-
PDF
- Split View
-
Views
-
Cite
Cite
Matthew Panagiotou, Athanasios Manginas, Charalampos Kyriazis, Dimos Karangelis, Distal embolization of a transcatheter valve in a valve complex: a bail-out surgical approach, European Journal of Cardio-Thoracic Surgery, Volume 52, Issue 6, December 2017, Pages 1229–1230, https://doi.org/10.1093/ejcts/ezx267
Close - Share Icon Share
Transcatheter aortic valve replacement has emerged as an alternative to surgical aortic valve replacement for high-risk and inoperable patients. Although transcatheter aortic valve replacement avoids the use of extracorporeal circulation and sternotomy, it is nonetheless associated with inherent complications. We aim to present an embolized valve-in-valve complex in the ascending aorta, which required emergency surgery with deep hypothermic circulatory arrest and proximal aortic cannulation.
INTRODUCTION
The increased worldwide experience with transcatheter valves has definitely improved the safety of the procedure. However, emergency conversion from transcatheter aortic valve replacement (TAVR) to open-heart surgery has been reported to be approximately 0.5–2.7% [1]. Herein, we present a case of an attempted TAVR, which required a salvage surgical intervention.
CASE REPORT
An 85-year-old man with a history of hypertension, chronic obstructive pulmonary disease and coronary artery disease underwent a transfemoral TAVR procedure due to symptomatic severe aortic stenosis. The patient’s left ventricular ejection fraction was 46%, the pulmonary systolic pressure was 65 mmHg and the peak aortic valve gradient was 66 mmHg, with an aortic valve area of 0.8 cm2. The logistic EuroSCORE I was 40.7%. Aortic annulus and ascending aorta diameters were 23.1 × 26.9 mm with an annular perimeter of 74.2 mm in the computed tomography angiogram. Left and right coronary ostia distances from the aortic annulus were measured at 10.9 and 15.3 mm, respectively, and the native aortic valve was significantly calcified (Fig. 1A). The procedure was performed in a hybrid room under mild sedation and a 29-mm self-expanding Portico valve (St. Jude Medical, Minneapolis, MN, USA) was implanted. Despite the necessary oversizing and the use of rapid ventricular pacing, the prosthesis migrated in a slightly supra-annular position, resulting in severe regurgitation and haemodynamic collapse with no signs of coronary obstruction. The patient was immediately intubated and resuscitated. A brief attempt to withdraw the bioprosthesis distally using a snare device was unsuccessful. A second Portico valve of the same size was rapidly introduced through the first prosthesis, but both valves migrated into the ascending aorta. At this point, a third valve was thought to be the solution, so a valve in valve (ViV) was captured with a loop wire in tension to permit a third implantation (Fig. 1B). Despite coaxial anchoring of the complex and the use of an extra stiff guide wire, the 2 valves did not permit the delivery system to bend, so the effort was abandoned. Additionally, the inferior struts of the first implant were identified to penetrate the aortic wall (Fig. 1C).
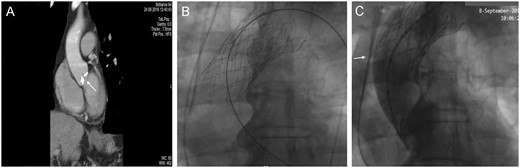
(A) Preoperative computed tomography scan showing the extent of the aortic valve calcification (white arrow). (B) Angiographic image. Projection of the valve in valve (ViV) after retrieval back to the ascending aorta. (C) Angiographic image. Projection of the ViV during contrast angiography. The inferior struts of the first implant are shown to penetrate the aortic wall (white arrow).
The elapsed time between the haemodynamic collapse and the abortion of TAVR was around 30 min. The surgical team was consulted, and within the next 20 min, an emergency median sternotomy was performed. Following pericardial dissection, a haematoma was identified on the ascending aorta (Fig. 2A). We came across a major dilemma in determining the optimal site of cannulation and cross-clamp. The ascending aorta was occupied by the ViV complex forcing us to cannulate proximally, as the prosthesis complex would not permit flow towards the coronary ostia and perfusion, if cannulation was done distally. In addition, there was also no option to cross-clamp, because the valve complex would be crushed causing unpredictable damage to the aorta. At that point, we felt it was more prudent to cannulate the aorta just above the sinotubular junction and cool the patient down to 22°C (deep hypothermic circulatory arrest).
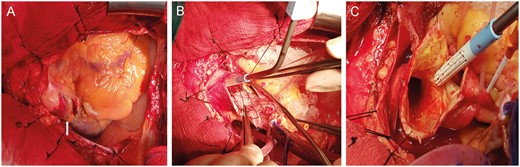
Intraoperative images: surgeon’s view. (A) Haematoma on the ascending aorta after dissection of the pericardium (white arrow). (B) Retrieval of the valve in valve from the ascending aorta. (C) The imprint of the valve skeleton in the aortic intima.
After aortotomy and removal of the valve complex (Fig. 2B and C), a second small aortic rupture associated with the upper struts was identified in the arch and sutured with 4/0 prolene. The aorta was recannulated distally and cross-clamped. Cardiopulmonary bypass was initiated, and during rewarming, the native valve cusps were removed, the annulus was decalcified and a self-expanding Perceval S (LivaNova Group, Milan, Italy) size L was implanted. The patient was weaned from cardiopulmonary bypass successfully and had an uneventful postoperative course and was discharged on the 14th postoperative day.
DISCUSSION
TAVR procedural complications resulted in emergency surgical involvement in up to 10.7% of the patients in the high-volume tertiary centre of Leipzig [1].
Hein et al. [2] reported that prosthesis embolization was the second most frequent cause of conversion from TAVI to emergency surgery after aortovalvular complications. Valve migration, in general, is usually well tolerated and usually treated with a new implant (ViV).
The occupation of a large portion of the ascending aorta by a bulky TAVR valve or a ViV complex represents a challenging scenario for every cardiac surgeon, as classical arterial cannulation sites, cross-clamp sites and myocardial protection protocols need to be revisited. Deep hypothermic circulatory arrest is an extremely useful technique for cardiac surgery cases, where inspection of the arch is mandatory and the cross-clamp use is considered precarious.
The use of sutureless valves confers the benefits of significant reduction in operative times and the provision of better effective orifice areas in comparison with any of the current available classic bioprostheses [1]. This case highlights that use of deep hypothermic circulatory arrest, and proximal cannulation can be successfully implemented in similar adverse scenarios.
Conflict of interest: none declared.




