 (37) |
| IV (M1) | 17 (40) |
| Median age at initial diagnosis (years) | 57 (range 10–77) |
| Median age at metastasectomy (years) | 66 (range 17–79) |
| Median interval until metastasectomy (months) | 96 (range 0–381) |
| Pulmonary metastases location | |
| Bilateral | 31 (72) |
| Positive lymph nodes | 9 (21) |
| Median number of metastases | 3 (range 1–60) |
| Median size of metastases in cm | 1 (range 0.3–10)a |
n = 41, no information regarding the size of metastases for 2 patients.
| Variables . | n (%) . |
|---|---|
| Gender | |
| Male | 19 (44) |
| Female | 24 (56) |
| Histopathology | |
| Follicular−Hurthle cell | 35 (81)−8 (23) |
| Papillary | 8 (19) |
| TNM stage of primary tumour <45 years | |
| I (M0) | 8 (19) |
| II (M1) | 2 (5) |
| TNM stage of primary tumour >45 years | |
| I–III (M0) | 16 (37) |
| IV (M1) | 17 (40) |
| Median age at initial diagnosis (years) | 57 (range 10–77) |
| Median age at metastasectomy (years) | 66 (range 17–79) |
| Median interval until metastasectomy (months) | 96 (range 0–381) |
| Pulmonary metastases location | |
| Bilateral | 31 (72) |
| Positive lymph nodes | 9 (21) |
| Median number of metastases | 3 (range 1–60) |
| Median size of metastases in cm | 1 (range 0.3–10)a |
| Variables . | n (%) . |
|---|---|
| Gender | |
| Male | 19 (44) |
| Female | 24 (56) |
| Histopathology | |
| Follicular−Hurthle cell | 35 (81)−8 (23) |
| Papillary | 8 (19) |
| TNM stage of primary tumour <45 years | |
| I (M0) | 8 (19) |
| II (M1) | 2 (5) |
| TNM stage of primary tumour >45 years | |
| I–III (M0) | 16 (37) |
| IV (M1) | 17 (40) |
| Median age at initial diagnosis (years) | 57 (range 10–77) |
| Median age at metastasectomy (years) | 66 (range 17–79) |
| Median interval until metastasectomy (months) | 96 (range 0–381) |
| Pulmonary metastases location | |
| Bilateral | 31 (72) |
| Positive lymph nodes | 9 (21) |
| Median number of metastases | 3 (range 1–60) |
| Median size of metastases in cm | 1 (range 0.3–10)a |
n = 41, no information regarding the size of metastases for 2 patients.
![Overall and DS survival post-pulmonary metastasectomy. (A) Overall survival: 5-year survival 75% and 10-year survival 48%. Median survival was 123 [95% confidence interval (CI) 70–176] months. (B) DS survival: 5-year survival 84% and 10-year survival 56%. Median survival was 143 (95% CI 107–179) months. DS: disease specific.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/53/3/10.1093_ejcts_ezx367/1/m_ezx367f1.jpeg?Expires=1747854143&Signature=lQCN~7SNIVKpjvBxdM1H5Kwcd0YAJeS54nUYAvzIqzlC6dbccIQLaoh3Ky-aStDvRtcFog~Jnc0z3W6Q9322x0fboqSfWn8O31Id2Hc~AkpXt60NA7qrNLxZEO7SJ1V2XgrB3bAmFfrI5DExXdhtk~T0kqTZiLPZJB97fSx2~no9ui7w93HQC4i0tYkv2jejq4AcxLna~Surx8wo67Utz4C2ONO8K0AYcf-4BVo2FU6XbhCDyxNvRCH0ZawcTDeNKcFqCTiDKZK2yj1oMewdcipP4z-GNLoVjr5xaYasdVZKueOIcYGwZzX3dh8EKqBHlzFy79WdbHpfQczP9L9rBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Overall and DS survival post-pulmonary metastasectomy. (A) Overall survival: 5-year survival 75% and 10-year survival 48%. Median survival was 123 [95% confidence interval (CI) 70–176] months. (B) DS survival: 5-year survival 84% and 10-year survival 56%. Median survival was 143 (95% CI 107–179) months. DS: disease specific.
The majority of the patients, that is 35 (81%), had undergone thoracotomy, with 5 (12%) patients having to undergo rethoracotomy for recurrence of metastases. Staged thoracotomy was performed in 29 (67%) cases for bilateral metastasis. In 2 cases, the patients opted against surgery for the contralateral side. Postoperative mortality was 0. Minor complications associated with surgery occurred in 7 (16%) patients (Table 2).
| Variables . | n (%) . |
|---|---|
| Surgical procedure | |
| Thoracotomy | 35 (81) |
| One-sided only | 4 (9) |
| Staged | 29 (67) |
| Bilateral | 2 (5) |
| Rethoracotomy | 5 (12) |
| Thoracoscopy | 6 (14) |
| Sternotomy | 5 (12) |
| Lymphadenectomy | |
| Systematic | 16 (37) |
| Sampling | 21 (49) |
| None | 6 (14) |
| Perioperative complications | |
| Transient atrial fibrillations | 3 (7) |
| Pneumonia | 1 (2) |
| Transient ischaemic attack | 2 (5) |
| Persistent pleural effusion | 1 (2) |
| Death | 0 (0.0) |
| None | 36 (84) |
| Variables . | n (%) . |
|---|---|
| Surgical procedure | |
| Thoracotomy | 35 (81) |
| One-sided only | 4 (9) |
| Staged | 29 (67) |
| Bilateral | 2 (5) |
| Rethoracotomy | 5 (12) |
| Thoracoscopy | 6 (14) |
| Sternotomy | 5 (12) |
| Lymphadenectomy | |
| Systematic | 16 (37) |
| Sampling | 21 (49) |
| None | 6 (14) |
| Perioperative complications | |
| Transient atrial fibrillations | 3 (7) |
| Pneumonia | 1 (2) |
| Transient ischaemic attack | 2 (5) |
| Persistent pleural effusion | 1 (2) |
| Death | 0 (0.0) |
| None | 36 (84) |
If a patient had undergone thoracotomy and sternotomy, both interventions are listed separately when performed separately.
| Variables . | n (%) . |
|---|---|
| Surgical procedure | |
| Thoracotomy | 35 (81) |
| One-sided only | 4 (9) |
| Staged | 29 (67) |
| Bilateral | 2 (5) |
| Rethoracotomy | 5 (12) |
| Thoracoscopy | 6 (14) |
| Sternotomy | 5 (12) |
| Lymphadenectomy | |
| Systematic | 16 (37) |
| Sampling | 21 (49) |
| None | 6 (14) |
| Perioperative complications | |
| Transient atrial fibrillations | 3 (7) |
| Pneumonia | 1 (2) |
| Transient ischaemic attack | 2 (5) |
| Persistent pleural effusion | 1 (2) |
| Death | 0 (0.0) |
| None | 36 (84) |
| Variables . | n (%) . |
|---|---|
| Surgical procedure | |
| Thoracotomy | 35 (81) |
| One-sided only | 4 (9) |
| Staged | 29 (67) |
| Bilateral | 2 (5) |
| Rethoracotomy | 5 (12) |
| Thoracoscopy | 6 (14) |
| Sternotomy | 5 (12) |
| Lymphadenectomy | |
| Systematic | 16 (37) |
| Sampling | 21 (49) |
| None | 6 (14) |
| Perioperative complications | |
| Transient atrial fibrillations | 3 (7) |
| Pneumonia | 1 (2) |
| Transient ischaemic attack | 2 (5) |
| Persistent pleural effusion | 1 (2) |
| Death | 0 (0.0) |
| None | 36 (84) |
If a patient had undergone thoracotomy and sternotomy, both interventions are listed separately when performed separately.
R0-resection was achieved in 31 (72%) cases. The median DS survival was not reached after 338 months. Patients with R1/R2-resection had a significantly reduced median DS survival of 78 months (95% confidence interval 47–108; P = 0.013) (Fig. 2A). The median recurrence-free DS survival after R0-resection was not reached after 275 months, 17 (55%) patients were recurrence free after 10 years (Fig. 2B). Systematic mediastinal lymphadenectomy (LAD) was performed in 16 (37.2%) patients. Statistically, there was a benefit in overall DS survival for these patients (P = 0.034) (Fig. 3). In the multivariate model, LAD covaries with R0 resection, which, when analysed separately, was associated with prolonged DS survival.
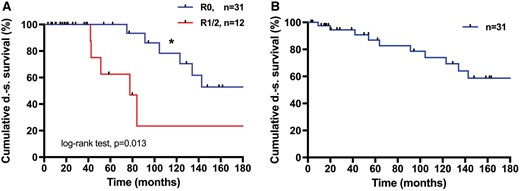
Survival after R0 vs R1/2 resection. (A) Five-year survival was 100% and 10-year survival was 77% vs 62% and 22%, respectively (*P = 0.013). Median not reached 338 months after R0 resection vs 78 months after R1/R2 resection. (B) Recurrence-free survival after R0 resection. Median not reached after 275 months. DS: disease specific.
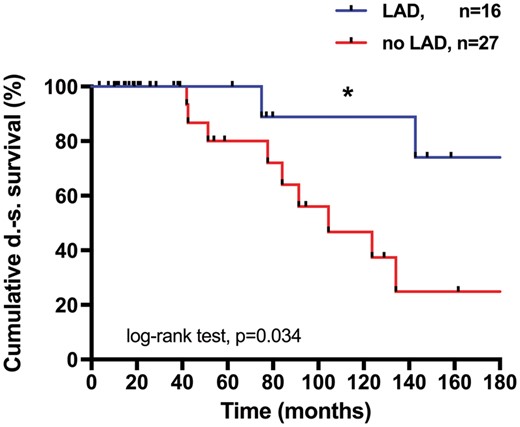
Survival after systematic lymph node dissection (LAD) (n = 43) vs no LAD/lymph node sampling. Five- and 10-year survival = 100%, vs 80% and 88% vs 46%, respectively (*P = 0.034). Median not reached 338 months after LAD vs 104 months without LAD/lymph node sampling. DS: disease-specific; LAD: lymphadenectomy.
DS survival was not dependent on gender (P = 0.53) or age <45 years (P = 0.25) at initial diagnosis of thyroid cancer. No statistically significant difference could be seen for patients who already presented with pulmonary metastases at initial diagnosis compared with patients who developed metastasis later in time (P = 0.4). A prolonged interval of more than 9 years between initial thyroidectomy and pulmonary metastases with subsequent metastasectomy also failed to reach statistical significance in our cohort (P = 0.1).
TG is an established biomarker for the follow-up of differentiated thyroid cancer. Increased serum levels have been associated with persistent disease or recurrence [2]. We compared serum TG levels before metastasectomy with those detected at the first regular follow-up about 3 months after surgery and found a decline of more than 80% to associate with prolonged DS survival (5- and 10-year survival 100% vs 69% and 82% vs 35%, respectively; P = 0.007) (Fig. 4). An absolute serum TG value of <10 ng/ml under thyroid-stimulating hormone withdrawal, which has been suggested as a cut-off before [22], did not reach statistical significance in our small study cohort (P = 0.13).
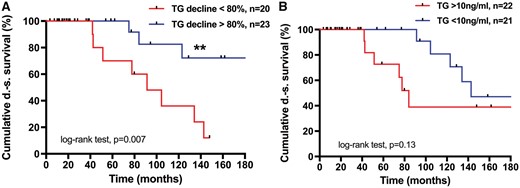
DS survival post-metastasectomy depends on serum TG levels. (A) Serum TG reduction by more than 80%. Five years 100%, 10 years 81% vs 69% and 36%, respectively (**P = 0.007). Median not reached after 338 months vs 91 months. (B) Serum TG reduction by more than 10 ng/ml. Five years 100%, 10 years 80% vs 66% and 36%, respectively (P = 0.13). Median not reached after 338 months vs 91 months. DS: disease specific; TG: thyroglobulin.
The decline in TG serum levels proved to be an independent predictor of long-term DS survival in multivariate analysis (P = 0.008, Table 3).
| Variables (n = 43) . | n (%) . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | ||
| Gender (female) | 24 (56) | 1.4 (0.49–4.03) | 0.53 | ||
| Age <45 years at initial diagnosis with SDCa | 10 (23) | 0.5 (0.16–1.58) | 0.25 | ||
| No metastases at initial diagnosis with SDCa (cM0) | 24 (56) | 0.65 (0.2–2.07) | 0.43 | ||
| Metastases after >3 years | 17 (40) | 0.44 (0.13–1.43) | 0.18 | ||
| Metastases after >9 years | 13 (30) | NAa | 0.1 | ||
| Single metastasis | 11 (26) | 0.69 (0.20–2.37) | 0.53 | ||
| R0-resection | 31 (72) | 0.27 (0.58–1.25) | 0.013 | 0.22 (0.047–1.02) | 0.053 |
| TG reduction >80% post-metastasectomy | 23 (53) | 0.23 (0.07–0.78) | 0.007 | 0.13 (0.027–0.59) | 0.008 |
| TG serum levels <10 ng/ml post-surgery | 21 (49) | 0.44 (0.13–1.51) | 0.13 | ||
| Systematic lymphadenectomy | 16 (37) | 0.29 (0.09–0.9) | 0.034 | 0.25 (0.047–1.30) | 0.1 |
| Variables (n = 43) . | n (%) . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | ||
| Gender (female) | 24 (56) | 1.4 (0.49–4.03) | 0.53 | ||
| Age <45 years at initial diagnosis with SDCa | 10 (23) | 0.5 (0.16–1.58) | 0.25 | ||
| No metastases at initial diagnosis with SDCa (cM0) | 24 (56) | 0.65 (0.2–2.07) | 0.43 | ||
| Metastases after >3 years | 17 (40) | 0.44 (0.13–1.43) | 0.18 | ||
| Metastases after >9 years | 13 (30) | NAa | 0.1 | ||
| Single metastasis | 11 (26) | 0.69 (0.20–2.37) | 0.53 | ||
| R0-resection | 31 (72) | 0.27 (0.58–1.25) | 0.013 | 0.22 (0.047–1.02) | 0.053 |
| TG reduction >80% post-metastasectomy | 23 (53) | 0.23 (0.07–0.78) | 0.007 | 0.13 (0.027–0.59) | 0.008 |
| TG serum levels <10 ng/ml post-surgery | 21 (49) | 0.44 (0.13–1.51) | 0.13 | ||
| Systematic lymphadenectomy | 16 (37) | 0.29 (0.09–0.9) | 0.034 | 0.25 (0.047–1.30) | 0.1 |
All patients who developed metastases after more than 9 years were still alive at the time of analysis.
CI: confidence interval; HR: hazard ratio; NA: not applicable; SDCa: differentiated thyroid cancer; TG: thyroglobulin.
| Variables (n = 43) . | n (%) . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | ||
| Gender (female) | 24 (56) | 1.4 (0.49–4.03) | 0.53 | ||
| Age <45 years at initial diagnosis with SDCa | 10 (23) | 0.5 (0.16–1.58) | 0.25 | ||
| No metastases at initial diagnosis with SDCa (cM0) | 24 (56) | 0.65 (0.2–2.07) | 0.43 | ||
| Metastases after >3 years | 17 (40) | 0.44 (0.13–1.43) | 0.18 | ||
| Metastases after >9 years | 13 (30) | NAa | 0.1 | ||
| Single metastasis | 11 (26) | 0.69 (0.20–2.37) | 0.53 | ||
| R0-resection | 31 (72) | 0.27 (0.58–1.25) | 0.013 | 0.22 (0.047–1.02) | 0.053 |
| TG reduction >80% post-metastasectomy | 23 (53) | 0.23 (0.07–0.78) | 0.007 | 0.13 (0.027–0.59) | 0.008 |
| TG serum levels <10 ng/ml post-surgery | 21 (49) | 0.44 (0.13–1.51) | 0.13 | ||
| Systematic lymphadenectomy | 16 (37) | 0.29 (0.09–0.9) | 0.034 | 0.25 (0.047–1.30) | 0.1 |
| Variables (n = 43) . | n (%) . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | ||
| Gender (female) | 24 (56) | 1.4 (0.49–4.03) | 0.53 | ||
| Age <45 years at initial diagnosis with SDCa | 10 (23) | 0.5 (0.16–1.58) | 0.25 | ||
| No metastases at initial diagnosis with SDCa (cM0) | 24 (56) | 0.65 (0.2–2.07) | 0.43 | ||
| Metastases after >3 years | 17 (40) | 0.44 (0.13–1.43) | 0.18 | ||
| Metastases after >9 years | 13 (30) | NAa | 0.1 | ||
| Single metastasis | 11 (26) | 0.69 (0.20–2.37) | 0.53 | ||
| R0-resection | 31 (72) | 0.27 (0.58–1.25) | 0.013 | 0.22 (0.047–1.02) | 0.053 |
| TG reduction >80% post-metastasectomy | 23 (53) | 0.23 (0.07–0.78) | 0.007 | 0.13 (0.027–0.59) | 0.008 |
| TG serum levels <10 ng/ml post-surgery | 21 (49) | 0.44 (0.13–1.51) | 0.13 | ||
| Systematic lymphadenectomy | 16 (37) | 0.29 (0.09–0.9) | 0.034 | 0.25 (0.047–1.30) | 0.1 |
All patients who developed metastases after more than 9 years were still alive at the time of analysis.
CI: confidence interval; HR: hazard ratio; NA: not applicable; SDCa: differentiated thyroid cancer; TG: thyroglobulin.
DISCUSSION
Thoracic metastasectomy has been established as a potentially curative treatment option for selected patients. Nevertheless, survival has been demonstrated to be highly dependent on the primary tumour entity, histology and differentiation, disease-free interval and number and size of metastases [19]. Metastases of differentiated thyroid cancer have been removed with curative intent [17], but only a small number of selected patients have been analysed, and there have not been any randomized controlled trials yet. Despite being one of the largest series to date, the cohort presented herein is still quite small with a total of only 43 patients. However, limited statistical analysis could be done. Our cohort contained significantly more patients with metastases arising from follicular thyroid cancer 35 (81%) compared with papillary thyroid cancer in 8 (19%), although papillary thyroid cancer comprises roughly 80% of all cases, with follicular thyroid cancer accounting for only about 10–20% [23]. An explanation for that could be that papillary tumours more commonly tend to metastasize to the regional lymph nodes, and only about 15% develop pulmonary or bone metastasis, whereas follicular and Hurthle cell carcinomas are more likely to spread to the lungs and bone in general [19].
The majority of patients had undergone thoracotomy for metastasectomy to palpate the lungs for nodules that could not be detected in the computed tomography scan. In a few earlier cases, partial sternotomy was performed. A minimally invasive approach was chosen for 6 (14%) patients. The extent and technique of surgery required for detecting all nodules remains highly controversial throughout the literature [24–27]. Equivalent survival could be shown after video-assisted thoracic surgery and open thoracotomy in case of a solitary lesion in the periphery of the lung [24].
Systematic mediastinal lymph node dissection (LAD) in the context of pulmonary metastasectomy is another avidly discussed topic. In most European institutions, only a lymph node sampling is done [12, 13, 25]. An adverse effect on the prognosis of patients with lymph node metastasis at the time of pulmonary metastasectomy for extra pulmonary carcinoma was found in several studies [12, 28]. Systemic mediastinal and hilar lymph node dissection in combination with pulmonary metastasectomy has been advocated for a more accurate staging of patients for clinical trials, with potentially beneficial adjuvant treatment [12]. Whether the benefit lies in the actual removal of the detected lymph node metastases or in the more precise information obtained regarding tumour spread has yet to be further elucidated. Interestingly, there are studies demonstrating ‘that histological work-up might not (always) show the true extent of tumour invasion’ [29]. Single-cell analysis was able to detect about 33% more actually tumour positive lymph nodes compared with lymph nodes detected as tumour positive in histopathology [29]. There might be a benefit for simultaneous systematic mediastinal lymphadectomy and pulmonary metastasectomy in this setting; however, this is a retrospective analysis, and even though our cohort is one of the largest for this unusual indication, it is very small in number and consists of selected patients. Thus, more and larger studies are needed to reach a conclusion.
Serum TG measurements are performed routinely as part of the follow-up for an early detection of recurrence of differentiated thyroid cancer [30]. A notable increase in TG levels leads to further investigation to localize remaining thyroid (tumour) tissue, especially if the serum TG level is higher than 10 ng/ml [22]. We found a relative decline in serum levels of more than 80% rather than a fixed value to be an independent factor associated with prolonged survival, indicating that there might be a positive effect of debulking surgery for selected patients in the context of further treatment.
The overall 10-year survival of patients with radioactive iodine ablation-refractory metastasis has been shown to be poor at only about 10–18% [5, 7]. In our study, a 10-year survival rate after thoracic metastasectomy of 56% could be attained.
CONCLUSION
We therefore conclude that salvage pulmonary metastasectomy is a safe therapeutic option, which could be shown to be associated with good long-term survival for selected patients after radioactive iodine ablation fails, especially if R0-resection can be achieved. There also appears to be a benefit if a significant reduction of tumour load, as indicated by TG serum levels, is possible. This study already provides one of the largest data sets worldwide up to date for this rare entity. However, to further corroborate these findings, larger and possibly prospective studies may be beneficial.
ACKNOWLEDGEMENTS
The authors thank S. Weber for expert statistical assistance. Part of this work was used for Raphael Kloeser’s MD thesis.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the Annual Meeting of the German Thoracic Surgery Society, Freiburg, Germany, 29 September–1 October 2016.




