-
PDF
- Split View
-
Views
-
Cite
Cite
Jason W. Greenberg, Timothy S. Lancaster, Richard B. Schuessler, Spencer J. Melby, Postoperative atrial fibrillation following cardiac surgery: a persistent complication, European Journal of Cardio-Thoracic Surgery, Volume 52, Issue 4, October 2017, Pages 665–672, https://doi.org/10.1093/ejcts/ezx039
Close - Share Icon Share
Abstract
Postoperative atrial fibrillation (POAF) is a common, expensive and potentially morbid complication following cardiac surgery. POAF occurs in around 35% of cardiac surgery cases and has a peak incidence on postoperative day 2. Patients who develop POAF incur on average $10 000–$20 000 in additional hospital treatment costs, 12–24 h of prolonged ICU time, and an additional 2 to 5 days in the hospital. POAF has been identified as an independent predictor of numerous adverse outcomes, including a 2- to 4-fold increased risk of stroke, reoperation for bleeding, infection, renal or respiratory failure, cardiac arrest, cerebral complications, need for permanent pacemaker placement, and a 2-fold increase in all-cause 30-day and 6-month mortality. The pathogenesis of POAF is incompletely understood but likely involves interplay between pre-existing physiological components and local and systemic inflammation. POAF is associated with numerous risk factors including advanced age, pre-existing conditions that cause cardiac remodelling and certain non-cardiovascular conditions. Clinical management of POAF includes both prophylactic and therapeutic measures, although the efficacy of many interventions remains in question. This review provides a comprehensive and up-to-date summary of the pathogenesis of POAF, outlines current clinical guidelines for POAF prophylaxis and management, and discusses new avenues for further investigation.
INTRODUCTION
Acute or new-onset AF (termed postoperative atrial fibrillation [POAF]) is a common postoperative complication that occurs in around 35% of cardiac surgery cases and is associated with numerous detrimental sequelae [1–4]. In contrast, the incidence of POAF following thoracic surgery is between 10 and 30% and the incidence following non-cardiac, non-thoracic surgery ranges from 1 to 15% [1, 2].
POAF is an independent predictor of numerous adverse outcomes, including a 2- to 4-fold increased risk of stroke, reoperation for bleeding, infection, renal or respiratory failure, cardiac arrest, cerebral complications, need for permanent pacemaker placement, and a 2-fold increase in all-cause 30-day and 6-month mortality [1–3, 5–9]. POAF may not be directly responsible for these poor outcomes but is likely contributory and is certainly a surrogate for increased morbidity and mortality following cardiac surgery [6]. Patients who develop POAF incur on average $10 000–$20 000 in additional hospital treatment costs, 12–24 h of prolonged ICU time, and an additional 2 to 5 days in the hospital [1, 3, 10, 11]. Healthcare expenditures related to the burden of POAF in the US are estimated at over $1 billion annually [4].
Clinical efforts to prevent and manage POAF following cardiac surgery have thus far presented a major challenge and results have been less than optimal. In spite of numerous trials examining prophylactic and treatment modalities, POAF incidence following cardiac surgery has not changed over the past several decades (Fig. 1) [3, 12].
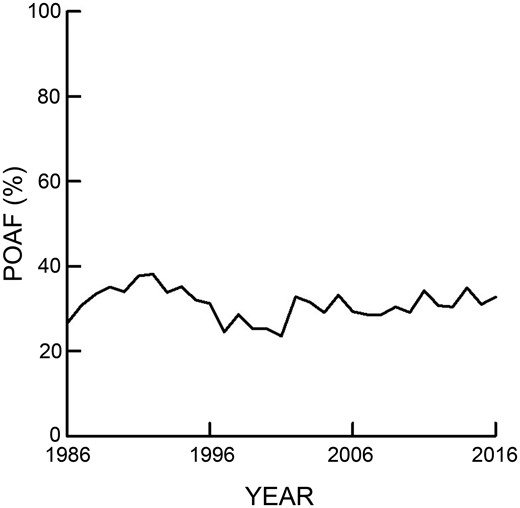
Incidence of new-onset postoperative atrial fibrillation (POAF) following cardiac surgery for all patients at a single institution over a 30-year period. The mean incidence of POAF was 31% with a standard deviation of 3.7%.
METHODS
In this classical review, a comprehensive literature search was performed using PubMed. Key search terms included ‘postoperative atrial fibrillation’, ‘management of postoperative atrial fibrillation’, and ‘inflammation’, ‘oxidative stress’, and ‘oxidative damage’ following cardiac surgery. The most recent pertinent additions to the POAF literature and the most current clinical guidelines for POAF prophylaxis and management were examined and summarized.
PATHOGENESIS OF POAF
Systemic inflammation and oxidative stress
Surgical trauma, ischaemia from the initiation and prolonged use of CPB, and reperfusion lead to oxidative stress and the production of pro-inflammatory molecules, resulting in endothelial and leucocyte activation, the release of NADPH oxidases, nitrous oxide production and reactive oxygen species generation [5, 7, 8, 13, 14]. Human studies have demonstrated an association between systemic inflammation and oxidative stress and the development of POAF [5, 13, 15, 16]. This association is supported by a demonstrated decrease in POAF from anti-inflammatory prophylaxis using corticosteroids [2, 5, 7, 14, 17, 18].
Local inflammation and oxidative stress
Pericardial disruption causes local inflammation around the heart and an increase in pericardial fluid (PCF) volume [1, 4]. Postoperative PCF is highly oxidative and contains blood, haemolyzed blood cells, haemoglobin and high levels of inflammatory markers that reflect leucocyte and platelet activation (Fig. 2) [15, 16]. The myocardium itself also produces pro-inflammatory molecules which contribute to local inflammation and directly alter cardiac function [5].
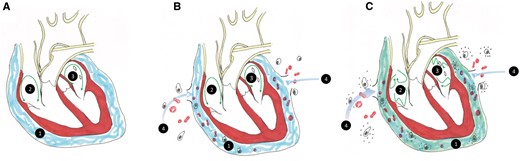
A (1) Pericardial fluid (blue) bathes the normal heart including the (2) right and (3) left atria. Green arrows represent atrial conduction. B Surgery disrupts the pericardium and blood and inflammatory cells enter the pericardial space (4). C (1) Pericardial fluid composition now includes inflammatory cells, cytokines, and highly oxidative proteins, causing atrial fibrillation (green arrows).
Inflammation within the pericardial space results in cardiomyocyte apoptosis and altered electrical activity, which allows heterogenous action potentials and arrhythmias to form and propagate [4, 5, 14]. Animal models have shown that the degree of atrial inflammation directly corresponds to the incidence of POAF [14–16]. Contact between inflammatory cells and cardiac tissue likely plays a role in the pathogenesis of POAF, although the exact mechanisms have not yet been elucidated [14].
Electrolytes
Hypomagnesemia (a serum magnesium level below normal [<1.2–2.0 mg/dl]) is common following surgery and has been identified by some studies as a predictor of POAF [4, 19]. Magnesium regulates calcium mobility, influences cardiomyocyte contractility, and has been shown to have anti-ischaemic effects [20, 21]. High levels of intracellular magnesium prolong atrioventricular (AV) node conduction time and may help prevent oxidative damage, while low magnesium levels increase sinus node automaticity [1, 22, 23]. Recent evaluation has called into question the presumed anti-arrhythmic properties of magnesium by showing that high serum levels were associated with POAF in a dose-dependent manner (Fig. 3) [24].
![Incidence of postoperative atrial fibrillation (POAF) based on quintile grouping of plasma potassium (red) and magnesium (blue) levels at AF onset. A higher potassium or magnesium quintile was associated with a higher probability of POAF (P < 0.001 for both). Numbers above bars represent potassium (mmol/l) or magnesium levels (mg/dl) for respective quintile group (adapted from ref. [24]).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/52/4/10.1093_ejcts_ezx039/2/m_ezx039f3.jpeg?Expires=1749227667&Signature=vMxYVflPyYOOYRjABfsMlGHKAbRqzb~iq-Momxrf3TXHx21wlEwlJXULqz-UBUFszXsGHpL~y2hd4XVqVouM2jqQiBn0rBWa8Sy-BU2b86XlnnEdGrRqTxfVwSwkJa3opKviN6qbjZOSeH51XIO1Wcbt-SiMNwLhQ0eIX3GJ17HUjFK24vjP92bJCg8FJLBG~1ONVqoB1QG0YlTbFuzS2W0YNfShuNN299ERA3KliIktGnH~4EfgWeaV-BYmLAaylUtYyaDnSQLB36386H926K91lbicF4YLYk74kEus2XoMADLf-r7-lAfvisFq69oXWcLpxEp7dRMWmDsuyeGFUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Incidence of postoperative atrial fibrillation (POAF) based on quintile grouping of plasma potassium (red) and magnesium (blue) levels at AF onset. A higher potassium or magnesium quintile was associated with a higher probability of POAF (P < 0.001 for both). Numbers above bars represent potassium (mmol/l) or magnesium levels (mg/dl) for respective quintile group (adapted from ref. [24]).
Hypokalemia (a serum potassium level below 3.5 mmol/l) leads to cellular hyperpolarity, higher resting potential, increased automaticity and excitability, and ventricular arrhythmias [8, 25]. Recent data call into question the widely held assumption that hypokalemia contributes to POAF [26, 27]. Our group has shown that low serum potassium levels were not associated with an increased risk of POAF and higher levels were not protective (Fig. 3) [24].
RISK FACTORS FOR POAF
Age
Advanced age is the most consistently reported and widely accepted risk factor for POAF [2–4, 8, 28]. The aging process leads to a loss of myocardial fibers, increased fibrosis and collagen deposition in the atria, particularly near the sinoatrial node, which alters atrial electrical properties [2, 4, 29]. Age-related physiological changes are a ‘setup’ for POAF, with acute surgical trauma and inflammation likely providing the inciting factors that induce POAF [8, 29].
A large single-institution retrospective study of 14 960 patients undergoing cardiac surgery over two decades found a non-linear trend between age and POAF incidence (Fig. 4) [3, 4]. The probability of developing POAF increases at a higher rate past the age of 55, and patients aged 72 or older are around 5 times more likely to develop POAF than patients younger than 55 [3].
![Estimated probability of developing postoperative atrial fibrillation (POAF) by age (adapted from ref. [3]).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/52/4/10.1093_ejcts_ezx039/2/m_ezx039f4.jpeg?Expires=1749227667&Signature=qd8d9dIRSJDSLSHXE5GSKFlSewqZS36NrcGnZpNH8SOj~0Ipvu4qm9Pw811ybOEzSKWWFtEZQva3loE-z-icVm3GY4a7UzNfcSSs19-mLIoqD32MI~txCAa-tHZFBZTABSAJMWJbEJv692nJ6bzd1P25ec-ke8~Yq3VZgX11EsJaoZx9qRedUtKSMIZXJZA4eC3SiKgqjGfvizCEwer3OQ6XWl3T9odKaATMIDB71Rh~IBxc6BBGf6nCSYgbJxVDxLMSlqHypZX-k~cJS8W-ReIpmaM7jRwgDnMqhG-K5n90SPiTxWdAcVQ985yU6yJYdcs5U44wJAfgU5T8PTIHng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Estimated probability of developing postoperative atrial fibrillation (POAF) by age (adapted from ref. [3]).
Cardiovascular risk factors
Cardiovascular risk factors for POAF include prior history of AF or other arrhythmias, non-coronary vascular disease, congestive heart failure, coronary artery disease, hypertension, left atrial enlargement and left ventricular dysfunction [1–4, 6, 7, 15]. Prior episodes of AF or electrophysiological abnormalities have consistently been identified as predictors of POAF, likely because the physiological factors necessary for the development and maintenance of arrhythmias already exist in these patients [2, 4, 10, 27].
Non-cardiovascular risk factors
Non-cardiovascular risk factors for POAF include: male gender, Caucasian race, chronic obstructive pulmonary disease, high cholesterol, hyperthyroidism, chronic kidney disease, diabetes, obesity and greater body surface area [1–4, 6, 27–29]. Individuals with a high body surface area (around 2.0 m2) often have larger atria and abnormal intrathoracic pressure, which may alter atrial electrophysiological properties and increase susceptibility to POAF [29]. POAF has also been associated with decreased mitochondrial function in patients with diabetes and metabolic syndrome [5]. A marker of mitochondrial function, the Bioenergetic Health Index, has recently been used to characterize bioenergetic changes following cardiac surgery and may serve as a predictor of POAF [15, 16, 30].
Cardiac surgery can also exacerbate inflammation in patients with a chronic inflammatory state, thereby increasing the probability of developing POAF [4, 5]. A genetic predisposition towards postoperative inflammation has recently been identified as a risk factor for POAF, but more research is required [2, 4, 5, 13].
PREDICTORS OF POAF
Timing of POAF onset
The risk of developing POAF follows a nonlinear trend and is highest on the second postoperative day [11]. Investigation into causes of POAF can be directed toward either of two phases of risk, each with distinct risk factors (Fig. 5). The first phase of risk occurs immediately after surgery and sharply declines during the first 18 h. The risk factors associated with this phase include advanced age, longer aortic cross-clamp time and mitral valve surgery. The second phase increases to a peak from hour zero to 36–48 h. The risk factors associated with this phase include advanced age, greater weight, Caucasian race and mitral valve surgery [11].
![Time related hazard for new-onset postoperative atrial fibrillation (POAF) after CABG +/− valve surgery with Phase I (red line) and Phase II (blue line) shown separately (reproduced from ref. [11] with permission from Elsevier).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/52/4/10.1093_ejcts_ezx039/2/m_ezx039f5.jpeg?Expires=1749227667&Signature=q37w3-PZdUMYBFKOMLu~aBx-CLp1O9N-tnWs~Eobm1hhumbWAnpLj3F9hiWZeSFNUNvEef9rT2XV6pC35WQATiWOCGT~~qkT5kd1~nWSV7f~EGocdZyrgXhgVOztTXpFTZToC7nSc9uRPiKyy2RyhqCqo-R96BwET3u15Fgt9K8i-hSycfkpuB35D8ng-NJv0exIaoqzLLJ16TpdlJ9M6k2eB4sCYiYpyIRTD-i-gQVoUIrwj5mHJdvOOP3tLRnT6967zdgu68HCijHYwlp5qxLlnUrkAtbTDTeV1xQzhUNUpoAcJw1rX5shmPHC-pG7lsHze7ylIbuDsdT3QgsYjA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Time related hazard for new-onset postoperative atrial fibrillation (POAF) after CABG +/− valve surgery with Phase I (red line) and Phase II (blue line) shown separately (reproduced from ref. [11] with permission from Elsevier).
Type of surgery
The overall incidence of POAF following cardiac surgery is approximately 35% but varies greatly depending on the type of procedure performed and patient characteristics [3–5, 28]. Isolated CABG has an incidence around 20–30% and isolated valve surgeries have an incidence around 35–40%. Combined CABG and valve surgery has a higher incidence of POAF, with various studies reporting incidences from 35% to greater than 60% [2–4, 28].
Intraoperative and perioperative factors
CPB and aortic cross-clamp duration have consistently been associated with POAF, and some studies have shown that reducing CPB and cross-clamp time may reduce POAF [6, 10, 28]. Off-pump cardiac surgery (primarily off-pump CABG and transcatheter approaches) has been shown to yield a lower incidence of POAF in most large studies [5, 7, 13, 31–33]. However, only about 20% of CABGs are currently performed off-pump, and most surgeons do not routinely perform this procedure [34].
Intra-aortic balloon pump usage, prolonged ventilation time, return to the ICU, cardiac tamponade and reoperation for bleeding are also predictors of POAF [3, 9, 28]. Retained blood in the chest cavity can lead to cardiac tamponade or form clots, which undergo inflammatory changes that amplify local and systemic inflammation. Drainage of blood from the pericardial space using chest tubes may therefore reduce the incidence of POAF and other postoperative complications associated with bleeding [35].
CHADS2 and CHA2DS2-VASc scores
CHADS2 and CHA2DS2-VASc scores combine cardiovascular and non-cardiovascular characteristics to determine a patient’s stroke risk. Recent studies have demonstrated a correlation between preoperative CHADS2 and CHA2DS2-VASc scores and POAF, making these tools potentially useful predictors of POAF [36, 37].
PREOPERATIVE POAF PROPHYLAXIS
β-Blockers
β-Blockers are Class II antiarrhythmic drugs (AADs) and are the most widely used prophylactic medication for patients undergoing cardiac surgery. Many studies have previously reported a link between preoperative β-blocker usage and decreased POAF incidence, and current guidelines list β-blocker administration for at least 24 h prior to surgery as a Class I recommendation for patients undergoing CABG or for those who have an ejection fraction greater than 30% [4, 28, 38–41]. However, reviews of the guidelines and a series of studies have called this recommendation into question. One large-scale retrospective review of over 500 000 patients undergoing CABG at 1107 hospitals found that preoperative β-blocker usage was associated with a slight but significant increase in POAF and an unchanged or slightly increased rate of postoperative mortality [40]. Some believe that postoperative β-blocker withdrawal may be responsible for these differences and it is recommended that administration of β-blockers to patients using them chronically should be resumed as soon as possible following surgery [1, 2, 28, 39]. As β-blockers carry a risk of bradycardia, hypotension, bronchospasm and heart failure exacerbation, administration to patients with poorly controlled asthma, heart failure, or other conditions that may become exacerbated by their use must be carefully considered [1].
Other antiarrhythmic drugs
Class III AADs such as amiodarone (the most widely used AAD), ibutilide, dofetilide and sotalol (which also has β-blocking effects) have also been widely used prophylactically in the preoperative period. Prophylactic amiodarone and sotalol are currently listed as Class II recommendations for POAF prevention [1, 4, 7, 8, 28, 39]. Studies have demonstrated that preoperative prophylactic oral amiodarone regimens (10 mg/kg started 6 days preoperatively in one large study, 600 mg/day for 7 days preoperatively in another) significantly decreased the incidence of postoperative atrial arrhythmias, stroke and hospital length of stay compared to placebo without any adverse complications other than occasional bradycardia [1, 2, 42–44]. Administration of sotalol 24–48 h before surgery has also been shown to significantly reduce the risk of POAF, but sotalol prophylaxis carries a higher rate of adverse side effects such as bradycardia and ventricular arrhythmias [7, 45]. Preoperative prophylactic administration of Class I AADs, including procainamide and propafenone, are not recommended due to their pro-arrhythmogenic properties [7, 41, 46, 47].
Corticosteroids
Corticosteroids decrease the heterogeneity of atrial conduction and reduce inflammation following cardiac surgery, and studies have shown that preoperative prophylactic corticosteroids reduced POAF incidence without an increased rate of postoperative infection [2, 14, 17, 18]. The most effective dosage and administration method of preoperative prophylactic corticosteroids remains unclear, however. One meta-analysis determined that doses of less than 1000 mg administered 24–72 h preoperatively significantly reduced the incidence of POAF, while another meta-analysis concluded that intermediate doses (4 days of 100 mg or one dose of 200–1000 mg of hydrocortisone, or one dose of 50–210 mg of dexamethasone) were more effective than lower (up to 8 mg) or higher (236–2850 mg) doses [17, 48]. Since corticosteroids carry the risk of hyperglycemia, immunosuppression, impaired wound healing, and increased gastrointestinal complications, they pose extra risk for certain patients and have not been widely adopted [17, 18].
Statins
Statins also reduce inflammation and oxidative stress following cardiac surgery, and early studies showed that preoperative statins significantly reduced POAF incidence [4, 49–51]. Dosage varied between these studies, from 20 to 80 mg, as did the time of administration, from 4 weeks preoperatively to the evening before [49]. More recent studies have shown conflicting data, however, and recommendations for preoperative statin prophylaxis cannot be made at present [1, 8, 52].
Other medications
The 2014 AATS guidelines suggest that administration of preoperative calcium channel blockers (i.e. diltiazem) is reasonable in high-risk patients who are not taking β-blockers, although their effect on POAF remains unclear [1, 7, 39]. Cardiac glycosides (i.e. digoxin) have not been recommended for use in preoperative prophylaxis [1]. The effects of prophylactic anticoagulants are also unclear; at least one institution has reported that preoperative warfarin reduced POAF incidence, but these results may have been confounded by other medications [3]. Interruption of anticoagulation for surgery in patients using chronic anticoagulants is acceptable for those with low stroke risk, based upon the patient’s CHA2DS2-VASc score [1]. Bridging with heparin, low molecular weight heparin or new oral anticoagulants (NOACs) including dabigatran, apixaban and rivaroxaban in patients without valvular disease may provide an alternative when complete interruption is not feasible [1, 2, 8]. While the administration of prophylactic angiotensin converting enzyme (ACE) inhibitors has increased drastically during the past several decades, preoperative ACE inhibitor usage has not been shown to decrease the incidence of POAF, although ACE inhibitor withdrawal has been linked with the development of POAF by some studies [3, 27].
INTRAOPERATIVE POAF PROPHYLAXIS
Intraoperative interventions aimed at preventing POAF include posterior pericardiotomy (in which an incision is made in the posterior pericardium from the left interior pulmonary vein to the diaphragm, parallel to the left phrenic nerve), anterior fat pad (AFP) preservation (in which the AFP, which contains parasympathetic ganglia, is not excised), and left atrial appendage (LAA) exclusion from circulation [7, 53].
Early evidence showed that posterior pericardiotomy significantly decreased the incidence of POAF, but additional trials have not corroborated these positive findings [54–56]. AFP preservation likely has no reductive effect on POAF [57, 58].
LAA exclusion has emerged as a target for prophylactic stroke prevention and an alternative to long-term anticoagulation therapy [53, 59]. Interest in this procedure has grown due to the low rates of stroke in patients who have had left atrial exclusion with the Cox-Maze procedure. Data from the Watchman device, which allows for percutaneous LAA exclusion, has provided some evidence that long-term stroke risk may be reduced with LAA exclusion alone [59, 60]. However, recent findings have shown that most elimination procedures fail to fully exclude the LAA from circulation, that percutaneous exclusion is associated with high rates of procedure-related harm, and that LAA exclusion increases POAF without influencing rates of stroke or long-term mortality [53, 59, 61]. It is therefore unclear whether cardiac surgery patients should undergo concomitant LAA exclusion, or whether anticoagulants may be safely discontinued in patients who do. Large studies are ongoing to evaluate whether LAA exclusion is protective against stroke in the long-term, but recommendation of this technique for a possible short-term advantage cannot be made at this time.
POSTOPERATIVE POAF PROPHYLAXIS
Medications
β-Blockers are the most common medication given for POAF prevention following surgery. While some studies have found that postoperative β-blockers significantly decreased the incidence of POAF and other postoperative complications, other analyses have attributed some β-blockers to excess deaths and disabling strokes [7, 38, 42, 40]. The Perioperative Ischemic Evaluation (POISE), reported that metoprolol prevented 15 myocardial infarctions but caused 8 excess deaths and 5 disabling strokes for every 1200 patients treated [40]. Despite such conflicting evidence, prescription of β-blockers following cardiac surgery remains a Class I recommendation and is performed in nearly 82% of cases [7, 39].
When the use of β-blockers is contraindicated, such as in patients with poorly controlled asthma or heart failure, postoperative administration of amiodarone is considered the second choice for POAF prevention [1, 28]. A recent Cochrane review of 118 studies reported that postoperative administration of amiodarone significantly reduced the incidence of POAF compared to control [42].
The efficacy of postoperative ACE inhibitors in POAF prevention is questionable; some studies have shown that postoperative ACE inhibitors decreased POAF incidence, while others showed no difference or an increase in comorbidities such as recurrent angina [39].
Electrolyte supplementation and repletion
Current clinical practice often includes intraoperative and postoperative repletion of magnesium and potassium, although its effects remain controversial [1, 19, 23, 25, 62–64]. Additionally, intraoperative and postoperative electrolyte supplementation has been proposed as a means of POAF prophylaxis, but this has also not been definitively shown to prevent POAF [1, 21, 22, 25, 27, 28, 42, 65]. Our recent single-institution retrospective observational study of over 2000 patients undergoing CABG and/or valve surgery found that, contrary to clinical dogma, magnesium supplementation was associated with an increased risk of POAF, while potassium supplementation was not protective against POAF and hypokalemia was not associated with POAF (Fig. 3) [24].
Atrial pacing
Various studies have reported mixed results regarding the effectiveness of single- or dual-chamber atrial pacing following cardiac surgery on POAF incidence [7, 10, 42, 66, 67]. Atrial pacing is, however, generally well tolerated [28, 67].
MANAGEMENT OF POAF
Episodes of POAF may resolve without intervention within minutes or hours, but persistent episodes of AF and those occurring in haemodynamically unstable patients require clinical intervention [8, 68]. Primary POAF management includes the use of one or more of the three mainstay medications: β-blockers, amiodarone and calcium channel blockers [8, 28]. Clinical practice typically involves using a single agent and addition of a second medication as needed. Concurrent use of all three may lead to bradycardia and hypotension, and should therefore be performed with caution. Special attention must also be given to prevent thromboembolic events [2].
Two general approaches for treatment of POAF exist: heart rate control and rhythm control [68]. Rate control focuses on slowing the heart rate and includes the use of β-blockers or calcium channel blockers. Rhythm control focuses on converting the arrhythmia into sinus rhythm by using Class I or III AADs or direct-current cardioversion for unstable patients or those with persistent episodes POAF. A recent prospective clinical study comparing rate versus rhythm control found that neither treatment strategy offered a significant clinical benefit over the other in terms of postoperative mortality, bleeding, thromboembolic events, length of stay, or freedom from AF at 60 days [68].
Antiarrhythmic drugs (AADs)
β-Blockers (Class II AADs) have rate control and antiarrhythmic effects and are listed as a Class I recommendation for treatment of POAF [1, 8, 28]. Due to the negative inotropic effects and heart rate depression caused by many traditional β-blockers, they should be used with caution in patients with hypotension, LV dysfunction, or heart failure [1, 12, 68]. In haemodynamically unstable patients, ultrashort-acting β-blockers such as esmolol and landiolol may provide better relief. Small-scale trials examining the effectiveness of landiolol have shown promising results, but further studies are warranted [12, 69].
Class III AADs (potassium channel blockers) such as amiodarone may also be used to convert patients to sinus rhythm, but are associated with bradycardia, hypotension and QT interval prolongation [1, 2, 7]. These agents are a Class II recommendation for POAF management and may be used independently or in conjunction with β-blockers, or as a first-line treatment in patients with hypotension, heart failure, or LV dysfunction [1, 8]. Discharge on any Class III AAD has been associated with a reduction in all-cause postoperative mortality, but long-term Class III AAD use has been linked with prolongation of atrial and ventricular refractory periods [43].
The 2014 AATS guidelines state as a Class II recommendation that AAD usage for patients discharged in sinus rhythm should be continued for 4 weeks after the last episode of POAF or until the first postoperative visit (2–6 weeks after discharge), while patients discharged in AF should continue AAD usage for 4 weeks following the first postoperative visit without POAF recurrence [1]. The 2010 Canadian Cardiovascular Society guidelines state that treatment may be discontinued between 6 and 12 weeks after restoration of sinus rhythm [28].
The effectiveness of Class I AADs (sodium channel blockers) such as procainamide, flecainide and propafenone in POAF treatment is unclear, but these medications carry a significant risk of pro-arrhythmic side effects and are therefore not recommended for treatment of POAF [1, 2, 7, 8].
Calcium channel blockers
Calcium channel blockers slow the propagation of action potentials at the AV node by altering cellular calcium flux. Calcium channel blockers are listed as a Class I recommendation for treatment of POAF when use of β-blockers is contraindicated or ineffective [1, 8, 12, 28].
Direct-current cardioversion
Direct-current cardioversion to sinus rhythm may become necessary in patients with episodes of POAF lasting over 48 h, in haemodynamically unstable patients, or when ventricular response is difficult to control [1, 2, 8]. Cardioversion is listed as a Class I recommendation by the AATS and a Class II recommendation by the 2014 AHA/ACC/HRS task force [1, 8]. The need for anticoagulation prior to cardioversion should be based upon the patient’s bleeding risk, CHA2DS2-VASc score and confirmation of the absence of intracardiac thrombus, particularly within the LAA, by TEE [1, 2]. When warranted, anticoagulation should be administered 3 weeks prior to cardioversion and continued for at least 4 weeks [1, 4]. In patients with a high bleeding risk, cardioversion without anticoagulation is listed as a Class II recommendation by the AATS [1].
Anticoagulants and antithrombotics
Within the first 48 h of POAF, anticoagulation to prevent thromboembolism is a Class I recommendation when the patient’s CHA2DS2-VASc score is higher than zero [1]. However, bleeding risk may outweigh the benefits of anticoagulation in patients with advanced age, uncontrolled hypertension, or a history of bleeding [4]. In such patients, postponing anticoagulation, implementing a rhythm control approach using pharmacological agents, or performing cardioversion without anticoagulation may be beneficial [1, 28].
Administration of anticoagulants and antithrombotics to patients without contraindications is listed as a Class I recommendation for episodes lasting longer than 48 h [1, 8, 28]. NOAC usage is listed as a Class II recommendation in patients where warfarin is contraindicated. NOACs are likely more effective than traditional warfarin at preventing stroke and major postoperative bleeding events, have fewer drug–drug interactions, and have rapid on/off action [1, 2, 8]. The short half-life of NOACs makes strict compliance important to ensure complete thromboembolism [8]. NOACs should not be used in patients with renal impairment (since no reversal agents exist to prevent renal damage if misused), those with a prosthetic heart valve, or those with impaired valve haemodynamics [1, 2]. Low molecular weight heparin may also serve as an alternative to warfarin in patients with high bleeding risk, low platelet counts and those who may require additional invasive procedures after discharge [1].
Anticoagulants and antithrombotics should be continued for a minimum of 4–6 weeks after return to sinus rhythm but may be continued for longer depending on the patient’s stroke risk [1, 2, 28]. Due to the self-limiting nature of POAF, long-term usage is often not warranted [23]. For patients who may require long-term anticoagulation due to POAF, the AATS recommends cardiology follow-up [1].
CONCLUSION
Postoperative atrial fibrillation is a common and expensive complication that occurs in about 35% of cardiac surgery patients and results in a prolonged hospital stay and approximately $10 000–$20 000 in additional hospital costs. POAF is an independent predictor of early and long-term complications including stroke, infection and cardiac arrest, and is associated with reoperation for bleeding and increased postoperative mortality. The etiology and risk factors for POAF are poorly understood, but advanced age, pre-existing cardiac conditions that cause restructuring, and susceptibility towards inflammation have been consistently linked with POAF. Decades of research has explored interventions to prevent or limit the incidence of POAF, but most are only partially effective. Due to the widespread incidence and numerous comorbidities associated with POAF, additional research focusing on the precise mechanisms of its pathogenesis is needed to yield a greater understanding of this complication and produce more effective prophylactic and treatment options.
Conflict of interest: none declared.
REFERENCES
- atrial fibrillation
- cardiac arrest
- cardiac surgery procedures
- hemorrhage
- inflammation
- cardiovascular system
- health care costs
- intensive care unit
- repeat surgery
- respiratory insufficiency
- infections
- brain
- heart
- kidney
- mortality
- persistence
- clinical practice guideline
- pacemaker, permanent
- stroke risk
- prevention




