-
PDF
- Split View
-
Views
-
Cite
Cite
Anita Nguyen, Hartzell V Schaff, Rick A Nishimura, Joseph A Dearani, Jeffrey B Geske, Brian D Lahr, Steve R Ommen, Does septal thickness influence outcome of myectomy for hypertrophic obstructive cardiomyopathy?, European Journal of Cardio-Thoracic Surgery, Volume 53, Issue 3, March 2018, Pages 582–589, https://doi.org/10.1093/ejcts/ezx398
Close - Share Icon Share
Abstract
Patients with hypertrophic obstructive cardiomyopathy and basal septal thickness <18 mm are often considered unsuitable candidates for myectomy. Mitral valve (MV) replacement is frequently performed instead. We aimed to determine whether septal thickness affects outcomes and adequacy of myectomy.
Clinical and echocardiographic data were reviewed for 1486 consecutive adult patients with hypertrophic obstructive cardiomyopathy who underwent transaortic septal myectomy from January 2005 through December 2014. Comparisons between patients, grouped by septal thickness (<18 mm, n = 369; 18–21 mm, n = 612 and >21 mm, n = 505), were performed with the Kruskal–Wallis and the Pearson χ2 tests and semiparametric analysis of covariance.
Median group ages were 57, 57 and 54 years (P = 0.007); men comprised 50.4%, 56.7% and 62.0%, respectively (P = 0.003). Intrinsic MV disease was present in 5.9%, 5.2% and 4.6%, respectively (P = 0.80). All patients underwent transaortic septal myectomy. Additional mitral procedures were performed in 7.6%, 7.8% and 8.1%, respectively (P = 0.90). Reasons for MV surgery included intrinsic MV disease (66.7%), residual mitral regurgitation (30.8%) and residual gradient (2.6%). All groups had postoperative gradient relief (median reduction: 51, 54 and 50 mmHg; P = 0.11). Ventricular septal defect occurred in 4 patients (0.3%), and risk did not differ by group (P = 0.24).
Adequate relief of left ventricular outflow tract obstruction can be achieved via transaortic septal myectomy without concomitant MV procedures when septal thickness is < 18 mm, and the risk of ventricular septal defect is minimal. Concomitant MV repair/replacement should be reserved for patients with intrinsic MV disease or inadequate relief of mitral regurgitation/left ventricular outflow tract obstruction following adequate extended septal myectomy.
INTRODUCTION
Hypertrophic obstructive cardiomyopathy (HOCM) is a genetic cardiac disease characterized by thickening of the ventricular septum and obstruction of the left ventricular outflow tract (LVOT), which can cause debilitating symptoms, including severe dyspnoea, syncope and chest pain [1, 2]. Medical management of HOCM may include beta-blockade, calcium channel blockers and disopyramide. Transaortic septal myectomy is the preferred treatment for HOCM refractory to medical management, and extended myectomy adequately relieves LVOT gradients, abolishes systolic anterior motion (SAM) and improves mitral regurgitation (MR) [3–6].
Most patients undergoing septal myectomy have marked hypertrophy of the basal septum. However, a subset of patients with dynamic LVOT obstruction have septal thickness <18 mm and typical symptoms related to HOCM (Fig. 1). Some authors have proposed that myectomy is not advisable in patients with a septal thickness <18 mm [7–9] because of a perceived increased risk of iatrogenic ventricular septal defect (VSD) as well as concerns regarding inadequate relief of LVOT obstruction. Instead of septal reduction therapy, mitral valve (MV) replacement has been recommended for patients with a basal septum <18–20 mm [7–11]. Other surgical groups have employed transaortic myectomy and concomitant MV procedures, such as anterior leaflet augmentation, reduction of posterior leaflet height and edge-to-edge leaflet repair [9, 12, 13].
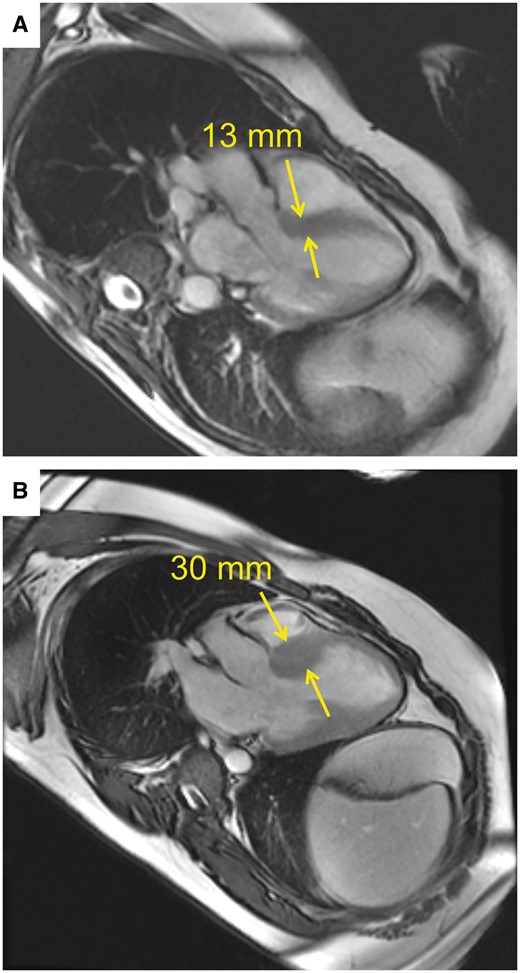
Preoperative cardiac magnetic resonance imaging in 2 patients. (A) Basal septal thickness measured 13 mm in the first patient. (B) Basal septal thickness measured 30 mm in the second patient. Yellow arrows mark where measurements were taken.
Our practice has been to perform extended septal myectomy as primary treatment for HOCM in all patients referred for operation, regardless of septal thickness. We have reserved adjunctive procedures on the MV for patients with intrinsic valvular disease and those who have persistent SAM and LVOT obstruction after adequate septectomy. In this study, we investigated short- and long-term outcomes of patients with HOCM stratified by basal septal thickness to evaluate whether myectomy is an adequate management option in patients with a basal septum <18 mm.
MATERIALS AND METHODS
Patient selection and stratification
A cohort of 1524 adult patients underwent transaortic septal myectomy between January 2005 and December 2014 at Mayo Clinic, Rochester, MN, USA. Patients who had previous MV surgery, those who did not have preoperative and postoperative echocardiographic studies available for review and those who did not provide research authorization were excluded. In total, 1486 (97.5%) patients were included in the study. Basal septal thickness was measured on preoperative transthoracic echocardiography (TTE) (Fig. 2), and the cohort was divided into 3 groups according to the thickness of the basal septum (<18 mm, 18–21 mm and >21 mm) (Fig. 3).
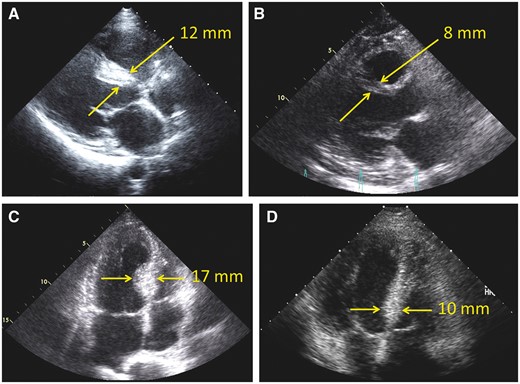
Preoperative and postoperative long-axis transthoracic echocardiography (TTE) in 1 patient (A, B) and preoperative and postoperative 4-chamber TTE in another patient (C, D). (A) Preoperative basal septal thickness of 12 mm. (B) Markedly reduced septal thickness of 8 mm postoperatively. (C) Preoperative basal septal thickness of 17 mm. (D) Markedly reduced septal thickness of 10 mm postoperatively. Yellow arrows mark where measurements were taken.
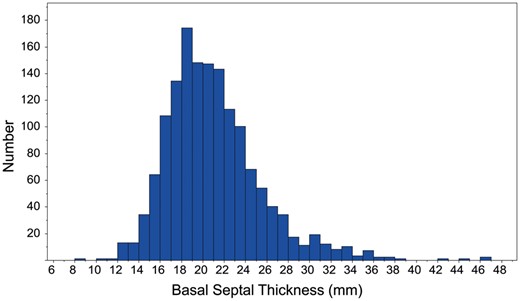
Histogram showing distribution of basal septal thickness among our cohort of 1486 patients.
Operative technique
We perform a median sternotomy and measure the pressure gradient between the left ventricle and the aorta before initiation of cardiopulmonary bypass [14, 15]. A dynamic gradient is also recorded after a premature ventricular contraction or with pharmacological provocation [16]. The aorta is cross-clamped and cold blood cardioplegia administered. An oblique aortotomy is made extending to the base of the non-coronary sinus. Pericardial stay sutures on the right side facilitate leftward rotation of the aorta and improve exposure of the subaortic area. The anterior mitral leaflet is displaced posteriorly by placing a cardiotomy sucker through the aortic valve. The fibrous scar tissue is visualized and a No. 10 knife blade used to incise the septum just to the right of the nadir of the right aortic sinus. The initial septal incision is carried counterclockwise to the anterior leaflet of the MV and then lengthened towards the apex of the left ventricle to excise the hypertrophied septum and endocardial scar, which generally weighs 3–12 g. When present, aberrant papillary muscles are excised if they contribute to obstruction and insert directly into the anterior mitral leaflet [14, 15, 17].
After bypass, the gradient between the left ventricle and the aorta is measured directly at rest and following premature ventricular contraction. Transoesophageal echocardiography is used to assess the myectomy site and mitral apparatus. In rare cases, where the gradient is still increased after myectomy or where SAM and associated MR are not adequately relieved, cardiopulmonary bypass may be reinstituted to extend the myectomy.
Data collection
Data were collated from our prospectively maintained cardiovascular surgery database; follow-up questionnaires were sent to all cardiovascular surgery patients at 1, 3, 5, 10, 15 and 20 years after surgery, and a retrospective review of the Mayo Clinic electronic health records was conducted. Vital status was determined using electronic health records, which include information from family members, physicians and Accurint for Health Care (LexisNexis Risk Solutions, Alpharetta, GA, USA).
Statistical analysis
Continuous variables were expressed as median (interquartile range) and categorical variables as frequencies (percentage). Comparisons of demographic, preoperative and operative measures across septal thickness groups were performed using the Kruskal–Wallis test for continuous variables and the Pearson’s χ2 test (or the Fisher’s exact test in cases of sparse data) for categorical variables. The Kaplan–Meier curves and the log-rank test were used to compare survival among the 3 groups, whereas the reverse Kaplan–Meier method was used to calculate median follow-up. A P-value of ≤0.05 was considered statistically significant. All analyses were carried out in SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Study outcomes based on postoperative echocardiographic measures were summarized both as follow-up values and as changes from preoperative assessment. Primary analyses were focused on the comparison of these changes between the 3 septal thickness groups. Because gradient measures were skewed with excessive ties at zero, semiparametric analysis of covariance was performed with the use of a proportional odds ordinal logistic regression model on the follow-up values (covariate adjusted for baseline values). When applied to a continuous outcome, the proportional odds model treats the data as ordinal using the rank values rather than raw measurements, making it more robust to distributional problems compared with linear regression [18]. Each model was formulated with the postoperative measurement as the dependent variable and the preoperative measurement and septal thickness group as independent variables. The latter term in the model was used to test whether changes were significantly different between groups after adjustment for baseline values. Because measurement of New York Heart Association (NYHA) class varied over late follow-up times, a 5-year estimate was obtained on each patient using linear interpolation of their observed baseline and follow-up measurements.
RESULTS
Baseline characteristics
Preoperative characteristics are listed in Table 1. Patients with septal thickness >21 mm were younger (P = 0.007), had greater body surface area (P < 0.001) and were more likely to be men (P = 0.003). Those with septal thickness >21 mm were also more likely to have a family history of HOCM or sudden cardiac death (P < 0.001) and to have an implantable cardioverter–defibrillator or permanent pacemaker (PPM) at presentation (P < 0.001). NYHA class III/IV dyspnoea was more likely to be present in patients with basal septal thickness >21 mm (P = 0.005).
| Characteristics . | Total (n = 1486) . | <18 mm (n = 369) . | 18–21 mm (n = 612) . | >21 mm (n = 505) . | P-value . |
|---|---|---|---|---|---|
| Age (years), median (IQR) | 56.1 (45.3, 65.2) | 57.0 (46.6, 65.2) | 57.2 (46.3, 65.7) | 54.2 (42.9, 64.0) | 0.007 |
| Male, n (%) | 846 (56.9) | 186 (50.4) | 347 (56.7) | 313 (62.0) | 0.003 |
| BSA (mm2), median (IQR) | 2.03 (1.85, 2.20) | 1.99 (1.82, 2.17) | 2.04 (1.85, 2.19) | 2.06 (1.90, 2.24) | <0.001 |
| Syncope, n (%) | 257 (17.3) | 69 (18.7) | 105 (17.2) | 83 (16.4) | 0.68 |
| Presyncope, n (%) | 451 (30.3) | 121 (32.8) | 162 (26.5) | 168 (33.3) | 0.02 |
| Hypertension, n (%) | 801 (53.9) | 205 (55.6) | 334 (54.6) | 262 (51.9) | 0.51 |
| Coronary artery disease, n (%) | 227 (15.3) | 53 (14.4) | 102 (16.7) | 72 (14.3) | 0.46 |
| NYHA class, n (%) | |||||
| I | 17 (1.1) | 10 (2.7) | 6 (1.0) | 1 (0.2) | 0.005 |
| II | 174 (11.7) | 56 (15.2) | 53 (8.7) | 65 (12.9) | |
| III | 1242 (83.6) | 290 (78.6) | 531 (86.9) | 421 (83.4) | |
| IV | 52 (3.5) | 13 (3.5) | 21 (3.4) | 18 (3.6) | |
| ICD, n (%) | 255 (17.2) | 33 (8.9) | 87 (14.2) | 135 (26.7) | <0.001 |
| PPM, n (%) | 141 (9.5) | 22 (6.0) | 51 (8.3) | 68 (13.5) | <0.001 |
| Family history (HOCM or SCD), n (%) | 364 (25.5) | 74 (21.6) | 123 (20.8) | 167 (33.9) | <0.001 |
| Characteristics . | Total (n = 1486) . | <18 mm (n = 369) . | 18–21 mm (n = 612) . | >21 mm (n = 505) . | P-value . |
|---|---|---|---|---|---|
| Age (years), median (IQR) | 56.1 (45.3, 65.2) | 57.0 (46.6, 65.2) | 57.2 (46.3, 65.7) | 54.2 (42.9, 64.0) | 0.007 |
| Male, n (%) | 846 (56.9) | 186 (50.4) | 347 (56.7) | 313 (62.0) | 0.003 |
| BSA (mm2), median (IQR) | 2.03 (1.85, 2.20) | 1.99 (1.82, 2.17) | 2.04 (1.85, 2.19) | 2.06 (1.90, 2.24) | <0.001 |
| Syncope, n (%) | 257 (17.3) | 69 (18.7) | 105 (17.2) | 83 (16.4) | 0.68 |
| Presyncope, n (%) | 451 (30.3) | 121 (32.8) | 162 (26.5) | 168 (33.3) | 0.02 |
| Hypertension, n (%) | 801 (53.9) | 205 (55.6) | 334 (54.6) | 262 (51.9) | 0.51 |
| Coronary artery disease, n (%) | 227 (15.3) | 53 (14.4) | 102 (16.7) | 72 (14.3) | 0.46 |
| NYHA class, n (%) | |||||
| I | 17 (1.1) | 10 (2.7) | 6 (1.0) | 1 (0.2) | 0.005 |
| II | 174 (11.7) | 56 (15.2) | 53 (8.7) | 65 (12.9) | |
| III | 1242 (83.6) | 290 (78.6) | 531 (86.9) | 421 (83.4) | |
| IV | 52 (3.5) | 13 (3.5) | 21 (3.4) | 18 (3.6) | |
| ICD, n (%) | 255 (17.2) | 33 (8.9) | 87 (14.2) | 135 (26.7) | <0.001 |
| PPM, n (%) | 141 (9.5) | 22 (6.0) | 51 (8.3) | 68 (13.5) | <0.001 |
| Family history (HOCM or SCD), n (%) | 364 (25.5) | 74 (21.6) | 123 (20.8) | 167 (33.9) | <0.001 |
BSA: body surface area; HOCM: hypertrophic obstructive cardiomyopathy; ICD: implantable cardioverter–defibrillator; IQR: interquartile range; NYHA: New York Heart Association; PPM: permanent pacemaker; SCD: sudden cardiac death.
| Characteristics . | Total (n = 1486) . | <18 mm (n = 369) . | 18–21 mm (n = 612) . | >21 mm (n = 505) . | P-value . |
|---|---|---|---|---|---|
| Age (years), median (IQR) | 56.1 (45.3, 65.2) | 57.0 (46.6, 65.2) | 57.2 (46.3, 65.7) | 54.2 (42.9, 64.0) | 0.007 |
| Male, n (%) | 846 (56.9) | 186 (50.4) | 347 (56.7) | 313 (62.0) | 0.003 |
| BSA (mm2), median (IQR) | 2.03 (1.85, 2.20) | 1.99 (1.82, 2.17) | 2.04 (1.85, 2.19) | 2.06 (1.90, 2.24) | <0.001 |
| Syncope, n (%) | 257 (17.3) | 69 (18.7) | 105 (17.2) | 83 (16.4) | 0.68 |
| Presyncope, n (%) | 451 (30.3) | 121 (32.8) | 162 (26.5) | 168 (33.3) | 0.02 |
| Hypertension, n (%) | 801 (53.9) | 205 (55.6) | 334 (54.6) | 262 (51.9) | 0.51 |
| Coronary artery disease, n (%) | 227 (15.3) | 53 (14.4) | 102 (16.7) | 72 (14.3) | 0.46 |
| NYHA class, n (%) | |||||
| I | 17 (1.1) | 10 (2.7) | 6 (1.0) | 1 (0.2) | 0.005 |
| II | 174 (11.7) | 56 (15.2) | 53 (8.7) | 65 (12.9) | |
| III | 1242 (83.6) | 290 (78.6) | 531 (86.9) | 421 (83.4) | |
| IV | 52 (3.5) | 13 (3.5) | 21 (3.4) | 18 (3.6) | |
| ICD, n (%) | 255 (17.2) | 33 (8.9) | 87 (14.2) | 135 (26.7) | <0.001 |
| PPM, n (%) | 141 (9.5) | 22 (6.0) | 51 (8.3) | 68 (13.5) | <0.001 |
| Family history (HOCM or SCD), n (%) | 364 (25.5) | 74 (21.6) | 123 (20.8) | 167 (33.9) | <0.001 |
| Characteristics . | Total (n = 1486) . | <18 mm (n = 369) . | 18–21 mm (n = 612) . | >21 mm (n = 505) . | P-value . |
|---|---|---|---|---|---|
| Age (years), median (IQR) | 56.1 (45.3, 65.2) | 57.0 (46.6, 65.2) | 57.2 (46.3, 65.7) | 54.2 (42.9, 64.0) | 0.007 |
| Male, n (%) | 846 (56.9) | 186 (50.4) | 347 (56.7) | 313 (62.0) | 0.003 |
| BSA (mm2), median (IQR) | 2.03 (1.85, 2.20) | 1.99 (1.82, 2.17) | 2.04 (1.85, 2.19) | 2.06 (1.90, 2.24) | <0.001 |
| Syncope, n (%) | 257 (17.3) | 69 (18.7) | 105 (17.2) | 83 (16.4) | 0.68 |
| Presyncope, n (%) | 451 (30.3) | 121 (32.8) | 162 (26.5) | 168 (33.3) | 0.02 |
| Hypertension, n (%) | 801 (53.9) | 205 (55.6) | 334 (54.6) | 262 (51.9) | 0.51 |
| Coronary artery disease, n (%) | 227 (15.3) | 53 (14.4) | 102 (16.7) | 72 (14.3) | 0.46 |
| NYHA class, n (%) | |||||
| I | 17 (1.1) | 10 (2.7) | 6 (1.0) | 1 (0.2) | 0.005 |
| II | 174 (11.7) | 56 (15.2) | 53 (8.7) | 65 (12.9) | |
| III | 1242 (83.6) | 290 (78.6) | 531 (86.9) | 421 (83.4) | |
| IV | 52 (3.5) | 13 (3.5) | 21 (3.4) | 18 (3.6) | |
| ICD, n (%) | 255 (17.2) | 33 (8.9) | 87 (14.2) | 135 (26.7) | <0.001 |
| PPM, n (%) | 141 (9.5) | 22 (6.0) | 51 (8.3) | 68 (13.5) | <0.001 |
| Family history (HOCM or SCD), n (%) | 364 (25.5) | 74 (21.6) | 123 (20.8) | 167 (33.9) | <0.001 |
BSA: body surface area; HOCM: hypertrophic obstructive cardiomyopathy; ICD: implantable cardioverter–defibrillator; IQR: interquartile range; NYHA: New York Heart Association; PPM: permanent pacemaker; SCD: sudden cardiac death.
Preoperative transthoracic echocardiography
All patients underwent preoperative Doppler TTE (Table 2). Among the 3 groups, the median (interquartile range) resting maximum instantaneous LVOT gradients (mmHg) were 44 (18, 77), 64 (27, 92) and 64 (36, 92) (P < 0.001). The median provoked LVOT maximum instantaneous gradients (mmHg) were 81 (55, 92), 74 (55, 100) and 75 (55, 95) (P = 0.83).
| Findings . | n . | Total (n = 1486) . | <188 mm (n = 369) . | 18–21 mm (n = 612) . | >21 mm (n = 505) . | P-value . |
|---|---|---|---|---|---|---|
| Preoperative resting LVOT gradient (TTE) (mmHg), median (IQR) | 1322 | 61 (26, 88) | 44 (18, 77) | 64 (27, 92) | 64 (36, 92) | <0.001 |
| Preoperative provoked LVOT gradient (TTE) (mmHg), median (IQR) | 763 | 77 (55, 96) | 81 (55, 92) | 74 (55, 100) | 75 (55, 95) | 0.83 |
| Prebypass resting LVOT gradient (mmHg),a median (IQR) | 1360 | 55 (30, 80) | 54 (29, 79) | 57 (31, 80) | 54 (31, 79) | 0.24 |
| Prebypass provoked LVOT gradient (mmHg),a median (IQR) | 935 | 108 (79, 150) | 108 (80, 150) | 117 (80, 161) | 102 (72, 139) | 0.007 |
| Moderate/severe MR, n (%) | 1474 | 422 (28.6) | 79 (21.7) | 192 (31.6) | 151 (30.1) | 0.003 |
| SAM, n (%) | 1383 | 1277 (92.3) | 293 (87.2) | 531 (92.8) | 453 (95.4) | <0.001 |
| IMVD, n (%) | 1468 | |||||
| Stenosis | 31 (2.1) | 9 (2.4) | 13 (2.1) | 9 (1.8) | 0.80 | |
| Prolapse | 46 (3.1) | 13 (3.6) | 19 (3.2) | 14 (2.8) | 0.82 | |
| MV surgery, n (%) | 1486 | |||||
| Repair | 91 (6.1) | 21 (5.7) | 38 (6.2) | 32 (6.3) | 0.92 | |
| Replacement | 26 (1.7) | 7 (1.9) | 10 (1.6) | 9 (1.8) | 0.96 | |
| Papillary muscle surgery | 81 (5.5) | 26 (7.0) | 31 (5.1) | 24 (4.8) | 0.29 | |
| Reasons for MV surgery, n (%) | 117 | 0.02 | ||||
| IMVD | 78 (66.7) | 21 (75.0) | 31 (64.6) | 26 (63.4) | ||
| Residual MR/SAM | 36 (30.8) | 4 (14.3) | 17 (35.4) | 15 (36.6) | ||
| Residual gradient | 3 (2.6) | 3 (10.7) | 0 (0.0) | 0 (0.0) |
| Findings . | n . | Total (n = 1486) . | <188 mm (n = 369) . | 18–21 mm (n = 612) . | >21 mm (n = 505) . | P-value . |
|---|---|---|---|---|---|---|
| Preoperative resting LVOT gradient (TTE) (mmHg), median (IQR) | 1322 | 61 (26, 88) | 44 (18, 77) | 64 (27, 92) | 64 (36, 92) | <0.001 |
| Preoperative provoked LVOT gradient (TTE) (mmHg), median (IQR) | 763 | 77 (55, 96) | 81 (55, 92) | 74 (55, 100) | 75 (55, 95) | 0.83 |
| Prebypass resting LVOT gradient (mmHg),a median (IQR) | 1360 | 55 (30, 80) | 54 (29, 79) | 57 (31, 80) | 54 (31, 79) | 0.24 |
| Prebypass provoked LVOT gradient (mmHg),a median (IQR) | 935 | 108 (79, 150) | 108 (80, 150) | 117 (80, 161) | 102 (72, 139) | 0.007 |
| Moderate/severe MR, n (%) | 1474 | 422 (28.6) | 79 (21.7) | 192 (31.6) | 151 (30.1) | 0.003 |
| SAM, n (%) | 1383 | 1277 (92.3) | 293 (87.2) | 531 (92.8) | 453 (95.4) | <0.001 |
| IMVD, n (%) | 1468 | |||||
| Stenosis | 31 (2.1) | 9 (2.4) | 13 (2.1) | 9 (1.8) | 0.80 | |
| Prolapse | 46 (3.1) | 13 (3.6) | 19 (3.2) | 14 (2.8) | 0.82 | |
| MV surgery, n (%) | 1486 | |||||
| Repair | 91 (6.1) | 21 (5.7) | 38 (6.2) | 32 (6.3) | 0.92 | |
| Replacement | 26 (1.7) | 7 (1.9) | 10 (1.6) | 9 (1.8) | 0.96 | |
| Papillary muscle surgery | 81 (5.5) | 26 (7.0) | 31 (5.1) | 24 (4.8) | 0.29 | |
| Reasons for MV surgery, n (%) | 117 | 0.02 | ||||
| IMVD | 78 (66.7) | 21 (75.0) | 31 (64.6) | 26 (63.4) | ||
| Residual MR/SAM | 36 (30.8) | 4 (14.3) | 17 (35.4) | 15 (36.6) | ||
| Residual gradient | 3 (2.6) | 3 (10.7) | 0 (0.0) | 0 (0.0) |
Prebypass LVOT gradient on transoesophageal echocardiography and/or direct needle measurement.
IMVD: intrinsic mitral valve disease; IQR: interquartile range; LVOT: left ventricular outflow tract; MR: mitral regurgitation; MV: mitral valve; SAM: systolic anterior motion; TTE: transthoracic echocardiography.
| Findings . | n . | Total (n = 1486) . | <188 mm (n = 369) . | 18–21 mm (n = 612) . | >21 mm (n = 505) . | P-value . |
|---|---|---|---|---|---|---|
| Preoperative resting LVOT gradient (TTE) (mmHg), median (IQR) | 1322 | 61 (26, 88) | 44 (18, 77) | 64 (27, 92) | 64 (36, 92) | <0.001 |
| Preoperative provoked LVOT gradient (TTE) (mmHg), median (IQR) | 763 | 77 (55, 96) | 81 (55, 92) | 74 (55, 100) | 75 (55, 95) | 0.83 |
| Prebypass resting LVOT gradient (mmHg),a median (IQR) | 1360 | 55 (30, 80) | 54 (29, 79) | 57 (31, 80) | 54 (31, 79) | 0.24 |
| Prebypass provoked LVOT gradient (mmHg),a median (IQR) | 935 | 108 (79, 150) | 108 (80, 150) | 117 (80, 161) | 102 (72, 139) | 0.007 |
| Moderate/severe MR, n (%) | 1474 | 422 (28.6) | 79 (21.7) | 192 (31.6) | 151 (30.1) | 0.003 |
| SAM, n (%) | 1383 | 1277 (92.3) | 293 (87.2) | 531 (92.8) | 453 (95.4) | <0.001 |
| IMVD, n (%) | 1468 | |||||
| Stenosis | 31 (2.1) | 9 (2.4) | 13 (2.1) | 9 (1.8) | 0.80 | |
| Prolapse | 46 (3.1) | 13 (3.6) | 19 (3.2) | 14 (2.8) | 0.82 | |
| MV surgery, n (%) | 1486 | |||||
| Repair | 91 (6.1) | 21 (5.7) | 38 (6.2) | 32 (6.3) | 0.92 | |
| Replacement | 26 (1.7) | 7 (1.9) | 10 (1.6) | 9 (1.8) | 0.96 | |
| Papillary muscle surgery | 81 (5.5) | 26 (7.0) | 31 (5.1) | 24 (4.8) | 0.29 | |
| Reasons for MV surgery, n (%) | 117 | 0.02 | ||||
| IMVD | 78 (66.7) | 21 (75.0) | 31 (64.6) | 26 (63.4) | ||
| Residual MR/SAM | 36 (30.8) | 4 (14.3) | 17 (35.4) | 15 (36.6) | ||
| Residual gradient | 3 (2.6) | 3 (10.7) | 0 (0.0) | 0 (0.0) |
| Findings . | n . | Total (n = 1486) . | <188 mm (n = 369) . | 18–21 mm (n = 612) . | >21 mm (n = 505) . | P-value . |
|---|---|---|---|---|---|---|
| Preoperative resting LVOT gradient (TTE) (mmHg), median (IQR) | 1322 | 61 (26, 88) | 44 (18, 77) | 64 (27, 92) | 64 (36, 92) | <0.001 |
| Preoperative provoked LVOT gradient (TTE) (mmHg), median (IQR) | 763 | 77 (55, 96) | 81 (55, 92) | 74 (55, 100) | 75 (55, 95) | 0.83 |
| Prebypass resting LVOT gradient (mmHg),a median (IQR) | 1360 | 55 (30, 80) | 54 (29, 79) | 57 (31, 80) | 54 (31, 79) | 0.24 |
| Prebypass provoked LVOT gradient (mmHg),a median (IQR) | 935 | 108 (79, 150) | 108 (80, 150) | 117 (80, 161) | 102 (72, 139) | 0.007 |
| Moderate/severe MR, n (%) | 1474 | 422 (28.6) | 79 (21.7) | 192 (31.6) | 151 (30.1) | 0.003 |
| SAM, n (%) | 1383 | 1277 (92.3) | 293 (87.2) | 531 (92.8) | 453 (95.4) | <0.001 |
| IMVD, n (%) | 1468 | |||||
| Stenosis | 31 (2.1) | 9 (2.4) | 13 (2.1) | 9 (1.8) | 0.80 | |
| Prolapse | 46 (3.1) | 13 (3.6) | 19 (3.2) | 14 (2.8) | 0.82 | |
| MV surgery, n (%) | 1486 | |||||
| Repair | 91 (6.1) | 21 (5.7) | 38 (6.2) | 32 (6.3) | 0.92 | |
| Replacement | 26 (1.7) | 7 (1.9) | 10 (1.6) | 9 (1.8) | 0.96 | |
| Papillary muscle surgery | 81 (5.5) | 26 (7.0) | 31 (5.1) | 24 (4.8) | 0.29 | |
| Reasons for MV surgery, n (%) | 117 | 0.02 | ||||
| IMVD | 78 (66.7) | 21 (75.0) | 31 (64.6) | 26 (63.4) | ||
| Residual MR/SAM | 36 (30.8) | 4 (14.3) | 17 (35.4) | 15 (36.6) | ||
| Residual gradient | 3 (2.6) | 3 (10.7) | 0 (0.0) | 0 (0.0) |
Prebypass LVOT gradient on transoesophageal echocardiography and/or direct needle measurement.
IMVD: intrinsic mitral valve disease; IQR: interquartile range; LVOT: left ventricular outflow tract; MR: mitral regurgitation; MV: mitral valve; SAM: systolic anterior motion; TTE: transthoracic echocardiography.
Intrinsic mitral valve disease (IMVD) was present in 5.2% of the entire cohort; among the 3 groups, mitral stenosis was present in 2.4%, 2.1% and 1.8% (P = 0.80) and MV leaflet prolapse in 3.6%, 3.2% and 2.8% (P = 0.81). At rest, SAM of the MV was observed in 87.2%, 92.8% and 95.4% (P < 0.001). Moderate or severe MR was more likely to be present in patients with septal thickness ≥18 mm (P = 0.003).
Operative details
All patients underwent transaortic septal myectomy. As listed in Table 2, additional MV surgery was performed in 117 (7.9%); MV repair in 91 (6.1% of the study cohort and 77.8% of mitral procedures) and replacement in 26 (1.8%) patients. Among the 3 groups, MV repair was performed in 5.7%, 6.2% and 6.3% (P = 0.92) and replacement in 1.9%, 1.6% and 1.8% (P = 0.95). The indications for mitral procedures (replacement or repair) were IMVD in 78 (66.7%), residual post-bypass MR in 36 (30.8%) and residual LVOT gradient in 3 (2.6%) patients. Patients with less severe septal hypertrophy (<18 mm) were less likely to undergo additional MV procedures (P = 0.02). Papillary muscle surgery was performed in 81 (5.5%) patients; no differences in the occurrence of papillary muscle operation were observed among the 3 groups (7.0%, 5.1% and 4.8%; P = 0.29).
Adequacy of myectomy
Following operation, resting and provoked LVOT gradients decreased on echocardiography (Table 3). In a subset analysis of patients for whom measurements were available, the resting LVOT gradient (mmHg) after bypass (n = 1131) changed by a median (interquartile range) of −35 (−72, −14), −55 (−86, −21) and −54 (−81, −27) (P < 0.001, between-group comparison of changes) and provoked LVOT (mmHg) after bypass (n = 150) changed by −61 (−76, −32), −60 (−89, −35) and −56 (−74, −29) (P = 0.04).
| Variables . | n . | <18 mm (n = 369) . | 18–21 mm (n = 612) . | >21 mm (n = 505) . | P-valueb . |
|---|---|---|---|---|---|
| Postoperative resting LVOT gradient (TTE) (mmHg), median (IQR) | 1330 | 0.11 | |||
| Follow-up value | 0 (0, 4) | 0 (0, 3) | 0 (0, 4) | ||
| Change from baseline | −51 (−76, −26) | −54 (−80, −29) | −50 (−76, −29) | ||
| Postoperative provoked LVOT gradient (TTE) (mmHg), median (IQR) | 799 | 0.005 | |||
| Follow-up value | 0 (0, 5) | 1 (0, 7) | 3 (0, 9) | ||
| Change from baseline | −114 (−155, −84) | −117 (−159, −84) | −102 (−138, −72) | ||
| Post-bypass resting LVOT gradient (mmHg),a median (IQR) | 1131 | <0.001 | |||
| Follow-up value | 0 (0, 9) | 0 (0, 12) | 3 (0, 16) | ||
| Change from baseline | −35 (−72, −14) | −55 (−86, −21) | −54 (−81, −27) | ||
| Post-bypass provoked LVOT gradient (mmHg),a median (IQR) | 150 | 0.04 | |||
| Follow-up value | 13 (10, 18) | 19 (13, 27) | 18 (13, 26) | ||
| Change from baseline | −61 (−76, −32) | −60 (−89, −35) | −56 (−74, −29) | ||
| LVEF (%), median (IQR) | 1461 | 0.44 | |||
| Follow-up value | 68 (63, 70) | 68 (64, 71) | 68 (63, 71) | ||
| Change from baseline | −4 (−9, 0) | −4 (−8, −1) | −4 (−9, 0) | ||
| MR grade, median (IQR) | 1433 | 0.11 | |||
| Follow-up value | 1 (1, 2) | 2 (1, 2) | 2 (1, 2) | ||
| Change from baseline | −1 (−1, 0) | −1 (−2, 0) | −1 (−2, 0) | ||
| Moderate/severe MR, n (%) | 1433 | 7 (2.0) | 9 (1.5) | 4 (0.8) | 0.20 |
| SAM, n (%) | 1016 | 67 (27.5) | 127 (30.5) | 127 (35.7) | 0.09 |
| Reoperation for bleeding, n (%) | 1486 | 13 (3.5) | 20 (3.3) | 15 (3.0) | 0.90 |
| VSD, n (%) | 1486 | 2 (0.5) | 2 (0.3) | 0 (0.0) | 0.24 |
| Operative mortality, n (%) | 1486 | 1 (0.3) | 2 (0.3) | 1 (0.2) | 1 |
| Permanent pacemaker for heart block, n (%) | 1486 | 9 (2.4) | 16 (2.6) | 11 (2.2) | 0.89 |
| Variables . | n . | <18 mm (n = 369) . | 18–21 mm (n = 612) . | >21 mm (n = 505) . | P-valueb . |
|---|---|---|---|---|---|
| Postoperative resting LVOT gradient (TTE) (mmHg), median (IQR) | 1330 | 0.11 | |||
| Follow-up value | 0 (0, 4) | 0 (0, 3) | 0 (0, 4) | ||
| Change from baseline | −51 (−76, −26) | −54 (−80, −29) | −50 (−76, −29) | ||
| Postoperative provoked LVOT gradient (TTE) (mmHg), median (IQR) | 799 | 0.005 | |||
| Follow-up value | 0 (0, 5) | 1 (0, 7) | 3 (0, 9) | ||
| Change from baseline | −114 (−155, −84) | −117 (−159, −84) | −102 (−138, −72) | ||
| Post-bypass resting LVOT gradient (mmHg),a median (IQR) | 1131 | <0.001 | |||
| Follow-up value | 0 (0, 9) | 0 (0, 12) | 3 (0, 16) | ||
| Change from baseline | −35 (−72, −14) | −55 (−86, −21) | −54 (−81, −27) | ||
| Post-bypass provoked LVOT gradient (mmHg),a median (IQR) | 150 | 0.04 | |||
| Follow-up value | 13 (10, 18) | 19 (13, 27) | 18 (13, 26) | ||
| Change from baseline | −61 (−76, −32) | −60 (−89, −35) | −56 (−74, −29) | ||
| LVEF (%), median (IQR) | 1461 | 0.44 | |||
| Follow-up value | 68 (63, 70) | 68 (64, 71) | 68 (63, 71) | ||
| Change from baseline | −4 (−9, 0) | −4 (−8, −1) | −4 (−9, 0) | ||
| MR grade, median (IQR) | 1433 | 0.11 | |||
| Follow-up value | 1 (1, 2) | 2 (1, 2) | 2 (1, 2) | ||
| Change from baseline | −1 (−1, 0) | −1 (−2, 0) | −1 (−2, 0) | ||
| Moderate/severe MR, n (%) | 1433 | 7 (2.0) | 9 (1.5) | 4 (0.8) | 0.20 |
| SAM, n (%) | 1016 | 67 (27.5) | 127 (30.5) | 127 (35.7) | 0.09 |
| Reoperation for bleeding, n (%) | 1486 | 13 (3.5) | 20 (3.3) | 15 (3.0) | 0.90 |
| VSD, n (%) | 1486 | 2 (0.5) | 2 (0.3) | 0 (0.0) | 0.24 |
| Operative mortality, n (%) | 1486 | 1 (0.3) | 2 (0.3) | 1 (0.2) | 1 |
| Permanent pacemaker for heart block, n (%) | 1486 | 9 (2.4) | 16 (2.6) | 11 (2.2) | 0.89 |
Post-bypass LVOT gradient on transoesophageal echocardiography and/or direct needle measurement.
For echocardiography measures assessed preoperatively and postoperatively, P-values are for the difference in changes across the 3 septal thickness groups.
IQR: interquartile range; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; MR: mitral regurgitation; SAM: systolic anterior motion; TTE: transthoracic echocardiography; VSD: ventricular septal defect.
| Variables . | n . | <18 mm (n = 369) . | 18–21 mm (n = 612) . | >21 mm (n = 505) . | P-valueb . |
|---|---|---|---|---|---|
| Postoperative resting LVOT gradient (TTE) (mmHg), median (IQR) | 1330 | 0.11 | |||
| Follow-up value | 0 (0, 4) | 0 (0, 3) | 0 (0, 4) | ||
| Change from baseline | −51 (−76, −26) | −54 (−80, −29) | −50 (−76, −29) | ||
| Postoperative provoked LVOT gradient (TTE) (mmHg), median (IQR) | 799 | 0.005 | |||
| Follow-up value | 0 (0, 5) | 1 (0, 7) | 3 (0, 9) | ||
| Change from baseline | −114 (−155, −84) | −117 (−159, −84) | −102 (−138, −72) | ||
| Post-bypass resting LVOT gradient (mmHg),a median (IQR) | 1131 | <0.001 | |||
| Follow-up value | 0 (0, 9) | 0 (0, 12) | 3 (0, 16) | ||
| Change from baseline | −35 (−72, −14) | −55 (−86, −21) | −54 (−81, −27) | ||
| Post-bypass provoked LVOT gradient (mmHg),a median (IQR) | 150 | 0.04 | |||
| Follow-up value | 13 (10, 18) | 19 (13, 27) | 18 (13, 26) | ||
| Change from baseline | −61 (−76, −32) | −60 (−89, −35) | −56 (−74, −29) | ||
| LVEF (%), median (IQR) | 1461 | 0.44 | |||
| Follow-up value | 68 (63, 70) | 68 (64, 71) | 68 (63, 71) | ||
| Change from baseline | −4 (−9, 0) | −4 (−8, −1) | −4 (−9, 0) | ||
| MR grade, median (IQR) | 1433 | 0.11 | |||
| Follow-up value | 1 (1, 2) | 2 (1, 2) | 2 (1, 2) | ||
| Change from baseline | −1 (−1, 0) | −1 (−2, 0) | −1 (−2, 0) | ||
| Moderate/severe MR, n (%) | 1433 | 7 (2.0) | 9 (1.5) | 4 (0.8) | 0.20 |
| SAM, n (%) | 1016 | 67 (27.5) | 127 (30.5) | 127 (35.7) | 0.09 |
| Reoperation for bleeding, n (%) | 1486 | 13 (3.5) | 20 (3.3) | 15 (3.0) | 0.90 |
| VSD, n (%) | 1486 | 2 (0.5) | 2 (0.3) | 0 (0.0) | 0.24 |
| Operative mortality, n (%) | 1486 | 1 (0.3) | 2 (0.3) | 1 (0.2) | 1 |
| Permanent pacemaker for heart block, n (%) | 1486 | 9 (2.4) | 16 (2.6) | 11 (2.2) | 0.89 |
| Variables . | n . | <18 mm (n = 369) . | 18–21 mm (n = 612) . | >21 mm (n = 505) . | P-valueb . |
|---|---|---|---|---|---|
| Postoperative resting LVOT gradient (TTE) (mmHg), median (IQR) | 1330 | 0.11 | |||
| Follow-up value | 0 (0, 4) | 0 (0, 3) | 0 (0, 4) | ||
| Change from baseline | −51 (−76, −26) | −54 (−80, −29) | −50 (−76, −29) | ||
| Postoperative provoked LVOT gradient (TTE) (mmHg), median (IQR) | 799 | 0.005 | |||
| Follow-up value | 0 (0, 5) | 1 (0, 7) | 3 (0, 9) | ||
| Change from baseline | −114 (−155, −84) | −117 (−159, −84) | −102 (−138, −72) | ||
| Post-bypass resting LVOT gradient (mmHg),a median (IQR) | 1131 | <0.001 | |||
| Follow-up value | 0 (0, 9) | 0 (0, 12) | 3 (0, 16) | ||
| Change from baseline | −35 (−72, −14) | −55 (−86, −21) | −54 (−81, −27) | ||
| Post-bypass provoked LVOT gradient (mmHg),a median (IQR) | 150 | 0.04 | |||
| Follow-up value | 13 (10, 18) | 19 (13, 27) | 18 (13, 26) | ||
| Change from baseline | −61 (−76, −32) | −60 (−89, −35) | −56 (−74, −29) | ||
| LVEF (%), median (IQR) | 1461 | 0.44 | |||
| Follow-up value | 68 (63, 70) | 68 (64, 71) | 68 (63, 71) | ||
| Change from baseline | −4 (−9, 0) | −4 (−8, −1) | −4 (−9, 0) | ||
| MR grade, median (IQR) | 1433 | 0.11 | |||
| Follow-up value | 1 (1, 2) | 2 (1, 2) | 2 (1, 2) | ||
| Change from baseline | −1 (−1, 0) | −1 (−2, 0) | −1 (−2, 0) | ||
| Moderate/severe MR, n (%) | 1433 | 7 (2.0) | 9 (1.5) | 4 (0.8) | 0.20 |
| SAM, n (%) | 1016 | 67 (27.5) | 127 (30.5) | 127 (35.7) | 0.09 |
| Reoperation for bleeding, n (%) | 1486 | 13 (3.5) | 20 (3.3) | 15 (3.0) | 0.90 |
| VSD, n (%) | 1486 | 2 (0.5) | 2 (0.3) | 0 (0.0) | 0.24 |
| Operative mortality, n (%) | 1486 | 1 (0.3) | 2 (0.3) | 1 (0.2) | 1 |
| Permanent pacemaker for heart block, n (%) | 1486 | 9 (2.4) | 16 (2.6) | 11 (2.2) | 0.89 |
Post-bypass LVOT gradient on transoesophageal echocardiography and/or direct needle measurement.
For echocardiography measures assessed preoperatively and postoperatively, P-values are for the difference in changes across the 3 septal thickness groups.
IQR: interquartile range; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; MR: mitral regurgitation; SAM: systolic anterior motion; TTE: transthoracic echocardiography; VSD: ventricular septal defect.
Compared with preoperative assessments, resting and provoked LVOT gradients improved at postoperative TTE (median 4 [3, 5] days after surgery). Resting LVOT gradients (mmHg) changed by a median of −51 (−76, −26), −54 (−80, −29) and −50 (−80, −29) (P = 0.17, between-group comparison of changes) and provoked gradient (mmHg) changed by −114 (−155, −84), −117 (−159, −84) and −102 (−138, −72) (P = 0.005). The MR grade improved by a median of 1 point (on an ordinal scale) in all 3 groups, with the overall rate of those with moderate/severe MR decreasing from 28.6% to 1.4% after surgery.
Complications
Postoperatively, 48 (3.2%) patients required reoperation, and rates were similar among all groups (3.5%, 3.3% and 3.0%; P = 0.90). Iatrogenic VSD occurred in 4 patients; of these, 2 were repaired during the initial operation and 1 at a later reoperation. The fourth patient’s VSD was very small and was seen only on postoperative TTE. This patient did not report any symptoms, and on serial follow-up TTEs, the VSD had not increased in size.
There were 4 postoperative deaths with no differences observed among the 3 groups (P = 1). Two of the 4 early deaths occurred in patients who had been discharged postoperatively and died of cardiac tamponade and subsequent arrest; both of these patients had been discharged on warfarin for anticoagulation. One early death occurred in a patient with a postoperative VSD repaired at operation.
In the early postoperative period, implantable cardioverter–defibrillator implantation was more frequently performed in patients with basal septal thickness >21 mm (4.3%, 4.4% and 10.1%; P < 0.001), but there was no significant difference in PPM insertion due to heart block among the 3 groups (2.4%, 2.6% and 2.2%; P = 0.89).
Symptomatic relief
A total of 547 (36.8%) patients had NYHA class recorded both preoperatively and on long-term follow-up surveys at a median of 5.0 (3.2, 5.1) years after surgery. Preoperatively, 84.5% of the 547 patients were in NYHA class III/IV, and postoperatively, only 24.0% reported dyspnoea classified as NYHA class III/IV (Fig. 4). When standardized to a 5-year follow-up measurement, NYHA class decreased from preoperative to postoperative assessment by a median of 1 (0, 2) point. No differences in symptomatic relief existed when 5-year decreases in NYHA class were compared among the 3 groups (P = 0.62).
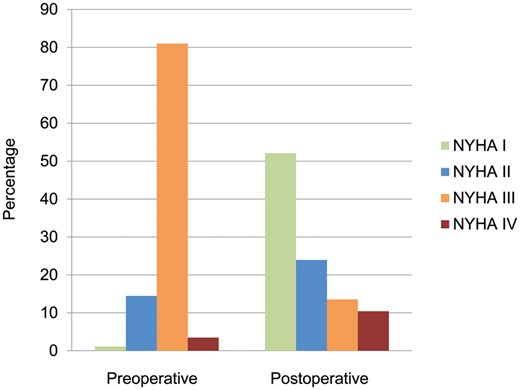
Preoperative versus postoperative NYHA functional class status (I–IV) in 547 patients at a median (interquartile range) follow-up of 5.0 (3.2, 5.1) years. NYHA: New York Heart Association.
Survival
The long-term survival in our study cohort is shown in Fig. 5. Median follow-up time was 3.0 (0.1, 5.2) years. Survival estimates at 1, 3 and 5 years were 99%, 97% and 95%. There were no significant differences in survival among the 3 groups (P = 0.29).
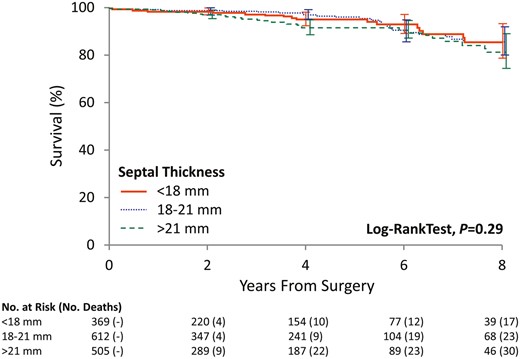
The Kaplan–Meier curve showing patient survival among the 3 groups, stratified by septal thickness.
DISCUSSION
Basal septal thickness has consistently been used as a criterion in selection of patients for transaortic septal myectomy, and many clinicians caution against surgery when basal septal thickness is <18–20 mm because of perceived increased risks of iatrogenic VSD and inadequate gradient relief [7–11]. In an often cited study by McIntosh et al [8], 29 patients with HOCM and basal septal thickness <18 mm underwent MV replacement instead of septal myectomy. The authors reported no postoperative LVOT gradients or iatrogenic VSDs. On the basis of these outcomes, they recommended MV replacement instead of transaortic septal myectomy in patients with septal thickness <18 mm.
The risk of VSD development after myectomy is low overall (<1% [19]) but may be increased for patients with relatively less basal septal hypertrophy. In this study, VSDs developed in 4 (0.3%) patients. Although there was no statistically significant association between VSD and basal septum on the prespecified wall thickness analysis, all VSDs occurred in patients with basal septal thickness ≤21 mm. However, it is important to emphasize how rarely this complication occurred, and even in patients with relatively less hypertrophy, the risk of VSD is very small. If an iatrogenic VSD is seen on postoperative echocardiography, additional surgical or percutaneous interventions are usually indicated. Patients with small VSDs can be monitored by TTE [20, 21].
In a recent study, Shingu et al. [9] described their experience with septal myectomy and concomitant MV procedures in 8 HOCM patients with thin interventricular septa (<20 mm). They performed transaortic myectomy with additional MV surgery if moderate/severe MR (n = 4) or IMVD (n = 3) was present. Isolated myectomy was limited to patients with mild MR (n = 1). The authors reported adequate gradient relief and decreased MR in all patients and concluded that combining myectomy with MV procedures should be considered for patients with a thin interventricular septum.
However, our practice has been to proceed with myectomy alone in most symptomatic adult patients with HOCM, regardless of measured basal septal thickness and degree of SAM-related MR. We reserve concomitant MV procedures for those patients with IMVD, such as mitral prolapse or stenosis [6, 22]. In rare cases, we also perform additional MV procedures if MR, SAM or LVOT gradient is not adequately relieved by myectomy alone.
In this study, concomitant MV procedures were rarely necessary; only 8% of our cohort required an additional MV operation. Of these, nearly 70% had IMVD, and rates of intrinsic disease were comparable among all 3 groups. However, reasons for additional MV procedures differed among the cohorts. In patients with basal septal thickness <18 mm, IMVD was the most common indication for concomitant MV procedures. In the 2 groups with basal thickness ≥18 mm, a significantly higher proportion of patients underwent additional MV operations because of residual MR or SAM.
There has been concern as to whether septal myectomy can achieve adequate gradient relief and abolish MR and SAM in patients with a relatively thin basal septum [8]. However, in this analysis of more than 1400 patients, significant reduction in LVOT gradient was seen on postoperative transesophageal echocardiography and TTE in all groups. Furthermore, marked reduction in MR and SAM occurred at rest in all patients. Our findings suggest that excellent outcomes for gradient relief and abolishing MR and SAM can be achieved in all patients, regardless of basal septal thickness.
Early postoperative mortality in our surgical cohort was very low (0.3%) and is consistent with outcomes reported in high-volume centres [23]. Although increased basal septal thickness has been found to be an independent predictor for sudden postprocedure death in patients undergoing alcohol septal ablation [24], this was not supported in our surgical cohort, as we did not observe any association between preoperative basal septal thickness and operative mortality.
Postoperative PPM insertion due to heart block is a known complication of septal myectomy. In a study using the Nationwide Inpatient Sample, Panaich et al [25] reported an overall postoperative PPM insertion rate of 9%. However, in our cohort, PPMs were necessary in only 2.4%, and there was no association between postoperative PPM insertion and basal septal thickness. Thus, our study suggests that patients with a relatively thin basal septum are not at increased risk of injury to conductive pathways during extended septal myectomy.
Late postoperative data confirm that myectomy results in significant symptomatic improvement in most patients, and long-term survival is excellent. No significant differences were observed for late postoperative symptomatic status or survival among the 3 groups stratified by basal septal thickness. Even though patients with a basal septum >21 mm had more severe symptoms at presentation and were more likely to have moderate or severe dyspnoea (NYHA class III/IV), symptom resolution was satisfactory in this group. Interestingly, patients with basal septal thickness >21 mm also more frequently had a family history of HOCM and sudden cardiac death, which is consistent with current beliefs that massive septal hypertrophy (≥30 mm) is a manifestation of more severe disease and an independent risk factor for sudden death [26].
Alternative septal reduction therapies, such as alcohol septal ablation, are favoured by some cardiologists [24]. In our practice, alcohol septal ablation is reserved for selected patients who are not candidates for myectomy because of older age or concomitant medical conditions, which might be expected to increase operative mortality [27, 28]. However, alcohol septal ablation has also been discouraged for patients with basal septal thickness <15–17 mm because of perceived risks of iatrogenic VSD [29, 30]. As shown in our study, extended septal myectomy is feasible in patients with a basal septum <18 mm, and surgery remains the gold standard for all patients with symptoms refractory to medical intervention.
Limitations
This was a retrospective study from a single tertiary centre experience that included a relatively large number of surgical HOCM patients. Therefore, our analysis was limited by inherent selection bias and may not be replicable in other centres. Although we had extensive follow-up information on survival, late follow-up information on symptomatic relief was not complete for all patients. However, there is no reason to believe that any follow-up bias exists in relation to preoperative septal thickness.
CONCLUSION
Surgical myectomy is an adequate treatment option for severely symptomatic patients with a basal septum <18 mm. The risk of iatrogenic VSD and operative mortality in this group are not increased compared with the risk for patients with basal septal thickness ≥18 mm. Concomitant MV procedures should only be performed if intrinsic MV disease exists or if extended myectomy does not adequately abolish MR, SAM or LVOT obstruction. Early outcomes in patients with a relatively thin basal septum are excellent, and long-term outcomes, including survival and symptomatic relief, are very encouraging.
Funding
This work was supported by the Paul and Ruby Tsai Family.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 31st Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 7–11 October 2017.




