-
PDF
- Split View
-
Views
-
Cite
Cite
Massimo A. Padalino, Anna Chiara Frigo, Marina Comisso, Martin Kostolny, Ikenna Omeje, Christian Schreiber, Jelena Pabst von Ohain, Julie Cleuziou, David J. Barron, Bart Meyns, Viktor Hraska, Bohdan Maruszewski, Michal Kozlowski, Luca A. Vricella, Narutoshi Hibino, Sarah Collica, Hakan Berggren, Mats Synnergren, Stojan Lazarov, David Kalfa, Emile Bacha, Christian Pizarro, Mark Hazekamp, Vlado Sojak, Jeffrey P. Jacobs, Matej Nosal, Jose Fragata, Sertac Cicek, George E. Sarris, Panayotis Zografos, Vladimiro L. Vida, Giovanni Stellin, Early and late outcomes after surgical repair of congenital supravalvular aortic stenosis: a European Congenital Heart Surgeons Association multicentric study, European Journal of Cardio-Thoracic Surgery, Volume 52, Issue 4, October 2017, Pages 789–797, https://doi.org/10.1093/ejcts/ezx245
Close - Share Icon Share
Abstract
Our goal was to evaluate the early and late results of the surgical management of congenital supravalvular aortic stenosis (SVAS).
We performed a retrospective, multicentre study using data from the European Congenital Heart Surgeons Association. Exclusion criteria were age >18 years, operation before 1990 and redo supravalvular aortic stenosis operations. Multivariate Cox regression analysis was performed to detect independent predictors of adverse events.
Of a total of 301 patients (male/female = 194/107; median age 3.9 years, range 13 days–17.9 years), 17.6% had a prior surgical or interventional procedure. Pulmonary artery stenosis was present in 41.5% and coronary anomalies in 13.6%. The operation consisted of a single patch repair in 36.7%, a pantaloon-shaped patch in 36.7%, a 3-patch technique in 14.3% and other techniques in 11.7%. Postoperative complications occurred in 14.9%, and the early mortality rate was 5%. At a median follow-up of 13 years (interquartile range 3.5–7.8; follow-up completed 79.1%), there were 10 late deaths (4.2%). A surgical reoperation or an interventional cardiology procedure occurred in 12.6% and 7.2%, respectively. No significant differences in outcomes between the techniques were found. Age at repair <12 months and pulmonary artery stenosis were associated with an increased risk of early (P = 0.0001) and overall mortality (P = 0.025), respectively. Having an operation after 2005 and co-existing pulmonary artery stenosis were significant predictors of late reintervention (P = 0.0110 and P = 0.001, respectively).
Surgical repair of congenital stenosis is an effective procedure with acceptable surgical risk and good late survival, but late morbidity is not negligible, especially in infants and when associated pulmonary artery stenosis is present.
Congenital supravalvular aortic stenosis (SVAS) is a rare form of aortic obstruction that may involve the aortic arch and also the brachiocephalic vessels in the most severe cases [1, 2]. The association of SVAS with mental retardation, characteristic facial features and pulmonary artery (PA) stenosis is known as Williams–Beuren syndrome (WBS) [3, 4], which is characterized by a microdeletion of the elastin gene on chromosome 7q11.23, which causes reduced elasticity, enhancing increased shear stress, collagen deposition and thickening of the aortic media [1, 5–7].
Several surgical approaches for SVAS repair have been described, but the optimal technique is still controversial. Most reports are of small clinical series covering a long interval [8–13].
The goal of this multicentre clinical study within the European Congenital Heart Surgeons Association was to analyse early results, associated procedures, late mortality rates and reinterventions across a large cohort of patients.
MATERIALS AND METHODS
This multicentre retrospective longitudinal study included patients who had surgical repair of SVAS. Exclusion criteria included operation before 1990, a previous surgical procedure involving the ascending aorta and age >18 years.
Review of medical records was approved by the local committee on clinical investigations from each hospital. Individual patients were not identified, and the need for patient consent was waived.
Preoperative data included the presence of associated WBS; distinction between focal (i.e. localized at the sinotubular junction) or diffuse (i.e. narrowing of the entire ascending aorta) stenosis; association of brachiocephalic vessels stenosis (as described in more severe cases [14]); aortic valve (AoV) anatomy and stenosis (i.e. peak echocardiography gradient >40 mmHg); coronary anomalies (origin or proximal course) identified at imaging (echocardiography, catheterization, computed tomography scan or magnetic resonance) and association with PA stenosis (central or peripheral, as revealed by echocardiography or catheterization), right or left ventricular outflow tract (R/LVOT) obstruction (including subvalvular and valvular stenosis) and aortic coarctation.
Intraoperative data included the type of repair and associated procedures, cardiopulmonary bypass and cross-clamp times, hospital stay, postoperative complications and early (in-hospital) death.
Surgical techniques included the McGoon repair (the single patch) [15]; the Doty technique (the pantaloon-shaped patch) [16] and the Brom technique (3 patches) (3) [17]. Miscellaneous techniques, such as the interdigitating technique and 2 separate patch repairs [18], were grouped together as other techniques.
Follow-up information was collected between January 2014 and December 2016 and recorded. All adverse events were recorded, including death (after discharge), surgical or interventional cardiology procedures, clinical status (expressed as New York Heart Association class) and detection of AoV regurgitation (AR) or AoV stenosis (AS).
Statistical analysis
Results regarding categorical variables report the number and percentage of subjects in each category; for quantitative variables, mean and standard deviation, or median, interquartile range and minimum and maximum are used.
Univariable logistic regression analysis was used to find predictors of early death; predictors of overall mortality, surgical reintervention and interventional cardiology procedure were analysed with the univariable Cox regression model. Those variables that were statistically significant at the 10% level were introduced in a multivariable logistic regression model with backward selection. The results are presented as P-value, hazard ratio and 95% confidence interval. Surgical and interventional cardiology reinterventions were analysed with death as a competing event. Firth’s penalized maximum likelihood estimation was used in case of separability.
Overall survival was estimated with the Kaplan–Meier method; surgical reinterventions and interventional cardiology procedures were estimated with the cumulative incidence function, considering death as a competing event, and were accompanied by 95% confidence intervals.
The cumulative incidence functions for surgical reintervention and interventional cardiology are also presented by surgical technique.
Finally, we compared patients’ characteristic by enrolment time (1990–2005 and 2006–2015) using the χ2 test, the Fisher’s exact test and the Wilcoxon rank-sum test.
Statistical analyses were performed with SAS 9.4 (SAS Institute, Inc., Cary, NC, USA) for Windows.
RESULTS
Patients
From January 1990 to December 2014, 301 patients from 18 European Congenital Heart Surgeons Association centres were included (Table 1); 73 patients (24.2%) were <12 months of age at the time of the operation.
| Variables . | Patients . | Percentage . |
|---|---|---|
| Total patients | 301 | 100 |
| Age at SVAS surgery (years), median (range) | 2.9 (0.03–17.97) | |
| Weight at SVAS surgery (kg), median (range) | 36.9 (4.1–72.5) | |
| Male , n | 194 | 64.4 |
| WBS, n | 176 | 58.5 |
| PA stenosis | 84 | |
| Coronary anomalies | 28 | |
| LVOTO | 12 | |
| RVOTO | 9 | |
| Dyspnoea | 29 | 9.6 |
| Signs and symptoms | 73 | 24.2 |
| CHF | 46 | |
| Syncope | 8 | |
| Chest pain | 2 | |
| Ventricular fibrillation/ cardiac arrest | 2 | |
| Other | 2 | |
| Hypertension | 1 | |
| Diffuse type | 76 | 25.2 |
| Familial history of SVAS | 23 | 7.6 |
| Patients who had prior cardiac procedure(s) | 53 | 17.6 |
| Interventional (catheter) procedures prior to SVAS | 35 | 11.6 |
| Diagnostic and interventional catheterization (unspecified) | 16 | |
| PA BD | 6 | |
| AoV BD | 5 | |
| PV BD | 3 | |
| RVOTO BD | 1 | |
| Peripheral PA BD | 1 | |
| Surgical procedures prior to SVAS | 24 | 8.0 |
| Coarctation repair | 15 | |
| PA surgical reconstruction | 3 | |
| AoV repair | 3 | |
| SAS resection | 1 | |
| Modified Konno procedure | 1 | |
| MV replacement | 1 | |
| Associated cardiovascular anomalies | ||
| PA stenosis | 125 | 41.5 |
| AoV stenosis | 69 | 22.9 |
| BCV stenosis | 42 | 13.9 |
| Coronary artery anomalies | 41 | 13.6 |
| LVOTO | 38 | 12.6 |
| Bicuspid AoV | 34 | 11.3 |
| RVOTO | 15 | 5.0 |
| Aortic arch hypoplasia | 14 | 4.6 |
| Aortic coarctation | 9 | 3.0 |
| Renal artery stenosis | 6 | 2.0 |
| MV abnormalities | 1 | 0.3 |
| Variables . | Patients . | Percentage . |
|---|---|---|
| Total patients | 301 | 100 |
| Age at SVAS surgery (years), median (range) | 2.9 (0.03–17.97) | |
| Weight at SVAS surgery (kg), median (range) | 36.9 (4.1–72.5) | |
| Male , n | 194 | 64.4 |
| WBS, n | 176 | 58.5 |
| PA stenosis | 84 | |
| Coronary anomalies | 28 | |
| LVOTO | 12 | |
| RVOTO | 9 | |
| Dyspnoea | 29 | 9.6 |
| Signs and symptoms | 73 | 24.2 |
| CHF | 46 | |
| Syncope | 8 | |
| Chest pain | 2 | |
| Ventricular fibrillation/ cardiac arrest | 2 | |
| Other | 2 | |
| Hypertension | 1 | |
| Diffuse type | 76 | 25.2 |
| Familial history of SVAS | 23 | 7.6 |
| Patients who had prior cardiac procedure(s) | 53 | 17.6 |
| Interventional (catheter) procedures prior to SVAS | 35 | 11.6 |
| Diagnostic and interventional catheterization (unspecified) | 16 | |
| PA BD | 6 | |
| AoV BD | 5 | |
| PV BD | 3 | |
| RVOTO BD | 1 | |
| Peripheral PA BD | 1 | |
| Surgical procedures prior to SVAS | 24 | 8.0 |
| Coarctation repair | 15 | |
| PA surgical reconstruction | 3 | |
| AoV repair | 3 | |
| SAS resection | 1 | |
| Modified Konno procedure | 1 | |
| MV replacement | 1 | |
| Associated cardiovascular anomalies | ||
| PA stenosis | 125 | 41.5 |
| AoV stenosis | 69 | 22.9 |
| BCV stenosis | 42 | 13.9 |
| Coronary artery anomalies | 41 | 13.6 |
| LVOTO | 38 | 12.6 |
| Bicuspid AoV | 34 | 11.3 |
| RVOTO | 15 | 5.0 |
| Aortic arch hypoplasia | 14 | 4.6 |
| Aortic coarctation | 9 | 3.0 |
| Renal artery stenosis | 6 | 2.0 |
| MV abnormalities | 1 | 0.3 |
AoV: aortic valve; BCV: brachiocephalic vessels; BD: balloon dilatation: CHF: congestive heart failure; LVOTO: left ventricular outflow tract obstruction; MV: mitral valve; PA: pulmonary artery; PV: pulmonary valve; RVOTO: right ventricular outflow tract obstruction; SAS: subaortic stenosis; SVAS: supravalvular aortic stenosis; WBS: Williams–Beuren syndrome.
| Variables . | Patients . | Percentage . |
|---|---|---|
| Total patients | 301 | 100 |
| Age at SVAS surgery (years), median (range) | 2.9 (0.03–17.97) | |
| Weight at SVAS surgery (kg), median (range) | 36.9 (4.1–72.5) | |
| Male , n | 194 | 64.4 |
| WBS, n | 176 | 58.5 |
| PA stenosis | 84 | |
| Coronary anomalies | 28 | |
| LVOTO | 12 | |
| RVOTO | 9 | |
| Dyspnoea | 29 | 9.6 |
| Signs and symptoms | 73 | 24.2 |
| CHF | 46 | |
| Syncope | 8 | |
| Chest pain | 2 | |
| Ventricular fibrillation/ cardiac arrest | 2 | |
| Other | 2 | |
| Hypertension | 1 | |
| Diffuse type | 76 | 25.2 |
| Familial history of SVAS | 23 | 7.6 |
| Patients who had prior cardiac procedure(s) | 53 | 17.6 |
| Interventional (catheter) procedures prior to SVAS | 35 | 11.6 |
| Diagnostic and interventional catheterization (unspecified) | 16 | |
| PA BD | 6 | |
| AoV BD | 5 | |
| PV BD | 3 | |
| RVOTO BD | 1 | |
| Peripheral PA BD | 1 | |
| Surgical procedures prior to SVAS | 24 | 8.0 |
| Coarctation repair | 15 | |
| PA surgical reconstruction | 3 | |
| AoV repair | 3 | |
| SAS resection | 1 | |
| Modified Konno procedure | 1 | |
| MV replacement | 1 | |
| Associated cardiovascular anomalies | ||
| PA stenosis | 125 | 41.5 |
| AoV stenosis | 69 | 22.9 |
| BCV stenosis | 42 | 13.9 |
| Coronary artery anomalies | 41 | 13.6 |
| LVOTO | 38 | 12.6 |
| Bicuspid AoV | 34 | 11.3 |
| RVOTO | 15 | 5.0 |
| Aortic arch hypoplasia | 14 | 4.6 |
| Aortic coarctation | 9 | 3.0 |
| Renal artery stenosis | 6 | 2.0 |
| MV abnormalities | 1 | 0.3 |
| Variables . | Patients . | Percentage . |
|---|---|---|
| Total patients | 301 | 100 |
| Age at SVAS surgery (years), median (range) | 2.9 (0.03–17.97) | |
| Weight at SVAS surgery (kg), median (range) | 36.9 (4.1–72.5) | |
| Male , n | 194 | 64.4 |
| WBS, n | 176 | 58.5 |
| PA stenosis | 84 | |
| Coronary anomalies | 28 | |
| LVOTO | 12 | |
| RVOTO | 9 | |
| Dyspnoea | 29 | 9.6 |
| Signs and symptoms | 73 | 24.2 |
| CHF | 46 | |
| Syncope | 8 | |
| Chest pain | 2 | |
| Ventricular fibrillation/ cardiac arrest | 2 | |
| Other | 2 | |
| Hypertension | 1 | |
| Diffuse type | 76 | 25.2 |
| Familial history of SVAS | 23 | 7.6 |
| Patients who had prior cardiac procedure(s) | 53 | 17.6 |
| Interventional (catheter) procedures prior to SVAS | 35 | 11.6 |
| Diagnostic and interventional catheterization (unspecified) | 16 | |
| PA BD | 6 | |
| AoV BD | 5 | |
| PV BD | 3 | |
| RVOTO BD | 1 | |
| Peripheral PA BD | 1 | |
| Surgical procedures prior to SVAS | 24 | 8.0 |
| Coarctation repair | 15 | |
| PA surgical reconstruction | 3 | |
| AoV repair | 3 | |
| SAS resection | 1 | |
| Modified Konno procedure | 1 | |
| MV replacement | 1 | |
| Associated cardiovascular anomalies | ||
| PA stenosis | 125 | 41.5 |
| AoV stenosis | 69 | 22.9 |
| BCV stenosis | 42 | 13.9 |
| Coronary artery anomalies | 41 | 13.6 |
| LVOTO | 38 | 12.6 |
| Bicuspid AoV | 34 | 11.3 |
| RVOTO | 15 | 5.0 |
| Aortic arch hypoplasia | 14 | 4.6 |
| Aortic coarctation | 9 | 3.0 |
| Renal artery stenosis | 6 | 2.0 |
| MV abnormalities | 1 | 0.3 |
AoV: aortic valve; BCV: brachiocephalic vessels; BD: balloon dilatation: CHF: congestive heart failure; LVOTO: left ventricular outflow tract obstruction; MV: mitral valve; PA: pulmonary artery; PV: pulmonary valve; RVOTO: right ventricular outflow tract obstruction; SAS: subaortic stenosis; SVAS: supravalvular aortic stenosis; WBS: Williams–Beuren syndrome.
Overall, 228 patients (75.7%) were asymptomatic or mildly symptomatic. Symptoms occurred in 73 patients: signs of congestive heart failure in 46 (15.3%), dyspnoea in 29 (9.6%) and syncope in 8 (2.65%). Two patients had an episode of ventricular fibrillation or cardiac arrest prior to the operation. WBS was present in 176 of 301 patients (58.5%).
Cardiac catheterization was indicated primarily in patients with WBS to rule out coronary artery anomalies and peripheral vessel stenosis. In non-WBS patients, it was performed if preoperative ischaemic symptoms or echocardiographic evidence of aortic or pulmonary vessels anomalies were present.
Native coronary artery anomalies were identified preoperatively in 41 (13.6%) patients, affecting mostly the left coronary artery branching vessels and included discrete ostial stenosis in 6 patients.
A prior cardiovascular procedure had been performed in 53 patients (17.6%), either surgical (24) or interventional (35). All preoperative data are listed in Table 1.
Early outcomes
All intraoperative and postoperative data are summarized in Table 2.
Intraoperative and postoperative data of patients undergoing surgery for SVAS
| Variables . | Patients . | Percentage . |
|---|---|---|
| Total patients, n | 301 | 100 |
| SVAS surgical procedure, n | 301 | 100 |
| Single patch | 110 | 36.7 |
| Pantaloon-shaped patch | 110 | 36.7 |
| Three patches | 44 | 14.3 |
| Other | 36 | 11.7 |
| Unknown | 2 | 0.6 |
| Cardiopulmonary bypass time (min), median (range) | 43.4 (25–356) | |
| Cross-clamping time (min), median (range) | 33 (9–180) | |
| Deep hypothermic circulatory arrest (patients) | 18 | 6.0 |
| Associated concomitant cardiovascular surgical procedures, n | 76 | 24.6 |
| Central PA plasty | 41 | |
| LVOT resective surgery | 13 | |
| RVOT enlargement | 13 | |
| Aortic arch repair | 10 | |
| Coronary artery surgery | 7 | |
| AoV replacement | 4 | |
| Coarctation repair | 3a | |
| Other | 4 | |
| Intensive care unit stay (days), median (range) | 2.4 (1–27) | |
| Hospital stay (days), median (range) | 9 (2–47) | |
| Postoperative complications, n | 45 | 14.9 |
| LCO | 9 | |
| Pleural or pericardial effusion | 7 | |
| Arterial hypertension | 4 | |
| Cardiac arrest | 4 | |
| Arrhythmias | 4 | |
| Neurological disorder | 4 | |
| Postoperative ischaemia | 4 | |
| RV dysfunction | 3 | |
| Sepsis | 2 | |
| Bleeding requiring re-exploration | 2 | |
| MOF | 1 | |
| Other | 1 | |
| Extracorporeal membrane oxygenator | 14 | 4.6 |
| Early death, n | 15 | 5.0 |
| LCO | 12 | |
| MOF | 1 | |
| CVA | 1 | |
| VF | 1 |
| Variables . | Patients . | Percentage . |
|---|---|---|
| Total patients, n | 301 | 100 |
| SVAS surgical procedure, n | 301 | 100 |
| Single patch | 110 | 36.7 |
| Pantaloon-shaped patch | 110 | 36.7 |
| Three patches | 44 | 14.3 |
| Other | 36 | 11.7 |
| Unknown | 2 | 0.6 |
| Cardiopulmonary bypass time (min), median (range) | 43.4 (25–356) | |
| Cross-clamping time (min), median (range) | 33 (9–180) | |
| Deep hypothermic circulatory arrest (patients) | 18 | 6.0 |
| Associated concomitant cardiovascular surgical procedures, n | 76 | 24.6 |
| Central PA plasty | 41 | |
| LVOT resective surgery | 13 | |
| RVOT enlargement | 13 | |
| Aortic arch repair | 10 | |
| Coronary artery surgery | 7 | |
| AoV replacement | 4 | |
| Coarctation repair | 3a | |
| Other | 4 | |
| Intensive care unit stay (days), median (range) | 2.4 (1–27) | |
| Hospital stay (days), median (range) | 9 (2–47) | |
| Postoperative complications, n | 45 | 14.9 |
| LCO | 9 | |
| Pleural or pericardial effusion | 7 | |
| Arterial hypertension | 4 | |
| Cardiac arrest | 4 | |
| Arrhythmias | 4 | |
| Neurological disorder | 4 | |
| Postoperative ischaemia | 4 | |
| RV dysfunction | 3 | |
| Sepsis | 2 | |
| Bleeding requiring re-exploration | 2 | |
| MOF | 1 | |
| Other | 1 | |
| Extracorporeal membrane oxygenator | 14 | 4.6 |
| Early death, n | 15 | 5.0 |
| LCO | 12 | |
| MOF | 1 | |
| CVA | 1 | |
| VF | 1 |
One case was treated interventionally intraoperatively.
SVAS: supravalvular aortic stenosis; PA: pulmonary artery; LVOT: left ventricular outflow tract; RVOT: right ventricular outflow tract; AoV: aortic valve; LCO: low cardiac output syndrome; RV: right ventricle; MOF: multiorgan failure; CVA: cerebrovascular accident; VF: ventricular fibrillation.
Intraoperative and postoperative data of patients undergoing surgery for SVAS
| Variables . | Patients . | Percentage . |
|---|---|---|
| Total patients, n | 301 | 100 |
| SVAS surgical procedure, n | 301 | 100 |
| Single patch | 110 | 36.7 |
| Pantaloon-shaped patch | 110 | 36.7 |
| Three patches | 44 | 14.3 |
| Other | 36 | 11.7 |
| Unknown | 2 | 0.6 |
| Cardiopulmonary bypass time (min), median (range) | 43.4 (25–356) | |
| Cross-clamping time (min), median (range) | 33 (9–180) | |
| Deep hypothermic circulatory arrest (patients) | 18 | 6.0 |
| Associated concomitant cardiovascular surgical procedures, n | 76 | 24.6 |
| Central PA plasty | 41 | |
| LVOT resective surgery | 13 | |
| RVOT enlargement | 13 | |
| Aortic arch repair | 10 | |
| Coronary artery surgery | 7 | |
| AoV replacement | 4 | |
| Coarctation repair | 3a | |
| Other | 4 | |
| Intensive care unit stay (days), median (range) | 2.4 (1–27) | |
| Hospital stay (days), median (range) | 9 (2–47) | |
| Postoperative complications, n | 45 | 14.9 |
| LCO | 9 | |
| Pleural or pericardial effusion | 7 | |
| Arterial hypertension | 4 | |
| Cardiac arrest | 4 | |
| Arrhythmias | 4 | |
| Neurological disorder | 4 | |
| Postoperative ischaemia | 4 | |
| RV dysfunction | 3 | |
| Sepsis | 2 | |
| Bleeding requiring re-exploration | 2 | |
| MOF | 1 | |
| Other | 1 | |
| Extracorporeal membrane oxygenator | 14 | 4.6 |
| Early death, n | 15 | 5.0 |
| LCO | 12 | |
| MOF | 1 | |
| CVA | 1 | |
| VF | 1 |
| Variables . | Patients . | Percentage . |
|---|---|---|
| Total patients, n | 301 | 100 |
| SVAS surgical procedure, n | 301 | 100 |
| Single patch | 110 | 36.7 |
| Pantaloon-shaped patch | 110 | 36.7 |
| Three patches | 44 | 14.3 |
| Other | 36 | 11.7 |
| Unknown | 2 | 0.6 |
| Cardiopulmonary bypass time (min), median (range) | 43.4 (25–356) | |
| Cross-clamping time (min), median (range) | 33 (9–180) | |
| Deep hypothermic circulatory arrest (patients) | 18 | 6.0 |
| Associated concomitant cardiovascular surgical procedures, n | 76 | 24.6 |
| Central PA plasty | 41 | |
| LVOT resective surgery | 13 | |
| RVOT enlargement | 13 | |
| Aortic arch repair | 10 | |
| Coronary artery surgery | 7 | |
| AoV replacement | 4 | |
| Coarctation repair | 3a | |
| Other | 4 | |
| Intensive care unit stay (days), median (range) | 2.4 (1–27) | |
| Hospital stay (days), median (range) | 9 (2–47) | |
| Postoperative complications, n | 45 | 14.9 |
| LCO | 9 | |
| Pleural or pericardial effusion | 7 | |
| Arterial hypertension | 4 | |
| Cardiac arrest | 4 | |
| Arrhythmias | 4 | |
| Neurological disorder | 4 | |
| Postoperative ischaemia | 4 | |
| RV dysfunction | 3 | |
| Sepsis | 2 | |
| Bleeding requiring re-exploration | 2 | |
| MOF | 1 | |
| Other | 1 | |
| Extracorporeal membrane oxygenator | 14 | 4.6 |
| Early death, n | 15 | 5.0 |
| LCO | 12 | |
| MOF | 1 | |
| CVA | 1 | |
| VF | 1 |
One case was treated interventionally intraoperatively.
SVAS: supravalvular aortic stenosis; PA: pulmonary artery; LVOT: left ventricular outflow tract; RVOT: right ventricular outflow tract; AoV: aortic valve; LCO: low cardiac output syndrome; RV: right ventricle; MOF: multiorgan failure; CVA: cerebrovascular accident; VF: ventricular fibrillation.
The most common indication for surgery was onset of symptoms ± associated coronary anomalies, followed by detection of aortic peak pressure gradient ≥40 mmHg and, less commonly, associated AR.
The most common surgical procedures were the single-patch technique (McGoon) and the pantaloon-shaped patch repair (Doty) in 110 patients each (36.8%). Deep hypothermic circulatory arrest was used in 24 cases (7.9%).
Associated surgical procedures occurred in 72 patients (23.9%). In particular, coronary artery surgery was performed in 7 of 41 patients with a preoperative diagnosis of a coronary anomaly (left coronary in 4 of 7) and consisted of coronary ridge resection (3), ostial plasty (2) and coronary artery bypass (2). There was 1 early death in this group (12.5%) in a patient who required postoperative extracorporeal membrane oxygenation.
Postoperative major complications occurred in 45 patients (14.9%); extracorporeal membrane oxygenation support was required in 14 (4.6%) patients, with a 50% survival. Early postoperative procedures were required in 14 (4.7%) patients: 11 underwent early reoperation (aortic arch plasty in 4, PA patch plasty in 2, right coronary ostial plasty in 1 and other minor procedures in 4), whereas 3 patients underwent right PA stenting (2) and PA dilatation (1) before discharge.
The early mortality rate was 5% (15 patients), caused mostly by postoperative cardiac dysfunction; 10 had a preoperative diagnosis of associated PA stenosis.
All 286 survivors were discharged home in good clinical condition, with residual supravalvular gradient <15 mmHg in 215 (71.4%) patients, and trivial or mild AR in 281 (93.3%) patients.
Late outcomes
Overall, the median follow-up time was 13 (interquartile range 3.6–7.8) years, with follow-up completeness of 79.1% (Table 3).
| Variables . | Patients . | Percentage . |
|---|---|---|
| Overall follow up (years, median, range) | 7.8 (0.1–26.4) | |
| Follow up (years, median, range) according to each surgical technique | ||
| Single patch | 10.7 (0.1–26.4) | |
| Pantaloon-shaped patch | 8.5 (0.1–23.7) | |
| Three patches | 3.2 (0.1–12.7) | |
| Other | 3.0 (0.1–14.2) | |
 | ||
| Lost to follow-up | 63 | 20.9 |
| Total, n | 238 | 79.1 |
| Late death, n | 10 | 4.2 |
| Sudden/cardiac | 6 | |
| Mechanical valve thrombosis | 1 | |
| Burkitt’s lymphoma | 1 | |
| Unknown | 2 | |
| Adverse events, n | 48 | 20.2 |
| Surgical reoperation (patientsa) | 30 | 12.6 |
| Aortic arch replacement/repair | 11 | |
| Redo SVAS | 8 | |
| AoV replacement | 7 | |
| SAS resection | 3 | |
| Recoarctation repair | 2 | |
| Mitral valve replacement | 2 | |
| Descending aorta replacement | 1 | |
| LVOT procedure | 1 | |
| Interventional cath procedure | 20 | 7.2 |
| RVOT/PA balloon dilatation | 9 | |
| Stenting of residual recoarctation | 8 | |
| AoV dilatation | 2 | |
| Dilatation of left iliac artery | 1 | |
| Aortic regurgitation, n | 228 | 100 |
| Mild | 208 | 91.2 |
| Moderate | 13 | 5.7 |
| Severe | 3 | 1.3 |
| Unknown | 4 | 1.7 |
| Aortic stenosis, n | 44 | 19.3 |
| Peak gradient >30 mmHg | 20 |
| Variables . | Patients . | Percentage . |
|---|---|---|
| Overall follow up (years, median, range) | 7.8 (0.1–26.4) | |
| Follow up (years, median, range) according to each surgical technique | ||
| Single patch | 10.7 (0.1–26.4) | |
| Pantaloon-shaped patch | 8.5 (0.1–23.7) | |
| Three patches | 3.2 (0.1–12.7) | |
| Other | 3.0 (0.1–14.2) | |
 | ||
| Lost to follow-up | 63 | 20.9 |
| Total, n | 238 | 79.1 |
| Late death, n | 10 | 4.2 |
| Sudden/cardiac | 6 | |
| Mechanical valve thrombosis | 1 | |
| Burkitt’s lymphoma | 1 | |
| Unknown | 2 | |
| Adverse events, n | 48 | 20.2 |
| Surgical reoperation (patientsa) | 30 | 12.6 |
| Aortic arch replacement/repair | 11 | |
| Redo SVAS | 8 | |
| AoV replacement | 7 | |
| SAS resection | 3 | |
| Recoarctation repair | 2 | |
| Mitral valve replacement | 2 | |
| Descending aorta replacement | 1 | |
| LVOT procedure | 1 | |
| Interventional cath procedure | 20 | 7.2 |
| RVOT/PA balloon dilatation | 9 | |
| Stenting of residual recoarctation | 8 | |
| AoV dilatation | 2 | |
| Dilatation of left iliac artery | 1 | |
| Aortic regurgitation, n | 228 | 100 |
| Mild | 208 | 91.2 |
| Moderate | 13 | 5.7 |
| Severe | 3 | 1.3 |
| Unknown | 4 | 1.7 |
| Aortic stenosis, n | 44 | 19.3 |
| Peak gradient >30 mmHg | 20 |
One patient can have more than 1 surgical procedure.
SVAS: supravalvular aortic stenosis; AoV: aortic valve; SAS: subaortic stenosis; LVOT: left ventricular outflow tract; RVOT: right ventricular outflow tract; PA: pulmonary artery.
| Variables . | Patients . | Percentage . |
|---|---|---|
| Overall follow up (years, median, range) | 7.8 (0.1–26.4) | |
| Follow up (years, median, range) according to each surgical technique | ||
| Single patch | 10.7 (0.1–26.4) | |
| Pantaloon-shaped patch | 8.5 (0.1–23.7) | |
| Three patches | 3.2 (0.1–12.7) | |
| Other | 3.0 (0.1–14.2) | |
 | ||
| Lost to follow-up | 63 | 20.9 |
| Total, n | 238 | 79.1 |
| Late death, n | 10 | 4.2 |
| Sudden/cardiac | 6 | |
| Mechanical valve thrombosis | 1 | |
| Burkitt’s lymphoma | 1 | |
| Unknown | 2 | |
| Adverse events, n | 48 | 20.2 |
| Surgical reoperation (patientsa) | 30 | 12.6 |
| Aortic arch replacement/repair | 11 | |
| Redo SVAS | 8 | |
| AoV replacement | 7 | |
| SAS resection | 3 | |
| Recoarctation repair | 2 | |
| Mitral valve replacement | 2 | |
| Descending aorta replacement | 1 | |
| LVOT procedure | 1 | |
| Interventional cath procedure | 20 | 7.2 |
| RVOT/PA balloon dilatation | 9 | |
| Stenting of residual recoarctation | 8 | |
| AoV dilatation | 2 | |
| Dilatation of left iliac artery | 1 | |
| Aortic regurgitation, n | 228 | 100 |
| Mild | 208 | 91.2 |
| Moderate | 13 | 5.7 |
| Severe | 3 | 1.3 |
| Unknown | 4 | 1.7 |
| Aortic stenosis, n | 44 | 19.3 |
| Peak gradient >30 mmHg | 20 |
| Variables . | Patients . | Percentage . |
|---|---|---|
| Overall follow up (years, median, range) | 7.8 (0.1–26.4) | |
| Follow up (years, median, range) according to each surgical technique | ||
| Single patch | 10.7 (0.1–26.4) | |
| Pantaloon-shaped patch | 8.5 (0.1–23.7) | |
| Three patches | 3.2 (0.1–12.7) | |
| Other | 3.0 (0.1–14.2) | |
 | ||
| Lost to follow-up | 63 | 20.9 |
| Total, n | 238 | 79.1 |
| Late death, n | 10 | 4.2 |
| Sudden/cardiac | 6 | |
| Mechanical valve thrombosis | 1 | |
| Burkitt’s lymphoma | 1 | |
| Unknown | 2 | |
| Adverse events, n | 48 | 20.2 |
| Surgical reoperation (patientsa) | 30 | 12.6 |
| Aortic arch replacement/repair | 11 | |
| Redo SVAS | 8 | |
| AoV replacement | 7 | |
| SAS resection | 3 | |
| Recoarctation repair | 2 | |
| Mitral valve replacement | 2 | |
| Descending aorta replacement | 1 | |
| LVOT procedure | 1 | |
| Interventional cath procedure | 20 | 7.2 |
| RVOT/PA balloon dilatation | 9 | |
| Stenting of residual recoarctation | 8 | |
| AoV dilatation | 2 | |
| Dilatation of left iliac artery | 1 | |
| Aortic regurgitation, n | 228 | 100 |
| Mild | 208 | 91.2 |
| Moderate | 13 | 5.7 |
| Severe | 3 | 1.3 |
| Unknown | 4 | 1.7 |
| Aortic stenosis, n | 44 | 19.3 |
| Peak gradient >30 mmHg | 20 |
One patient can have more than 1 surgical procedure.
SVAS: supravalvular aortic stenosis; AoV: aortic valve; SAS: subaortic stenosis; LVOT: left ventricular outflow tract; RVOT: right ventricular outflow tract; PA: pulmonary artery.
Among the 238 early survivors, there were 10 late deaths (4.2%), 6 of which were sudden or of proven cardiac cause (Table 3).
Overall, major adverse events occurred in 48 patients (20.2%): 30 patients (12.6%) required reoperation after a median time of 4.4 years (range 0.1–22.6 years). The most common procedure was aortic arch replacement or patch plasty in 11 patients, as an isolated procedure in 8 (1 due to aortitis) or associated with recurrent SVAS in 2 patients and PA reconstruction in 1. Of note, the initial operation in these patients was associated with a high frequency of associated PA stenosis (8 of 11) and with diffuse SVAS (5 of 11); LVOT obstruction was present in 3 of 11 patients. The median interval from the first operation to aortic arch plasty was 3.8 (range 0.1–9) years, with 1 reoperation occurring within 1 month of discharge.
Eight patients (2.7%) required a patch reoperation at the site of the previous SVAS repair after a single-patch technique in 4, 3-patch technique in 2 and other, an interdigitating technique, in 2; 3 of the 8 patients required concomitant ascending aorta replacement. There were no deaths at reoperation.
In 7 patients (2.3%), AoV replacement was required after a median interval from the first operation of 5.7 (0.8–22.3) years: a mechanical valve in 2, a homograft in 1 and the Ross procedure in 4 (3 of whom required late re-reoperation with mechanical prosthesis in 2 and right ventricle-PA conduit replacement in 1). All but 1 patient presented with a significant degree of AS at the first operation (6 of 7 were tricuspid), LVOT obstruction was present in 5 of 7, SVAS was focal in all and 1 had initial AoV prosthesis at the first operation. At reoperation, they all had progression of stenosis, with mild AR in 4. One additional patient had bicuspid AoV repair (with associated subaortic stenosis resection).
An interventional cardiology procedure was required in 20 patients (6.7%) after a median time of 27.4 (range 1.17–271.2) months, most commonly after PA plasty procedures and when outflow tract obstruction was present; in 9 patients, central and/or peripheral PAs were balloon dilated.
All late survivors (228) were in New York Heart Association Class ≤II. At echocardiography, AS was present in 44 (19.3%) patients, but the peak gradient was >30 mmHg in 20 patients; AR was less than mild in 208 (91.2%) patients.
Among the 238 patients with complete follow-up, 104 patients were operated on before 2006 and 134 underwent repair after 2006; there was a trend towards increased use of the 3-patch technique with decreased use of the single-patch techique. All follow-up data are summarized in detail in Table 3.
Statistical analysis
Results of univariable analysis showed that age at repair <12 months, diffuse SVAS, associated PA stenosis and AS were significant risk factors for death (Table 4). Age at repair <12 months, brachiocephalic vessels stenosis, PA stenosis, PA patch plasty and LVOT obstruction were significant risk factors for late reintervention or reoperations (Table 4).
| . | Logistic regression . | Cox regression . | ||
|---|---|---|---|---|
| Early death . | Death . | Catheter procedures at FU . | Redo operation at FU . | |
| P-value . | P-value . | |||
| OR (95% CI) . | HR (95% CI) . | |||
| Enrolment year (2006–2015) | 0.5879 | 0.8695 | 0.9764 | 0.0391 |
| 0.750 (0.265–2.123) | 1.082 (0.422–2.777) | 1.015 (0.383–2.689) | 2.303 (1.042–5.088) | |
| Age <1 year | 0.0001 | 0.0001 | 0.0076 | 0.0032 |
| 9.936 (3.058–32.284) | 6.194 (2.425–15.826) | 3.674 (1.412–9.558) | 3.237 (1.482–7.072) | |
| Gender, female | 0.1482 | 0.0511 | 0.3000 | 0.1288 |
| 2.159 (0.761–6.127) | 2.452 (0.996–6.035) | 0.568 (0.195–1.656) | 0.469 (0.176–1.246) | |
| WBS | 0.9019 | 0.2791 | 0.7869 | 0.1956 |
| 1.069 (0.371–3.083) | 1.706 (0.648–4.489) | 0.877 (0.337–2.278) | 0.593 (0.269–1.308) | |
| Coronary anomalies | 0.4640 | 0.1918 | 0.8712 | 0.2874 |
| 1.632 (0.440–6.049) | 2.088 (0.691–6.307) | 1.127 (0.267–4.756) | 0.349 (0.050–2.428) | |
| SVAS familiar | 0.4928 | 0.4769 | 0.2378 | 0.1648 |
| 0.361 (0.020–6.622) | 0.351 (0.020–6.274) | 2.381 (0.564–10.048) | 2.335 (0.706–7.722) | |
| SVAS focal | 0.1055 | 0.1856 | 0.4138 | 0.1232 |
| 0.421 (0.148–1.200) | 0.541 (0.217–1.344) | 0.665 (0.250–1.768) | 0.531 (0.238–1.187) | |
| SVAS diffuse | 0.0588 | 0.0923 | 0.3243 | 0.1792 |
| 2.752 (0.963–7.864) | 2.186 (0.879–5.437) | 1.637 (0.614–4.361) | 1.753 (0.773–3.974) | |
| BCV stenosis | 0.4640 | 0.2686 | 0.0030 | 0.0008 |
| 1.632 (0.440–6.049) | 1.864 (0.618–5.617) | 4.431 (1.659–11.838) | 4.154 (1.802–9.577) | |
| PA stenosis | 0.0520 | 0.0044 | 0.0363 | 0.0937 |
| 2.974 (0.991–8.927) | 4.415 (1.590–12.261) | 2.841 (1.069–7.551) | 1.949 (0.893–4.252) | |
| AoV stenosis | 0.0321 | 0.0511 | 0.2900 | 0.2882 |
| 3.161 (1.103–9.058) | 2.477 (0.996–6.161) | 1.664 (0.648–4.271) | 1.546 (0.692–3.454) | |
| Type of surgical procedure | 0.2068 | 0.1674 | 0.3832 | 0.8192 |
| Pantaloon vs single | 0.208 (0.044–0.985) | 0.320 (0.100–1.019) | 0.647 (0.212–1.974) | 0.971 (0.401–2.347) |
| Three patches vs single | 0.821 (0.212–3.187) | 1.133 (0.351–3.652) | 0.507 (0.064–4.046) | 1.263 (0.351–4.549) |
| Other vs single | 0.330 (0.040–2.701) | 0.334 (0.043–2.629) | 2.096 (0.555–7.913) | 1.748 (0.492–6.209) |
| PA patch plasty | 0.4640 | 0.2431 | <0.0001 | 0.1400 |
| 1.632 (0.440–6.049) | 1.929 (0.640–5.818) | 7.324 (2.780–19.294) | 2.126 (0.781–5.786) | |
| LVOT obstruction | 0.4854 | 0.6808 | 0.0426 | 0.0021 |
| 0.481 (0.061–3.764) | 0.735 (0.170–3.184) | 2.805 (1.035–7.601) | 3.522 (1.577–7.866) | |
| . | Logistic regression . | Cox regression . | ||
|---|---|---|---|---|
| Early death . | Death . | Catheter procedures at FU . | Redo operation at FU . | |
| P-value . | P-value . | |||
| OR (95% CI) . | HR (95% CI) . | |||
| Enrolment year (2006–2015) | 0.5879 | 0.8695 | 0.9764 | 0.0391 |
| 0.750 (0.265–2.123) | 1.082 (0.422–2.777) | 1.015 (0.383–2.689) | 2.303 (1.042–5.088) | |
| Age <1 year | 0.0001 | 0.0001 | 0.0076 | 0.0032 |
| 9.936 (3.058–32.284) | 6.194 (2.425–15.826) | 3.674 (1.412–9.558) | 3.237 (1.482–7.072) | |
| Gender, female | 0.1482 | 0.0511 | 0.3000 | 0.1288 |
| 2.159 (0.761–6.127) | 2.452 (0.996–6.035) | 0.568 (0.195–1.656) | 0.469 (0.176–1.246) | |
| WBS | 0.9019 | 0.2791 | 0.7869 | 0.1956 |
| 1.069 (0.371–3.083) | 1.706 (0.648–4.489) | 0.877 (0.337–2.278) | 0.593 (0.269–1.308) | |
| Coronary anomalies | 0.4640 | 0.1918 | 0.8712 | 0.2874 |
| 1.632 (0.440–6.049) | 2.088 (0.691–6.307) | 1.127 (0.267–4.756) | 0.349 (0.050–2.428) | |
| SVAS familiar | 0.4928 | 0.4769 | 0.2378 | 0.1648 |
| 0.361 (0.020–6.622) | 0.351 (0.020–6.274) | 2.381 (0.564–10.048) | 2.335 (0.706–7.722) | |
| SVAS focal | 0.1055 | 0.1856 | 0.4138 | 0.1232 |
| 0.421 (0.148–1.200) | 0.541 (0.217–1.344) | 0.665 (0.250–1.768) | 0.531 (0.238–1.187) | |
| SVAS diffuse | 0.0588 | 0.0923 | 0.3243 | 0.1792 |
| 2.752 (0.963–7.864) | 2.186 (0.879–5.437) | 1.637 (0.614–4.361) | 1.753 (0.773–3.974) | |
| BCV stenosis | 0.4640 | 0.2686 | 0.0030 | 0.0008 |
| 1.632 (0.440–6.049) | 1.864 (0.618–5.617) | 4.431 (1.659–11.838) | 4.154 (1.802–9.577) | |
| PA stenosis | 0.0520 | 0.0044 | 0.0363 | 0.0937 |
| 2.974 (0.991–8.927) | 4.415 (1.590–12.261) | 2.841 (1.069–7.551) | 1.949 (0.893–4.252) | |
| AoV stenosis | 0.0321 | 0.0511 | 0.2900 | 0.2882 |
| 3.161 (1.103–9.058) | 2.477 (0.996–6.161) | 1.664 (0.648–4.271) | 1.546 (0.692–3.454) | |
| Type of surgical procedure | 0.2068 | 0.1674 | 0.3832 | 0.8192 |
| Pantaloon vs single | 0.208 (0.044–0.985) | 0.320 (0.100–1.019) | 0.647 (0.212–1.974) | 0.971 (0.401–2.347) |
| Three patches vs single | 0.821 (0.212–3.187) | 1.133 (0.351–3.652) | 0.507 (0.064–4.046) | 1.263 (0.351–4.549) |
| Other vs single | 0.330 (0.040–2.701) | 0.334 (0.043–2.629) | 2.096 (0.555–7.913) | 1.748 (0.492–6.209) |
| PA patch plasty | 0.4640 | 0.2431 | <0.0001 | 0.1400 |
| 1.632 (0.440–6.049) | 1.929 (0.640–5.818) | 7.324 (2.780–19.294) | 2.126 (0.781–5.786) | |
| LVOT obstruction | 0.4854 | 0.6808 | 0.0426 | 0.0021 |
| 0.481 (0.061–3.764) | 0.735 (0.170–3.184) | 2.805 (1.035–7.601) | 3.522 (1.577–7.866) | |
Bold values underline significant results.
OR: odds ratio; CI: confidence interval; HR: hazard ratio; FU: follow-up; WBS: Williams–Beuren syndrome; SVAS: supravalvular aortic stenosis; BCV: brachiocephalic vessels: PA: pulmonary artery; AoV: aortic valve; LVOT: left ventricular outflow tract.
| . | Logistic regression . | Cox regression . | ||
|---|---|---|---|---|
| Early death . | Death . | Catheter procedures at FU . | Redo operation at FU . | |
| P-value . | P-value . | |||
| OR (95% CI) . | HR (95% CI) . | |||
| Enrolment year (2006–2015) | 0.5879 | 0.8695 | 0.9764 | 0.0391 |
| 0.750 (0.265–2.123) | 1.082 (0.422–2.777) | 1.015 (0.383–2.689) | 2.303 (1.042–5.088) | |
| Age <1 year | 0.0001 | 0.0001 | 0.0076 | 0.0032 |
| 9.936 (3.058–32.284) | 6.194 (2.425–15.826) | 3.674 (1.412–9.558) | 3.237 (1.482–7.072) | |
| Gender, female | 0.1482 | 0.0511 | 0.3000 | 0.1288 |
| 2.159 (0.761–6.127) | 2.452 (0.996–6.035) | 0.568 (0.195–1.656) | 0.469 (0.176–1.246) | |
| WBS | 0.9019 | 0.2791 | 0.7869 | 0.1956 |
| 1.069 (0.371–3.083) | 1.706 (0.648–4.489) | 0.877 (0.337–2.278) | 0.593 (0.269–1.308) | |
| Coronary anomalies | 0.4640 | 0.1918 | 0.8712 | 0.2874 |
| 1.632 (0.440–6.049) | 2.088 (0.691–6.307) | 1.127 (0.267–4.756) | 0.349 (0.050–2.428) | |
| SVAS familiar | 0.4928 | 0.4769 | 0.2378 | 0.1648 |
| 0.361 (0.020–6.622) | 0.351 (0.020–6.274) | 2.381 (0.564–10.048) | 2.335 (0.706–7.722) | |
| SVAS focal | 0.1055 | 0.1856 | 0.4138 | 0.1232 |
| 0.421 (0.148–1.200) | 0.541 (0.217–1.344) | 0.665 (0.250–1.768) | 0.531 (0.238–1.187) | |
| SVAS diffuse | 0.0588 | 0.0923 | 0.3243 | 0.1792 |
| 2.752 (0.963–7.864) | 2.186 (0.879–5.437) | 1.637 (0.614–4.361) | 1.753 (0.773–3.974) | |
| BCV stenosis | 0.4640 | 0.2686 | 0.0030 | 0.0008 |
| 1.632 (0.440–6.049) | 1.864 (0.618–5.617) | 4.431 (1.659–11.838) | 4.154 (1.802–9.577) | |
| PA stenosis | 0.0520 | 0.0044 | 0.0363 | 0.0937 |
| 2.974 (0.991–8.927) | 4.415 (1.590–12.261) | 2.841 (1.069–7.551) | 1.949 (0.893–4.252) | |
| AoV stenosis | 0.0321 | 0.0511 | 0.2900 | 0.2882 |
| 3.161 (1.103–9.058) | 2.477 (0.996–6.161) | 1.664 (0.648–4.271) | 1.546 (0.692–3.454) | |
| Type of surgical procedure | 0.2068 | 0.1674 | 0.3832 | 0.8192 |
| Pantaloon vs single | 0.208 (0.044–0.985) | 0.320 (0.100–1.019) | 0.647 (0.212–1.974) | 0.971 (0.401–2.347) |
| Three patches vs single | 0.821 (0.212–3.187) | 1.133 (0.351–3.652) | 0.507 (0.064–4.046) | 1.263 (0.351–4.549) |
| Other vs single | 0.330 (0.040–2.701) | 0.334 (0.043–2.629) | 2.096 (0.555–7.913) | 1.748 (0.492–6.209) |
| PA patch plasty | 0.4640 | 0.2431 | <0.0001 | 0.1400 |
| 1.632 (0.440–6.049) | 1.929 (0.640–5.818) | 7.324 (2.780–19.294) | 2.126 (0.781–5.786) | |
| LVOT obstruction | 0.4854 | 0.6808 | 0.0426 | 0.0021 |
| 0.481 (0.061–3.764) | 0.735 (0.170–3.184) | 2.805 (1.035–7.601) | 3.522 (1.577–7.866) | |
| . | Logistic regression . | Cox regression . | ||
|---|---|---|---|---|
| Early death . | Death . | Catheter procedures at FU . | Redo operation at FU . | |
| P-value . | P-value . | |||
| OR (95% CI) . | HR (95% CI) . | |||
| Enrolment year (2006–2015) | 0.5879 | 0.8695 | 0.9764 | 0.0391 |
| 0.750 (0.265–2.123) | 1.082 (0.422–2.777) | 1.015 (0.383–2.689) | 2.303 (1.042–5.088) | |
| Age <1 year | 0.0001 | 0.0001 | 0.0076 | 0.0032 |
| 9.936 (3.058–32.284) | 6.194 (2.425–15.826) | 3.674 (1.412–9.558) | 3.237 (1.482–7.072) | |
| Gender, female | 0.1482 | 0.0511 | 0.3000 | 0.1288 |
| 2.159 (0.761–6.127) | 2.452 (0.996–6.035) | 0.568 (0.195–1.656) | 0.469 (0.176–1.246) | |
| WBS | 0.9019 | 0.2791 | 0.7869 | 0.1956 |
| 1.069 (0.371–3.083) | 1.706 (0.648–4.489) | 0.877 (0.337–2.278) | 0.593 (0.269–1.308) | |
| Coronary anomalies | 0.4640 | 0.1918 | 0.8712 | 0.2874 |
| 1.632 (0.440–6.049) | 2.088 (0.691–6.307) | 1.127 (0.267–4.756) | 0.349 (0.050–2.428) | |
| SVAS familiar | 0.4928 | 0.4769 | 0.2378 | 0.1648 |
| 0.361 (0.020–6.622) | 0.351 (0.020–6.274) | 2.381 (0.564–10.048) | 2.335 (0.706–7.722) | |
| SVAS focal | 0.1055 | 0.1856 | 0.4138 | 0.1232 |
| 0.421 (0.148–1.200) | 0.541 (0.217–1.344) | 0.665 (0.250–1.768) | 0.531 (0.238–1.187) | |
| SVAS diffuse | 0.0588 | 0.0923 | 0.3243 | 0.1792 |
| 2.752 (0.963–7.864) | 2.186 (0.879–5.437) | 1.637 (0.614–4.361) | 1.753 (0.773–3.974) | |
| BCV stenosis | 0.4640 | 0.2686 | 0.0030 | 0.0008 |
| 1.632 (0.440–6.049) | 1.864 (0.618–5.617) | 4.431 (1.659–11.838) | 4.154 (1.802–9.577) | |
| PA stenosis | 0.0520 | 0.0044 | 0.0363 | 0.0937 |
| 2.974 (0.991–8.927) | 4.415 (1.590–12.261) | 2.841 (1.069–7.551) | 1.949 (0.893–4.252) | |
| AoV stenosis | 0.0321 | 0.0511 | 0.2900 | 0.2882 |
| 3.161 (1.103–9.058) | 2.477 (0.996–6.161) | 1.664 (0.648–4.271) | 1.546 (0.692–3.454) | |
| Type of surgical procedure | 0.2068 | 0.1674 | 0.3832 | 0.8192 |
| Pantaloon vs single | 0.208 (0.044–0.985) | 0.320 (0.100–1.019) | 0.647 (0.212–1.974) | 0.971 (0.401–2.347) |
| Three patches vs single | 0.821 (0.212–3.187) | 1.133 (0.351–3.652) | 0.507 (0.064–4.046) | 1.263 (0.351–4.549) |
| Other vs single | 0.330 (0.040–2.701) | 0.334 (0.043–2.629) | 2.096 (0.555–7.913) | 1.748 (0.492–6.209) |
| PA patch plasty | 0.4640 | 0.2431 | <0.0001 | 0.1400 |
| 1.632 (0.440–6.049) | 1.929 (0.640–5.818) | 7.324 (2.780–19.294) | 2.126 (0.781–5.786) | |
| LVOT obstruction | 0.4854 | 0.6808 | 0.0426 | 0.0021 |
| 0.481 (0.061–3.764) | 0.735 (0.170–3.184) | 2.805 (1.035–7.601) | 3.522 (1.577–7.866) | |
Bold values underline significant results.
OR: odds ratio; CI: confidence interval; HR: hazard ratio; FU: follow-up; WBS: Williams–Beuren syndrome; SVAS: supravalvular aortic stenosis; BCV: brachiocephalic vessels: PA: pulmonary artery; AoV: aortic valve; LVOT: left ventricular outflow tract.
Multivariable logistic regression analysis showed that age at repair <12 months and associated PA stenosis were risk factors for early death and late death (OR 3.456). In addition, PA stenosis, AS and LVOT obstruction were significantly associated with risk for reoperation or reintervention at follow-up (Table 5).
| . | Logistic regression . | Cox regression . | ||
|---|---|---|---|---|
| Early death . | Death . | Catheter procedures at FU . | Redo surgery at FU . | |
| P-value . | P-value . | |||
| OR (95% CI) . | HR (95% CI) . | |||
| Enrolment year (2006–2015) | 0.0110 | |||
| 2.875 (1.271–6.419) | ||||
| Age <1 year | 0.0001 | 0.0047 | ||
| 9.936 (3.058–32.284) | 4.100 (1.540–10.911) | |||
| Gender, male | 0.0494 | |||
| 2.491 (1.002–6.190) | ||||
| BCV stenosis (supra-aortic stenosis) | 0.0004 | 0.0013 | ||
| 2.652 (1.057–6.653) | 4.353 (1.778–10.659) | |||
| PA stenosis | 0.0225 | 0.0001 | ||
| 3.456 (1.191–10.028) | 9.536 (2.983–30.482) | |||
| AoV stenosis | 0.0491 | |||
| 2.517 (1.004–6.311) | ||||
| LVOT obstruction | 0.0059 | 0.0030 | ||
| 5.389 (1.625–17.866) | 3.714 (1.561–8.839) | |||
| . | Logistic regression . | Cox regression . | ||
|---|---|---|---|---|
| Early death . | Death . | Catheter procedures at FU . | Redo surgery at FU . | |
| P-value . | P-value . | |||
| OR (95% CI) . | HR (95% CI) . | |||
| Enrolment year (2006–2015) | 0.0110 | |||
| 2.875 (1.271–6.419) | ||||
| Age <1 year | 0.0001 | 0.0047 | ||
| 9.936 (3.058–32.284) | 4.100 (1.540–10.911) | |||
| Gender, male | 0.0494 | |||
| 2.491 (1.002–6.190) | ||||
| BCV stenosis (supra-aortic stenosis) | 0.0004 | 0.0013 | ||
| 2.652 (1.057–6.653) | 4.353 (1.778–10.659) | |||
| PA stenosis | 0.0225 | 0.0001 | ||
| 3.456 (1.191–10.028) | 9.536 (2.983–30.482) | |||
| AoV stenosis | 0.0491 | |||
| 2.517 (1.004–6.311) | ||||
| LVOT obstruction | 0.0059 | 0.0030 | ||
| 5.389 (1.625–17.866) | 3.714 (1.561–8.839) | |||
Bold values underline significant results.
OR: odds ratio; CI: confidence interval; HR: hazard ratio; FU: follow-up; BCV: brachiocephalic vessels: PA: pulmonary artery; AoV: aortic valve; LVOT: left ventricular outflow tract.
| . | Logistic regression . | Cox regression . | ||
|---|---|---|---|---|
| Early death . | Death . | Catheter procedures at FU . | Redo surgery at FU . | |
| P-value . | P-value . | |||
| OR (95% CI) . | HR (95% CI) . | |||
| Enrolment year (2006–2015) | 0.0110 | |||
| 2.875 (1.271–6.419) | ||||
| Age <1 year | 0.0001 | 0.0047 | ||
| 9.936 (3.058–32.284) | 4.100 (1.540–10.911) | |||
| Gender, male | 0.0494 | |||
| 2.491 (1.002–6.190) | ||||
| BCV stenosis (supra-aortic stenosis) | 0.0004 | 0.0013 | ||
| 2.652 (1.057–6.653) | 4.353 (1.778–10.659) | |||
| PA stenosis | 0.0225 | 0.0001 | ||
| 3.456 (1.191–10.028) | 9.536 (2.983–30.482) | |||
| AoV stenosis | 0.0491 | |||
| 2.517 (1.004–6.311) | ||||
| LVOT obstruction | 0.0059 | 0.0030 | ||
| 5.389 (1.625–17.866) | 3.714 (1.561–8.839) | |||
| . | Logistic regression . | Cox regression . | ||
|---|---|---|---|---|
| Early death . | Death . | Catheter procedures at FU . | Redo surgery at FU . | |
| P-value . | P-value . | |||
| OR (95% CI) . | HR (95% CI) . | |||
| Enrolment year (2006–2015) | 0.0110 | |||
| 2.875 (1.271–6.419) | ||||
| Age <1 year | 0.0001 | 0.0047 | ||
| 9.936 (3.058–32.284) | 4.100 (1.540–10.911) | |||
| Gender, male | 0.0494 | |||
| 2.491 (1.002–6.190) | ||||
| BCV stenosis (supra-aortic stenosis) | 0.0004 | 0.0013 | ||
| 2.652 (1.057–6.653) | 4.353 (1.778–10.659) | |||
| PA stenosis | 0.0225 | 0.0001 | ||
| 3.456 (1.191–10.028) | 9.536 (2.983–30.482) | |||
| AoV stenosis | 0.0491 | |||
| 2.517 (1.004–6.311) | ||||
| LVOT obstruction | 0.0059 | 0.0030 | ||
| 5.389 (1.625–17.866) | 3.714 (1.561–8.839) | |||
Bold values underline significant results.
OR: odds ratio; CI: confidence interval; HR: hazard ratio; FU: follow-up; BCV: brachiocephalic vessels: PA: pulmonary artery; AoV: aortic valve; LVOT: left ventricular outflow tract.
Cox regression analyses showed that patients operated on after 2005 were at increased risk for reoperations compared with those operated on in the earlier era.
The overall survival rate was 90.5% at 25 years (Fig. 1), with the cumulative incidence of surgical reoperation or reintervention shown in Fig. 2A and B. The incidence of both reoperation and reintervention was low across the whole population and was similar in all groups, regardless of the surgical techniques used for repair (Fig. 3A and B).
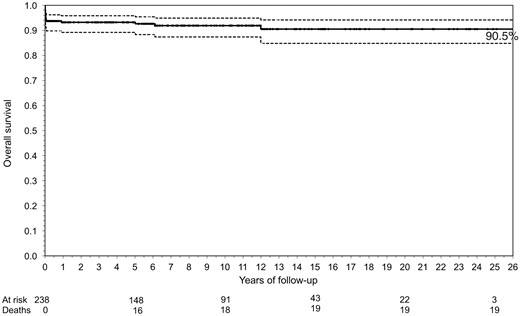
Kaplan–Meier curve showing the overall survival rate after supravalvular aortic stenosis repair. The solid line is the survival curve; the dashed lines represent 95% confidence intervals and dots are censored observations (middle line).
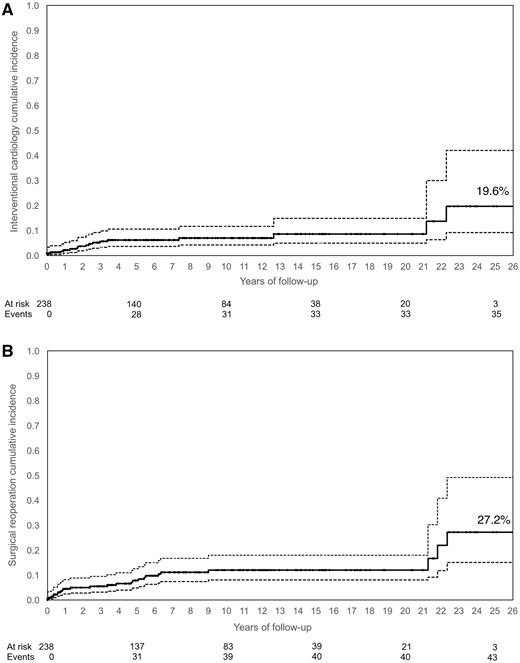
The cumulative incidence of interventional cardiology procedures (A) or surgical reoperation procedures (B). The solid line is the cumulative incidence curve; the dashed lines represent 95% confidence intervals and the dots are censored observations (middle line).
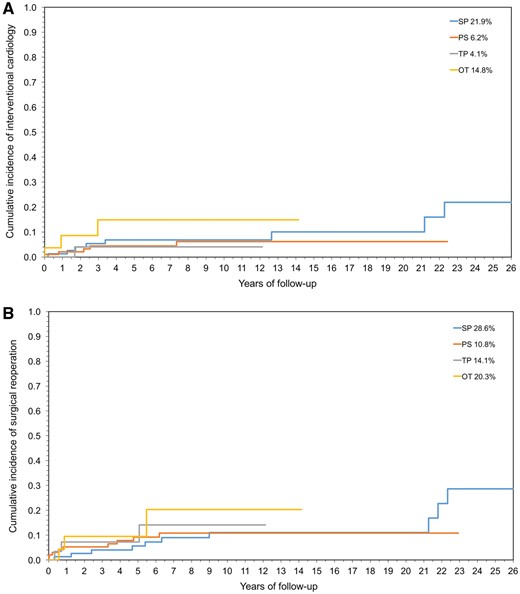
Cumulative incidence by surgical technique of interventional cardiology procedures (A) or surgical reoperation procedures (B). SP: single patch; PS: pantaloon-shaped patch; TP: 3 patches; OT: other techniques.
DISCUSSION
This multicentre retrospective clinical study evaluated the outcomes after surgery for congenital SVAS since 1990 and demonstrated that there is a good overall survival rate, with an acceptable operative risk and no significant difference in survival or reintervention with the different surgical techniques.
McGoon et al. [15] in 1961 first reported the single-patch repair for SVAS. Doty et al. [16] in 1977 modified the technique with a pantaloon-shaped patch that extended into 2 aortic valve sinuses. Some years later, Brom [17] described a technique with 3 separated triangular patches into the 3 aortic valve sinuses, the goal being to reconstruct a symmetrical ascending aorta. In 1993, Myers described the ‘interdigitating’ technique, a patchless but more demanding procedure [18]. However, which surgical technique is optimal remains unclear. Hazekamp et al. [12] could not demonstrate any difference in outcome among surgical techniques in their series of 29 patients. Stamm et al. [8] reported 75 patients since 1957, undergoing 3 different techniques, showing that the 3-patch technique had superior haemodynamics and a lower mortality rate and need for reoperation. In contrast, in 2012, Kawarana et al. [10] reported a series of 22 patients treated with extended single-patch repair, with 0% mortality and 0% reoperation rate at mid-term. In the same year, Deo et al. [11] reported a 26% late mortality rate and freedom from reoperation at 20 years of 86 ± 10% in 78 patients after a single-patch repair from 1956. More recently, Fricke et al. [9] reported no reoperations or death at 5 years in 28 patients treated with 3 patches, whereas the group receiving the pantaloon-shaped patch presented with significant late mortality and reoperation rates (24% and 25%, respectively) over a longer (14.7 years) follow-up period.
The European Congenital Heart Surgeons Association undertook this multicentre study, because definitive conclusions could not be drawn. In this series, the various surgical techniques neither are significantly different in terms of late reintervention rate (Fig. 3A and B) nor are they significant predictors of outcomes (Tables 4 and 5), as reported elsewhere [12]. On the contrary, outcomes were more related to patients’ preoperative characteristics (age <12 months, diffuse SVAS) rather than to techniques. The detrimental effect of the high proximal aortic pressure on the coronary arteries and the possibility for accelerated atherosclerotic changes are well known, implying a benefit of surgery at an early age. However, in this and others’ experience [11], repair in infancy was shown to be a significant risk factor for survival (Table 5), which justifies a strategy of delaying surgery until after infancy, if possible.
In this series, surgical reoperations occurred at an acceptable rate (12.6%) compared to other studies [9], with no operative deaths. Interestingly, only 8 patients (3% of the whole series) required a surgical procedure at the site of a previous SVAS repair, consisting of redo patch plasty in 7 and aortic root and ascending aorta replacement in 1, demonstrating the anatomical effectiveness of the procedures.
Previous studies have suggested that patients with SVAS and WBS have a worse prognosis [19]. In a recent series of 447 patients with WBS having operations, there was a 9% incidence of major late cardiac adverse events (mostly sudden death or arrhythmias), especially after major surgery on the LVOT and consisting mostly of coronary adverse events [13]. In this series, WBS was not associated with early death or worse late outcomes. In addition, some studies have shown WBS to be associated with a high incidence of preoperative PA stenosis or ventricular outflow obstruction [20], but this was not the case in this study, which may partly explain why the patients with WBS in this series had favourable outcomes.
As reported elsewhere [20], although PA stenosis is frequently diagnosed preoperatively in SVAS, an operation on the pulmonary arterial tree is not always required at repair, often because stenoses can affect the peripheral vessels. In this series, only 41 of 125 patients with PA stenosis required an associated PA procedure during SVAS repair, all being performed on the central pulmonary arteries. Furthermore, the overall incidence of procedures on the PA at follow-up was low. This finding could be related to some degree of development of the pulmonary arterial tree during somatic growth. Nevertheless, the associated presence of preoperative PA stenosis was a risk factor for late death and catheter intervention (odds ratio 3.456 and 9.536, respectively) in this study, reinforcing the importance of close follow-up in these patients.
It is known that patients with SVAS typically have abnormal thickening of the aortic wall at the sinotubular junction that may extend even to the coronary ostia level [14, 19]. Coronary stenosis and ischaemia may be associated with SVAS, with multiple aetiologies (isolation of the coronary ostia from fused cusps, arteriosclerosis resulting from intracoronary hypertension and discrete or diffuse ostial stenosis) [13, 21]. Stamm et al. [14] reported an incidence of 23% of marked dilation or tortuosity of coronary arteries and 45% of coronary arterial orifice stenosis. In addition, severe left ventricular hypertrophy further compounds coronary subendocardial malperfusion, causing myocardial ischaemia. The incidence of the preoperative diagnosis of some kinds of coronary anomalies ranges between 9% and 18% [10–12]. Deo et al. [11] reported a preoperative rate of 18% coronary ostial obstruction that was not treated at surgery. In this series, coronary anomalies were detected preoperatively in 41 patients (13.6%). Nevertheless, only 7 patients underwent simultaneous surgery on coronary arteries, with 1 operative death. In addition, 3 patients in this group (not treated for coronary issues previously) required a subsequent emergent coronary procedure (interventional stenting in 2 and surgical coronary ostial plasty in 1). Furthermore, 60% of late deaths were described as ‘cardiac’ or ‘sudden’, suggesting a probable coronary event. Thus, even though this study showed no direct association between coronary involvement and operative deaths, the follow-up findings would support the data in the literature that suggest that coronary anomalies may have an impact on the outcomes in patients with SVAS. The presence of coronary anomalies that may be amenable to effective surgical treatment needs to be ruled out preoperatively, because they may play a role in causing early and late deaths. However, the high diagnostic procedural risk needs to be carefully considered, and the goal of the surgeon should be to carefully assess the coronary anatomy in all cases.
In this series, the presence of LVOT obstruction was associated with an increased risk of reintervention at follow-up. These cases were associated with the diffuse form of SVAS and with aortic arch stenosis (hypoplasia or coarctation) that often required a late reintervention (either surgical or interventional). Nevertheless, there was no increased risk of death in these patients. These findings were consistent with the report by Hornik et al. [13] that outlined that patients with WBS and complex LVOT had a higher risk of major cardiac adverse events.
Finally, in this series of operations performed over more than 2 decades, multivariable analysis indicated no differences in outcomes before or after 2005. However, the incidence of late overall reoperations increased in patients operated on after 2005. This finding may be related to a change in the techniques used (Table 3), but the significance of this finding remains limited because the overall number of reinterventions at the site of previous SVAS repair was minimal (8), regardless of the surgical technique.
Limitations
The major limitations are the intrinsic retrospective nature of this multicentre study, with evident inter-centre/intracentre variability regarding indications for surgery, choice of surgical technique and the collection of follow-up data. In addition, the differences in the surgical techniques that have evolved over the decades make a reliable comparison difficult.
CONCLUSIONS
The surgical repair of congenital SVAS is an effective procedure with an acceptably low surgical risk and good long-term survival rates. However, the occurrence of late adverse events is not negligible. In this analysis, there was no significant difference in outcomes among techniques, but the study did suggest that repair in early infancy was a significant risk factor for early death and reoperation in the long term. Finally, the presence of native LVOT obstruction and PA stenosis is associated with increased risk of late reoperation.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 30th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 1–5 October 2016.
Christian Schreiber passed away after data collection and during paper submission.




