-
PDF
- Split View
-
Views
-
Cite
Cite
Mauro Iafrancesco, Nora Goebel, Jorge Mascaro, Ulrich F.W. Franke, Davide Pacini, Roberto Di Bartolomeo, Gabriel Weiss, Martin Grabenwöger, Sergey A. Leontyev, Friedrich-Wilhelm Mohr, Thanos Sioris, Heinz Jakob, Konstantinos Tsagakis, on behalf of the International E-vita Open Registry Group, Aortic diameter remodelling after the frozen elephant trunk technique in aortic dissection: results from an international multicentre registry†, European Journal of Cardio-Thoracic Surgery, Volume 52, Issue 2, August 2017, Pages 310–318, https://doi.org/10.1093/ejcts/ezx131
Close - Share Icon Share
Abstract
OBJECTIVES: The frozen elephant trunk (FET) technique allows one-stage hybrid repair approach in aortic dissection (AoD). Even if the effect of the FET technique on promoting false lumen (FL) thrombosis has been proved in the past, the relative importance of FL thrombosis on aortic remodelling at different levels of the distal aorta and the magnitude of this effect is not well known. The aim of the study was to evaluate aortic remodelling following a FET technique for AoD.
METHODS: A multicentre international registry database was searched to identify all patients who underwent a FET procedure for an AoD. A total of 383 patients with AoD were operated on between January 2005 and March 2014 with the FET technique; 137 patients (65 acute AoD and 72 chronic AoD) who survived the initial repair with at least a 1-year follow-up CT scan were included in the study.
RESULTS: The rate of FL thrombosis was higher in the mid-descending thoracic aorta (99.3%) and lower in the distal abdominal aorta (13.9%) but similar between acute and chronic AoDs. The negative remodelling rate was similar between acute and chronic AoDs in the abdominal aorta, but chronic AoD exhibited a higher rate of negative remodelling in the descending thoracic aorta (33% vs 17.5%, P = 0.040).
CONCLUSIONS: The FET technique provides an effective treatment for AoD, promoting FL thrombosis and remodelling in the descending thoracic aorta. Changes in the diameter of the aortic lumen depend mainly on the status of the FL and are similar between acute and chronic AoD. Changes in the diameter of true lumen are affected by both the FL status and the timing of the presentation. However, increased FL thrombosis and positive remodelling rates are not maintained at the level of the abdominal aorta, and strict follow-up is mandatory to detect early changes in the aortic dimensions, which may warrant further interventions.
INTRODUCTION
Since its first description in 1996 [1], the frozen elephant trunk (FET) technique has become an important tool in the treatment of aortic disease by providing one-stage repair of complex aortic conditions and a landing zone for further downstream stent graft procedures, if necessary. Furthermore, the FET technique has been shown to promote aortic remodelling and false lumen (FL) thrombosis in aortic dissections (AoD), but the mechanism of this process remains unclear and unpredictable as well as the role of aortic dimensions and FL status. To elucidate the impact of aortic diameters and FL lumen thrombosis on aortic remodelling after AoD treated with a FET technique, we analysed data from the multicentre International E-vita Open Registry (IEOR), the largest registry of implantations of the first commercially available FET hybrid prosthesis.
MATERIALS AND METHODS
Patients and inclusion criteria
Between January 2005 and March 2014, 383 patients with an AoD were operated on with a FET procedure with the E-vita Open Plus stent graft hybrid prosthesis (Jotec GmbH, Hechingen, Germany). Operative survivors were selected for the study if at least 1 year of follow-up data along with good quality preoperative and follow-up CT scans were available. Exclusion criteria included a dissection flap limited to the aortic arch or to the proximal descending aorta and deployment of the stent graft in the FL. Operative mortality for the entire cohort was 16.5% (63 patients). Among the 320 patients discharged alive from the hospital, 137 patients operated on at 7 centres in Europe (Birmingham, UK; Stuttgart, Germany; Bologna, Italy; Vienna, Austria; Leipzig, Germany; Tampere, Finland; and Essen, Germany) met the inclusion criteria and represent the population of this study. Indications for surgery were acute type A AoD in 61 patients (44.5%), acute type B AoD in 4 patients (2.9%), chronic type A AoD in 51 patients (37.2%) and chronic type B AoD in 21 patients (15.3%). The preoperative characteristics of the patients are shown in Table 1. There was no significant difference between the patients included and excluded from the study in terms of median age (59 vs 60 years, P = 0.039), acuity of disease (acute dissection 47.4% vs 57.6%, P: 0.236), preoperative characteristics (i.e. chronic obstructive pulmonary disease 15.3% vs 15.5%, P = 0.731, renal failure 7.3% vs 11%, P:0.458) or associated intervention (i.e. aortic root surgery 27% vs 37%, P = 0.113).
| . | n (%)/median [IQR] . |
|---|---|
| Preoperative characteristics | |
| Age | 59 [49–66] |
| Sex (male) | 109 (76.6) |
| Hypertension | 112 (81.8) |
| Hypercholesterolaemia | 57 (41.6) |
| Diabetes | 9 (6.6) |
| COPD | 261 (15.3) |
| Renal failure | 10 (7.3) |
| PVD | 10 (7.3) |
| LVEF <40 | 3 (2.2) |
| LVEF 40–60% | 59 (43.1) |
| Previous stroke | 2 (1.5) |
| Marfan syndrome | 19 (6.6) |
| CAD | 15 (10.9) |
| AV regurgitation >2+ | 50 (36.5) |
| Reoperation (previous sternotomy) | 40 (29.2) |
| Previous endovascular intervention | |
| Arch stenting | 3 (2.2) |
| DTA stenting | 7 (5.1) |
| AA stenting | 3 (2.2) |
| Clinical presentation | |
| TL collapse | 32 (23.4) |
| Malperfusion | 38 (27.7) |
| Neurological deficit | 16 (11.7) |
| Cardiac tamponade | 10 (7.3) |
| Haemodynamic instability | 18 (13.1) |
| Preoperative inotropes | 14 (10.2) |
| Preoperative acute renal failure | 11 (8.0) |
| Ventilated | 8 (5.8) |
| . | n (%)/median [IQR] . |
|---|---|
| Preoperative characteristics | |
| Age | 59 [49–66] |
| Sex (male) | 109 (76.6) |
| Hypertension | 112 (81.8) |
| Hypercholesterolaemia | 57 (41.6) |
| Diabetes | 9 (6.6) |
| COPD | 261 (15.3) |
| Renal failure | 10 (7.3) |
| PVD | 10 (7.3) |
| LVEF <40 | 3 (2.2) |
| LVEF 40–60% | 59 (43.1) |
| Previous stroke | 2 (1.5) |
| Marfan syndrome | 19 (6.6) |
| CAD | 15 (10.9) |
| AV regurgitation >2+ | 50 (36.5) |
| Reoperation (previous sternotomy) | 40 (29.2) |
| Previous endovascular intervention | |
| Arch stenting | 3 (2.2) |
| DTA stenting | 7 (5.1) |
| AA stenting | 3 (2.2) |
| Clinical presentation | |
| TL collapse | 32 (23.4) |
| Malperfusion | 38 (27.7) |
| Neurological deficit | 16 (11.7) |
| Cardiac tamponade | 10 (7.3) |
| Haemodynamic instability | 18 (13.1) |
| Preoperative inotropes | 14 (10.2) |
| Preoperative acute renal failure | 11 (8.0) |
| Ventilated | 8 (5.8) |
IQR: interquartile range; COPD: chronic obstructive pulmonary disease; PVD: peripheral vascular disease; LVEF: left ventricular ejection fraction; CAD: coronary artery disease; AV: aortic valve; DTA: descending thoracic aorta; AA: abdominal aorta; TL: true lumen.
| . | n (%)/median [IQR] . |
|---|---|
| Preoperative characteristics | |
| Age | 59 [49–66] |
| Sex (male) | 109 (76.6) |
| Hypertension | 112 (81.8) |
| Hypercholesterolaemia | 57 (41.6) |
| Diabetes | 9 (6.6) |
| COPD | 261 (15.3) |
| Renal failure | 10 (7.3) |
| PVD | 10 (7.3) |
| LVEF <40 | 3 (2.2) |
| LVEF 40–60% | 59 (43.1) |
| Previous stroke | 2 (1.5) |
| Marfan syndrome | 19 (6.6) |
| CAD | 15 (10.9) |
| AV regurgitation >2+ | 50 (36.5) |
| Reoperation (previous sternotomy) | 40 (29.2) |
| Previous endovascular intervention | |
| Arch stenting | 3 (2.2) |
| DTA stenting | 7 (5.1) |
| AA stenting | 3 (2.2) |
| Clinical presentation | |
| TL collapse | 32 (23.4) |
| Malperfusion | 38 (27.7) |
| Neurological deficit | 16 (11.7) |
| Cardiac tamponade | 10 (7.3) |
| Haemodynamic instability | 18 (13.1) |
| Preoperative inotropes | 14 (10.2) |
| Preoperative acute renal failure | 11 (8.0) |
| Ventilated | 8 (5.8) |
| . | n (%)/median [IQR] . |
|---|---|
| Preoperative characteristics | |
| Age | 59 [49–66] |
| Sex (male) | 109 (76.6) |
| Hypertension | 112 (81.8) |
| Hypercholesterolaemia | 57 (41.6) |
| Diabetes | 9 (6.6) |
| COPD | 261 (15.3) |
| Renal failure | 10 (7.3) |
| PVD | 10 (7.3) |
| LVEF <40 | 3 (2.2) |
| LVEF 40–60% | 59 (43.1) |
| Previous stroke | 2 (1.5) |
| Marfan syndrome | 19 (6.6) |
| CAD | 15 (10.9) |
| AV regurgitation >2+ | 50 (36.5) |
| Reoperation (previous sternotomy) | 40 (29.2) |
| Previous endovascular intervention | |
| Arch stenting | 3 (2.2) |
| DTA stenting | 7 (5.1) |
| AA stenting | 3 (2.2) |
| Clinical presentation | |
| TL collapse | 32 (23.4) |
| Malperfusion | 38 (27.7) |
| Neurological deficit | 16 (11.7) |
| Cardiac tamponade | 10 (7.3) |
| Haemodynamic instability | 18 (13.1) |
| Preoperative inotropes | 14 (10.2) |
| Preoperative acute renal failure | 11 (8.0) |
| Ventilated | 8 (5.8) |
IQR: interquartile range; COPD: chronic obstructive pulmonary disease; PVD: peripheral vascular disease; LVEF: left ventricular ejection fraction; CAD: coronary artery disease; AV: aortic valve; DTA: descending thoracic aorta; AA: abdominal aorta; TL: true lumen.
The E-vita Open Plus hybrid prosthesis
The E-vita Open Plus hybrid prosthesis (Jotec, Hechingen, Germany) consists of a single polyester graft encapsulating circumferential Z-shaped nitinol rings along its distal length with a proximal crimped segment for conventional arch replacement. Indications, technique and risk factor analysis for postoperative complications were described previously [2]. The size and length of the stent graft were selected according to preoperative measurements. A guidewire was used for stent graft deployment in 109 patients (79.6%). The median [interquartile range (IQR)] of the prosthesis diameter used was 28 mm [24–30 mm].
Operative strategy
No standard surgical protocol for aortic arch surgery or stent-graft placement and implantation was applied. In all patients, surgical access was obtained through a median sternotomy; surgical repair was performed with the patient on cardiopulmonary bypass and hypothermic cerebral arrest (HCA) with either deep (≤20°C, n = 1, 0.7%), moderate (20–25°C, n = 82, 59.9%) or mild hypothermia (>25°C, n = 54, 39.4%). Selective antegrade cerebral perfusion (SACP) was used in 136 cases (99.3%) through bilateral (135, 98.6%) or unilateral (1, 0.7%) perfusion. Arterial return sites were the ascending aorta in 38 patients (27.7%), the aortic arch in 1 (0.7%), the femoral artery in 4 (2.9%), the right axillary/subclavian artery in 81 (59.1%) and other sites in 13 (9.5%). Cerebrospinal fluid drainage was used in 32 patients (23.4%). Reimplantation of epiaortic vessels was performed as islands in 72 patients (52.6%) or separately in 65 patients (47.4%), and the left subclavian artery was sacrificed in 5 patients (3.6%).
Definitions of variables and outcomes
Renal failure was defined as a creatinine level >2 mg/dl or the need for dialysis or haemofiltration. HCA was defined as the time from the cessation of cardiopulmonary bypass to the institution of SACP (period of no flow to the brain). Visceral ischaemia was defined by the sum of the HCA and the SACP times (period of no flow beyond the left subclavian artery). Proximal anastomosis levels were defined according to the Ishimaru classification [3]. Distal landing zones were defined by the level of the adjacent thoracic vertebra. Endoleaks were defined according to reporting standards as recommended by the Society of Vascular Surgery [4]. Prosthesis oversize was defined as a ratio between diameter of the stent graft and the true lumen (TL) >20%.
Cross-sectional imaging analysis
Aortic diameters were measured at specific levels:
Level 1 (L1), in the mid descending thoracic aorta (DTA) at the level of pulmonary artery bifurcation, which is usually covered by the stent graft. If the aorta was not covered by the stent at the level of the pulmonary artery bifurcation, L1 was measured as the midpoint between the proximal and distal ends of the stent graft.
Level 2 (L2), just below the end of the stent graft.
Level 3 (L3), in the distal DTA at the level of the 10th thoracic vertebra, which is usually not covered by the stent graft. If the aorta was covered by the stent at the level of the 10th thoracic vertebra, L3 was measured as the midpoint between the distal end of the stent graft and the diaphragm.
Level 4 (L4), as the proximal abdominal aorta (AA) at the level of the celiac artery.
Level 5 (L5), as the distal AA at the level of the third lumbar vertebra.
Aortic diameters were measured as follows:
For every level, the maximum diameter of the aortic lumen (AL) and of the TL was recorded.
Aortic diameters were generally taken from axial sections of the CT scans. If there was severe tortuosity of the aorta, making it impossible to obtain adequate measurements, these were taken from multiplanar reconstructions.
The AL diameter was measured as the distance between the outer borders of the vessel.
The diameter of the TL was measured from the outer border of the aorta to the outer border of the dissection flap.
The status of the FL (patent/thrombosed) was also recorded for every level. FL patency was defined by complete absence of thrombus in the FL.
Remodelling
Aortic remodelling was defined as positive if there was significant decrease in the diameter of the AL and/or increase in the diameter of the TL, stable if the diameters of the AL and the TL did not change significantly, negative if there was a significant increase in the diameter of the AL or a significant decrease in the diameter of the TL. A change in the diameters of the AL or the TL was considered significant if it was greater than 10% [5].
In particular:
A significant increase in the AL was considered negative remodelling, irrespective of a change in the diameter of the TL.
For patients with a stable AL diameter, remodelling was considered:
positive, if there was a significant increase the diameter of the TL;
stable, if the TL remained stable;
negative, if there was a significant decrease in the diameter of the TL.
For patients with a significant reduction in the diameter of the AL, remodelling was considered:
positive, if there was a significant increase in the diameter of the TL or if the diameter of the TL remained stable;
positive, if the diameter of the TL decreased but the decrease in the diameter of the AL was greater than that in the TL;
negative, if the diameter of the TL decreased but the decrease in the AL was less than the decrease in the TL.
Follow-up
All patients had a CT scan prior to discharge and were followed up in their own centres according to a standardized protocol involving clinical and CT scan examinations every 6 months for the first 2 years and annually afterwards. Follow-up was complete in 100% of patients. The median [IQR] follow-up was 32 months [21–53 months]. The median [IQR] interval between the operation and the last CT scan was 24 months [18–45 months]
Statistical methods
Categorical data are reported as numbers and percentages. Continuous variables are reported as medians and IQRs. Assumption of normality of continuous data was tested with the Shapiro–Wilk test. Categorical data were compared by χ2 tests for independent variables and by the McNemar test for paired variables. If the assumption of normality was not met, continuous variables were compared using the Mann–Whitney test for independent variables and the Wilcoxon matched-pair signed-rank test for paired variables. If the assumption of normality was met, continuous variables were compared using the Student’s t-test for independent or paired variables as appropriate. Correlation between ordinal variables was calculated with the Spearman coefficient. All tests were 2 sided, with a statistical significance set by an α level of 0.05. Long-term survival was assessed using the Kaplan–Meier method, and the differences between the groups were compared with the log-rank test. All calculations were performed using SPSS statistical software version 20.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
Operative data
The stent graft was successfully deployed in all patients. The median [IQR] times for cardiopulmonary bypass, cross-clamp, HCA, SACP and visceral ischaemia were 231 [199–280], 135 [104–179], 6 [2–16], 60 [49–76] and 73 [56–90] min, respectively. The level of the proximal anastomosis was in Zone 1 in 2 patients (1.5%), in Zone 2 in 29 (21.1%), in Zone 3 in 104 (75.9%) and in Zone 4 in 2 (1.5%). The distal landing zone was higher than T5 in 3 patients (2.2%), between T6 and T7 in 40 (29.2%), between T8 and T9 in 77 (56.2%) and lower than T10 in 17 (12.4%). The TL was oversized in 30 patients (21.9%). The aortic arch was replaced with the integrated prosthesis in 102 patients (74.4%), with a separate tubular graft in 20 patients (14.7%) and with a separate branched graft in 15 (10.9%). Concomitant procedures included ascending aorta replacement in 114 patients (83.2%), aortic valve resuspension in 10 (7.3%), aortic valve repair in 12 (8.8%), aortic valve replacement in 7 (5.1%), aortic root replacement with a valved conduit in 21 (15.3%), valve-sparing aortic root replacement in 16 (11.7%) and mitral valve repair or replacement in 6 (4.4%).
Early outcome
All patients selected for this study were discharged alive from the hospital. The incidence of permanent stroke, permanent spinal cord ischaemia, renal failure and mechanical ventilator support longer than 72 h was 2.2%, 3.6%, 19.7% and 24.1%, respectively. The incidence of major complications was significantly higher in the deceased group than in the survivor group. The median [IQR] hospital stay was 17 [12–29] days. Seven patients developed an early type Ib endoleak (5.1%).
ANALYSIS OF AORTIC DIAMETERS AND FALSE LUMEN STATUS
Changes in the aortic diameters of the entire cohort, including subgroup analysis according to the FL status, are shown in Fig. 1. The rate of the different statuses of FL at different levels preoperatively and at follow-up are reported in Table 2.
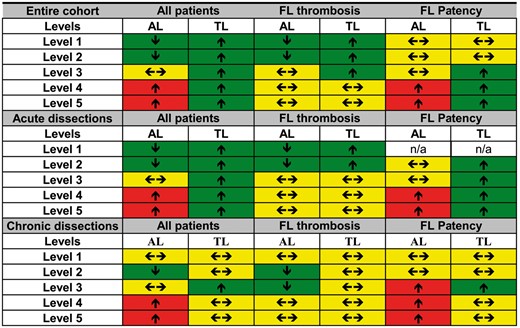
Changes in diameter for the entire cohort and for acute and chronic dissections, according to false lumen status. Arrows indicate changes in aortic diameter (↑ = increase in diameter, ←→ = no changes in diameter and ↓ = decrease in diameter). Colours indicate the effect of diametric changes in relation to remodelling (green: favours positive remodelling, yellow: no effect on remodelling and red: favours negative remodelling).
| . | Preoperative . | Follow-up . | P-value . | ||||
|---|---|---|---|---|---|---|---|
| . | FL patency . | FL thrombosis . | FL absent . | FL patency . | FL thrombosis . | FL absent . | . |
| Level 1 | 117 (85.4%) | 20 (14.6%) | 0 (0%) | 1 (0.7%) | 136 (99.3%) | 0 (0%) | <0.001 |
| Level 2 | 115 (83.9%) | 22 (16.1%) | 0 (0%) | 30 (21.9%) | 107 (78.1%) | 0 (0%) | <0.001 |
| Level 3 | 116 (84.7%) | 20 (14.6%) | 1 (0.7%) | 62 (45.3%) | 72 (52.6%) | 3 (2.1%) | <0.001 |
| Level 4 | 118 (86.2%) | 15 (10.9%) | 4 (2.9%) | 98 (71.6%) | 34 (24.8%) | 5 (3.6%) | <0.001 |
| Level 5 | 109 (79.6%) | 13 (9.5%) | 15 (10.9%) | 101 (73.7%) | 19 (13.9%) | 17 (12.4%) | 0.210 |
| . | Preoperative . | Follow-up . | P-value . | ||||
|---|---|---|---|---|---|---|---|
| . | FL patency . | FL thrombosis . | FL absent . | FL patency . | FL thrombosis . | FL absent . | . |
| Level 1 | 117 (85.4%) | 20 (14.6%) | 0 (0%) | 1 (0.7%) | 136 (99.3%) | 0 (0%) | <0.001 |
| Level 2 | 115 (83.9%) | 22 (16.1%) | 0 (0%) | 30 (21.9%) | 107 (78.1%) | 0 (0%) | <0.001 |
| Level 3 | 116 (84.7%) | 20 (14.6%) | 1 (0.7%) | 62 (45.3%) | 72 (52.6%) | 3 (2.1%) | <0.001 |
| Level 4 | 118 (86.2%) | 15 (10.9%) | 4 (2.9%) | 98 (71.6%) | 34 (24.8%) | 5 (3.6%) | <0.001 |
| Level 5 | 109 (79.6%) | 13 (9.5%) | 15 (10.9%) | 101 (73.7%) | 19 (13.9%) | 17 (12.4%) | 0.210 |
FL: false lumen.
| . | Preoperative . | Follow-up . | P-value . | ||||
|---|---|---|---|---|---|---|---|
| . | FL patency . | FL thrombosis . | FL absent . | FL patency . | FL thrombosis . | FL absent . | . |
| Level 1 | 117 (85.4%) | 20 (14.6%) | 0 (0%) | 1 (0.7%) | 136 (99.3%) | 0 (0%) | <0.001 |
| Level 2 | 115 (83.9%) | 22 (16.1%) | 0 (0%) | 30 (21.9%) | 107 (78.1%) | 0 (0%) | <0.001 |
| Level 3 | 116 (84.7%) | 20 (14.6%) | 1 (0.7%) | 62 (45.3%) | 72 (52.6%) | 3 (2.1%) | <0.001 |
| Level 4 | 118 (86.2%) | 15 (10.9%) | 4 (2.9%) | 98 (71.6%) | 34 (24.8%) | 5 (3.6%) | <0.001 |
| Level 5 | 109 (79.6%) | 13 (9.5%) | 15 (10.9%) | 101 (73.7%) | 19 (13.9%) | 17 (12.4%) | 0.210 |
| . | Preoperative . | Follow-up . | P-value . | ||||
|---|---|---|---|---|---|---|---|
| . | FL patency . | FL thrombosis . | FL absent . | FL patency . | FL thrombosis . | FL absent . | . |
| Level 1 | 117 (85.4%) | 20 (14.6%) | 0 (0%) | 1 (0.7%) | 136 (99.3%) | 0 (0%) | <0.001 |
| Level 2 | 115 (83.9%) | 22 (16.1%) | 0 (0%) | 30 (21.9%) | 107 (78.1%) | 0 (0%) | <0.001 |
| Level 3 | 116 (84.7%) | 20 (14.6%) | 1 (0.7%) | 62 (45.3%) | 72 (52.6%) | 3 (2.1%) | <0.001 |
| Level 4 | 118 (86.2%) | 15 (10.9%) | 4 (2.9%) | 98 (71.6%) | 34 (24.8%) | 5 (3.6%) | <0.001 |
| Level 5 | 109 (79.6%) | 13 (9.5%) | 15 (10.9%) | 101 (73.7%) | 19 (13.9%) | 17 (12.4%) | 0.210 |
FL: false lumen.
Acute aortic dissection
Changes in aortic diameters
Changes in aortic diameters for patients with acute dissections are presented in Table 3. In segments with FL thrombosis, there was a significant decrease in the diameter of the AL and an increase in that of the TL at L1 and L2, whereas the diameters remained stable at L3, L4 and L5. In segments with FL patency, the diameter of the AL remained stable at L2 and L3 and increased at L4 and L5, whereas that of the TL increased at L2, L3, L4 and L5 (L1 was not analysed because there were no patients with FL patency at this level).
Changes in diameter of the AL and TL for the entire cohort in acute dissections
| . | AL PO . | AL FU . | P-value . | TL PO . | TL FU . | P-value . |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Level 1 | 36 [33–38] | 32 [30–36] | <0.0001 | 26 [20–30] | 28 [25–30] | 0.009 |
| Level 2 | 33 [30–36] | 31 [28–36] | 0.006 | 25 [20–30] | 27 [24–30] | <0.001 |
| Level 3 | 31 [28–34] | 31 [29–36] | 0.214 | 24 [19–28] | 26 [22–29] | 0.002 |
| Level 4 | 29 [27–30] | 31 [28–34] | <0.0001 | 22 [17–26] | 24 [19–27] | 0.014 |
| Level 5 | 23 [22–26] | 26 [23–28] | <0.0001 | 18 [16–21] | 20 [17–23] | 0.014 |
| FL patency | ||||||
| Level 1 | n/a | n/a | n/a | n/a | n/a | n/a |
| Level 2 | 32 [30–38] | 30 [30–38] | 0.665 | 23 [18–26] | 25 [22–29] | 0.041 |
| Level 3 | 29 [28–33] | 31 [30–36] | 0.072 | 23 [18–27] | 25 [22–28] | 0.002 |
| Level 4 | 29 [27–30] | 31 [28–35] | <0.0001 | 22 [17–26] | 24 [19–27] | 0.015 |
| Level 5 | 23 [21–25] | 26 [23–28] | <0.0001 | 18 [16–21] | 20 [16–22] | 0.004 |
| FL thrombosis | ||||||
| Level 1 | 36 [33–38] | 32 [30–36] | <0.001 | 26 [20–30] | 28 [25–30] | 0.009 |
| Level 2 | 33 [31–36] | 31 [28–36] | 0.003 | 25 [20–30] | 28 [25–31] | <0.001 |
| Level 3 | 32 [31–35] | 31 [30–35] | 0.877 | 27 [22–30] | 28 [26–30] | 0.462 |
| Level 4 | 30 [28–32] | 30 [28–34] | 0.233 | 27 [21–28] | 27 [23–28] | 1.000 |
| Level 5 | 26 [22–30] | 24 [23–30] | 0.677 | 24 [18–30] | 21 [19–24] | 0.715 |
| . | AL PO . | AL FU . | P-value . | TL PO . | TL FU . | P-value . |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Level 1 | 36 [33–38] | 32 [30–36] | <0.0001 | 26 [20–30] | 28 [25–30] | 0.009 |
| Level 2 | 33 [30–36] | 31 [28–36] | 0.006 | 25 [20–30] | 27 [24–30] | <0.001 |
| Level 3 | 31 [28–34] | 31 [29–36] | 0.214 | 24 [19–28] | 26 [22–29] | 0.002 |
| Level 4 | 29 [27–30] | 31 [28–34] | <0.0001 | 22 [17–26] | 24 [19–27] | 0.014 |
| Level 5 | 23 [22–26] | 26 [23–28] | <0.0001 | 18 [16–21] | 20 [17–23] | 0.014 |
| FL patency | ||||||
| Level 1 | n/a | n/a | n/a | n/a | n/a | n/a |
| Level 2 | 32 [30–38] | 30 [30–38] | 0.665 | 23 [18–26] | 25 [22–29] | 0.041 |
| Level 3 | 29 [28–33] | 31 [30–36] | 0.072 | 23 [18–27] | 25 [22–28] | 0.002 |
| Level 4 | 29 [27–30] | 31 [28–35] | <0.0001 | 22 [17–26] | 24 [19–27] | 0.015 |
| Level 5 | 23 [21–25] | 26 [23–28] | <0.0001 | 18 [16–21] | 20 [16–22] | 0.004 |
| FL thrombosis | ||||||
| Level 1 | 36 [33–38] | 32 [30–36] | <0.001 | 26 [20–30] | 28 [25–30] | 0.009 |
| Level 2 | 33 [31–36] | 31 [28–36] | 0.003 | 25 [20–30] | 28 [25–31] | <0.001 |
| Level 3 | 32 [31–35] | 31 [30–35] | 0.877 | 27 [22–30] | 28 [26–30] | 0.462 |
| Level 4 | 30 [28–32] | 30 [28–34] | 0.233 | 27 [21–28] | 27 [23–28] | 1.000 |
| Level 5 | 26 [22–30] | 24 [23–30] | 0.677 | 24 [18–30] | 21 [19–24] | 0.715 |
All measurements are in millimetres. Values are reported as median [interquartile range].
AL: aortic lumen; PO: preoperative; FU: follow-up; FL: false lumen; TL: true lumen; n/a: not applicable.
Changes in diameter of the AL and TL for the entire cohort in acute dissections
| . | AL PO . | AL FU . | P-value . | TL PO . | TL FU . | P-value . |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Level 1 | 36 [33–38] | 32 [30–36] | <0.0001 | 26 [20–30] | 28 [25–30] | 0.009 |
| Level 2 | 33 [30–36] | 31 [28–36] | 0.006 | 25 [20–30] | 27 [24–30] | <0.001 |
| Level 3 | 31 [28–34] | 31 [29–36] | 0.214 | 24 [19–28] | 26 [22–29] | 0.002 |
| Level 4 | 29 [27–30] | 31 [28–34] | <0.0001 | 22 [17–26] | 24 [19–27] | 0.014 |
| Level 5 | 23 [22–26] | 26 [23–28] | <0.0001 | 18 [16–21] | 20 [17–23] | 0.014 |
| FL patency | ||||||
| Level 1 | n/a | n/a | n/a | n/a | n/a | n/a |
| Level 2 | 32 [30–38] | 30 [30–38] | 0.665 | 23 [18–26] | 25 [22–29] | 0.041 |
| Level 3 | 29 [28–33] | 31 [30–36] | 0.072 | 23 [18–27] | 25 [22–28] | 0.002 |
| Level 4 | 29 [27–30] | 31 [28–35] | <0.0001 | 22 [17–26] | 24 [19–27] | 0.015 |
| Level 5 | 23 [21–25] | 26 [23–28] | <0.0001 | 18 [16–21] | 20 [16–22] | 0.004 |
| FL thrombosis | ||||||
| Level 1 | 36 [33–38] | 32 [30–36] | <0.001 | 26 [20–30] | 28 [25–30] | 0.009 |
| Level 2 | 33 [31–36] | 31 [28–36] | 0.003 | 25 [20–30] | 28 [25–31] | <0.001 |
| Level 3 | 32 [31–35] | 31 [30–35] | 0.877 | 27 [22–30] | 28 [26–30] | 0.462 |
| Level 4 | 30 [28–32] | 30 [28–34] | 0.233 | 27 [21–28] | 27 [23–28] | 1.000 |
| Level 5 | 26 [22–30] | 24 [23–30] | 0.677 | 24 [18–30] | 21 [19–24] | 0.715 |
| . | AL PO . | AL FU . | P-value . | TL PO . | TL FU . | P-value . |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Level 1 | 36 [33–38] | 32 [30–36] | <0.0001 | 26 [20–30] | 28 [25–30] | 0.009 |
| Level 2 | 33 [30–36] | 31 [28–36] | 0.006 | 25 [20–30] | 27 [24–30] | <0.001 |
| Level 3 | 31 [28–34] | 31 [29–36] | 0.214 | 24 [19–28] | 26 [22–29] | 0.002 |
| Level 4 | 29 [27–30] | 31 [28–34] | <0.0001 | 22 [17–26] | 24 [19–27] | 0.014 |
| Level 5 | 23 [22–26] | 26 [23–28] | <0.0001 | 18 [16–21] | 20 [17–23] | 0.014 |
| FL patency | ||||||
| Level 1 | n/a | n/a | n/a | n/a | n/a | n/a |
| Level 2 | 32 [30–38] | 30 [30–38] | 0.665 | 23 [18–26] | 25 [22–29] | 0.041 |
| Level 3 | 29 [28–33] | 31 [30–36] | 0.072 | 23 [18–27] | 25 [22–28] | 0.002 |
| Level 4 | 29 [27–30] | 31 [28–35] | <0.0001 | 22 [17–26] | 24 [19–27] | 0.015 |
| Level 5 | 23 [21–25] | 26 [23–28] | <0.0001 | 18 [16–21] | 20 [16–22] | 0.004 |
| FL thrombosis | ||||||
| Level 1 | 36 [33–38] | 32 [30–36] | <0.001 | 26 [20–30] | 28 [25–30] | 0.009 |
| Level 2 | 33 [31–36] | 31 [28–36] | 0.003 | 25 [20–30] | 28 [25–31] | <0.001 |
| Level 3 | 32 [31–35] | 31 [30–35] | 0.877 | 27 [22–30] | 28 [26–30] | 0.462 |
| Level 4 | 30 [28–32] | 30 [28–34] | 0.233 | 27 [21–28] | 27 [23–28] | 1.000 |
| Level 5 | 26 [22–30] | 24 [23–30] | 0.677 | 24 [18–30] | 21 [19–24] | 0.715 |
All measurements are in millimetres. Values are reported as median [interquartile range].
AL: aortic lumen; PO: preoperative; FU: follow-up; FL: false lumen; TL: true lumen; n/a: not applicable.
False lumen thrombosis
FL thrombosis rates according to the mode of presentation are presented in Table 4. At the last CT scan, thrombosis of the FL at L1 was present in all patients. The rate of thrombosis at L2, L3, L4 and L5 was 80%, 49.2%, 29.2% and 18.5%, respectively. The rate of FL thrombosis increased at all levels, and the increase was statistically significant at all levels except L5.
False lumen thrombosis rate according to the mode of presentation (acute/chronic)
| . | All patients . | Acute dissections . | Chronic dissections . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | PO . | FU . | P-value . | PO . | FU . | P-value . | PO . | FU . | P-value . |
| Level 1 | 14.6% | 99.3% | <0.001 | 15.4% | 100% | n/a | 13.9% | 98.6% | <0.001 |
| Level 2 | 16.1% | 77.4% | <0.001 | 20% | 80% | <0.001 | 12.5% | 75% | <0.001 |
| Level 3 | 14.6% | 52.6% | <0.001 | 18.5% | 49.2% | <0.001 | 11.1% | 55.6% | <0.001 |
| Level 4 | 10.9% | 24.8% | <0.001 | 15.4% | 29.2% | 0.022 | 6.9% | 20.8% | 0.002 |
| Level 5 | 9.5% | 13.9% | 0.271 | 15.4% | 18.5% | 0.754 | 4.2% | 9.7% | 0.219 |
| . | All patients . | Acute dissections . | Chronic dissections . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | PO . | FU . | P-value . | PO . | FU . | P-value . | PO . | FU . | P-value . |
| Level 1 | 14.6% | 99.3% | <0.001 | 15.4% | 100% | n/a | 13.9% | 98.6% | <0.001 |
| Level 2 | 16.1% | 77.4% | <0.001 | 20% | 80% | <0.001 | 12.5% | 75% | <0.001 |
| Level 3 | 14.6% | 52.6% | <0.001 | 18.5% | 49.2% | <0.001 | 11.1% | 55.6% | <0.001 |
| Level 4 | 10.9% | 24.8% | <0.001 | 15.4% | 29.2% | 0.022 | 6.9% | 20.8% | 0.002 |
| Level 5 | 9.5% | 13.9% | 0.271 | 15.4% | 18.5% | 0.754 | 4.2% | 9.7% | 0.219 |
PO: preoperative; FU: follow-up; n/a: not applicable.
False lumen thrombosis rate according to the mode of presentation (acute/chronic)
| . | All patients . | Acute dissections . | Chronic dissections . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | PO . | FU . | P-value . | PO . | FU . | P-value . | PO . | FU . | P-value . |
| Level 1 | 14.6% | 99.3% | <0.001 | 15.4% | 100% | n/a | 13.9% | 98.6% | <0.001 |
| Level 2 | 16.1% | 77.4% | <0.001 | 20% | 80% | <0.001 | 12.5% | 75% | <0.001 |
| Level 3 | 14.6% | 52.6% | <0.001 | 18.5% | 49.2% | <0.001 | 11.1% | 55.6% | <0.001 |
| Level 4 | 10.9% | 24.8% | <0.001 | 15.4% | 29.2% | 0.022 | 6.9% | 20.8% | 0.002 |
| Level 5 | 9.5% | 13.9% | 0.271 | 15.4% | 18.5% | 0.754 | 4.2% | 9.7% | 0.219 |
| . | All patients . | Acute dissections . | Chronic dissections . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | PO . | FU . | P-value . | PO . | FU . | P-value . | PO . | FU . | P-value . |
| Level 1 | 14.6% | 99.3% | <0.001 | 15.4% | 100% | n/a | 13.9% | 98.6% | <0.001 |
| Level 2 | 16.1% | 77.4% | <0.001 | 20% | 80% | <0.001 | 12.5% | 75% | <0.001 |
| Level 3 | 14.6% | 52.6% | <0.001 | 18.5% | 49.2% | <0.001 | 11.1% | 55.6% | <0.001 |
| Level 4 | 10.9% | 24.8% | <0.001 | 15.4% | 29.2% | 0.022 | 6.9% | 20.8% | 0.002 |
| Level 5 | 9.5% | 13.9% | 0.271 | 15.4% | 18.5% | 0.754 | 4.2% | 9.7% | 0.219 |
PO: preoperative; FU: follow-up; n/a: not applicable.
Remodelling
Aortic remodelling, according to the classification proposed, was calculated for 57 patients with acute dissections. The aortic remodelling rates are presented in Fig. 2. The negative remodelling rate increased from L1 to L5. There was significant correlation between FL thrombosis and positive or stable aortic remodelling at L2 (r = 0.36, P = 0.006), L3 (r = 0.33, P = 0.011) L4 (r = 0.29, P = 0.029) but not at L5 (r = 0.127, P = 0.347). Correlation at L1 was not calculated because all patients presented a thrombosed FL at this level.

Aortic remodelling rate for acute (A) and chronic (B) dissections.
Chronic dissection
Change in aortic diameters
The changes in aortic diameters for patients with chronic dissections are presented in Table 5. In segments with FL thrombosis, the diameter of the AL decreased in L2 and L3 and remained stable in L1, L4 and L5 whereas that of the TL remained stable at all levels. In segments with FL patency, the diameter of the AL remained stable at L1 and L2 and increased in L3, L4 and L5 whereas that of the TL remained stable at all levels except in L3 where it increased.
Changes in the diameters of the aortic lumen and the true lumen for the entire cohort with chronic dissections
| . | AL PO . | AL FU . | P-value . | TL PO . | TL FU . | P-value . |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Level 1 | 50 [36–63] | 47 [39–60] | 0.221 | 29 [25–33] | 30 [26–32] | 0.448 |
| Level 2 | 40 [35–51] | 38 [32–46] | 0.019 | 25 [21–30] | 27 [25–30] | 0.082 |
| Level 3 | 49 [43–55] | 35 [33–47] | 0.169 | 25 [21–28] | 26 [24–29] | <0.001 |
| Level 4 | 34 [29–41] | 36 [31–43] | 0.002 | 26 [22–29] | 25 [22–29] | 0.261 |
| Level 5 | 27 [24–33] | 29 [24–34] | 0.031 | 19 [19–22] | 20 [17–22] | 0.534 |
| FL patency | ||||||
| Level 1 | 45 [37–54] | 42 [35–51] | 0.103 | 29 [23–33] | 30 [26–32] | 0.191 |
| Level 2 | 40 [31–58] | 41 [32–50] | 0.670 | 24 [20–29] | 25 [22–29] | 0.270 |
| Level 3 | 37 [32–46] | 38 [33–49] | 0.011 | 24 [20–27] | 26 [24–29] | <0.001 |
| Level 4 | 34 [29–39] | 36 [30–41] | 0.007 | 25 [21–28] | 25 [22–28] | 0.078 |
| Level 5 | 27 [23–32] | 29 [24–34] | 0.007 | 19 [18–22] | 20 [17–22] | 0.094 |
| FL thrombosis | ||||||
| Level 1 | 50 [35–63] | 47 [39–60] | 0.483 | 31 [26–34] | 30 [25–31] | 0.084 |
| Level 2 | 40 [35–51] | 37 [32–45] | 0.002 | 26 [23–30] | 28 [25–30] | 0.156 |
| Level 3 | 49 [44–56] | 35 [33–47] | 0.028 | 28 [25–51] | 26 [23–33] | 0.223 |
| Level 4 | 40 [32–47] | 43 [33–51] | 0.146 | 26 [26–34] | 26 [24–31] | 0.296 |
| Level 5 | 29 [24–37] | 30 [23–33] | 0.397 | 20 [18–27] | 20 [17–23] | 0.070 |
| . | AL PO . | AL FU . | P-value . | TL PO . | TL FU . | P-value . |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Level 1 | 50 [36–63] | 47 [39–60] | 0.221 | 29 [25–33] | 30 [26–32] | 0.448 |
| Level 2 | 40 [35–51] | 38 [32–46] | 0.019 | 25 [21–30] | 27 [25–30] | 0.082 |
| Level 3 | 49 [43–55] | 35 [33–47] | 0.169 | 25 [21–28] | 26 [24–29] | <0.001 |
| Level 4 | 34 [29–41] | 36 [31–43] | 0.002 | 26 [22–29] | 25 [22–29] | 0.261 |
| Level 5 | 27 [24–33] | 29 [24–34] | 0.031 | 19 [19–22] | 20 [17–22] | 0.534 |
| FL patency | ||||||
| Level 1 | 45 [37–54] | 42 [35–51] | 0.103 | 29 [23–33] | 30 [26–32] | 0.191 |
| Level 2 | 40 [31–58] | 41 [32–50] | 0.670 | 24 [20–29] | 25 [22–29] | 0.270 |
| Level 3 | 37 [32–46] | 38 [33–49] | 0.011 | 24 [20–27] | 26 [24–29] | <0.001 |
| Level 4 | 34 [29–39] | 36 [30–41] | 0.007 | 25 [21–28] | 25 [22–28] | 0.078 |
| Level 5 | 27 [23–32] | 29 [24–34] | 0.007 | 19 [18–22] | 20 [17–22] | 0.094 |
| FL thrombosis | ||||||
| Level 1 | 50 [35–63] | 47 [39–60] | 0.483 | 31 [26–34] | 30 [25–31] | 0.084 |
| Level 2 | 40 [35–51] | 37 [32–45] | 0.002 | 26 [23–30] | 28 [25–30] | 0.156 |
| Level 3 | 49 [44–56] | 35 [33–47] | 0.028 | 28 [25–51] | 26 [23–33] | 0.223 |
| Level 4 | 40 [32–47] | 43 [33–51] | 0.146 | 26 [26–34] | 26 [24–31] | 0.296 |
| Level 5 | 29 [24–37] | 30 [23–33] | 0.397 | 20 [18–27] | 20 [17–23] | 0.070 |
All measurements are in millimetres. Values are reported as median [interquartile range].
AL: aortic lumen; PO: preoperative; FU: follow-up; TL: true lumen.
Changes in the diameters of the aortic lumen and the true lumen for the entire cohort with chronic dissections
| . | AL PO . | AL FU . | P-value . | TL PO . | TL FU . | P-value . |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Level 1 | 50 [36–63] | 47 [39–60] | 0.221 | 29 [25–33] | 30 [26–32] | 0.448 |
| Level 2 | 40 [35–51] | 38 [32–46] | 0.019 | 25 [21–30] | 27 [25–30] | 0.082 |
| Level 3 | 49 [43–55] | 35 [33–47] | 0.169 | 25 [21–28] | 26 [24–29] | <0.001 |
| Level 4 | 34 [29–41] | 36 [31–43] | 0.002 | 26 [22–29] | 25 [22–29] | 0.261 |
| Level 5 | 27 [24–33] | 29 [24–34] | 0.031 | 19 [19–22] | 20 [17–22] | 0.534 |
| FL patency | ||||||
| Level 1 | 45 [37–54] | 42 [35–51] | 0.103 | 29 [23–33] | 30 [26–32] | 0.191 |
| Level 2 | 40 [31–58] | 41 [32–50] | 0.670 | 24 [20–29] | 25 [22–29] | 0.270 |
| Level 3 | 37 [32–46] | 38 [33–49] | 0.011 | 24 [20–27] | 26 [24–29] | <0.001 |
| Level 4 | 34 [29–39] | 36 [30–41] | 0.007 | 25 [21–28] | 25 [22–28] | 0.078 |
| Level 5 | 27 [23–32] | 29 [24–34] | 0.007 | 19 [18–22] | 20 [17–22] | 0.094 |
| FL thrombosis | ||||||
| Level 1 | 50 [35–63] | 47 [39–60] | 0.483 | 31 [26–34] | 30 [25–31] | 0.084 |
| Level 2 | 40 [35–51] | 37 [32–45] | 0.002 | 26 [23–30] | 28 [25–30] | 0.156 |
| Level 3 | 49 [44–56] | 35 [33–47] | 0.028 | 28 [25–51] | 26 [23–33] | 0.223 |
| Level 4 | 40 [32–47] | 43 [33–51] | 0.146 | 26 [26–34] | 26 [24–31] | 0.296 |
| Level 5 | 29 [24–37] | 30 [23–33] | 0.397 | 20 [18–27] | 20 [17–23] | 0.070 |
| . | AL PO . | AL FU . | P-value . | TL PO . | TL FU . | P-value . |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Level 1 | 50 [36–63] | 47 [39–60] | 0.221 | 29 [25–33] | 30 [26–32] | 0.448 |
| Level 2 | 40 [35–51] | 38 [32–46] | 0.019 | 25 [21–30] | 27 [25–30] | 0.082 |
| Level 3 | 49 [43–55] | 35 [33–47] | 0.169 | 25 [21–28] | 26 [24–29] | <0.001 |
| Level 4 | 34 [29–41] | 36 [31–43] | 0.002 | 26 [22–29] | 25 [22–29] | 0.261 |
| Level 5 | 27 [24–33] | 29 [24–34] | 0.031 | 19 [19–22] | 20 [17–22] | 0.534 |
| FL patency | ||||||
| Level 1 | 45 [37–54] | 42 [35–51] | 0.103 | 29 [23–33] | 30 [26–32] | 0.191 |
| Level 2 | 40 [31–58] | 41 [32–50] | 0.670 | 24 [20–29] | 25 [22–29] | 0.270 |
| Level 3 | 37 [32–46] | 38 [33–49] | 0.011 | 24 [20–27] | 26 [24–29] | <0.001 |
| Level 4 | 34 [29–39] | 36 [30–41] | 0.007 | 25 [21–28] | 25 [22–28] | 0.078 |
| Level 5 | 27 [23–32] | 29 [24–34] | 0.007 | 19 [18–22] | 20 [17–22] | 0.094 |
| FL thrombosis | ||||||
| Level 1 | 50 [35–63] | 47 [39–60] | 0.483 | 31 [26–34] | 30 [25–31] | 0.084 |
| Level 2 | 40 [35–51] | 37 [32–45] | 0.002 | 26 [23–30] | 28 [25–30] | 0.156 |
| Level 3 | 49 [44–56] | 35 [33–47] | 0.028 | 28 [25–51] | 26 [23–33] | 0.223 |
| Level 4 | 40 [32–47] | 43 [33–51] | 0.146 | 26 [26–34] | 26 [24–31] | 0.296 |
| Level 5 | 29 [24–37] | 30 [23–33] | 0.397 | 20 [18–27] | 20 [17–23] | 0.070 |
All measurements are in millimetres. Values are reported as median [interquartile range].
AL: aortic lumen; PO: preoperative; FU: follow-up; TL: true lumen.
False lumen thrombosis
FL thrombosis rates according to the mode of presentation are presented in Table 4. At the last CT scan, thrombosis of the FL occurred in 98.6%, 75%, 55.6%, 20.8% and 9.7% at L1, L2, L3, L4 and L5, respectively. The rate of FL thrombosis increased at all levels; the increase was statistically significant at all levels except L5.
Remodelling
Aortic remodelling, according to the classification proposed, was calculated for 60 patients with chronic dissections. The aortic remodelling rates are presented in Fig. 2. The negative remodelling rates increased from L1 to L5. There was no significant correlation between FL thrombosis and positive or stable aortic remodelling at L2, L2, L3 or L4. Correlation at L1 was not calculated in all patients who presented a thrombosed FL.
Follow-up
Ten patients died during the follow-up period, all in the chronic dissections group. The cause of death was aortic rupture in 1 patient (0.7%), heart failure in 1 patient (0.7%), respiratory failure in 1 patient (0.7%), pulmonary embolism in 1 patient (0.7%), sepsis in 1 patient (0.7%), multiorgan failure in 1 patient (0.7%), suicide in 1 patient (0.7%), unknown in 2 patients (1.4%); 1 patient (0.7%) died postoperatively after distal reintervention. One-, 3- and 5-year estimates of survival in the acute and chronic groups were 100%, 100% and 100% and 100%, 92.3% and 82.3%, respectively (Fig. 3) with a statically significant difference (P = 0.03).
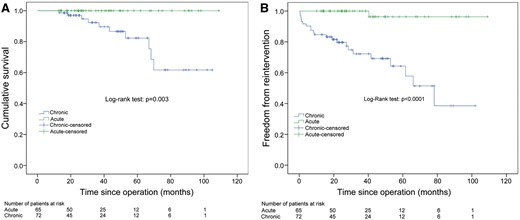
Kaplan–Meier curve for survival (A) and for freedom from distal reintervention (B) in acute and chronic dissections.
A total of 23 (16.8%) patients had reintervention on the distal aorta: open surgical thoraco-abdominal aortic replacement in 4 patients (3.0%), open surgical abdominal aortic replacement in 2 (1.5%), thoracic endovascular aortic repair in 16 (11.7%), abdominal endovascular aortic repair in 1 (0.7%). Only 1 patient underwent distal reintervention in the acute dissection group. One-, 3- and 5-year estimates of freedom from distal reintervention in the acute and chronic groups were 100%, 100% and 96.3%, and 84.7%, 79.7% and 64.3%, respectively (Fig. 3) with a statically significant difference (P < 0.001).
Five patients underwent open or endovascular reintervention on aortic branches, and 2 patients underwent proximal reoperation (1 on the aortic root and 1 on the aortic, mitral and tricuspid valves).
DISCUSSION
In AoD, the FET technique is used to close intimal tears in the proximal DTA and exclude the FL from antegrade perfusion-initiating thrombosis. The FL thrombosis activates aortic remodelling downstream, which may induce FL shrinkage and obliteration. However, the natural history of AoD after the FET technique is not yet well known, and evaluation of aortic remodelling, implicating the changes in the AL, TL and FL, could provide more information about the influence of this technique on aortic changes over time. However, there is no consensus on which parameters (diameter, area, volume or absolute ratio between AL and TL growth) or limits (10% increase, 20% increase) should be used to define aortic remodelling. Dohle et al. [5] proposed to use a 10% increase of volumetric change to define significant changes in the measurements of AL and TL, according to the standards defined by the Society of Vascular Surgeons [4]. We decided to follow this method, applying this cut-off to diametric measurements. Patterson et al. demonstrated previously that the measurements of the diameter correlate well with cross-sectional areas and represent an adequate tool to assess aortic remodelling [6]. It must be noted that the process of aortic dilatation or remodelling in AoD has not been fully explained. Several authors have shown a direct link between FL thrombosis and AL dilatation [7–9], but others have also pointed out the importance of different risk factors like patency of entry tear, race, initial aortic diameter and the presence of ulcer-like projections [10–12]. There is evidence that aortic remodelling affects the fate of the distal aorta, influencing the need for distal reintervention [5, 13–15].
Our data confirmed that the FET technique is extremely effective in promoting FL thrombosis in the DTA, but this effect is weaker in the AA. However, even if the absolute rate of FL thrombosis decreases below the stent graft, it is still significantly higher than the preoperative value up to the level of the celiac artery. In contrast to previous experience with conventional thoracic endovascular aortic repair [16], we found no significant difference in the rate of FL thrombosis between acute and chronic AoDs. However, more data are needed before being able to compare the medium- and long-term results of the FET technique versus thoracic endovascular aortic repair in patients with chronic AoD.
Analysis of the diametric changes reveals important findings. Giving the complexity of the data interpretation, it is useful to consider the different groups separately.
Aortic lumen changes in acute and chronic dissections
The diameter of the AL presented similar changes in both acute and chronic AoDs and did not increase at the level of the stent graft, remained stable just below it and increased distally whereas the diameter of the TL increased at all levels in acute AoD and remained stable in chronic AoD. The AL showed similar changes between acute and chronic AoDs, even when considering the different FL status. In both groups, the diameter of the AL decreased proximally and remained stable distally in segments with FL thrombosis whereas the diameter of the AL remained stable proximally and increased distally in segments with FL patency.
True lumen changes in acute and chronic dissections
On the other hand, the diameter of the TL presented different changes between acute and chronic AoDs according to the FL status. In segments with FL thrombosis, the diameter of the TL increased proximally and remained stable distally in acute AoD, whereas it remained stable both proximally and distally in chronic AoD. In segments with FL patency, the diameter of the TL increased in all segments in patients with acute AoD and remained stable in all segments in patients with chronic AoD.
Two conclusions may be drawn from these data:
Timing of presentation (acute versus chronic AoD) has different effects on diametric changes in AL and TL. The changes in the diameter of the AL are dependent on the status of the FL but do not differ between acute and chronic AoDs: This finding suggests that the external aortic wall retains its ability to remodel even in the chronic phase and that this ability is influenced positively by thrombosis of the FL. Changes in the diameter of the TL are also dependent on the status of the FL but, in contrast to the AL, the TL showed a more favourable remodelling in acute than in chronic AoD, suggesting that the dissection flap, which is thicker and stiffer in the chronic phase, reduces its ability to expand with time and that the TL is therefore less likely to expand in chronic than acute AoD.
The second interesting finding is the stability of the AL in the thoracic aorta even in patients with FL patency. This apparent paradox may be explained by the presence of the stent graft in the TL. Zhang demonstrated that blood pressure variability increases the risk of a late aortic event after AoD [17] and that a reduction of the dP/dT on the aortic wall may prevent aortic dilatation [18]. It is possible that stabilization of the TL, which markedly reduces the movement of the dissection flap, may reduce the pressure pulsatility in the FL, thereby protecting it from further expansion. However, other factors, including closure of the entry tear and change from antegrade to retrograde flow in the FL, may also be important causative factors. More data are needed to clarify this.
The rate of aortic remodelling did not differ significantly between acute and chronic AoDs in the abdominal aorta. However, patients with chronic AoD presented an increased rate of negative remodelling at L2 (33% vs 17.5%, P = 0.040), which may explain the higher rate of secondary intervention in these patients. Dohle et al. already demonstrated [5] that negative remodelling in the distal aorta is a risk factor for reintervention. In fact, most of the reinterventions (16 of 23, 69.5%) involved the descending thoracic aorta, which is the level with a higher rate of negative remodelling. However, one should also consider that enlargement of the thoraco-abdominal aorta after type A dissection takes several years to become critical, and it is possible that longer follow-up will show a rate of reintervention for acute dissection closer to that of chronic dissection.
FL patency correlated with negative remodelling at all levels, except L5 in patients with acute AoD. However, in those patients with chronic AoD, we were unable to demonstrate such a correlation. Whether different methods of defining aortic remodelling may be more accurate to demonstrate a correlation with FL thrombosis in all patients remains to be elucidated.
Limitations
Limitations of the present study include the retrospective nature of the study and the lack of a control group. The number of patients included in the study is relatively low compared with the total number of patients operated. However, given the similar characteristics between patients included and excluded from the analysis, we believe that the patients included in the present study closely represent the entire cohort of the patients operated with the E-vita Open Plus graft. The absence of standardized surgical indications and protocol may have led to a selection bias not possible to identify. The lack of detailed information regarding the number and site of persistently patent entry tears and the origin of collateral tributaries of the distal aorta emerging from the FL does not allow speculation as to their role as risk factors for FL patency or aortic dilatation. Ideally, the results from this study should be confirmed by an analysis of a similar cohort with analysis of cross-sectional areas of AL, TL and FL. However, given the multicentre nature of this study and the availability of data on diametric measures and FL status at multiple levels in the distal aorta, this study also represents a valuable opportunity to better clarify the evolution of the distal aorta after the FET technique and the relative importance of FL status in patients with acute and chronic AoDs.
CONCLUSION
The present study offers an insight into the fate of the distal aorta after the FET technique. Our results indicated that the FET technique is an effective treatment for patients with acute and chronic AoDs and promotes FL thrombosis and positive remodelling but that this effect is small in the AA. Changes in the diameter of the AL depend mainly on the status of the FL and are similar between patients with acute and chronic AoDs. In segments with FL thrombosis, the diameter of the AL either decreases or remains stable throughout the thoraco-abdominal aorta but in segments with FL patency the diameter of the AL tends to enlarge in the distal aorta. Changes in the diameter of the TL are affected by both the FL status and the timing of presentation. Different pathophysiological characteristics of the dissection flap at the stented and non-stented aortic segments may be responsible for these variations. Close follow-up of all patients who underwent dissection repair with the FET technique remains mandatory to identify patients with expansion of the AL who may need a secondary intervention.
Conflict of interest: Heinz Jakob is a consultant to JOTEC GmbH, Hechingen, Germany.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr Bachet(Nogent sur Marne, France): Your international and multicentre studies seem to confirm what was intuitively and also objectively demonstrated by previous analysis of the same kind already reported in the literature. That is, the use of the frozen elephant trunk technique, associated to the total replacement of the aortic arch in surgery of the aortic dissection, leads to favourable remodelling of the distal thoracic aorta in a large proportion of patients, but that this beneficial evolution does not concern the abdominal aorta in most patients.
Your demonstration is convincing enough and brings another argument in favour of the use of the frozen elephant trunk technique in this setting. Yet, some points of your study seem to me to be questionable, or even open to criticism. It is a little surprising and somewhat disappointing that among 383 patients operated on with the technique in 7 centres, only 137, that is about one-third, could be included. I suppose that this is linked to the multicentre origin of your data, but even if we take into account the hospital mortality, it is for me, quite difficult to admit that a large proportion of patients who had undergone and survived such a heavy and risky procedure, could not be followed up regularly with a proper CT scan during the first postoperative years. Do you think if a much larger number of patients had been included, the results would have been similar, or possibly different?
It is also stated that 1 exclusion criterion was a dissection flap limited to the arch or the very beginning of the descending aorta. So what was the indication for a frozen elephant trunk in those patients?
In your study, you have reported acute and chronic type A dissections. It may be inferred that most of the chronic type A dissections were in fact residual distal dissections after initial emergency surgery. So what was the delay between that initial surgery and the second late operation using the elephant trunk technique? Similarly, you report acute and chronic type B dissections. As far as I know, type B dissections start, by definition, beyond the origin of the left subclavian artery and develop along the thoracic and thoracic-abdominal aorta. So what was the indication of replacing the transverse arch and using a frozen elephant trunk in those case? Were they all retrograde dissections?
More importantly, your methodology may appear somewhat disappointing. You state indeed, and I will quote here, ‘while measurement of the total aortic diameter is a relatively easy task, assessment of the diameters of the true lumen, TL, and the false lumen, FL, can be definitely more demanding, especially in the case of the FL, the false lumen, which can assume a half-moon shape in particular when thrombosed’. This reason seems somewhat questionable as such studies have already been carried out without any difficulty.
So, you have only considered the patent or thrombosed condition of the false lumen and not its possible dilatation or shrinking. Indeed, you have measured only the evolution of the true lumen and of the whole aorta, regardless of the false lumen. The non-measurement of the false lumen, and therefore, the absence of actual knowledge of its evolution deprives the reader of a clear analysis of the reciprocal influence of those various elements on the whole aortic remodelling. One may observe that only 1 reintervention was performed in the group of acute dissections, and 22 in the group of chronic dissections. This is not surprising if we observe that the mean follow-up duration was 3 years and that the maximum was less than 5 years. Generally speaking, this is too short a delay to observe late complications after surgery of acute type A dissection.
Nevertheless, your comparison between acute and chronic dissections is quite interesting and its results are not very surprising. The fact that the distal remodelling, and in particular, the increase in the diameter of the true lumen was more important in acute cases than in chronic ones may most likely be linked to the fact that histologically the vascular structures, and in particular, the intimal flap, are much more fixed and resistant in the latter setting.
Do you think, finally, that a fine analysis through angio CT scans or MRI studies of the collateral tributaries of the distal aorta emerging from the false lumen could give some idea and prospect concerning the evolution of the distal aorta and some indication for late reintervention or surgery?
In conclusion, your study is quite interesting and contributory. The several points of criticism that I have developed here for the sake of the discussion, I hope you will address. They should not mask the excellent work carried out by your multicentre group and overall, should not negatively influence our colleagues regarding the use of frozen elephant trunk in surgery of acute type A dissection.
Dr Iafrancesco: The first question is about the numbers. While the number of patients in the study is smaller compared to the total number of patients operated, there are some reasons for it. Some patients did not have a CT scan and were operated on the basis of the TOE and some patients have a pre op CT scan which involves only the thoracic aorta, so they were excluded. Patients who died in the hospital were excluded, and most of the patients have been operated in the recent years, because obviously at the beginning, the experience was limited, so did not meet the criteria of 1 follow-up. So, therefore the number of patients is limited.
I agree that possibly with bigger numbers, we could have different results, but we do not know this for sure. However, at least the rate of remodelling seems reasonable, according to the previous study.
What, I think, is important, is that despite the fact that in type B chronic dissection, we have a higher rate of reintervention we have demonstrated that in the follow-up, the rate of remodelling is the same. That means that distal intervention should be carried out as it has a low mortality and has the power to remodel the distal aorta. Regarding the measurement of the diameter, there is evidence that measurement of the diameter is well related to the cross-sectional area. I agree, probably volumetric measurements are the best to define the remodelling. However, it is not very easy in a multicentre trial and it does not allow to study the aorta at several levels. So we, for the first time, wanted to have measurement of 5 different levels to see exactly what was happening.
Regarding the exclusion criteria, there were patients who had an isolated dissection flap up to the proximal aorta, in association with a not dissected aneurysm, of the aortic arch, and this was the main indication for surgery in these patients. Regarding type A and type B definition, for this study, type B is defined as not involving the ascending aorta. So if the patient has a primary tear in the aortic arch, and the dissection involves of the arch and the descending aorta then it is defined as type B. I forgot the next question.
Dr Bachet: It is about type B dissections. What was the reason for those type B dissections to be operated?
Dr Iafrancesco: Oh, for the type A. For the type A, large part of the patients with chronic type A dissection were in natural history. So there were 51 patients with chronic type A dissection, and the redo were 30. So a large part of the patients came without the previous operation, with a primary chronic type A dissection. So the difference between timing of intervention loses a little bit of importance, because almost half of them did not have a previous intervention.
Dr Bachet: But I keep on believing that if you want to compare both evolution in acute type B dissection and chronic type A dissection, you should give the delays of the evolution of the type B dissection.
Dr Iafrancesco: Yes.
Dr Bachet: Maybe there was a delay of 7 years or something like this; whereas for acute dissection, it was less than 3 years. This would make a big difference.
Dr Iafrancesco: Yes.
Dr Urbanski(Bad Neustadt, Germany): I just have a technical question. Regarding your results in chronic dissection, would you consider resecting the membrane before expanding the stent-graft, in order to expand it within a single lumen, and directing the flow into both luminal below the distal edge of the stent?
Dr Iafrancesco: Every centre has its own strategy, so I cannot comment for what happened in other centres. In our centre, we do not fenestrate with a frozen.
Dr Urbanski: Yes, but would you consider it in the future, to resect the membrane and to implant the elephant trunk into a single lumen?
Dr Iafrancesco: To be honest, I do not think so, because if you implant the frozen without the membrane of the dissection, you will actually perfuse the false lumen, and the whole point is to try to get it thrombosed. The thrombosed rate at the level of L1–L2 is extremely high, and is in the region of 95–100%, so that part is actually protected.
Dr Urbanski: But the thrombosis of the false lumen is not always good for the patients, because they can suffer paraplegia if there are visceral arteries and intercostal arteries originating from the false lumen.
Dr Iafrancesco: Yes, it is possible.
Author notes
Presented at the 29th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Amsterdam, Netherlands, 3–7 October 2015.
‡The first two authors contributed equally to this study.




