-
PDF
- Split View
-
Views
-
Cite
Cite
Maximilian Kreibich, Tim Berger, Julia Morlock, Stoyan Kondov, Johannes Scheumann, Fabian A Kari, Bartosz Rylski, Matthias Siepe, Friedhelm Beyersdorf, Martin Czerny, The frozen elephant trunk technique for the treatment of acute complicated Type B aortic dissection, European Journal of Cardio-Thoracic Surgery, Volume 53, Issue 3, March 2018, Pages 525–530, https://doi.org/10.1093/ejcts/ezx281
Close - Share Icon Share
Abstract
Our goal was to report our preliminary results in patients with acute complicated Type B aortic dissection without a suitable landing zone for primary thoracic endovascular aortic repair who were treated with the frozen elephant trunk (FET) technique.
Within a 25-month period, 14 patients with acute complicated Type B aortic dissection underwent surgical repair using the FET technique. The reasons to perform the FET procedure were an ectatic ascending aorta/arch in 6 patients and the lack of an adequate landing zone in 8 patients.
No deaths were observed. A non-disabling stroke occurred in 2 patients. Symptomatic spinal cord injury was not observed. The closure of the primary entry tear was successfully achieved in all patients. In 3 patients, a secondary distal thoracic endovascular aortic repair extension was performed during the same hospital stay. The median follow-up period was 6 ± 5 months.
The FET technique is an attractive method for the repair of acute complicated Type B aortic dissection without a suitable landing zone for primary thoracic endovascular aortic repair. It should be considered as an alternative in patients who are at high risk for retrograde Type A aortic dissection, in patients with an unfavourable anatomy or in patients with connective tissue disease.
INTRODUCTION
The standard treatment for acute complicated Type B aortic dissection is thoracic endovascular aortic repair (TEVAR) [1]. However, some patients do not have a sufficient proximal landing zone and therefore require various degrees of supra-aortic transposition to achieve stable stent graft deployment. The most complete variant of supra-aortic transposition, total arch rerouting, is associated with an excessive incidence of retrograde Type A aortic dissection in patients with acute and chronic Type B aortic syndromes [2]. In addition, unfavourable aortic anatomy or connective tissue disease may prevent TEVAR from being an advisable therapeutic option. The frozen elephant trunk (FET) technique has emerged as a safe and an effective treatment modality in various proximal thoracic aortic diseases [3, 4]. Consequently, this method may also be appropriate for the treatment of acute complicated Type B aortic dissection in the aforementioned group of patients.
Our goal was to report our preliminary results in patients with acute complicated Type B aortic dissection without an adequate landing zone for primary TEVAR who were treated with the FET technique.
PATIENTS AND METHODS
Patients
The study population comprised 14 patients (10 men and 4 women) aged 63 ± 10 years operated on between January 2015 and February 2017. All patients presented with acute complicated Type B aortic dissection and were treated by total aortic arch replacement with the FET technique by median sternotomy. Early and mid-term outcomes were evaluated.
Definition of clinical parameters
Mortality was defined as in-hospital death. Neurological injury was defined according to the Valve Academic Research Consortium (VARC) and was sub-classified as disabling and non-disabling strokes [5, 6]. Complicated Type B aortic dissection was defined as clinical malperfusion involving the visceral, renal or iliac axis arteries, rupture or impending rupture (mediastinal haematoma formation, Hounsfield units of pleural effusion >30 and rapid morphological alterations such as transition from a standard distal arch formation to an eccentric bulging, wall weakening or discontinuity), uncontrolled hypertension, persistent pain or findings of rapid early diameter expansion (increase of ≥6 mm) on computed tomography (CT) imaging [7]. Acute aortic dissection was diagnosed when the onset of symptoms occurred fewer than 14 days before the clinical presentation [7]. Coronary angiography was performed preoperatively via the right radial artery when there was significant evidence for coronary calcification in the CT scans to diagnose coronary artery disease.
Data collection and follow-up protocol
Data were collected retrospectively using our clinical database. After discharge, regular follow-up examinations were scheduled in our outpatient aortic clinic, including clinical examinations and CT scans. Aortic diameters and segmental lengths were analysed from electrocardiography-gated CT angiography scans. A slice thickness of 3 mm or less was accepted and present in all patients. Analysis was performed using the Impax EE (Agfa HealthCare N.V., Mortsel, Belgium). All the measurements were taken using multiplanar reconstruction, always in the plane perpendicular to the manually corrected local aortic centre line. The aortic diameter was calculated as the mean of the maximum and minimal diameters. Aortic segments were defined as the level of the stented segment (L1 segment), the level of the thoraco-abdominal transition (L2 segment) and the level of the celiac trunk (L3 segment). We analysed the CT scans obtained before and after the operation and at the last follow-up visit.
Technique of the frozen elephant trunk implantation, perfusion management and neuroprotection
The Thoraflex hybrid prosthesis (Vascutec, a Terumo Company, Inchinnan, UK) was used in all patients. It consists of a 4-branched conventional gelatin-coated woven polyester prosthesis and an arch graft with a self-expanding stent graft (thin-walled polyester and nitinol ring stents) at the distal end (Fig. 1). Patients undergoing the FET procedure were monitored with bilateral radial and left-sided femoral blood pressure monitoring. Near-infrared spectroscopy was used in all cases. Cerebrospinal fluid drainage was not used in this cohort. The 100-mm stent grafts were used in all patients. The diameters of the graft portion varied between 26 mm and 30 mm, and the diameters of the stent graft portion of the prosthesis varied between 28 mm and 32 mm. A calculated surrogate diameter was used to size the FET prosthesis: The anticipated predissection aortic diameters were calculated at the level of the left sub-clavian artery (LSA); a 20% increase in aortic diameter after dissection was assumed. In case of doubt, under-sizing was performed to avoid potential stent graft-induced new entries.
![Intraoperative situs after the frozen elephant trunk procedure using the Thoraflex hybrid graft. Reproduced with permission from Czerny et al. [3].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/53/3/10.1093_ejcts_ezx281/1/m_ezx281f1.jpeg?Expires=1747916026&Signature=qPmWciF1JFewzWOtMTCavnj2TZeOF0-k4gIH0p9tYQnzvp09JEX8JRYaA1R4RvbuePWmll1uCAeelbG9a98FRWmtrxkMGqJxCbJw1276PsKBWeMsypFyvvKjIwWDWOQox8lnxWTZc5cJo8avY5pyU~LkUhF0Vajb0YGRGaoSna6DvVy0wqAkPegeSyde3ONEueYe0LbwANfkGmwUHVKBmtwrBXO732prqNXSWka5MJBzkFnyRdcAEZIf8krWp6ZsaKbnzDs0Qf8yJpcZIihrxVeadjxfsGom0PQrSw97MU1XtnFxbUI2Iuf8h80YpZa4RjcMAHz-c-eFm5F4EUo3fQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Intraoperative situs after the frozen elephant trunk procedure using the Thoraflex hybrid graft. Reproduced with permission from Czerny et al. [3].
The right sub-clavian artery was routinely cannulated for arterial return. The target core body temperature for lower body hypothermic circulatory arrest was 25–26 °C. In all patients, selective antegrade cerebral perfusion (SACP) and antegrade and retrograde cold blood cardioplegia were used. The brachiocephalic trunk and the left common carotid artery were dissected, and the LSA was exposed during the cooling period. After having reached the intended core temperature, the brachiocephalic trunk was clamped, and the right-sided SACP was begun together with lower body hypothermic circulatory arrest. As a next step, the left common carotid artery was released from the arch tissue, and the left-sided SACP was added. The LSA was circumferentially dissected and mobilized as cephalad as possible. Afterwards, the Thoraflex hybrid prosthesis was deployed. Transoesophageal echocardiography was used before circulatory arrest to confirm the orientation of the true lumen and to compare it with the CT scans. When a level of uncertainty remained during the FET deployment, aortoscopy was liberally used. Because of the short, flexible delivery system of the prosthesis, direct insertion is, in our opinion, as safe as the previously used standard method of placing a wire in a retrograde fashion when using other devices with a longer, less flexible introduction system. After deployment, the circumferential distal arch/descending aorta and the LSA anastomosis were performed. Antegrade systemic perfusion was re-established through the perfusion branch of the prosthesis. An additional pump was used for separate cerebral and systemic circulation. Thereafter, the left SACP was stopped, the cannulas were removed and the left common carotid artery and the brachiocephalic trunk anastomoses were performed. Finally, the adequate length to the sinotubular junction was determined, the graft was shortened accordingly and the last anastomosis was performed. The patient was weaned from cardiopulmonary bypass when the remaining anastomoses were completed, and he or she was rewarmed.
Statistical analysis
All values were expressed as mean ± standard deviation. Parameters between groups were compared using an unpaired t-test; exact P-values were calculated with IBM SPSS Statistic 20 software. The P-values of < 0.05 were considered significant.
RESULTS
Chronic health conditions, risk factors and previous aortic procedures
Patient demographics are summarized in Table 1. Ten men and 4 women, aged 63 ± 10 years, were treated with the FET technique. Hypertension was the most frequent risk factor in the cohort. Three patients presented with significant coronary artery disease, and 1 patient presented with chronic obstructive lung disease. Finally, 2 patients (1 with connective tissue disease) had previous ascending aortic replacement and sustained their Type B aortic dissection as an independent secondary aortic event.
| Demographics | |
| Age (years), median ± SD | 63 ± 10 |
| Female, n | 4 |
| Chronic health conditions and risk factors, n | |
| Arterial hypertension | 13 |
| Chronic obstructive pulmonary disease | 1 |
| Diabetes mellitus | 0 |
| Chronic renal impairment | 0 |
| Coronary artery disease | 3 |
| Extracardiac arteriopathy | 0 |
| History of stroke | 0 |
| Redo surgery | 2 |
| Surgical indications (multiple causes possible), n | |
| Malperfusion | 4 |
| Impending rupture | 10 |
| Persistent pain | 1 |
| Rapid diameter expansion | 5 |
| Uncontrolled hypertension | 0 |
| Demographics | |
| Age (years), median ± SD | 63 ± 10 |
| Female, n | 4 |
| Chronic health conditions and risk factors, n | |
| Arterial hypertension | 13 |
| Chronic obstructive pulmonary disease | 1 |
| Diabetes mellitus | 0 |
| Chronic renal impairment | 0 |
| Coronary artery disease | 3 |
| Extracardiac arteriopathy | 0 |
| History of stroke | 0 |
| Redo surgery | 2 |
| Surgical indications (multiple causes possible), n | |
| Malperfusion | 4 |
| Impending rupture | 10 |
| Persistent pain | 1 |
| Rapid diameter expansion | 5 |
| Uncontrolled hypertension | 0 |
SD: standard deviation.
| Demographics | |
| Age (years), median ± SD | 63 ± 10 |
| Female, n | 4 |
| Chronic health conditions and risk factors, n | |
| Arterial hypertension | 13 |
| Chronic obstructive pulmonary disease | 1 |
| Diabetes mellitus | 0 |
| Chronic renal impairment | 0 |
| Coronary artery disease | 3 |
| Extracardiac arteriopathy | 0 |
| History of stroke | 0 |
| Redo surgery | 2 |
| Surgical indications (multiple causes possible), n | |
| Malperfusion | 4 |
| Impending rupture | 10 |
| Persistent pain | 1 |
| Rapid diameter expansion | 5 |
| Uncontrolled hypertension | 0 |
| Demographics | |
| Age (years), median ± SD | 63 ± 10 |
| Female, n | 4 |
| Chronic health conditions and risk factors, n | |
| Arterial hypertension | 13 |
| Chronic obstructive pulmonary disease | 1 |
| Diabetes mellitus | 0 |
| Chronic renal impairment | 0 |
| Coronary artery disease | 3 |
| Extracardiac arteriopathy | 0 |
| History of stroke | 0 |
| Redo surgery | 2 |
| Surgical indications (multiple causes possible), n | |
| Malperfusion | 4 |
| Impending rupture | 10 |
| Persistent pain | 1 |
| Rapid diameter expansion | 5 |
| Uncontrolled hypertension | 0 |
SD: standard deviation.
Surgical characteristics of the cohort
Surgical characteristics and operative times are summarized in Table 2. The median period between acute onset of initial symptoms and treatment by FET was 6 ± 5 days, and the repeat CT scan was performed immediately before the operation. The main indications for the operation were newly developing signs of impending rupture or early diameter progression. In the 5 patients with early diameter progression, the mean increase in diameter was 7.0 ± 5.3 mm at the proximal descending aortic level, 6.8 ± 6.3 mm at the level of the thoraco-abdominal transition and 4.5 ± 4.3 mm at the level of the celiac trunk. Four patients presented with true-lumen collapse malperfusion due to dynamic occlusion of the vessels of visceral organs, the renal vessels, the iliac axis or any combination of these. One patient with impending rupture also presented with refractory pain. Concomitant procedures were performed in 6 patients, including coronary artery bypass grafting, aortic valve replacement or repair including replacement of the ascending aorta. One patient was also treated with tricuspid valve annuloplasty.
| Time between diagnosis and FET procedure in days, median ± SD | 6 ± 5 |
| Additional cardiac and vascular procedures, n | |
| CABG | 4 |
| Aortic valve replacement | 3 |
| David procedure | 1 |
| Bentall procedure | 1 |
| Tricuspid valve repair | 1 |
| Operative times (min), median ± SD | |
| CPB | 212 ± 48 |
| CX | 112 ± 34 |
| HCA, lower body | 37 ± 22 |
| SACP | 71 ± 12 |
| Time between diagnosis and FET procedure in days, median ± SD | 6 ± 5 |
| Additional cardiac and vascular procedures, n | |
| CABG | 4 |
| Aortic valve replacement | 3 |
| David procedure | 1 |
| Bentall procedure | 1 |
| Tricuspid valve repair | 1 |
| Operative times (min), median ± SD | |
| CPB | 212 ± 48 |
| CX | 112 ± 34 |
| HCA, lower body | 37 ± 22 |
| SACP | 71 ± 12 |
CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; CX: cross-clamp; HCA: hypothermic circulatory arrest; FET: frozen elephant trunk; SACP: selective antegrade cerebral perfusion; SD: standard deviation.
| Time between diagnosis and FET procedure in days, median ± SD | 6 ± 5 |
| Additional cardiac and vascular procedures, n | |
| CABG | 4 |
| Aortic valve replacement | 3 |
| David procedure | 1 |
| Bentall procedure | 1 |
| Tricuspid valve repair | 1 |
| Operative times (min), median ± SD | |
| CPB | 212 ± 48 |
| CX | 112 ± 34 |
| HCA, lower body | 37 ± 22 |
| SACP | 71 ± 12 |
| Time between diagnosis and FET procedure in days, median ± SD | 6 ± 5 |
| Additional cardiac and vascular procedures, n | |
| CABG | 4 |
| Aortic valve replacement | 3 |
| David procedure | 1 |
| Bentall procedure | 1 |
| Tricuspid valve repair | 1 |
| Operative times (min), median ± SD | |
| CPB | 212 ± 48 |
| CX | 112 ± 34 |
| HCA, lower body | 37 ± 22 |
| SACP | 71 ± 12 |
CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; CX: cross-clamp; HCA: hypothermic circulatory arrest; FET: frozen elephant trunk; SACP: selective antegrade cerebral perfusion; SD: standard deviation.
Outcome characteristics
No deaths were observed in the cohort. Our strategy resolved malperfusion in all cases. Haemofiltration was not warranted in any patient, and all patients were weaned from the respirator without tracheotomy. No cases of paraplegia or myocardial infarction were observed. Two patients presented with a non-disabling right hemispheric ischaemic stroke. One patient presented with a left-sided recurrent nerve palsy. In 3 patients, secondary distal TEVAR extension was performed after 5 ± 2 days. In those patients, multiple downstream communications between the true lumen and the false lumen at the thoracic level were detectable, and TEVAR was performed to enhance complete false-lumen thrombosis down to the level of the diaphragm. The median follow-up period was 6 ± 5 months. The outcome characteristics are summarized in Table 3, and the representative computed tomography scans before and after the operation are shown in Fig. 2.
| In-hospital complications, n | |
| In-hospital deaths | 0 |
| Dialysis | 0 |
| Acute myocardial infarction | 0 |
| Non-disabling stroke | 2 |
| Disabling stroke | 0 |
| Paraplegia | 0 |
| Recurrent nerve palsy | 1 |
| Tracheotomy | 0 |
| Late outcome | |
| Follow-up (months), median ± SD | 6 ± 5 |
| Late deaths, n | 0 |
| Distal stent graft implantation, n | 3 |
| In-hospital complications, n | |
| In-hospital deaths | 0 |
| Dialysis | 0 |
| Acute myocardial infarction | 0 |
| Non-disabling stroke | 2 |
| Disabling stroke | 0 |
| Paraplegia | 0 |
| Recurrent nerve palsy | 1 |
| Tracheotomy | 0 |
| Late outcome | |
| Follow-up (months), median ± SD | 6 ± 5 |
| Late deaths, n | 0 |
| Distal stent graft implantation, n | 3 |
SD: standard deviation.
| In-hospital complications, n | |
| In-hospital deaths | 0 |
| Dialysis | 0 |
| Acute myocardial infarction | 0 |
| Non-disabling stroke | 2 |
| Disabling stroke | 0 |
| Paraplegia | 0 |
| Recurrent nerve palsy | 1 |
| Tracheotomy | 0 |
| Late outcome | |
| Follow-up (months), median ± SD | 6 ± 5 |
| Late deaths, n | 0 |
| Distal stent graft implantation, n | 3 |
| In-hospital complications, n | |
| In-hospital deaths | 0 |
| Dialysis | 0 |
| Acute myocardial infarction | 0 |
| Non-disabling stroke | 2 |
| Disabling stroke | 0 |
| Paraplegia | 0 |
| Recurrent nerve palsy | 1 |
| Tracheotomy | 0 |
| Late outcome | |
| Follow-up (months), median ± SD | 6 ± 5 |
| Late deaths, n | 0 |
| Distal stent graft implantation, n | 3 |
SD: standard deviation.
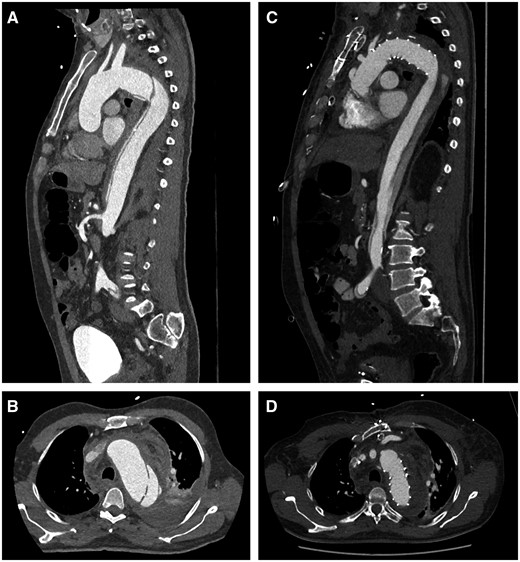
Representative computer tomography scan: sagittal plane view of an acute Stanford Type B aortic dissection with a significant true-lumen collapse (A) and a rupture site causing a left haemothorax (B). Postoperative images following the frozen elephant trunk procedure: a significant true-lumen expansion and almost complete false-lumen thrombosis in the sagittal plane view (C) with successful closure of the entry (D).
Aortic characteristics
The majority of visceral and renal vessels originated from the true lumen. In 6 patients, the left renal artery originated from the false lumen; in 1 patient, the right renal artery originated from the false lumen. In 3 patients, the celiac trunk originated from the false lumen, and in 1 patient, the superior mesenteric artery originated from the false lumen. Visceral and/or renal vessels originating from both lumina were observed in 1 patient in this cohort (Supplementary Material, Table S1). The dissection extended into the descending thoracic aorta in 2 patients, into the abdominal aorta in 4 patients and into the iliac arteries in 8 patients. In 10 patients, the dissection extended to the LSA, and in 4 patients, the dissection included supra-aortic vessels and the aortic arch. One patient of the cohort was diagnosed with Marfan syndrome, and 3 patients presented with bicuspid aortic valve. One patient had a bovine trunk. No other aortic arch anomalies were observed. Ascending aortic and aortic arch diameters are summarized in Table 4.
| Marfan syndrome, n | 1 |
| Cuspidity of aortic valve, n | |
| Bicuspid | 3 |
| Tricuspid | 6 |
| Ascending aorta, median ± SD | |
| Diameter at ST junction (mm) | 35.8 ± 3.6 |
| Mid-ascending diameter (mm) | 38.9 ± 6.6 |
| Distance ST junction to BCT (mm) | 81.2 ± 12.5 |
| Arch vessel abnormalities, n | |
| Bovine trunk | 1 |
| Isolated vertebral offspring | 0 |
| Aortic arch diameter, median ± SD | |
| Distal BCT (mm) | 38.0 ± 5.4 |
| Distal LCCA (mm) | 37.2 ± 5.2 |
| Distal LSA (mm) | 37.1 ± 6.9 |
| Marfan syndrome, n | 1 |
| Cuspidity of aortic valve, n | |
| Bicuspid | 3 |
| Tricuspid | 6 |
| Ascending aorta, median ± SD | |
| Diameter at ST junction (mm) | 35.8 ± 3.6 |
| Mid-ascending diameter (mm) | 38.9 ± 6.6 |
| Distance ST junction to BCT (mm) | 81.2 ± 12.5 |
| Arch vessel abnormalities, n | |
| Bovine trunk | 1 |
| Isolated vertebral offspring | 0 |
| Aortic arch diameter, median ± SD | |
| Distal BCT (mm) | 38.0 ± 5.4 |
| Distal LCCA (mm) | 37.2 ± 5.2 |
| Distal LSA (mm) | 37.1 ± 6.9 |
BCT: brachiocephalic trunk; LCCA: left common carotid artery; LSA: left sub-clavian artery; SD: standard deviation; ST: sinotubular.
| Marfan syndrome, n | 1 |
| Cuspidity of aortic valve, n | |
| Bicuspid | 3 |
| Tricuspid | 6 |
| Ascending aorta, median ± SD | |
| Diameter at ST junction (mm) | 35.8 ± 3.6 |
| Mid-ascending diameter (mm) | 38.9 ± 6.6 |
| Distance ST junction to BCT (mm) | 81.2 ± 12.5 |
| Arch vessel abnormalities, n | |
| Bovine trunk | 1 |
| Isolated vertebral offspring | 0 |
| Aortic arch diameter, median ± SD | |
| Distal BCT (mm) | 38.0 ± 5.4 |
| Distal LCCA (mm) | 37.2 ± 5.2 |
| Distal LSA (mm) | 37.1 ± 6.9 |
| Marfan syndrome, n | 1 |
| Cuspidity of aortic valve, n | |
| Bicuspid | 3 |
| Tricuspid | 6 |
| Ascending aorta, median ± SD | |
| Diameter at ST junction (mm) | 35.8 ± 3.6 |
| Mid-ascending diameter (mm) | 38.9 ± 6.6 |
| Distance ST junction to BCT (mm) | 81.2 ± 12.5 |
| Arch vessel abnormalities, n | |
| Bovine trunk | 1 |
| Isolated vertebral offspring | 0 |
| Aortic arch diameter, median ± SD | |
| Distal BCT (mm) | 38.0 ± 5.4 |
| Distal LCCA (mm) | 37.2 ± 5.2 |
| Distal LSA (mm) | 37.1 ± 6.9 |
BCT: brachiocephalic trunk; LCCA: left common carotid artery; LSA: left sub-clavian artery; SD: standard deviation; ST: sinotubular.
An increase in the diameter of the true lumen was observed after the FET procedure compared to preoperative diameters at all levels: at the level of the stented segment (L1 segment), at the level of the thoraco-abdominal transition (L2 segment) and at the level of the celiac trunk (L3 segment). The increase in the diameter of the true lumen reached statistical significance at the L1 segment in the postoperative and in the latest follow-up CT scans and did not reach statistical significance at the L2 or L3 segment. Correspondingly, the postoperative diameter of the false lumen decreased in all segments without reaching statistical significance. During later follow-up visits, the diameter of the false lumen decreased significantly in the L1 segment, whereas it was constant compared to the postoperative controls in the L2 and L3 segments. The maximal aortic diameter decreased in the L1 segment during late follow-up, whereas it was relatively stable in the L2 and L3 segments. All changes in aortic diameter are tabulated in Table 5. Postoperatively, complete false-lumen thrombosis was detected in 69% in the L1 segment, in 23% in the L2 segment and in 38% in the L3 segment. During later follow-up, complete false-lumen thrombosis was observed in 100% in the L1 segment and in 50% in the L2 and L3 segments.
| . | Preoperatively (n = 12) . | Postoperatively (n = 13) . | P-value . | Latest FU (n = 6) . | P-value . |
|---|---|---|---|---|---|
| L1 | |||||
| True lumen | 17.8 ± 8.5 | 27.6 ± 4.6 | <0.01 | 33.5 ± 5.0 | <0.01 |
| False lumen | 21.7 ± 5.3 | 16.5 ± 5.7 | 0.07 | 5.2 ± 7.8 | <0.01 |
| Maximum diameter (mm) | 41.0 ± 7.5 | 45.7 ± 7.6 | 0.17 | 38.7 ± 6.8 | 0.53 |
| L2 | |||||
| True lumen | 14.8 ± 9.1 | 21.6 ± 10. | 1.00 | 23.8 ± 10.6 | 0.09 |
| False lumen | 17.4 ± 8.6 | 12.6 ± 8.0 | 0.13 | 13.3 ± 12.5 | 0.21 |
| Maximum diameter (mm) | 35.5 ± 6.8 | 36.5 ± 5.5 | 0.70 | 37.1 ± 6.8 | 0.67 |
| L3 | |||||
| True lumen | 13.3 ± 6.8 | 19.1 ± 7.3 | 0.07 | 20.7 ± 8.7 | 0.10 |
| False lumen | 19.9 ± 2.4 | 12.8 ± 7.7 | 0.10 | 11.1 ± 11.2 | 0.16 |
| Maximum diameter (mm) | 31.0 ± 5.4 | 31.4 ± 4.4 | 0.88 | 31.7 ± 3.5 | 0.78 |
| . | Preoperatively (n = 12) . | Postoperatively (n = 13) . | P-value . | Latest FU (n = 6) . | P-value . |
|---|---|---|---|---|---|
| L1 | |||||
| True lumen | 17.8 ± 8.5 | 27.6 ± 4.6 | <0.01 | 33.5 ± 5.0 | <0.01 |
| False lumen | 21.7 ± 5.3 | 16.5 ± 5.7 | 0.07 | 5.2 ± 7.8 | <0.01 |
| Maximum diameter (mm) | 41.0 ± 7.5 | 45.7 ± 7.6 | 0.17 | 38.7 ± 6.8 | 0.53 |
| L2 | |||||
| True lumen | 14.8 ± 9.1 | 21.6 ± 10. | 1.00 | 23.8 ± 10.6 | 0.09 |
| False lumen | 17.4 ± 8.6 | 12.6 ± 8.0 | 0.13 | 13.3 ± 12.5 | 0.21 |
| Maximum diameter (mm) | 35.5 ± 6.8 | 36.5 ± 5.5 | 0.70 | 37.1 ± 6.8 | 0.67 |
| L3 | |||||
| True lumen | 13.3 ± 6.8 | 19.1 ± 7.3 | 0.07 | 20.7 ± 8.7 | 0.10 |
| False lumen | 19.9 ± 2.4 | 12.8 ± 7.7 | 0.10 | 11.1 ± 11.2 | 0.16 |
| Maximum diameter (mm) | 31.0 ± 5.4 | 31.4 ± 4.4 | 0.88 | 31.7 ± 3.5 | 0.78 |
Values are represented as median ± standard deviation.
FU: follow-up; L1: at the level of the stented segment; L2: at the level of the thoraco-abdominal transition; L3: at the level of the celiac trunk.
| . | Preoperatively (n = 12) . | Postoperatively (n = 13) . | P-value . | Latest FU (n = 6) . | P-value . |
|---|---|---|---|---|---|
| L1 | |||||
| True lumen | 17.8 ± 8.5 | 27.6 ± 4.6 | <0.01 | 33.5 ± 5.0 | <0.01 |
| False lumen | 21.7 ± 5.3 | 16.5 ± 5.7 | 0.07 | 5.2 ± 7.8 | <0.01 |
| Maximum diameter (mm) | 41.0 ± 7.5 | 45.7 ± 7.6 | 0.17 | 38.7 ± 6.8 | 0.53 |
| L2 | |||||
| True lumen | 14.8 ± 9.1 | 21.6 ± 10. | 1.00 | 23.8 ± 10.6 | 0.09 |
| False lumen | 17.4 ± 8.6 | 12.6 ± 8.0 | 0.13 | 13.3 ± 12.5 | 0.21 |
| Maximum diameter (mm) | 35.5 ± 6.8 | 36.5 ± 5.5 | 0.70 | 37.1 ± 6.8 | 0.67 |
| L3 | |||||
| True lumen | 13.3 ± 6.8 | 19.1 ± 7.3 | 0.07 | 20.7 ± 8.7 | 0.10 |
| False lumen | 19.9 ± 2.4 | 12.8 ± 7.7 | 0.10 | 11.1 ± 11.2 | 0.16 |
| Maximum diameter (mm) | 31.0 ± 5.4 | 31.4 ± 4.4 | 0.88 | 31.7 ± 3.5 | 0.78 |
| . | Preoperatively (n = 12) . | Postoperatively (n = 13) . | P-value . | Latest FU (n = 6) . | P-value . |
|---|---|---|---|---|---|
| L1 | |||||
| True lumen | 17.8 ± 8.5 | 27.6 ± 4.6 | <0.01 | 33.5 ± 5.0 | <0.01 |
| False lumen | 21.7 ± 5.3 | 16.5 ± 5.7 | 0.07 | 5.2 ± 7.8 | <0.01 |
| Maximum diameter (mm) | 41.0 ± 7.5 | 45.7 ± 7.6 | 0.17 | 38.7 ± 6.8 | 0.53 |
| L2 | |||||
| True lumen | 14.8 ± 9.1 | 21.6 ± 10. | 1.00 | 23.8 ± 10.6 | 0.09 |
| False lumen | 17.4 ± 8.6 | 12.6 ± 8.0 | 0.13 | 13.3 ± 12.5 | 0.21 |
| Maximum diameter (mm) | 35.5 ± 6.8 | 36.5 ± 5.5 | 0.70 | 37.1 ± 6.8 | 0.67 |
| L3 | |||||
| True lumen | 13.3 ± 6.8 | 19.1 ± 7.3 | 0.07 | 20.7 ± 8.7 | 0.10 |
| False lumen | 19.9 ± 2.4 | 12.8 ± 7.7 | 0.10 | 11.1 ± 11.2 | 0.16 |
| Maximum diameter (mm) | 31.0 ± 5.4 | 31.4 ± 4.4 | 0.88 | 31.7 ± 3.5 | 0.78 |
Values are represented as median ± standard deviation.
FU: follow-up; L1: at the level of the stented segment; L2: at the level of the thoraco-abdominal transition; L3: at the level of the celiac trunk.
DISCUSSION
The FET technique is an attractive method for the repair of acute complicated Type B aortic dissection in patients without a suitable landing zone for primary TEVAR and may be considered as an alternative in patients who are at high risk for retrograde Type A aortic dissection, in patients with an unfavourable anatomy or in patients with connective tissue disease.
Chronic health conditions and risk factors in this cohort were comparable to those noted in previous reports addressing the issue of complicated Type B aortic dissection. One patient with connective tissue disease sustained acute complicated Type B aortic dissection as an unrelated new event after previous repair of acute Type A aortic dissection showing morphologically regular diameters in downstream aortic segments in all CT scans that were completed before onset.
Several factors may obviate primary TEVAR in acute uncomplicated Type B aortic dissection [1, 2]. The need for supra-aortic transpositions to create a sufficient proximal landing zone for safe, accurate TEVAR is a frequently observed issue [2]. Sub-clavian-to-carotid transposition or bypass has proved to be an excellent method without an increased incidence of retrograde Type A aortic dissection. Also, double transposition is efficient in this clinical scenario. However, in patients in whom total arch rerouting is needed to create a sufficient proximal landing zone for TEVAR, the risk for retrograde Type A aortic dissection becomes extremely high [8–10]. The mechanisms behind this clinical observation are manifold. The most important factors seem to be an inherently diseased proximal thoracic aorta despite regular diameters in patients with underlying aortic dissection; injury incurred while tangentially clamping the ascending aorta to create a central anastomosis for total arch rerouting and finally compliance mismatch between a highly elastic ascending aorta and the relatively rigid nitinol frame of the stent graft [11]. Aortic diameters are another issue: It has been shown that, at a threshold of 40 mm of the ascending aorta, patients undergoing total arch rerouting, even when they have other underlying diagnoses, are at increased risk of retrograde Type A aortic dissection [12].
Clinical outcome in this series was excellent and more than comparable to outcomes in other series in which patients with acute complicated Type B aortic dissection were treated with primary TEVAR [13–15]. Because this approach has a disruptive component, it is extremely important to clearly define the cohort and to deliver results comparable to those with the standard treatment. Our conceptual approach for stratifying patients with acute Type B aortic dissection is as follows: If patients present with an acute complicated aortic dissection, they undergo immediate treatment. If they present with an uncomplicated aortic dissection, they are screened for morphological risk factors, for malperfusion and for early diameter increase as described previously, because there are sub-cohorts at risk of developing secondary complications after initially uncomplicated presentation, as was the case in this series [16–19].
If one or more of these parameters are present, patients are scheduled for TEVAR. The minimal required length of the proximal landing zone for safe, accurate stent graft deployment is 2 cm. If this criterion is not available, sub-clavian revascularization is performed prior to TEVAR. If the surgeon will be unable to create a sufficient proximal landing zone using sub-clavian revascularization or even double transposition, or if the ascending aorta or aortic arch diameter is >40 mm, then the patients are scheduled for the FET technique with the main intention to prevent retrograde Type A aortic dissection. Because it is our standard practice to exclusively use the short version of the prosthesis and because the majority of primary entry tears in patients with malperfusion or in patients at risk for early diameter increase are very close to the offspring of the LSA, the short version of the stent graft will be able to seal the majority of primary entry tears.
An increase in the diameter of the true lumen and a concomitant decrease in the diameter of the false lumen, in association with false-lumen thrombosis at several levels, were observed in all patients. The long-term efficiency is highly dependent on the number and the sizes of communications between the lumina. Yet, the ideal scenario of 1 entry tear, 1 re-entry tear, all visceral and renal arteries originating from the true lumen and no communication between lumina is the exception not the rule. This scenario will heal completely if the surgeon simply closes the primary entry tear [19, 20]. Any other scenario will leave false-lumen perfusion at any level. Yet, if there are multiple communications between lumina, which is a risk factor for diameter increase or if an increase in the diameter occurs late, TEVAR for distal extension remains a pragmatic and simple option. Using the short version of the stent graft also seems to be the main reason for the absence of symptomatic spinal cord injury. If a classical surgical extension is needed, it is the personal experience of the authors and the published experience of others that anastomoses between a stent graft and a classical Dacron prosthesis are feasible [21].
Interestingly, we did not note a relationship between true, false or true-lumen and false-lumen offspring of the renal or visceral arteries and the perfusion of the respective end organs. This association has been described previously, and it has been hypothesized that most vessels originate from the true lumen at the onset of disease and that secondary intimal–medial membrane rupture causes communication of both lumina, thereby creating the appearance of false-lumen offspring [22].
Limitations and strengths
This series was small but with a disruptive approach that enriches the treatment armamentarium for patients with acute complicated Type B aortic dissection. It is obvious that an aortic centre that treats the entire ‘organ aorta’ with both endovascular and open techniques is needed to provide this option and that, when one is confronted with such clinical scenarios, this approach represents the end rather than the beginning of a decision-making algorithm.
CONCLUSION
The FET technique is an attractive method for the repair of acute complicated Type B aortic dissection without a suitable landing zone for primary TEVAR and may be considered as an alternative for patients who are at high risk for retrograde Type A aortic dissection, in patients with an unfavourable anatomy or in patients with connective tissue disease. Also, the FET procedure provided an optimal proximal landing zone for later TEVAR. The mid- and long-term stability has to be re-evaluated.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Conflict of interest: none declared.
REFERENCES
Author notes
Maximilian Kreibich and Tim Berger contributed equally to this work.




