-
PDF
- Split View
-
Views
-
Cite
Cite
Luigi Pirelli, Jacob S Scheinerman, Derek R Brinster, Nirav C Patel, Alaeldin Eltom, Jonathan M Hemli, Chad A Kliger, Transinnominate approach for transcatheter aortic valve replacement: single-centre experience of minimally invasive alternative access, European Journal of Cardio-Thoracic Surgery, Volume 53, Issue 3, March 2018, Pages 545–551, https://doi.org/10.1093/ejcts/ezx361
Close - Share Icon Share
Abstract
Iliofemoral arteries have been the preferred access for transcatheter aortic valve replacement (TAVR). When these arteries are too small, calcified or tortuous, an alternative access must be considered. Transinnominate (TI) access is an extrathoracic approach that does not require manipulation of major neurovascular structures or the apex. The aim of this study is to evaluate the efficacy and safety of TI TAVR as an alternative access in patients with severe aortic stenosis not amenable to a transfemoral approach.
Thirteen patients with severe aortic stenosis underwent TI TAVR between February 2016 and January 2017 at our institution. The average Society of Thoracic Surgeons (STS) score was 7.7 ± 4.5%. Eight patients had previous surgical revascularization, 7 of which involved the left thoracic artery. All patients underwent preoperative computed tomography angiography that revealed significant atheromatous and calcific disease of the iliofemoral vessels and/or the descending aorta. The innominate artery was found to be of appropriate calibre (>10 mm), free of plaque and easy to access via surgical incision. Fusion multimodality imaging was utilized in all cases to guide the procedure.
The innominate artery was accessed via a 2-inch right parasternal supraclavicular incision. Nine self-expandable valves and 4 balloon-expandable valves were implanted. Procedural success occurred in all cases without intraprocedural and in-hospital mortality. No neurological deficits or vascular complications were recorded; postoperative bleeding was trivial. Ten patients were discharged on Day 3 and 3 patients who required PPM on Day 5.
TI approach represents a safe, reproducible and minimally invasive hybrid technique for TAVR in high-risk patients. In our early experience, surgical trauma and perioperative complications are minimal with rapid patient recovery.
INTRODUCTION
Transcatheter aortic valve replacement (TAVR) has revolutionized the treatment of aortic stenosis [1]. Patients deemed to be at intermediate, and high risk or inoperable for surgery now have an alternative that is less invasive and does not require the use of cardiopulmonary bypass and aortic cross-clamping, portending good mid-term prognosis [2]. Self-expandable and balloon-expandable aortic transcatheter heart valves (THVs) can be implanted via different access approaches, depending on patient-specific anatomy and comorbidities [3]. Transfemoral is the most commonly used approach; it represents an established and safe access if the iliofemoral vessels can accommodate the appropriate sheath size (≥14 Fr) and are free of significant calcification and/or tortuosity. Transapical (TA), transaortic (TAo), transcarotid (TC), subclavian/axillary and transcaval are all valid alternative options, each having inherent risks.
TA access was originally considered the first option for alternative access during the initial experience of TAVR. The need for a left thoracotomy, particularly in patients with chronic pulmonary disease, the presence of a friable apex and the proximity to the left internal mammary artery (LIMA) and left anterior descending artery (LAD), rendered this route morbid and a surgical challenge. The TAo approach was subsequently utilized, given the procedural simplicity and comparable outcomes to transfemoral TAVR. Nonetheless, partial sternotomy can interfere with respiratory function, and the risk of re-entry in redo cases, especially in patients with previous revascularization, is a concern.
The transition to extrathoracic alternative access has the potential for faster procedure times and ventilator use and shorter intensive care unit and overall length of stay. TC and subclavian/axillary approaches provide easier surgical access, but carry a risk of increased neurological events and brachial plexus injury. Newer techniques, such as transcaval access, have high rates of bleeding: major or life-threatening bleeding in 13% of patients, covered stents use in over 60% and 35% of patients requiring a median 2-unit transfusion [4]. Rates of life-threatening or major bleeding are similar to intrathoracic alternative access, in the major pivotal TAVR trials ranging from 8.7% to 22.6% [5–7].
The transinnominate (TI) access is a novel approach for delivering a THV. The brachiocephalic artery is the first large branch of the aortic arch. It arises at the level of the right second costal cartilage and traverses superiorly, posteriorly and laterally towards the right sternoclavicular articulation, where it finally divides. Its diameter is usually large, greater than 10 mm. Moreover, the innominate artery is rarely subject to atheromatous and calcific disease, unlike the carotid arteries. Although its trajectory is rightward, it typically maintains a straight line to the aortic valve. Compared with other alternative routes for valve delivery and implantation, TI access is less traumatic without violating the thoracic cavity and manipulating major neurovascular structures or the apex and can potentially allow for early extubation and local bleeding control. To our knowledge, TI access has not been the subject of any large series in the literature. We describe our single-centre experience of patients with severe aortic stenosis requiring alternative transcatheter access via the innominate artery.
MATERIALS AND METHODS
Study population
Thirteen consecutive patients with severe trileaflet aortic stenosis who underwent TI TAVR from February 2016 to January 2017 at Lenox Hill Heart and Vascular Institute-Northwell Health were included. This study was approved by the Northwell institutional review board. All patients were evaluated by an interventional/structural cardiologist and 2 cardiac surgeons, and the cases were discussed by the heart valve team. All patients were deemed to be at high risk or inoperable for surgical aortic valve replacement due to age, comorbidities, frailty index and/or presence of a ‘hostile mediastinum’. Preprocedural left heart catheterization was performed. In patients with previous revascularization, especially if LIMA was patent, the risk of resternotomy was deemed high. Patients with a bovine aortic arch were excluded from this approach and alternative means of access considered. All procedures were performed in a dedicated structural heart catheterization laboratory under general anaesthesia.
Computed tomographic angiography
As part of preprocedural planning, cardiac computed tomography angiography (CTA) and CTA abdomen and pelvis (256-slice iCT scanner, Philips Healthcare, Cleveland, OH, USA) were performed to evaluate cardiac anatomy and plan for optimal approach. Patients were positioned supine with both arms adducted, in an effort to reproduce the same anatomical conditions that operators would encounter on the catheterization table during THV implantation. CTA slices were 3D/4D volume rendered and evaluated using postprocessing software (TeraRecon, Foster City, CA, USA). Although the diameter of the iliofemoral arteries was adequate in 8 patients (>5 mm), CTA showed severe calcification with tortuosity that precluded safe introduction of the delivery system; the remaining patients had diameters <5 mm bilaterally and/or dense mobile plaque within the descending aorta or aortic arch.
The relationship between the ascending aorta and the sternum and surrounding structures, including the right ventricle, LAD graft and proximal vein graft anastomosis on the ascending aorta was evaluated, which is of particular importance to patients with a history of coronary artery bypass grafting. Although the distance of the ascending aorta to the sternum was greater than 1 cm and the LIMA lateralized to the left of the sternal bone and other grafts were non-adherent in all cases, the risk of resternotomy was deemed high by 2 cardiac surgeons.
The arch branches were then analysed; none of the patients had a bovine aortic arch and the brachiocephalic, the left carotid and the left subclavian arteries emerged from the aortic arch in the usual fashion. Depth and relation of the innominate artery to the sternal notch and clavicle were assessed. The 3D reconstructed images showed that only a small parasternal supraclavicular incisions without the need for sternotomy and/or thoracotomy was required to access the vessel (Fig. 1).
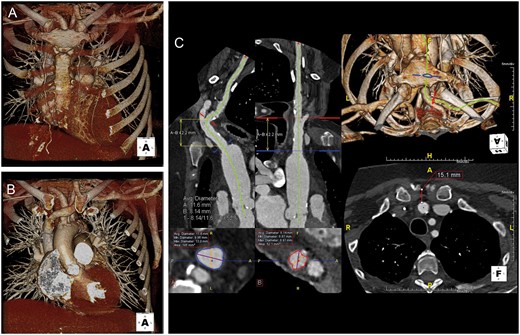
Gated chest computed tomography angiography showing anatomical relations of the innominate artery with surrounding structures. 3D volume-rendered image (A) with and (B) without the sternum. Centre line generation with multiplanar reformation performed to evaluate innominate artery diameter and distance of access to annular plane. (C) Repositioning of 3D reconstruction is performed to mimic intraprocedural position on the table to evaluate the relationship between the innominate artery and the adjacent bony structures and mediastinum.
Computed tomographic angiography–fluoroscopy fusion imaging
CTA-fluoroscopy fusion imaging (HeartNavigator system, Philips Healthcare, Best, Netherlands) utilized end-diastolic images and segmented the following structures: aortic valve, aorta, coronary arteries, innominate artery extending into the proximal common carotid and subclavian arteries, clavicle and ribs/sternum. Landmarks were placed on the innominate artery away from atherosclerotic disease at the optimal site of puncture as well as at the base of the aortic cusps, ostium of the coronary arteries and centre of the aortic valve. The CTA images were coregistered and overlaid onto live fluoroscopy using an aortogram (10 ml of diluted non-ionic contrast media) in 2 different projections, performed 20° apart. Volume-rendered 3D images were replaced with an outlined view of the aorta/aortic valve and bony landmarks; these images and landmarks were displayed with the same perspective as the X-ray system used to guide entry into the innominate artery.
Hybrid technique
Intervention was performed in a hybrid operating room (OR) suite within the cardiac catheterization laboratory. The patients were positioned supine with their neck extended and head tilted to the left. The anaesthesiologists were positioned at the head of the table with the C-arm and the transoesophageal echocardiogram (TOE) machine on the left side of the patient. Bilateral cerebral oximetry was monitored for the whole duration of the procedure. Endotracheal intubation was performed using a single-lumen tube, and transvenous pacemaker was introduced and positioned into the right ventricular apex via a left internal jugular vein with thresholds <1 mA. TOE was utilized for all procedures.
A 5-Fr sheath was introduced into the common femoral artery and a 5-Fr pigtail advanced in the ascending aorta and placed at the level of the non-coronary cusp for aortography. We performed a 5-cm right lower anterior supraclavicular neck incision, along the medial border of the sternocleidomastoid muscle, just lateral to the sternal notch. Soft tissue was dissected, and the right common carotid and subclavian arteries identified. The internal jugular vein was identified and retracted aside with sylastic tape, if necessary. With further medial and caudal dissection, the origin of the innominate trunk was exposed. Two 4-0 Prolene purse strings were placed at the site of CTA-fluoroscopy fusion landmark, typically at the base of the brachiocephalic artery. Heparin was administered per protocol with goal activated clotting time (ACT) >250 s.
A 6-Fr sheath was introduced, and the aortic valve was crossed using a 5-Fr JR4.0 catheter/0.035″ straight guidewire. A 0.035″ stiff support guidewire (Confida, Medtronic, Minneapolis, MN, USA and Lunderquist, Cook Medical, Bloomington, IN, USA) was positioned within the left ventricle and 8-Fr sheath upsized to an 18-Fr sheath (Cook Medical) for self-expandable THVs and 16-Fr E-sheath (Edwards, Irvine, CA, USA) for balloon-expandable THVs.
Initial experience of this technique started with self-expandable THVs (CoreValve Classic, Medtronic) due to its ease of deliverability and ability to manipulate the wire and delivery system for coaxial alignment. The subsequent introduction of the Evolut R system (Medtronic) allowed for retrievability, providing an opportunity to reposition the device if needed. With the newest balloon-expandable THV platform transition to the Commander delivery catheter, our team began utilizing the SAPIEN 3 THVs (Edwards). This delivery catheter, compared with the previous NovaFlex+ generation, affords greater flexibility allowing for improved coaxial alignment, in the setting of concerns regarding aortic angulation and trajectory and the potential for THV malalignment within the aortic annulus. In addition, the Commander delivery balloon inflates serially from distal/proximal to central regions in a ‘dog-bone’ shape that allows for secure engagement within the left ventricular outflow tract and ascending aorta and improved centring of the SAPIEN 3 THV, irrespective of aortic angulation.
The valve was positioned under fluoroscopy, fusion imaging and TOE guidance (Fig. 2). Heparin was reversed using protamine sulphate standard infusion, 1 mg/100 U of heparin. The delivery system and sheaths were removed under rapid ventricular pacing, purse string tied and haemostasis achieved. No drains were utilized, and the soft tissue and skin were reapproximated in usual fashion.
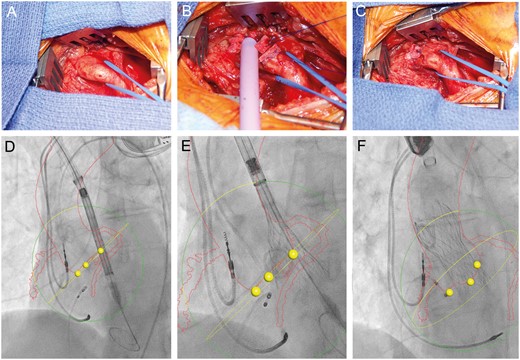
Surgical exposure of the innominate artery via a supraclavicular, parasternal exposure. (A) Insertion of an 18-Fr sheath (Cook Medical) within the 4-0 Prolene-pledgeted purse string. (B) Sheath removed and purse string secured. (C) Computed tomography angiography–fluoroscopy fusion images (HeartNavigator System, Philips Healthcare, Best, Netherlands) with outline of the aorta, innominate artery and coronary arteries identified in red, base of the coronary cusps as yellow dots and annular plane as yellow circle. Medtronic CoreValve THV is identified crossing the aortic valve (D), during partial (E) and final (F) deployment.
Statistical analysis
Statistical analyses were performed using SPSS statistical package (version 20, IBM SPSS Inc., Chicago, IL, USA). Continuous variables were reported as mean ± standard deviation or as median (interquartile range) where appropriate. The unpaired Student’s t-test was used for the comparison of continuous, normally distributed variables. Statistical significance was defined as P <0.05.
RESULTS
Baseline patient characteristics are summarized in Table 1. Patients were 80 ± 7.8 years of age. Male-to-female ratio was 9/4; all were symptomatic with dyspnoea on minimal exertion and in New York Heart Association Functional Class (NYHA-FC) III–IV. The Society of Thoracic Surgeons (STS) score for surgical aortic valve replacement was 7.7 ± 4.5%. Seven of 8 patients with a history of surgical revascularization had anastomosis of the LIMA to the LAD; this graft was patent in 5 patients on CTA and coronary angiography. In the remaining 2 patients, the LIMA was atretic or severely stenosed. The eighth patient had a saphenous vein graft to the LAD. One patient had end-stage renal disease on haemodialysis via a left arteriovenous fistula.
| . | n = 13 . |
|---|---|
| Age (years) | 80 ± 7.8 |
| Males, n (%) | 9 (69) |
| NYHA Class III–IV, n (%) | 13 (100) |
| Previous cardiac operations, n (%) | 8 (62) |
| Previous CABG, n (%) | 8 (62) |
| Baseline creatinine (mg/dl), mean ± SD | 1.4 ± 0.7 |
| Preoperative Hb (g/dl), mean ± SD | 11.5 ± 1.6 |
| STS score (%), mean ± SD | 7.7 ± 4.5 |
| Transthoracic echocardiography, mean ± SD | |
| LVEF | 52.7 ± 14.2 |
| Aortic transvalvular peak gradient (mmHg) | 69.7 ± 20.3 |
| Aortic transvalvular mean gradient (mmHg) | 42.4 ± 15.2 |
| Computed tomographic angiography, mean ± SD | |
| AVA on CTA (cm2) | 0.64 ± 0.2 |
| Aortic valve perimeter (mm) | 77.7 ± 7.7 |
| Distance aortic annulus to RCA (mm) | 17.7 ± 4.1 |
| Distance aortic annulus to LM (mm) | 14.4 ± 3.7 |
| Diameter of ascending aorta (mm) | 30.7 ± 3.2 |
| Diameter of brachiocephalic artery (mm) | 10.4 ± 2.4 |
| Distance innominate artery–aortic annulus (mm) | 91 ± 9.2 |
| . | n = 13 . |
|---|---|
| Age (years) | 80 ± 7.8 |
| Males, n (%) | 9 (69) |
| NYHA Class III–IV, n (%) | 13 (100) |
| Previous cardiac operations, n (%) | 8 (62) |
| Previous CABG, n (%) | 8 (62) |
| Baseline creatinine (mg/dl), mean ± SD | 1.4 ± 0.7 |
| Preoperative Hb (g/dl), mean ± SD | 11.5 ± 1.6 |
| STS score (%), mean ± SD | 7.7 ± 4.5 |
| Transthoracic echocardiography, mean ± SD | |
| LVEF | 52.7 ± 14.2 |
| Aortic transvalvular peak gradient (mmHg) | 69.7 ± 20.3 |
| Aortic transvalvular mean gradient (mmHg) | 42.4 ± 15.2 |
| Computed tomographic angiography, mean ± SD | |
| AVA on CTA (cm2) | 0.64 ± 0.2 |
| Aortic valve perimeter (mm) | 77.7 ± 7.7 |
| Distance aortic annulus to RCA (mm) | 17.7 ± 4.1 |
| Distance aortic annulus to LM (mm) | 14.4 ± 3.7 |
| Diameter of ascending aorta (mm) | 30.7 ± 3.2 |
| Diameter of brachiocephalic artery (mm) | 10.4 ± 2.4 |
| Distance innominate artery–aortic annulus (mm) | 91 ± 9.2 |
AVA: stands for aortic valve area; CABG: coronary artery bypass grafting; CTA: computed tomography angiography; Hb: haemoglobin; LM: left main; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; RCA: right coronary artery; SD: standard deviation; STS: Society of Thoracic Surgeons.
| . | n = 13 . |
|---|---|
| Age (years) | 80 ± 7.8 |
| Males, n (%) | 9 (69) |
| NYHA Class III–IV, n (%) | 13 (100) |
| Previous cardiac operations, n (%) | 8 (62) |
| Previous CABG, n (%) | 8 (62) |
| Baseline creatinine (mg/dl), mean ± SD | 1.4 ± 0.7 |
| Preoperative Hb (g/dl), mean ± SD | 11.5 ± 1.6 |
| STS score (%), mean ± SD | 7.7 ± 4.5 |
| Transthoracic echocardiography, mean ± SD | |
| LVEF | 52.7 ± 14.2 |
| Aortic transvalvular peak gradient (mmHg) | 69.7 ± 20.3 |
| Aortic transvalvular mean gradient (mmHg) | 42.4 ± 15.2 |
| Computed tomographic angiography, mean ± SD | |
| AVA on CTA (cm2) | 0.64 ± 0.2 |
| Aortic valve perimeter (mm) | 77.7 ± 7.7 |
| Distance aortic annulus to RCA (mm) | 17.7 ± 4.1 |
| Distance aortic annulus to LM (mm) | 14.4 ± 3.7 |
| Diameter of ascending aorta (mm) | 30.7 ± 3.2 |
| Diameter of brachiocephalic artery (mm) | 10.4 ± 2.4 |
| Distance innominate artery–aortic annulus (mm) | 91 ± 9.2 |
| . | n = 13 . |
|---|---|
| Age (years) | 80 ± 7.8 |
| Males, n (%) | 9 (69) |
| NYHA Class III–IV, n (%) | 13 (100) |
| Previous cardiac operations, n (%) | 8 (62) |
| Previous CABG, n (%) | 8 (62) |
| Baseline creatinine (mg/dl), mean ± SD | 1.4 ± 0.7 |
| Preoperative Hb (g/dl), mean ± SD | 11.5 ± 1.6 |
| STS score (%), mean ± SD | 7.7 ± 4.5 |
| Transthoracic echocardiography, mean ± SD | |
| LVEF | 52.7 ± 14.2 |
| Aortic transvalvular peak gradient (mmHg) | 69.7 ± 20.3 |
| Aortic transvalvular mean gradient (mmHg) | 42.4 ± 15.2 |
| Computed tomographic angiography, mean ± SD | |
| AVA on CTA (cm2) | 0.64 ± 0.2 |
| Aortic valve perimeter (mm) | 77.7 ± 7.7 |
| Distance aortic annulus to RCA (mm) | 17.7 ± 4.1 |
| Distance aortic annulus to LM (mm) | 14.4 ± 3.7 |
| Diameter of ascending aorta (mm) | 30.7 ± 3.2 |
| Diameter of brachiocephalic artery (mm) | 10.4 ± 2.4 |
| Distance innominate artery–aortic annulus (mm) | 91 ± 9.2 |
AVA: stands for aortic valve area; CABG: coronary artery bypass grafting; CTA: computed tomography angiography; Hb: haemoglobin; LM: left main; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; RCA: right coronary artery; SD: standard deviation; STS: Society of Thoracic Surgeons.
All patients had severe calcific aortic valve stenosis. Calculated aortic valve peak and mean gradients were 69.7 ± 20.3 and 42.4 ± 15.2 mmHg, respectively, on transthoracic echocardiogram. The average aortic valve area based on CTA was 0.64 ± 0.2 cm2, and the aortic annular perimeter was 77 ± 7.7 mm. Distance of the annulus to the left main and the right coronary arteries was 14.4 ± 3.7 and 17.7 ± 4.1 mm, respectively. These measurements were utilized to select the appropriate prosthesis type and size. A Medtronic Corevalve was implanted in 9 cases. Four of them received a retrievable Corevalve Evolut R transcatheter aortic valve prosthesis. A balloon-expandable valve (Edwards SAPIEN 3) was used in the remaining 4 patients. The ascending aorta measured 30.7 ± 3.2 mm on average. The minimal diameter of innominate arteries was 10.4 ± 2.4 mm. The CTA did not reveal any atheromatous or calcific plaque along its course. The distance of the artery from the aortic annular plane was 91 ± 9.2 mm. Left ventricular ejection fraction was 52.7 ± 14.2%. Two patients had chronic systolic heart failure associated with reduced ejection fraction (20 and 30%, respectively). None of the patients had significant concomitant significant mitral or tricuspid valvular pathology.
There was no intraprocedural and hospital mortality (Table 2). The valves were implanted successfully in all cases. Transvalvular gradients postimplantation were negligible, and the degree of intravalvular aortic regurgitation was trivial. Intraprocedural TOE did not show any significant paravalvular leak. All patients were extubated in the hybrid OR suite. No neurological deficits were reported. All patients were transferred to the cardiac cardiothoracic intensive care unit (CTICU) for monitoring with the transvenous pacemaker (TVP) in place. One patient with preoperative first-degree atrioventricular block required implantation of permanent pacemaker (PPM) on postoperative day (POD) 4 for persistent bradycardia. Two more patients developed a new left bundle branch block and were implanted with a PPM on POD 3. All the other patients had their TVP removed on POD 2. Dual antiplatelet therapy was resumed the day after surgery unless contraindicated.
| . | n = 13 . |
|---|---|
| Valve type, n (%) | |
| CoreValve Classic | 5 (38) |
| Corevalve Evolut R | 4 (31) |
| Edwards SAPIEN 3 | 4 (31) |
| Success of implant, n (%) | 13 (100) |
| In-hospital and 30-day mortality (%) | 0 |
| Degree of aortic regurgitation postimplant (0–IV) | 0 |
| Postimplant PPM, n (%) | 3 (23) |
| Postimplant NYHA Class I–II (%) | 100 |
| . | n = 13 . |
|---|---|
| Valve type, n (%) | |
| CoreValve Classic | 5 (38) |
| Corevalve Evolut R | 4 (31) |
| Edwards SAPIEN 3 | 4 (31) |
| Success of implant, n (%) | 13 (100) |
| In-hospital and 30-day mortality (%) | 0 |
| Degree of aortic regurgitation postimplant (0–IV) | 0 |
| Postimplant PPM, n (%) | 3 (23) |
| Postimplant NYHA Class I–II (%) | 100 |
NYHA: New York Heart Association.
| . | n = 13 . |
|---|---|
| Valve type, n (%) | |
| CoreValve Classic | 5 (38) |
| Corevalve Evolut R | 4 (31) |
| Edwards SAPIEN 3 | 4 (31) |
| Success of implant, n (%) | 13 (100) |
| In-hospital and 30-day mortality (%) | 0 |
| Degree of aortic regurgitation postimplant (0–IV) | 0 |
| Postimplant PPM, n (%) | 3 (23) |
| Postimplant NYHA Class I–II (%) | 100 |
| . | n = 13 . |
|---|---|
| Valve type, n (%) | |
| CoreValve Classic | 5 (38) |
| Corevalve Evolut R | 4 (31) |
| Edwards SAPIEN 3 | 4 (31) |
| Success of implant, n (%) | 13 (100) |
| In-hospital and 30-day mortality (%) | 0 |
| Degree of aortic regurgitation postimplant (0–IV) | 0 |
| Postimplant PPM, n (%) | 3 (23) |
| Postimplant NYHA Class I–II (%) | 100 |
NYHA: New York Heart Association.
As a routine follow-up, we repeated a transthoracic echocardiography on POD 1 for assessment of valve and ventricular function. All THVs were in an adequate position and functioning well with no new findings of regurgitant jets. All patients were discharged from the hospital on POD 3 in good condition except for 3 patients who received the PPM and were discharged home on POD 5. Short-term follow-up at 30 days and 3 months was complete. Patients are all alive and experienced an improvement in symptoms and quality of life. All patients were in NYHA Class I–II (Fig. 3).
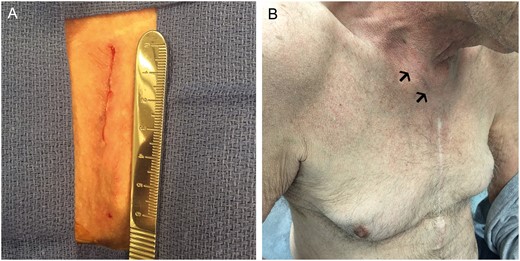
Result after closure of supraclavicular, parasternal incision, intraprocedural (A) and at 3-month follow-up (B).
DISCUSSION
Aortic valve replacement is the only definitive treatment available for severe symptomatic aortic stenosis. Improvement in surgical techniques, haemodynamics and performance of valve prostheses and newer and more efficient heart lung machines have rendered surgical aortic valve replacement a more controlled and safe procedure with overall low intra- and periprocedural mortality. Nevertheless, patient comorbidities make surgical aortic valve replacement a morbid procedure with risk of complications that are not negligible in the short and middle term, especially in certain categories of patients [8, 9].
New technologies and minimally invasive approaches have been developed over the past decade to minimize surgical trauma, invasiveness, operating time and improving overall recovery and prognosis. Upper ministernotomies or right parasternal thoracotomies are now widely used to preserve respiratory function and reduce pain to accelerate recuperation of postoperative functional [10]. Sutureless valves are slowly gaining popularity because of faster implantation process and drastic reduction of clamp and bypass times [11]. Despite improvements in techniques and devices, some patients portend a significant risk of mortality and morbidity. TAVR is now a surgical alternative with transfemoral access being the preferred approach for valve introduction and deployment. However, if the iliofemoral vessels are small, calcified or severely diseased, alternative access requires consideration [12]. The apex of the left ventricle, the ascending aorta, the carotid artery and the inferior vena cava (coupled with the descending aorta) are all viable options, depending on the anatomy and the quality/size of the vessels; nonetheless, each has its own risks.
The TA approach was considered during the initial experience of balloon-expandable TAVRs. Surgeons feel comfortable enough to work on the LV apex, even through a small lateral thoracotomy incision [13]. Despite the vast experience described in the literature with TA, this remains an invasive and morbid procedure, not ideal in patients with a low left ventricular ejection fraction, those prone to arrhythmias, or in reoperations with coronary artery disease and lateral/anterior grafts, where the apex of the left ventricle can be ‘frail’ and weak [14–16]. Rahnavardi et al. [17] published a meta-analysis on over 2700 cases focused on clinical outcomes and major complications in TA TAVI from 2000 until 2012. In these large series, implantation success rates varied between 93% and 100% with a 30-day and 3-year mortality rates of 10% and almost 60%, respectively; major complications were frequent, including cardiac tamponade (0–11%); major bleeding (1–17%); myocardial infarction (0–6%); cardiopulmonary bypass support (0–15%) and conversion to surgery (up to 9.5%). Moreover, the authors pointed out that a learning curve had a key role in improving the operator’s and institutions’ overall outcome. Many centres including ours have moved away from the TA approach, because there are options that are safer, less invasive and equally efficient and reproducible.
Upper ministernotomy for a TAo approach is a safe and widely used technique to reconstruct the ascending aorta and aortic root. Surgeons are accustomed to cannulating a soft area of the aorta as a routine for establishment of cardiopulmonary bypass. Cases in which the mediastinum has already been violated may increase the risk of re-entry. Eight of our patients had coronary artery bypass grafting in the past, with LIMA and vein grafts in proximity of the sternum. Avoidance of resternotomy, either full or partial, would decrease the overall risk of the procedure. Moreover, despite the fact that the majority of the respiratory muscles and the diaphragm remain intact during upper ministernotomy, this remains an invasive procedure with a higher morbidity and longer recovery time when compared with procedures that do not violate the chest. Furthermore, right parasternal thoracotomy in the second intercostal space is another option to access the ascending aorta. Depending on the anatomical configuration of the aorta and its orientation, this approach may also be problematic, especially in reoperations and previous coronary artery bypass grafting s [13].
TC approach has been considered by some centres as an alternative access for valve delivery and deployment, although it has only been the subject of small series in patients considered not suitable for TF, TA and TAo approaches. Modine et al. [18] described results on 12 patients who underwent TC TAVR: implantation success was 100%, 30-day mortality was 0% and only 1 patient experienced a minor embolic neurological impairment, albeit on the opposite site of the carotid access. Carotids can be accessed through a lateral neck incision and have a smaller calibre compared with the innominate arteries. Moreover, neck anatomy is complex: nerves, arteries, veins and muscles are grouped in a very small area, and exposure can be troublesome and difficult if not performed by experienced vascular surgeons. Moreover, subclinical plaques and calcifications can be present, and the risk of mobilization of these plaques or even simple manipulation of the conduit might significantly increase the risk of stroke.
We believe that the brachiocephalic artery is a good compromise of all these options. The vessel typically is of good calibre, wide enough to accommodate a 16-Fr or 18-Fr sheath used for valve introduction. Atheromatous and calcific disease, if present, is usually limited to the proximal portion of the arterial tree. Exposure is easy and a wide portion of the artery can be visualized, from its origin to the bifurcation, which helps in maintaining proximal and distal control. Neither bone fracture (as in upper sternotomies) nor rib spreading (as in right or left thoracotomies for direct aortic or TA procedures) is necessary, and none of the pleural spaces are violated. Since chest tube/drains are not required, early mobilization and improved respiratory effort are enhanced and minimal pain or discomfort has been reported.
Surgeons are confident in dealing with the brachiocephalic artery, because it is manipulated in the same way as the aortic arch or the subclavian artery. Preoperative CTA well delineates the anatomy of the innominate artery in relation to the sternum and surrounding structures. A TI approach is feasible if the vessel is superficial and not too deep beneath the manubrium. Bail out in case of difficult control of the proximal segment of the artery is a ministernotomy with a J incision down to the second or third right intercostal space. Placement of purse-string sutures and providing haemostasis require the same skill set that cardiac surgeons use to manipulate the aorta, the femoral vessels or the subclavian arteries. To date, we have not had any minor or major vascular complications, and none of the patients experienced any neurological deficit. We tend to secure haemostasis after the sheaths are removed without leaving any drain in the small pocket. None of the patients experienced postoperative pain or discomfort. The incision is small enough to promote early mobilization, and since respiratory mechanics is not affected, recovery is fast and uncomplicated. Also, since the entry point on the vessel is only a few centimetres away and typically in direct line with the aortic valve annulus, operators have direct control over the valve delivery system, and fine adjustments can be made with ease.
Improvement of transcatheter technologies and smaller size delivery systems give the operators several safe options for valve implantation. Our team strongly believes that no alternative access should be universally used but should be personalized to each patient, depending on the surgical history, anatomy and preoperative imaging. We find the TI a good first alternative access option, but there are patients in whom this approach is not ideal, for example in cases of previous neck surgery or a diseased/calcified brachiocephalic artery. In these rare instances, our team prefers a subclavian/axillary (preferably on the left) or a direct aortic approach.
Recently published 2016 STS/ACC Transcatheter Valve Therapy Registry shows that approximately 25% of all TAVR from 2012 until 2015 were conducted via a non-femoral approach. Despite a clear trend of increasing transfemoral cases due to smaller size catheters and delivery systems, alternative route TAVR was still utilized in 13% of patients in 2015 alone [19]. We feel that among this population with poor peripheral vascular access requiring an alternative route for valve delivery, a majority of these cases would be good candidates for a TI approach.
Limitations
A limitation of this case series is population size. Given improvements in TAVR technologies, the majority of TAVR procedures are performed transfemorally. Our centre does have significant experience with all alternative approaches; nonetheless, this is a novel approach for our team. The TI approach was not randomized to any other alternative access. Follow-up, although complete, was short. A longer follow-up is needed, including clinical assessment and imaging evaluation of access.
CONCLUSIONS
TI approach represents a safe, reproducible and minimally invasive hybrid technique for TAVR in high-risk patients. In our early experience, surgical trauma and perioperative complications were minimal with rapid patient recovery. With smaller devices and advances in pre- and intraprocedural imaging technologies, the need for non-transfemoral access will continue to decrease. Nevertheless, this need will remain in this complex patient population; larger studies are needed comparing TI with these other alternative access approaches.
Conflict of interest: none declared.




