-
PDF
- Split View
-
Views
-
Cite
Cite
Suguru Ohira, Hiroaki Miyata, Kiyoshi Doi, Noboru Motomura, Shinichi Takamoto, Hitoshi Yaku, Risk model of aortic valve replacement after cardiovascular surgery based on a National Japanese Database, European Journal of Cardio-Thoracic Surgery, Volume 51, Issue 2, February 2017, Pages 347–353, https://doi.org/10.1093/ejcts/ezw247
Close - Share Icon Share
Abstract
The aims of this study were to investigate early results of aortic valve replacement (AVR) after cardiovascular surgery and create a risk model using a national database in Japan.
We used the Japan Adult Cardiovascular Surgery Database. Between 2008 and 2013, 2157 patients who underwent AVR for aortic stenosis after cardiovascular surgery or redo AVR were retrospectively analysed.
The background of prior surgery (including overlapping cases) was as follows: coronary artery bypass grafting (CABG), 31.9%; valve, 67.5% and thoracic aorta, 9.0%. The mean age was 70.4. Concomitant procedures were as follows: CABG, 14.5%; mitral valve surgery, 29.9% and aortic surgery, 5.9%. The 30-day and operative mortality rates were 5.5 and 8.5%, respectively. Major morbidity occurred in 25.7%. The incidence rate of stroke was 3.8%, and that of pacemaker implantation was 3.7%. There were seven risk factors for both the operative mortality and composite outcome: age, active endocarditis, ejection fraction <30%, New York Heart Association classification IV, mitral regurgitation ≥2, renal failure and other concomitant cardiac procedure. The C-indexes of operative mortality and the composite outcome were 0.761 and 0.709, respectively.
We could identify risk factors predicting the operative mortality and composite outcome associated with AVR after prior cardiovascular surgery based on a national Japanese database. Early outcomes were acceptable despite these operations being associated with a higher risk than primary AVR. Our results may be informative when treating such high-risk patients.
INTRODUCTION
The number of patients who require aortic valve replacement (AVR) after prior cardiovascular surgery has grown due to increasing numbers of people suffering from aortic stenosis (AS) or requiring reintervention after prior AVR [1–7]. These are technically demanding procedures when compared with primary AVR [3, 5] due to older age, greater prevalence of comorbidities, other cardiac lesions requiring concomitant procedures, longer cardiopulmonary bypass and clamping times, incident rates of intraoperative organ injury including a patent graft, lethal bleeding and frequent need for transfusion [2, 7]. Operative mortality associated with AVR after prior cardiovascular surgery has been reported to be ∼5% in isolated cases [3, 4, 8] and 5–10% in combined procedures [1, 9, 10]. There have been only two multicentre or national studies regarding AVR after prior cardiovascular surgery. The RECORD initiative, involving seven centres in Europe, investigated the short- and mid-term outcomes of more than 700 patients [6, 11]. The other one was a study using the Society of Thoracic Surgeons (STS) National Database [5]. It investigated the short-term outcomes of 3380 patients receiving isolated AVR for a failed aortic bioprosthesis. However, both of them did not perform risk analysis for early outcomes. In addition, there has been no such study involving Asian races whose average stature is smaller than that of Western populations [12, 13]. One of the important issues regarding the smaller stature is the consequently smaller prosthesis to be implanted, which would make AVR a more difficult procedure. Indeed, a previous multicentre study from Japan demonstrated that a size of 19 or 21 mm comprises ∼50% of AVRs using a bioprosthesis [13].
Recently, transcatheter aortic valve replacement (TAVR) has been developed to treat high-risk patients with AS [14]. In addition, TAVR has the potential in the redo scenario to avoid repeat sternotomy, such as in patients who previously received other types of valve surgery [15] or coronary artery bypass grafting (CABG) with a patent graft [16–18], who had deep sternal wound infection, or who require reintervention for a failed bioprosthesis, the so-called ‘valve-in-valve’ procedure [8, 19–21]. Since indications for TAVR will be expanded with the advancement of techniques and devices, an appropriate risk assessment of patients undergoing intervention for the aortic valve after cardiovascular surgery is important to ensure quality control and will be helpful when considering whether surgical AVR or TAVR is more appropriate for a patient who received prior cardiovascular surgery. Based on this background, the aims of this study were to investigate the early results and create risk models of the outcomes of AVR after prior cardiovascular surgery using a national database of Japan: the Japan Adult Cardiovascular Surgery Database (JACVSD).
Study population
The JACVSD is a national database, which currently involves nearly all cardiovascular units in Japan [12]. The JACVSD started in 2000 to estimate surgical outcomes after cardiovascular procedures in Japan. The number of institutions has been increasing, and the database incorporated clinical information from 260 000 individual datasets from 533 hospitals in 2013 [12]. The data registration project was approved by the institutional review board, and informed consent was obtained from all patients in each participating hospital. Any JACVSD records that had been obtained without patient consent were excluded from this study. The data collection form has a total of 255 variables (definitions are available online at www.jacvsd.umin.jp), and these are almost identical to those in the STS National Database (available online at www.sts.org). The JACVSD incorporates software for a web-based data collection system, and through this system, the data manager of each participating hospital was responsible for forwarding their data electronically to the central office. The rate of data entry was monitored in the central office to fully input the data. The accuracy of submitted data was maintained through a data audit; administrative office members and investigators who previously used the JACVSD for clinical studies randomly visited a participating hospital every month.
Concomitant procedures such as CABG or mitral valve surgery were included. Exclusion criteria were aortic root replacement or surgery for acute aortic dissection or rupture. We examined cardiovascular surgery procedures relating to AVR after prior cardiovascular surgery between 1 January 2008 and 31 December 2013. Using this database, we collected 56 100 AVR cases. Of these, redo procedures were performed in 4291 patients. Patients with aortic regurgitation without AS or missing data at discharge were excluded (n = 2081). Aortic root replacement and surgery for acute aortic dissection or rupture were also excluded (n = 53). Consequently, the population for this risk model analysis comprised 2157 cases.
Surgical procedures
Surgical AVR was performed through a full or hemi-sternotomy in this study. The size and type of aortic valve prosthesis, position of implantation (intra- or supra-annular position) and method of implanted sutures (interrupted or buttress sutures) were determined at each institute.
End-points and definition
The primary end-points of this study were 30-day and operative mortality rates. Operative mortality was exactly the same as the 30-day operative mortality as expressed in the STS National Database [5]. This includes any patient who died within the index period of hospitalization, regardless of the length of hospital stay, as well as any patient who died after being discharged from hospital up to 30 days from the date of the operation. In this analysis, both the operative mortality and composite operative mortality or major complications (the composite outcome) were used as the end-points. Major morbidity was defined as any of the following five postoperative outcomes: stroke, cardiovascular reoperation irrespective of the reason, mechanical ventilation for more than 72 h after surgery, renal failure or deep sternal wound infection. Stroke was defined as a new neurological deficit persisting for more than 72 h. Postoperative renal failure was defined as a patient with newly required haemodialysis or showing a creatinine increase greater than or equal to 2-fold the preoperative baseline and greater than or equal to 2.0 mg/dl. Gastrointestinal bleeding was bleeding from the stomach or intestine. The definitions of preoperative complications were as follows: liver dysfunction was defined as (i) liver enzymes ≥100 I.U., (ii) total bilirubin ≥1.5 mg/dl or (iii) cirrhosis; renal failure was defined as (i) the presence of proteinuria, (ii) a serum creatinine ≥1.3 mg/dl or (iii) an estimated glomerular filtration rate <60 ml/min/1.73 m2; moderate degree of chronic lung disease was defined as follows: (i) forced expiratory volume 1.0 <60%, (ii) steroid use, (iii) PO2 ≤60 mmHg or (iv) PCO2 ≥50 mmHg and congestive heart failure was defined as clinical symptoms including dyspnoea on effort, orthopnoea, respiratory wheeze and objective data showing fluid overload within 2 weeks before surgery. Other cardiac procedures included a left ventricular procedure, congenital repair, arrhythmia correction surgery, pericardiectomy or mechanical device implantation.
Statistical analysis
The statistical model was multiple logistic regression; the variables entered in the model were selected using bivariate tests, χ2 tests for categorical covariates, and unpaired t-tests or Wilcoxon rank sum tests for continuous covariates. All variables significant at the P< 0.2 level were entered into the model provided they were present in at least 1% of the sample. A multivariate stepwise logistic regression analysis was then performed for each of the two outcomes. The stability of the model was checked every time a variable was eliminated. In the case of continuous variables, in which the relationship with the outcome was not linear, such as for preoperative creatinine, we determined cut-off points. The area under the receiver operating characteristic curve (C-index) was used to assess how well the model could discriminate between patients who survived from those who had died (0.5–1.0). We considered a C-index of <0.7 as poor, 0.7–0.8 as acceptable and greater than 0.8 as excellent [22]. The SPSS version 20.0J software program (SPSS Japan, Tokyo, Japan) was used for all analyses, and a P-value <0.05 was considered to be significant.
RESULTS
Table 1 demonstrates the basic characteristics of patients. The background of prior surgery (including overlapping cases) was CABG, 31.9%; valve, 67.5% and thoracic aorta, 9.0%. Table 2 presents operative details. Types of prosthesis used were bioprosthesis, 51.3% and mechanical valve, 48.7%. The prosthesis size most often used was 19 mm (37.6%), followed by 21 mm (27.5%). Concomitant procedures were performed in 40.5%: CABG, 14.5%; mitral valve surgery, 29.9% and aortic surgery, 5.9% (including overlapping cases).
| . | All (n = 2157) . | Mortality (−) (n = 1974) . | Mortality (+) (n = 183) . | P-value . |
|---|---|---|---|---|
| Age, years | 70.7 ± 10.8 | 70.4 ± 10.9 | 73.3 ± 8.8 | <0.001 |
| Body surface area, m2 | 1.50 ± 0.18 | 1.50 ± 0.18 | 1.51 ± 0.19 | 0.714 |
| Body mass index, kg/m2 | 21.7 ± 3.5 | 21.8 ± 3.5 | 21.5 ± 3.9 | 0.339 |
| <20 | 1700 (78.8) | 1563 (79.2) | 137 (74.9) | |
| 20–25 | 334 (15.5) | 299 (15.1) | 35 (19.1) | |
| 25–30 | 105 (4.9) | 98 (5.0) | 7 (3.8) | |
| ≥30 | 18 (0.8) | 14 (0.7) | 4 (2.2) | 0.074 |
| Male sex | 932 (43.2) | 831 (42.1) | 101 (55.2) | <0.001 |
| Previous surgery | ||||
| Coronary | 689 (31.9) | 612 (31) | 77 (42.1) | 0.003 |
| Valve | 1456 (67.5) | 1338 (67.8) | 118 (64.4) | 0.407 |
| Aorta | 194 (9.0) | 182 (10.5) | 12 (6.6) | 0.285 |
| Hypertension | 1238 (57.4) | 1115 (56.5) | 123 (67.2) | 0.006 |
| Hyperlipidaemia | 803 (37.2) | 727 (36.8) | 76 (41.5) | 0.239 |
| Diabetes mellitus | 577 (26.8) | 513 (26.0) | 64 (35.0) | 0.011 |
| Insulin user | 154 (7.1) | 141 (7.1) | 13 (7.1) | 0.984 |
| PAD | 250 (11.56) | 210 (10.6) | 40 (21.9) | <0.001 |
| Smoking | 609 (28.2) | 547 (27.7) | 62 (33.9) | 0.141 |
| Chronic lung disease ≥ moderate | 97 (4.5) | 83 (4.2) | 14 (7.7) | 0.049 |
| Renal failure | 412 (19.1) | 336 (17.0) | 76 (41.5) | <0.001 |
| Haemodialysis | 224 (10.4) | 178 (9.0) | 46 (25.1) | <0.001 |
| Creatinine, mg/dl | 1.66 ± 2.30 | 1.55 ± 2.2 | 2.8 ± 3.1 | <0.001 |
| Liver dysfunction | 99 (4.6) | 84 (4.3) | 15 (8.2) | 0.005 |
| Carotid stenosis | 117 (5.5) | 100 (5.1) | 17 (9.3) | 0.025 |
| Cerebrovascular disease | 266 (12.3) | 233 (11.8) | 33 (18.0) | 0.020 |
| Recent stroke | 24 (1.1) | 15 (0.8) | 9 (4.9) | <0.001 |
| Active endocarditis | 93 (4.2) | 73 (3.7) | 20 (10.9) | <0.001 |
| Atrial fibrillation | 748 (34.7) | 686 (34.9) | 62 (36.6) | 0.876 |
| Operation ≥ emergency | 38 (1.8) | 25 (1.3) | 13 (7.4) | <0.001 |
| Congestive heart failure | 777 (36.0) | 678 (34.3) | 99 (54.1) | <0.001 |
| NYHA classification IV | 169 (7.8) | 131 (6.6) | 38 (20.8) | <0.001 |
| CCS classification ≥III | 154 (7.1) | 132 (6.7) | 22 (12.0) | 0.011 |
| Cardiogenic shock | 45 (2.1) | 28 (1.4) | 17 (9.3) | <0.001 |
| Inotropic agent use | 71 (3.3) | 54 (2.7) | 17 (9.3) | <0.001 |
| Left main disease | 179 (8.3) | 150 (7.6) | 29 (14.5) | <0.001 |
| Triple-vessel disease | 269 (12.5) | 230 (11.7) | 39 (21.3) | <0.001 |
| Unstable angina | 61 (2.8) | 45 (2.3) | 16 (8.7) | <0.001 |
| Previous PCI | 316 (14.6) | 277 (14.0) | 39 (21.3) | 0.011 |
| Previous myocardial infarction | 240 (11.1) | 206 (10.4) | 34 (18.6) | 0.001 |
| Ejection fraction 30–60% | 826 (38.3) | 746 (37.8) | 80 (43.7) | 0.134 |
| Ejection fraction <30% | 117 (5.4) | 91 (1.4) | 26 (14.2) | <0.001 |
| Aortic regurgitation ≥2 | 1019 (47.2) | 917 (46.1) | 102 (55.8) | 0.020 |
| Mitral regurgitation ≥2 | 759 (35.2) | 674 (34.1) | 85 (46.4) | <0.001 |
| Tricuspid regurgitation ≥2 | 930 (43.1) | 852 (43.2) | 78 (42.6) | 0.950 |
| . | All (n = 2157) . | Mortality (−) (n = 1974) . | Mortality (+) (n = 183) . | P-value . |
|---|---|---|---|---|
| Age, years | 70.7 ± 10.8 | 70.4 ± 10.9 | 73.3 ± 8.8 | <0.001 |
| Body surface area, m2 | 1.50 ± 0.18 | 1.50 ± 0.18 | 1.51 ± 0.19 | 0.714 |
| Body mass index, kg/m2 | 21.7 ± 3.5 | 21.8 ± 3.5 | 21.5 ± 3.9 | 0.339 |
| <20 | 1700 (78.8) | 1563 (79.2) | 137 (74.9) | |
| 20–25 | 334 (15.5) | 299 (15.1) | 35 (19.1) | |
| 25–30 | 105 (4.9) | 98 (5.0) | 7 (3.8) | |
| ≥30 | 18 (0.8) | 14 (0.7) | 4 (2.2) | 0.074 |
| Male sex | 932 (43.2) | 831 (42.1) | 101 (55.2) | <0.001 |
| Previous surgery | ||||
| Coronary | 689 (31.9) | 612 (31) | 77 (42.1) | 0.003 |
| Valve | 1456 (67.5) | 1338 (67.8) | 118 (64.4) | 0.407 |
| Aorta | 194 (9.0) | 182 (10.5) | 12 (6.6) | 0.285 |
| Hypertension | 1238 (57.4) | 1115 (56.5) | 123 (67.2) | 0.006 |
| Hyperlipidaemia | 803 (37.2) | 727 (36.8) | 76 (41.5) | 0.239 |
| Diabetes mellitus | 577 (26.8) | 513 (26.0) | 64 (35.0) | 0.011 |
| Insulin user | 154 (7.1) | 141 (7.1) | 13 (7.1) | 0.984 |
| PAD | 250 (11.56) | 210 (10.6) | 40 (21.9) | <0.001 |
| Smoking | 609 (28.2) | 547 (27.7) | 62 (33.9) | 0.141 |
| Chronic lung disease ≥ moderate | 97 (4.5) | 83 (4.2) | 14 (7.7) | 0.049 |
| Renal failure | 412 (19.1) | 336 (17.0) | 76 (41.5) | <0.001 |
| Haemodialysis | 224 (10.4) | 178 (9.0) | 46 (25.1) | <0.001 |
| Creatinine, mg/dl | 1.66 ± 2.30 | 1.55 ± 2.2 | 2.8 ± 3.1 | <0.001 |
| Liver dysfunction | 99 (4.6) | 84 (4.3) | 15 (8.2) | 0.005 |
| Carotid stenosis | 117 (5.5) | 100 (5.1) | 17 (9.3) | 0.025 |
| Cerebrovascular disease | 266 (12.3) | 233 (11.8) | 33 (18.0) | 0.020 |
| Recent stroke | 24 (1.1) | 15 (0.8) | 9 (4.9) | <0.001 |
| Active endocarditis | 93 (4.2) | 73 (3.7) | 20 (10.9) | <0.001 |
| Atrial fibrillation | 748 (34.7) | 686 (34.9) | 62 (36.6) | 0.876 |
| Operation ≥ emergency | 38 (1.8) | 25 (1.3) | 13 (7.4) | <0.001 |
| Congestive heart failure | 777 (36.0) | 678 (34.3) | 99 (54.1) | <0.001 |
| NYHA classification IV | 169 (7.8) | 131 (6.6) | 38 (20.8) | <0.001 |
| CCS classification ≥III | 154 (7.1) | 132 (6.7) | 22 (12.0) | 0.011 |
| Cardiogenic shock | 45 (2.1) | 28 (1.4) | 17 (9.3) | <0.001 |
| Inotropic agent use | 71 (3.3) | 54 (2.7) | 17 (9.3) | <0.001 |
| Left main disease | 179 (8.3) | 150 (7.6) | 29 (14.5) | <0.001 |
| Triple-vessel disease | 269 (12.5) | 230 (11.7) | 39 (21.3) | <0.001 |
| Unstable angina | 61 (2.8) | 45 (2.3) | 16 (8.7) | <0.001 |
| Previous PCI | 316 (14.6) | 277 (14.0) | 39 (21.3) | 0.011 |
| Previous myocardial infarction | 240 (11.1) | 206 (10.4) | 34 (18.6) | 0.001 |
| Ejection fraction 30–60% | 826 (38.3) | 746 (37.8) | 80 (43.7) | 0.134 |
| Ejection fraction <30% | 117 (5.4) | 91 (1.4) | 26 (14.2) | <0.001 |
| Aortic regurgitation ≥2 | 1019 (47.2) | 917 (46.1) | 102 (55.8) | 0.020 |
| Mitral regurgitation ≥2 | 759 (35.2) | 674 (34.1) | 85 (46.4) | <0.001 |
| Tricuspid regurgitation ≥2 | 930 (43.1) | 852 (43.2) | 78 (42.6) | 0.950 |
Values are n (%) or mean ± SD.
CCS: Canadian Cardiovascular Society; NYHA: New York Heart Association; PAD: peripheral artery disease; PCI: percutaneous coronary intervention.
| . | All (n = 2157) . | Mortality (−) (n = 1974) . | Mortality (+) (n = 183) . | P-value . |
|---|---|---|---|---|
| Age, years | 70.7 ± 10.8 | 70.4 ± 10.9 | 73.3 ± 8.8 | <0.001 |
| Body surface area, m2 | 1.50 ± 0.18 | 1.50 ± 0.18 | 1.51 ± 0.19 | 0.714 |
| Body mass index, kg/m2 | 21.7 ± 3.5 | 21.8 ± 3.5 | 21.5 ± 3.9 | 0.339 |
| <20 | 1700 (78.8) | 1563 (79.2) | 137 (74.9) | |
| 20–25 | 334 (15.5) | 299 (15.1) | 35 (19.1) | |
| 25–30 | 105 (4.9) | 98 (5.0) | 7 (3.8) | |
| ≥30 | 18 (0.8) | 14 (0.7) | 4 (2.2) | 0.074 |
| Male sex | 932 (43.2) | 831 (42.1) | 101 (55.2) | <0.001 |
| Previous surgery | ||||
| Coronary | 689 (31.9) | 612 (31) | 77 (42.1) | 0.003 |
| Valve | 1456 (67.5) | 1338 (67.8) | 118 (64.4) | 0.407 |
| Aorta | 194 (9.0) | 182 (10.5) | 12 (6.6) | 0.285 |
| Hypertension | 1238 (57.4) | 1115 (56.5) | 123 (67.2) | 0.006 |
| Hyperlipidaemia | 803 (37.2) | 727 (36.8) | 76 (41.5) | 0.239 |
| Diabetes mellitus | 577 (26.8) | 513 (26.0) | 64 (35.0) | 0.011 |
| Insulin user | 154 (7.1) | 141 (7.1) | 13 (7.1) | 0.984 |
| PAD | 250 (11.56) | 210 (10.6) | 40 (21.9) | <0.001 |
| Smoking | 609 (28.2) | 547 (27.7) | 62 (33.9) | 0.141 |
| Chronic lung disease ≥ moderate | 97 (4.5) | 83 (4.2) | 14 (7.7) | 0.049 |
| Renal failure | 412 (19.1) | 336 (17.0) | 76 (41.5) | <0.001 |
| Haemodialysis | 224 (10.4) | 178 (9.0) | 46 (25.1) | <0.001 |
| Creatinine, mg/dl | 1.66 ± 2.30 | 1.55 ± 2.2 | 2.8 ± 3.1 | <0.001 |
| Liver dysfunction | 99 (4.6) | 84 (4.3) | 15 (8.2) | 0.005 |
| Carotid stenosis | 117 (5.5) | 100 (5.1) | 17 (9.3) | 0.025 |
| Cerebrovascular disease | 266 (12.3) | 233 (11.8) | 33 (18.0) | 0.020 |
| Recent stroke | 24 (1.1) | 15 (0.8) | 9 (4.9) | <0.001 |
| Active endocarditis | 93 (4.2) | 73 (3.7) | 20 (10.9) | <0.001 |
| Atrial fibrillation | 748 (34.7) | 686 (34.9) | 62 (36.6) | 0.876 |
| Operation ≥ emergency | 38 (1.8) | 25 (1.3) | 13 (7.4) | <0.001 |
| Congestive heart failure | 777 (36.0) | 678 (34.3) | 99 (54.1) | <0.001 |
| NYHA classification IV | 169 (7.8) | 131 (6.6) | 38 (20.8) | <0.001 |
| CCS classification ≥III | 154 (7.1) | 132 (6.7) | 22 (12.0) | 0.011 |
| Cardiogenic shock | 45 (2.1) | 28 (1.4) | 17 (9.3) | <0.001 |
| Inotropic agent use | 71 (3.3) | 54 (2.7) | 17 (9.3) | <0.001 |
| Left main disease | 179 (8.3) | 150 (7.6) | 29 (14.5) | <0.001 |
| Triple-vessel disease | 269 (12.5) | 230 (11.7) | 39 (21.3) | <0.001 |
| Unstable angina | 61 (2.8) | 45 (2.3) | 16 (8.7) | <0.001 |
| Previous PCI | 316 (14.6) | 277 (14.0) | 39 (21.3) | 0.011 |
| Previous myocardial infarction | 240 (11.1) | 206 (10.4) | 34 (18.6) | 0.001 |
| Ejection fraction 30–60% | 826 (38.3) | 746 (37.8) | 80 (43.7) | 0.134 |
| Ejection fraction <30% | 117 (5.4) | 91 (1.4) | 26 (14.2) | <0.001 |
| Aortic regurgitation ≥2 | 1019 (47.2) | 917 (46.1) | 102 (55.8) | 0.020 |
| Mitral regurgitation ≥2 | 759 (35.2) | 674 (34.1) | 85 (46.4) | <0.001 |
| Tricuspid regurgitation ≥2 | 930 (43.1) | 852 (43.2) | 78 (42.6) | 0.950 |
| . | All (n = 2157) . | Mortality (−) (n = 1974) . | Mortality (+) (n = 183) . | P-value . |
|---|---|---|---|---|
| Age, years | 70.7 ± 10.8 | 70.4 ± 10.9 | 73.3 ± 8.8 | <0.001 |
| Body surface area, m2 | 1.50 ± 0.18 | 1.50 ± 0.18 | 1.51 ± 0.19 | 0.714 |
| Body mass index, kg/m2 | 21.7 ± 3.5 | 21.8 ± 3.5 | 21.5 ± 3.9 | 0.339 |
| <20 | 1700 (78.8) | 1563 (79.2) | 137 (74.9) | |
| 20–25 | 334 (15.5) | 299 (15.1) | 35 (19.1) | |
| 25–30 | 105 (4.9) | 98 (5.0) | 7 (3.8) | |
| ≥30 | 18 (0.8) | 14 (0.7) | 4 (2.2) | 0.074 |
| Male sex | 932 (43.2) | 831 (42.1) | 101 (55.2) | <0.001 |
| Previous surgery | ||||
| Coronary | 689 (31.9) | 612 (31) | 77 (42.1) | 0.003 |
| Valve | 1456 (67.5) | 1338 (67.8) | 118 (64.4) | 0.407 |
| Aorta | 194 (9.0) | 182 (10.5) | 12 (6.6) | 0.285 |
| Hypertension | 1238 (57.4) | 1115 (56.5) | 123 (67.2) | 0.006 |
| Hyperlipidaemia | 803 (37.2) | 727 (36.8) | 76 (41.5) | 0.239 |
| Diabetes mellitus | 577 (26.8) | 513 (26.0) | 64 (35.0) | 0.011 |
| Insulin user | 154 (7.1) | 141 (7.1) | 13 (7.1) | 0.984 |
| PAD | 250 (11.56) | 210 (10.6) | 40 (21.9) | <0.001 |
| Smoking | 609 (28.2) | 547 (27.7) | 62 (33.9) | 0.141 |
| Chronic lung disease ≥ moderate | 97 (4.5) | 83 (4.2) | 14 (7.7) | 0.049 |
| Renal failure | 412 (19.1) | 336 (17.0) | 76 (41.5) | <0.001 |
| Haemodialysis | 224 (10.4) | 178 (9.0) | 46 (25.1) | <0.001 |
| Creatinine, mg/dl | 1.66 ± 2.30 | 1.55 ± 2.2 | 2.8 ± 3.1 | <0.001 |
| Liver dysfunction | 99 (4.6) | 84 (4.3) | 15 (8.2) | 0.005 |
| Carotid stenosis | 117 (5.5) | 100 (5.1) | 17 (9.3) | 0.025 |
| Cerebrovascular disease | 266 (12.3) | 233 (11.8) | 33 (18.0) | 0.020 |
| Recent stroke | 24 (1.1) | 15 (0.8) | 9 (4.9) | <0.001 |
| Active endocarditis | 93 (4.2) | 73 (3.7) | 20 (10.9) | <0.001 |
| Atrial fibrillation | 748 (34.7) | 686 (34.9) | 62 (36.6) | 0.876 |
| Operation ≥ emergency | 38 (1.8) | 25 (1.3) | 13 (7.4) | <0.001 |
| Congestive heart failure | 777 (36.0) | 678 (34.3) | 99 (54.1) | <0.001 |
| NYHA classification IV | 169 (7.8) | 131 (6.6) | 38 (20.8) | <0.001 |
| CCS classification ≥III | 154 (7.1) | 132 (6.7) | 22 (12.0) | 0.011 |
| Cardiogenic shock | 45 (2.1) | 28 (1.4) | 17 (9.3) | <0.001 |
| Inotropic agent use | 71 (3.3) | 54 (2.7) | 17 (9.3) | <0.001 |
| Left main disease | 179 (8.3) | 150 (7.6) | 29 (14.5) | <0.001 |
| Triple-vessel disease | 269 (12.5) | 230 (11.7) | 39 (21.3) | <0.001 |
| Unstable angina | 61 (2.8) | 45 (2.3) | 16 (8.7) | <0.001 |
| Previous PCI | 316 (14.6) | 277 (14.0) | 39 (21.3) | 0.011 |
| Previous myocardial infarction | 240 (11.1) | 206 (10.4) | 34 (18.6) | 0.001 |
| Ejection fraction 30–60% | 826 (38.3) | 746 (37.8) | 80 (43.7) | 0.134 |
| Ejection fraction <30% | 117 (5.4) | 91 (1.4) | 26 (14.2) | <0.001 |
| Aortic regurgitation ≥2 | 1019 (47.2) | 917 (46.1) | 102 (55.8) | 0.020 |
| Mitral regurgitation ≥2 | 759 (35.2) | 674 (34.1) | 85 (46.4) | <0.001 |
| Tricuspid regurgitation ≥2 | 930 (43.1) | 852 (43.2) | 78 (42.6) | 0.950 |
Values are n (%) or mean ± SD.
CCS: Canadian Cardiovascular Society; NYHA: New York Heart Association; PAD: peripheral artery disease; PCI: percutaneous coronary intervention.
| . | All (n = 2157) . | Mortality (−) (n = 1974) . | Mortality (+) (n = 183) . | P-value . |
|---|---|---|---|---|
| Full sternotomy | 2143 (99.3) | 1960 (99.3) | 183 (100) | 0.508 |
| Partial sternotomy | 14 (0.7) | 14 (0.7) | 0 | 0.508 |
| Aortic valve prosthesis | ||||
| Mechanical | 1041 (48.7) | 970 (49.1) | 81 (43.5) | 0.155 |
| Bioprosthesis | 1106 (51.3) | 1004 (50.9) | 102 (56.5) | 0.155 |
| ≤18 mm | 316 (14.8) | 293 (15.0) | 23 (12.7) | |
| 19 mm | 803 (37.6) | 730 (37.4) | 73 (40.3) | |
| 20 mm | 81 (3.8) | 73 (3.4) | 8 (4.4) | |
| 21 mm | 586 (27.5) | 540 (27.7) | 46 (25.4) | |
| 22 mm | 24 (1.1) | 23 (1.2) | 1 (0.6) | |
| 23 mm | 258 (12.1) | 231 (11.8) | 27 (14.8) | |
| 24 mm | 2 (0.1) | 2 (0.1) | 0 (0) | |
| 25 mm | 41 (1.9) | 40 (2.0) | 1 (0.5) | |
| ≥26 mm | 22 (1.0) | 20 (1.0) | 2 (1.1) | 0.705 |
| Isolated AVR | 1285 (59.6) | 1172 (59.5) | 113 (61.7) | 0.584 |
| Concomitant procedure | 872 (40.4) | 802 (40.6) | 70 (38.3) | |
| CABG | 313 (14.5) | 272 (13.8) | 41 (22.4) | 0.002 |
| Mitral valve | 645 (29.9) | 590 (29.9) | 55 (30.1) | 0.963 |
| Repair | 148 (6.9) | 132 (6.7) | 16 (8.7) | 0.368 |
| Replacement | 497 (23.0) | 458 (23.2) | 39 (21.3) | 0.625 |
| Tricuspid valve repair | 543 (25.2) | 506 (25.6) | 37 (20.2) | 0.127 |
| Aortic replacement | 127 (5.9) | 116 (5.9) | 11 (6.0) | 0.941 |
| Other procedures | 125 (5.8) | 110 (5.6) | 15 (8.2) | 0.198 |
| Circulatory arrest | 29 (1.3) | 21 (1.1) | 8 (4.4) | 0.001 |
| Clamp time, min | 137 ± 62 | 134 ± 57 | 170 ± 95 | <0.001 |
| Perfusion time, min | 208 ± 94 | 200 ± 57 | 298 ± 163 | <0.001 |
| . | All (n = 2157) . | Mortality (−) (n = 1974) . | Mortality (+) (n = 183) . | P-value . |
|---|---|---|---|---|
| Full sternotomy | 2143 (99.3) | 1960 (99.3) | 183 (100) | 0.508 |
| Partial sternotomy | 14 (0.7) | 14 (0.7) | 0 | 0.508 |
| Aortic valve prosthesis | ||||
| Mechanical | 1041 (48.7) | 970 (49.1) | 81 (43.5) | 0.155 |
| Bioprosthesis | 1106 (51.3) | 1004 (50.9) | 102 (56.5) | 0.155 |
| ≤18 mm | 316 (14.8) | 293 (15.0) | 23 (12.7) | |
| 19 mm | 803 (37.6) | 730 (37.4) | 73 (40.3) | |
| 20 mm | 81 (3.8) | 73 (3.4) | 8 (4.4) | |
| 21 mm | 586 (27.5) | 540 (27.7) | 46 (25.4) | |
| 22 mm | 24 (1.1) | 23 (1.2) | 1 (0.6) | |
| 23 mm | 258 (12.1) | 231 (11.8) | 27 (14.8) | |
| 24 mm | 2 (0.1) | 2 (0.1) | 0 (0) | |
| 25 mm | 41 (1.9) | 40 (2.0) | 1 (0.5) | |
| ≥26 mm | 22 (1.0) | 20 (1.0) | 2 (1.1) | 0.705 |
| Isolated AVR | 1285 (59.6) | 1172 (59.5) | 113 (61.7) | 0.584 |
| Concomitant procedure | 872 (40.4) | 802 (40.6) | 70 (38.3) | |
| CABG | 313 (14.5) | 272 (13.8) | 41 (22.4) | 0.002 |
| Mitral valve | 645 (29.9) | 590 (29.9) | 55 (30.1) | 0.963 |
| Repair | 148 (6.9) | 132 (6.7) | 16 (8.7) | 0.368 |
| Replacement | 497 (23.0) | 458 (23.2) | 39 (21.3) | 0.625 |
| Tricuspid valve repair | 543 (25.2) | 506 (25.6) | 37 (20.2) | 0.127 |
| Aortic replacement | 127 (5.9) | 116 (5.9) | 11 (6.0) | 0.941 |
| Other procedures | 125 (5.8) | 110 (5.6) | 15 (8.2) | 0.198 |
| Circulatory arrest | 29 (1.3) | 21 (1.1) | 8 (4.4) | 0.001 |
| Clamp time, min | 137 ± 62 | 134 ± 57 | 170 ± 95 | <0.001 |
| Perfusion time, min | 208 ± 94 | 200 ± 57 | 298 ± 163 | <0.001 |
Values are n (%) or mean ± SD.
AVR: aortic valve replacement; CABG: coronary artery bypass grafting.
| . | All (n = 2157) . | Mortality (−) (n = 1974) . | Mortality (+) (n = 183) . | P-value . |
|---|---|---|---|---|
| Full sternotomy | 2143 (99.3) | 1960 (99.3) | 183 (100) | 0.508 |
| Partial sternotomy | 14 (0.7) | 14 (0.7) | 0 | 0.508 |
| Aortic valve prosthesis | ||||
| Mechanical | 1041 (48.7) | 970 (49.1) | 81 (43.5) | 0.155 |
| Bioprosthesis | 1106 (51.3) | 1004 (50.9) | 102 (56.5) | 0.155 |
| ≤18 mm | 316 (14.8) | 293 (15.0) | 23 (12.7) | |
| 19 mm | 803 (37.6) | 730 (37.4) | 73 (40.3) | |
| 20 mm | 81 (3.8) | 73 (3.4) | 8 (4.4) | |
| 21 mm | 586 (27.5) | 540 (27.7) | 46 (25.4) | |
| 22 mm | 24 (1.1) | 23 (1.2) | 1 (0.6) | |
| 23 mm | 258 (12.1) | 231 (11.8) | 27 (14.8) | |
| 24 mm | 2 (0.1) | 2 (0.1) | 0 (0) | |
| 25 mm | 41 (1.9) | 40 (2.0) | 1 (0.5) | |
| ≥26 mm | 22 (1.0) | 20 (1.0) | 2 (1.1) | 0.705 |
| Isolated AVR | 1285 (59.6) | 1172 (59.5) | 113 (61.7) | 0.584 |
| Concomitant procedure | 872 (40.4) | 802 (40.6) | 70 (38.3) | |
| CABG | 313 (14.5) | 272 (13.8) | 41 (22.4) | 0.002 |
| Mitral valve | 645 (29.9) | 590 (29.9) | 55 (30.1) | 0.963 |
| Repair | 148 (6.9) | 132 (6.7) | 16 (8.7) | 0.368 |
| Replacement | 497 (23.0) | 458 (23.2) | 39 (21.3) | 0.625 |
| Tricuspid valve repair | 543 (25.2) | 506 (25.6) | 37 (20.2) | 0.127 |
| Aortic replacement | 127 (5.9) | 116 (5.9) | 11 (6.0) | 0.941 |
| Other procedures | 125 (5.8) | 110 (5.6) | 15 (8.2) | 0.198 |
| Circulatory arrest | 29 (1.3) | 21 (1.1) | 8 (4.4) | 0.001 |
| Clamp time, min | 137 ± 62 | 134 ± 57 | 170 ± 95 | <0.001 |
| Perfusion time, min | 208 ± 94 | 200 ± 57 | 298 ± 163 | <0.001 |
| . | All (n = 2157) . | Mortality (−) (n = 1974) . | Mortality (+) (n = 183) . | P-value . |
|---|---|---|---|---|
| Full sternotomy | 2143 (99.3) | 1960 (99.3) | 183 (100) | 0.508 |
| Partial sternotomy | 14 (0.7) | 14 (0.7) | 0 | 0.508 |
| Aortic valve prosthesis | ||||
| Mechanical | 1041 (48.7) | 970 (49.1) | 81 (43.5) | 0.155 |
| Bioprosthesis | 1106 (51.3) | 1004 (50.9) | 102 (56.5) | 0.155 |
| ≤18 mm | 316 (14.8) | 293 (15.0) | 23 (12.7) | |
| 19 mm | 803 (37.6) | 730 (37.4) | 73 (40.3) | |
| 20 mm | 81 (3.8) | 73 (3.4) | 8 (4.4) | |
| 21 mm | 586 (27.5) | 540 (27.7) | 46 (25.4) | |
| 22 mm | 24 (1.1) | 23 (1.2) | 1 (0.6) | |
| 23 mm | 258 (12.1) | 231 (11.8) | 27 (14.8) | |
| 24 mm | 2 (0.1) | 2 (0.1) | 0 (0) | |
| 25 mm | 41 (1.9) | 40 (2.0) | 1 (0.5) | |
| ≥26 mm | 22 (1.0) | 20 (1.0) | 2 (1.1) | 0.705 |
| Isolated AVR | 1285 (59.6) | 1172 (59.5) | 113 (61.7) | 0.584 |
| Concomitant procedure | 872 (40.4) | 802 (40.6) | 70 (38.3) | |
| CABG | 313 (14.5) | 272 (13.8) | 41 (22.4) | 0.002 |
| Mitral valve | 645 (29.9) | 590 (29.9) | 55 (30.1) | 0.963 |
| Repair | 148 (6.9) | 132 (6.7) | 16 (8.7) | 0.368 |
| Replacement | 497 (23.0) | 458 (23.2) | 39 (21.3) | 0.625 |
| Tricuspid valve repair | 543 (25.2) | 506 (25.6) | 37 (20.2) | 0.127 |
| Aortic replacement | 127 (5.9) | 116 (5.9) | 11 (6.0) | 0.941 |
| Other procedures | 125 (5.8) | 110 (5.6) | 15 (8.2) | 0.198 |
| Circulatory arrest | 29 (1.3) | 21 (1.1) | 8 (4.4) | 0.001 |
| Clamp time, min | 137 ± 62 | 134 ± 57 | 170 ± 95 | <0.001 |
| Perfusion time, min | 208 ± 94 | 200 ± 57 | 298 ± 163 | <0.001 |
Values are n (%) or mean ± SD.
AVR: aortic valve replacement; CABG: coronary artery bypass grafting.
Table 3 presents postoperative characteristics. Raw 30-day and operative mortality rates were 5.5% (118 patients) and 8.5% (183 patients), respectively. The incidence of major morbidity was recorded in 554 patients (25.7%): 231 cases of cardiac reoperation (10.7%); 315 cases of prolonged ventilation (14.6%); 205 cases of renal failure (9.5%) including 119 cases of newly required dialysis (5.5%); 81 cases of permanent stroke (3.8%) and 18 cases of deep sternal wound infection (0.8%). Blood transfusion was required in 2013 patients (93.3%). Pacemaker implantation (PMI) for heart block was required in 79 patients (3.7%).
| . | All (n = 2157) . | Mortality (+) (n = 183) . |
|---|---|---|
| 30-day mortality | 118 (5.5) | - |
| Operative mortality | 183 (8.5) | - |
| Major morbidity including | 554 (25.7) | 183 (100) |
| Permanent stroke | 81 (3.8) | 26 (14.2) |
| Prolonged ventilation | 315 (14.6) | 100 (54.6) |
| Renal failure | 205 (9.5) | 84 (45.9) |
| Deep sternal infection | 18 (0.8) | 3 (1.6) |
| Cardiac reoperation | 231 (10.7) | 79 (43.2) |
| Pacemaker implantation | 79 (3.7) | 16 (8.7) |
| Renal failure requiring dialysis | 119 (5.5) | 68 (37.2) |
| Reoperation for bleeding | 152 (7.0) | 56 (30.6) |
| Blood transfusion | 2013 (93.3) | 183 (100) |
| Atrial fibrillation | 371 (17.2) | 35 (19.1) |
| Pneumonia | 137 (6.4) | 54 (29.5) |
| Gastrointestinal bleeding | 83 (2.5) | 35 (19.1) |
| Lower limb ischaemia | 9 (0.4) | 7 (3.8) |
| Iliac dissection | 1 (0.05) | 0 (0) |
| . | All (n = 2157) . | Mortality (+) (n = 183) . |
|---|---|---|
| 30-day mortality | 118 (5.5) | - |
| Operative mortality | 183 (8.5) | - |
| Major morbidity including | 554 (25.7) | 183 (100) |
| Permanent stroke | 81 (3.8) | 26 (14.2) |
| Prolonged ventilation | 315 (14.6) | 100 (54.6) |
| Renal failure | 205 (9.5) | 84 (45.9) |
| Deep sternal infection | 18 (0.8) | 3 (1.6) |
| Cardiac reoperation | 231 (10.7) | 79 (43.2) |
| Pacemaker implantation | 79 (3.7) | 16 (8.7) |
| Renal failure requiring dialysis | 119 (5.5) | 68 (37.2) |
| Reoperation for bleeding | 152 (7.0) | 56 (30.6) |
| Blood transfusion | 2013 (93.3) | 183 (100) |
| Atrial fibrillation | 371 (17.2) | 35 (19.1) |
| Pneumonia | 137 (6.4) | 54 (29.5) |
| Gastrointestinal bleeding | 83 (2.5) | 35 (19.1) |
| Lower limb ischaemia | 9 (0.4) | 7 (3.8) |
| Iliac dissection | 1 (0.05) | 0 (0) |
Values are n (%) or mean ± SD.
| . | All (n = 2157) . | Mortality (+) (n = 183) . |
|---|---|---|
| 30-day mortality | 118 (5.5) | - |
| Operative mortality | 183 (8.5) | - |
| Major morbidity including | 554 (25.7) | 183 (100) |
| Permanent stroke | 81 (3.8) | 26 (14.2) |
| Prolonged ventilation | 315 (14.6) | 100 (54.6) |
| Renal failure | 205 (9.5) | 84 (45.9) |
| Deep sternal infection | 18 (0.8) | 3 (1.6) |
| Cardiac reoperation | 231 (10.7) | 79 (43.2) |
| Pacemaker implantation | 79 (3.7) | 16 (8.7) |
| Renal failure requiring dialysis | 119 (5.5) | 68 (37.2) |
| Reoperation for bleeding | 152 (7.0) | 56 (30.6) |
| Blood transfusion | 2013 (93.3) | 183 (100) |
| Atrial fibrillation | 371 (17.2) | 35 (19.1) |
| Pneumonia | 137 (6.4) | 54 (29.5) |
| Gastrointestinal bleeding | 83 (2.5) | 35 (19.1) |
| Lower limb ischaemia | 9 (0.4) | 7 (3.8) |
| Iliac dissection | 1 (0.05) | 0 (0) |
| . | All (n = 2157) . | Mortality (+) (n = 183) . |
|---|---|---|
| 30-day mortality | 118 (5.5) | - |
| Operative mortality | 183 (8.5) | - |
| Major morbidity including | 554 (25.7) | 183 (100) |
| Permanent stroke | 81 (3.8) | 26 (14.2) |
| Prolonged ventilation | 315 (14.6) | 100 (54.6) |
| Renal failure | 205 (9.5) | 84 (45.9) |
| Deep sternal infection | 18 (0.8) | 3 (1.6) |
| Cardiac reoperation | 231 (10.7) | 79 (43.2) |
| Pacemaker implantation | 79 (3.7) | 16 (8.7) |
| Renal failure requiring dialysis | 119 (5.5) | 68 (37.2) |
| Reoperation for bleeding | 152 (7.0) | 56 (30.6) |
| Blood transfusion | 2013 (93.3) | 183 (100) |
| Atrial fibrillation | 371 (17.2) | 35 (19.1) |
| Pneumonia | 137 (6.4) | 54 (29.5) |
| Gastrointestinal bleeding | 83 (2.5) | 35 (19.1) |
| Lower limb ischaemia | 9 (0.4) | 7 (3.8) |
| Iliac dissection | 1 (0.05) | 0 (0) |
Values are n (%) or mean ± SD.
| . | Operative mortality . | Composite outcomes . | ||
|---|---|---|---|---|
| OR . | 95% CI . | OR . | 95% CI . | |
| Age (reference ≤60 years old, per 5 years) | 1.23 | 1.10–1.379 | 1.20 | 1.116–1.287 |
| Male sex | 1.36 | 0.974–1.890 | ||
| Prior CABG | 1.64 | 1.11–2.43 | ||
| Prior valve surgery | 2.0 | 1.378–2.875 | ||
| Bilateral carotid stenosis | 2.48 | 1.24–5.298 | ||
| Recent stroke (≤2 weeks) | 4.14 | 1.590–10.765 | ||
| Chronic lung disease ≥moderate | 1.61 | 1.018–2.543 | ||
| Liver dysfunction | 2.08 | 1.103–3.908 | ||
| Renal failure | 1.73 | 1.083–2.776 | 1.99 | 1.552–2.556 |
| Haemodialysis | 1.89 | 1.068–3.324 | ||
| Peripheral artery disease | 1.50 | 0.984–2.298 | ||
| Active endocarditis | 1.83 | 0.99–3.392 | 1.99 | 1.312–3.023 |
| Operation ≥ emergency | 3.35 | 1.504–7.474 | ||
| Shock | 2.14 | 1.038–4.338 | ||
| Unstable angina | 2.09 | 1.085–4.008 | ||
| Congestive heart failure | 1.42 | 1.004–2.007 | ||
| NYHA classification IV | 1.69 | 1.037–2.753 | 1.80 | 1.240–2.608 |
| CCS classification ≥III | 1.46 | 0.997–2.144 | ||
| Ejection fraction <30% | 2.39 | 1.440–3.980 | 1.92 | 1.232–2.980 |
| Ejection fraction 30–60% | 1.33 | 1.062–1.861 | ||
| Previous myocardial infarction | 1.41 | 1.005–1.976 | ||
| Atrial fibrillation | 1.24 | 0.977–1.569 | ||
| Aortic regurgitation ≥2 | 1.41 | 1.008–1.961 | ||
| Mitral regurgitation ≥2 | 1.34 | 0.960–1.875 | 2.0 | 1.197–3.372 |
| Concomitant CABG | 1.38 | 1.019–1.861 | ||
| Concomitant valve surgery | 1.45 | 1.140–1.852 | ||
| Other procedures | 1.73 | 0.943–3.172 | 1.82 | 1.212–2.719 |
| Surgical volume (1-case increment) | 0.997 | 0.993–1.000 | ||
| . | Operative mortality . | Composite outcomes . | ||
|---|---|---|---|---|
| OR . | 95% CI . | OR . | 95% CI . | |
| Age (reference ≤60 years old, per 5 years) | 1.23 | 1.10–1.379 | 1.20 | 1.116–1.287 |
| Male sex | 1.36 | 0.974–1.890 | ||
| Prior CABG | 1.64 | 1.11–2.43 | ||
| Prior valve surgery | 2.0 | 1.378–2.875 | ||
| Bilateral carotid stenosis | 2.48 | 1.24–5.298 | ||
| Recent stroke (≤2 weeks) | 4.14 | 1.590–10.765 | ||
| Chronic lung disease ≥moderate | 1.61 | 1.018–2.543 | ||
| Liver dysfunction | 2.08 | 1.103–3.908 | ||
| Renal failure | 1.73 | 1.083–2.776 | 1.99 | 1.552–2.556 |
| Haemodialysis | 1.89 | 1.068–3.324 | ||
| Peripheral artery disease | 1.50 | 0.984–2.298 | ||
| Active endocarditis | 1.83 | 0.99–3.392 | 1.99 | 1.312–3.023 |
| Operation ≥ emergency | 3.35 | 1.504–7.474 | ||
| Shock | 2.14 | 1.038–4.338 | ||
| Unstable angina | 2.09 | 1.085–4.008 | ||
| Congestive heart failure | 1.42 | 1.004–2.007 | ||
| NYHA classification IV | 1.69 | 1.037–2.753 | 1.80 | 1.240–2.608 |
| CCS classification ≥III | 1.46 | 0.997–2.144 | ||
| Ejection fraction <30% | 2.39 | 1.440–3.980 | 1.92 | 1.232–2.980 |
| Ejection fraction 30–60% | 1.33 | 1.062–1.861 | ||
| Previous myocardial infarction | 1.41 | 1.005–1.976 | ||
| Atrial fibrillation | 1.24 | 0.977–1.569 | ||
| Aortic regurgitation ≥2 | 1.41 | 1.008–1.961 | ||
| Mitral regurgitation ≥2 | 1.34 | 0.960–1.875 | 2.0 | 1.197–3.372 |
| Concomitant CABG | 1.38 | 1.019–1.861 | ||
| Concomitant valve surgery | 1.45 | 1.140–1.852 | ||
| Other procedures | 1.73 | 0.943–3.172 | 1.82 | 1.212–2.719 |
| Surgical volume (1-case increment) | 0.997 | 0.993–1.000 | ||
Surgical volume indicates the number of aortic valve replacement procedures after prior cardiovascular surgery at each institute.
CABG: coronary artery bypass grafting; CCS: Canadian Cardiovascular Society; NYHA: New York Heart Association.
| . | Operative mortality . | Composite outcomes . | ||
|---|---|---|---|---|
| OR . | 95% CI . | OR . | 95% CI . | |
| Age (reference ≤60 years old, per 5 years) | 1.23 | 1.10–1.379 | 1.20 | 1.116–1.287 |
| Male sex | 1.36 | 0.974–1.890 | ||
| Prior CABG | 1.64 | 1.11–2.43 | ||
| Prior valve surgery | 2.0 | 1.378–2.875 | ||
| Bilateral carotid stenosis | 2.48 | 1.24–5.298 | ||
| Recent stroke (≤2 weeks) | 4.14 | 1.590–10.765 | ||
| Chronic lung disease ≥moderate | 1.61 | 1.018–2.543 | ||
| Liver dysfunction | 2.08 | 1.103–3.908 | ||
| Renal failure | 1.73 | 1.083–2.776 | 1.99 | 1.552–2.556 |
| Haemodialysis | 1.89 | 1.068–3.324 | ||
| Peripheral artery disease | 1.50 | 0.984–2.298 | ||
| Active endocarditis | 1.83 | 0.99–3.392 | 1.99 | 1.312–3.023 |
| Operation ≥ emergency | 3.35 | 1.504–7.474 | ||
| Shock | 2.14 | 1.038–4.338 | ||
| Unstable angina | 2.09 | 1.085–4.008 | ||
| Congestive heart failure | 1.42 | 1.004–2.007 | ||
| NYHA classification IV | 1.69 | 1.037–2.753 | 1.80 | 1.240–2.608 |
| CCS classification ≥III | 1.46 | 0.997–2.144 | ||
| Ejection fraction <30% | 2.39 | 1.440–3.980 | 1.92 | 1.232–2.980 |
| Ejection fraction 30–60% | 1.33 | 1.062–1.861 | ||
| Previous myocardial infarction | 1.41 | 1.005–1.976 | ||
| Atrial fibrillation | 1.24 | 0.977–1.569 | ||
| Aortic regurgitation ≥2 | 1.41 | 1.008–1.961 | ||
| Mitral regurgitation ≥2 | 1.34 | 0.960–1.875 | 2.0 | 1.197–3.372 |
| Concomitant CABG | 1.38 | 1.019–1.861 | ||
| Concomitant valve surgery | 1.45 | 1.140–1.852 | ||
| Other procedures | 1.73 | 0.943–3.172 | 1.82 | 1.212–2.719 |
| Surgical volume (1-case increment) | 0.997 | 0.993–1.000 | ||
| . | Operative mortality . | Composite outcomes . | ||
|---|---|---|---|---|
| OR . | 95% CI . | OR . | 95% CI . | |
| Age (reference ≤60 years old, per 5 years) | 1.23 | 1.10–1.379 | 1.20 | 1.116–1.287 |
| Male sex | 1.36 | 0.974–1.890 | ||
| Prior CABG | 1.64 | 1.11–2.43 | ||
| Prior valve surgery | 2.0 | 1.378–2.875 | ||
| Bilateral carotid stenosis | 2.48 | 1.24–5.298 | ||
| Recent stroke (≤2 weeks) | 4.14 | 1.590–10.765 | ||
| Chronic lung disease ≥moderate | 1.61 | 1.018–2.543 | ||
| Liver dysfunction | 2.08 | 1.103–3.908 | ||
| Renal failure | 1.73 | 1.083–2.776 | 1.99 | 1.552–2.556 |
| Haemodialysis | 1.89 | 1.068–3.324 | ||
| Peripheral artery disease | 1.50 | 0.984–2.298 | ||
| Active endocarditis | 1.83 | 0.99–3.392 | 1.99 | 1.312–3.023 |
| Operation ≥ emergency | 3.35 | 1.504–7.474 | ||
| Shock | 2.14 | 1.038–4.338 | ||
| Unstable angina | 2.09 | 1.085–4.008 | ||
| Congestive heart failure | 1.42 | 1.004–2.007 | ||
| NYHA classification IV | 1.69 | 1.037–2.753 | 1.80 | 1.240–2.608 |
| CCS classification ≥III | 1.46 | 0.997–2.144 | ||
| Ejection fraction <30% | 2.39 | 1.440–3.980 | 1.92 | 1.232–2.980 |
| Ejection fraction 30–60% | 1.33 | 1.062–1.861 | ||
| Previous myocardial infarction | 1.41 | 1.005–1.976 | ||
| Atrial fibrillation | 1.24 | 0.977–1.569 | ||
| Aortic regurgitation ≥2 | 1.41 | 1.008–1.961 | ||
| Mitral regurgitation ≥2 | 1.34 | 0.960–1.875 | 2.0 | 1.197–3.372 |
| Concomitant CABG | 1.38 | 1.019–1.861 | ||
| Concomitant valve surgery | 1.45 | 1.140–1.852 | ||
| Other procedures | 1.73 | 0.943–3.172 | 1.82 | 1.212–2.719 |
| Surgical volume (1-case increment) | 0.997 | 0.993–1.000 | ||
Surgical volume indicates the number of aortic valve replacement procedures after prior cardiovascular surgery at each institute.
CABG: coronary artery bypass grafting; CCS: Canadian Cardiovascular Society; NYHA: New York Heart Association.
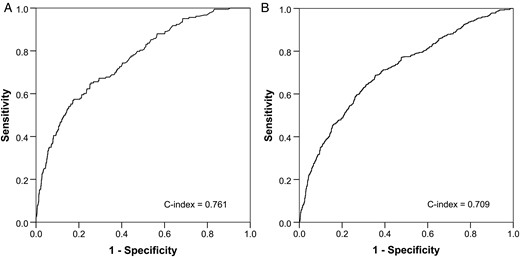
The areas under the receiver operating characteristic curve for (A) operative mortality and (B) composite outcome.
DISCUSSION
In this study, we showed the early results of AVR after prior cardiovascular surgery using a national database of Japan and demonstrated risk factors for the operative mortality and composite outcome that were not analysed in previous multicentre [6, 11] or national database studies [5]. In addition, although there have been many studies with favourable results from leading institutes [3, 4, 7], these reports lack significant statistical power to investigate the outcome of AVR after prior cardiovascular surgery and do not reflect outcomes in the real world. Therefore, evidence from a national database with a large cohort is meaningful and necessary. Currently, a risk model of AVR is available from some databases to estimate operative risks. However, one of the scoring-related problems is calibration in high-risk patients, particularly with a history of previous cardiovascular surgery [23].
TAVR is an alternative procedure to treat high-risk populations including patients with a previous history of cardiovascular surgery. Patients with a failed bioprosthesis would be treated with the valve-in-valve technique, and the others after prior cardiovascular surgery would be treated with TAVR involving the native aortic valve. The mortality rate associated with TAVR after previous cardiac surgery is 4.2–10%, after prior CABG [16–18], or 7.6–17% with the valve-in-valve technique [19–21]. A recent meta-analysis showed that TAVR after prior CABG facilitated similar early and 1-year mortality rates compared with AVR after prior CABG [18], and the transcatheter valve-in-valve technique achieves comparable mortality rates, with lower risk of strokes and bleeding but higher paravalvular leakage rates compared with redo AVR [19]. Since TAVR will become more popular with the accumulation of experiences and advancement of devices, our results provide a significant insight as a reference when considering and determining which patients may benefit from each procedure.
Although 40% of patients received a concomitant procedure, the mortality rate in this study is considered to be acceptable (30-day mortality: 5.5%, operative mortality: 8.5%). These mortality rates were higher than recent results of primary AVR in Japan: isolated AVR, 30-day mortality: 2.0% and operative mortality: 3.0%; combined AVR, 30-day mortality: 3.6–4.7% and operative mortality: 4.9–7.3% in 2012 [24]. The mortality rates in the two previous studies were 4.6% in the STS National Database [5] and 5.5% in RECORD cohorts [6, 11]. The backgrounds of our cohort regarding prior surgery were similar to those of the RECORD cohort, such as CABG 30% and valve surgery 65%. However, our cohort received more concomitant procedures, including CABG 14.5% and mitral valve surgery 29.9%, whereas the RECORD cohort included CABG 8.0% and mitral valve surgery 10.8%. In addition, concomitant CABG or valve surgery was risk factor for the composite outcome, and preoperative mitral regurgitation was a risk factor for both outcomes in the present study. Another feature of our cohort was the higher prevalence of haemodialysis (10.4%) than that in the study using the STS National Database (3.8%) [5]. Multiple logistic regression analysis demonstrated that both renal failure and haemodialysis were independent risk factors for operative mortality in the present study. Therefore, we considered that our database might include more complicated patients compared with previous multicentre studies [5, 6, 11].
The average stature of Asians is smaller than that of Western populations, with an average body mass index of 21–23 kg/m2 and body surface area of ∼1.6–1.7 m2 [12, 13]. These factors may be operative risks when undergoing cardiac surgery. In the present study, however, these variables were not independent risk factors for operative mortality.
The case volume of participating hospitals is a significant factor, because AVR after prior cardiovascular surgery is a challenging procedure [3, 4, 10]. In the present study, multiple logistic regression analysis showed that institutional experiences of AVR after prior cardiovascular surgery was associated with a lower incidence of the composite outcome. Therefore, our results suggest that high-level skill sets and extensive experience are important to achieve an excellent outcome.
Postoperative stroke and PMI are major concerns in both surgical AVR and TAVR [8, 25, 26]. The incidence of stroke in this study was comparable with that of TAVR (1.7–7.5% with the valve-in-valve technique [15, 19–21], and 2.9–6.5% in TAVR after previous cardiac surgery) [14, 16–18]. The incidence of PMI (3.8%) in the present study was excellent compared with studies regarding redo AVR or AVR after cardiovascular surgery (11.0–11.4%) [5, 6] and TAVR (7.4–8.3% in the valve-in-valve and 3.5–7.5% in the redo scenario) [17, 18].
The rate of using a bioprosthesis (∼50%) was lower than expected, because the mean age at operation in this study was over 70 and the current rate of using an aortic bioprosthesis in primary AVR is more than 70% in Japan [24]. One of the primary reasons for this discrepancy is the mean life expectancy of the Japanese (men: 80.5 and women: 86.8 years old) being higher than in other nations. Based on this background, surgeons might place an emphasis on durability when performing AVR after prior cardiovascular surgery to avoid a subsequent redo AVR. Another possibility is a limitation of the implanted valve size due to the small stature of this cohort. This means that surgeons may choose a mechanical prosthesis as a safer way to prevent patient–prosthesis mismatch rather than performing annular dilatation to implant a larger bioprosthesis. Indeed, a valve of 21 mm or smaller was implanted in 69.0% of our cohort. This rate was higher than in previous studies, in which the same sizes of valve (≦21 mm) comprised 20–36.3% [6, 7, 11]. In TAVR, a valve size of 23 mm or larger is currently used to treat an annular size of 18–27 mm [14–21]. Since a smaller prosthesis was used at a high rate in this study, a further investigation will be needed to compare the mid-term results of patients receiving AVR or TAVR using a smaller prosthesis after prior cardiovascular surgery.
The incidence rates of peripheral vascular complications were low in our cohort (iliac dissection: 0.04% and lower limb ischaemia: 0.4%), whereas those incidence rates in the works in the literature are higher in TAVR with a transfemoral approach (3.3–15.3%) [16, 20]. TAVR via a transfemoral artery is a favourable option in the redo scenario. However, vascular complications could be fatal with a femoral approach, and the risk would be higher in Asians due to the narrower femoro-iliac arteries [27]. Therefore, a lower incidence of vascular injury is one of the advantages of surgical AVR.
A lower ejection fraction, congestive heart failure and emergency status were independent risks for both major outcomes. The type of previous surgery was not a risk for mortality but was an independent risk for the composite outcome. Patients with previous CABG comprised ∼30% in this study. There has been controversy over whether previous CABG could be a risk of reoperation due to risks of injury of the patent graft, and a diseased coronary artery that is associated with the susceptibility to ischaemia, an impaired cardiac function or requirement of planned or unplanned revascularization [9, 10]. A history of previous valve surgery was also a risk associated with the composite outcome. Unfortunately, the exact breakdown of the types of previous valve surgery was uncertain because of this database's entry system, and this is a clear limitation of this study. Recent stroke (<2 weeks) was a risk factor for operative mortality. Patients with recent stroke are obviously challenging due to the risk of recurrent stroke or intracranial haemorrhage after cardiopulmonary bypass. Although we did not have any data on the aetiology of preoperative stroke, there is a possibility that these patients would suffer from stroke due to infective endocarditis or a thrombosed prosthesis requiring urgent or emergency surgery.
The C-indexes of the operative mortality and composite outcome were 0.761 (95% confidence interval: 0.725–0.797) and 0.709 (95% confidence interval: 0.696–0.722), respectively, which indicate acceptable risk models (C-indexes ≥ 0.7). The discriminatory ability of predicted operative mortality was comparable with those reported in the recent meta-analyses of EuroSCORE II performance regarding isolated or combined valve surgery: C-indexes from 0.72 to 0.81 (the results of a multicentre database) [27]. One possible criticism is that isolated AVR and AVR with a concomitant procedure should be analysed separately, as in the STS database. However, there are high rates of concomitant coronary artery, mitral valve and aortic disease in the real world when undergoing AVR after prior cardiovascular surgery, as shown in the present study. Therefore, we analysed one risk model to assess the operative risk.
Study limitations
This study has several limitations. Firstly, since it was retrospective, the results are weaker than those from a randomized prospective study. Secondly, our cohort did not precisely correspond to potential candidates for TAVR because this study included those who had received prior mechanical AVR, or patients undergoing concomitant CABG, mitral valve surgery or an aortic procedure. Although the former ones are not candidates for the valve-in-valve procedure, it was difficult to exclude such patients from our cohort due to the data entry system of the JACVSD. TAVR for the latter patients is now restricted in the guidelines, but the indication for these patients might be expanded in the future. Thirdly, precise information on the surgical procedure was not available, including the cannulation strategy or the method of implantation. Fourthly, the exact number of patients who were excluded from the analysis was not available in this database because the information of patients not providing consent had not been entered into the database from the beginning. Finally, the JACVSD does not provide any data on the pre- and postoperative pressure gradient of the aortic valve, postoperative valve status including paravalvular leakage, or mid- or long-term clinical follow-ups.
CONCLUSIONS
Although AVR after prior cardiovascular surgery is a challenging procedure, the early results including operative mortality, the incidence of major morbidity, stroke and PMI were acceptable. We could identify risk factors for both the operative mortality and the composite outcome after AVR in the redo scenario using a national database in Japan. Our results may be informative when treating such high-risk patients.
ACKNOWLEDGEMENTS
The authors thank Chieko Fujimura and Eriko Fukuchi (Department of Healthcare Quality Assessment, The University of Tokyo, Graduate School of Medicine, Tokyo, Japan) for providing assistance with data collection.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 80th Annual Scientific Meeting of the Japanese Circulation Society, Sendai, Japan, 18–20 March 2016.
- aortic valve stenosis
- coronary artery bypass surgery
- endocarditis
- mitral valve insufficiency
- cardiac pacemaker implantation
- cerebrovascular accident
- aortic valve replacement
- kidney failure
- adult
- descending thoracic aorta
- cardiovascular surgical procedures
- objective (goal)
- heart
- morbidity
- ejection fraction
- previous surgery
- aortic surgery
- mitral valve procedures
- new york heart association classification
- surgical mortality
- japanese




