-
PDF
- Split View
-
Views
-
Cite
Cite
Prashant N. Mohite, Mohamed Zeriouh, Diana G. Sáez, Aron-Frederik Popov, Anton Sabashnikov, Bartlomiej Zych, Ashok Padukone, Levente Fazekas, Olga Ananiadou, Fabio De Robertis, Simona Soresi, Anna Reed, Martin Carby, André R. Simon, Influence of history of cannabis smoking in selected donors on the outcomes of lung transplantation, European Journal of Cardio-Thoracic Surgery, Volume 51, Issue 1, January 2017, Pages 142–147, https://doi.org/10.1093/ejcts/ezw255
Close - Share Icon Share
Cannabis is the most commonly abused illicit drug and the smokers are at the risk of lung infections, bullous emphysema and lung cancer. However, no evidence about the outcomes of lung transplantation (LTx) utilizing the lungs from such donors is available in the literature.
We retrospectively analysed lung ‘organ offers’ and LTx at our centre between January 2007 and November 2013. The outcomes of LTx utilizing lungs from donors with a history of cannabis smoking were compared with the outcomes of those with no such history using unadjusted model as well as propensity score matching.
A total of 302 LTxs were performed during this period and were grouped depending on the history of cannabis smoking in donors—‘cannabis’ (n = 19) and control group (n = 283). All the donors in ‘cannabis’ group were tobacco smokers compared with 43% in the control group. Preoperative characteristics in recipients in both groups were comparable. Intraoperative and post-LTx variables including 1- and 3-year survivals were comparable in both groups.
The history of donor cannabis smoking does not appear to affect early and mid-term outcomes after LTx and potentially improve the donor pool. As it does not seem to negatively affect the outcomes after LTx, it should not be per se considered a contraindication for lung donation.
INTRODUCTION
Donor organ shortage leads to significant mortality on the waiting list for lung transplantation (LTx). To overcome this donor shortage, several techniques such as optimization of donor management, use of extended criteria donors, use of cardiocirculatory arrest donors and ex vivo lung perfusion are being utilized [1–3]. One aspect of this liberalization encompasses lungs from donors with a history of cannabis smoking.
Cannabis is by far the most widely cultivated, trafficked and abused illicit drug especially among the adolescents [4]. Cannabis is derived from the Cannabis sativa plant and the drug is prepared using the dried flower tops and leaves. The drug is most commonly smoked in a hand-rolled cigarette (referred to as a joint) or in a designed water pipe (referred to as a bong). About 147 million people, 2.5% of the world population, consume cannabis (annual prevalence), whereas 0.2% consume cocaine and 0.2% consume opiates. The most rapid growth in cannabis abuse since the 1960s has been in developed countries in North America, Western Europe and Australia [5]. Approximately 10% individuals who ever smoke cannabis experience a dependence syndrome [6]. Cannabis smokers are prone to lung infections, bullous emphysema and lung cancer although its association with lung cancer is not fully established [7].
Given the prevalence of smoking in the general population, and consequently in the organ donation pool, a policy of refusal to use lung allografts from smokers would have a profound impact on the number of available organs [8]. Conversely, the risk that a positive smoking history in lung donors could adversely affect transplant outcome causes concern [9]. Bhorade et al. mention about the utilization of lungs from donors with cannabis smoking in a bid to expand the donor pool [10]. Limited evidence is available about safety in the utilization of lungs from donor with a history of cannabis smoking and generally such donors are considered even more riskier due to additive bad effect of cannabis over smoking. In an attempt to address this pertinent issue, we collected and analysed patient and donor characteristics, as well as outcome data, for all LTxs performed at our centre over a 7-year period and investigated the effect of donor cannabis smoking history on the outcomes of LTx. To the best of our knowledge, this study is the first analysis comparing the influence of donor cannabis smoking on the outcomes of LTx to date.
MATERIALS AND METHODS
The study design was a retrospective review of the prospectively collected data, whereas the Institutional Review Board at our centre approved this study and waived the need for individual patient consent. An analysis of all lung offers (n = 3362) to our institute between January 2007 and November 2013 was performed with a focus on the history of cannabis smoking in the donors. A total of 302 LTxs were performed at our institute between January 2007 and November 2013. Recipients were divided into two groups depending on the history of cannabis smoking in the donors from whom they received organs: ‘cannabis’ group consisting of recipients who received lungs from donors with a history of cannabis smoking (n = 19) and control group consisting of recipients with donors with no such history (n = 283). History of cannabis smoking was obtained from next of kin and/or patient's GP. Donors were considered to have a history of cannabis smoking if they have smoked cannabis more than ‘occasional’ and are current cannabis smokers. One pack-year was defined as 20 cigarettes (one pack) smoked per day for 1 year. We segregated donors with smoking history as smokers who had smoked less than 20 pack-years and heavy smokers who had smoked more than 20 pack-years.
End-points of the study
Overall survival after LTx and bronchiolitis obliterans syndrome (BOS)-free survival were the primary end-points, whereas PaO2/FiO2 ratio at the end of the transplant; 24, 48 and 72 h after transplant; duration of mechanical ventilation; intensive care unit (ICU) and total hospital stay; as well as the need for postoperative use of extracorporeal membrane oxygenation (ECMO) were the secondary end-points.
Organ procurement and lung transplant protocol
Donor organ assessments performed at donor hospitals included radiological assessment, fibreoptic bronchoscopy, gross organ inspection and palpation, assessment of compliance using deflation test and selective blood gas analysis from each pulmonary vein. The standard preservation solution used was low potassium dextran (Perfadex, Medisan, Uppsala, Sweden). As mentioned in our previous papers, the lungs were matched to the recipients according to blood group, height, total lung capacity, time already spent on the LTx waiting list and the clinical status of the recipient at the time of the transplantation [3, 11]. BOS was diagnosed when post-transplant fraction of expired volume in 1 s (FEV1) measured on a regular basis permanently dropped >20% of the best FEV1 achieved after the LTx. Demographics and perioperative recipient and donor data as well as long-term outcomes were compared.
Statistical analysis
IBM SPSS Statistics for Windows, Version 21 (IBM Corp. Released 2012. IBM Corp., Armonk, NY, USA) was used for data analysis. The data were presented as continuous or categorical variables. Continuous data were evaluated for normality using one-sample Kolmogorov–Smirnov test and confirmed by histograms. Continuous variables were expressed as the mean ± standard deviation in cases of normal distributed variables or median (interquartile range) in cases of non-normal distributed variables. Categorical variables are presented as total numbers of patients and percentages. In continuous data, unpaired t-test was used for normally distributed variables, whereas Mann–Whitney U-test was used for non-normally distributed variables. In categorical data, Pearson's χ2 or Fisher's exact tests were used depending on the minimum expected count in each cross tab. Survival analysis was performed with Kaplan–Meier survival estimation. Log-rank (Mantel–Cox) test was applied for the comparison of cumulative survival and BOS-free survival estimates of patients from both groups. P-values <0.05 were considered statistically significant. Propensity score matching was performed using the SPSS software. A propensity score for each patient was estimated using a logistic regression model with preoperative characteristics that showed statistically significant differences between the two groups as independent variables. Matching was based on one-to-three nearest neighbour matching method with a tolerance level on the maximum propensity score distance (callipers of width 0.2 SD of the logit of the PS) that resulted in a total number of 76 patients who were well matched for baseline characteristics.
RESULTS
Between January 2007 and November 2013, a total of 3362 lung offers were made to our institute, and of them, 189 donors (5.62%) had a history of cannabis smoking. A total of 135 donors were declined at the time of offer due to a combination of old age, heavy smoking and cannabis smoking (n = 7); a combination of heavy smoking and cannabis smoking (n = 19); cannabis smoking along with other drug abuse (n = 26); poor function (n = 14); lack of suitable recipient (n = 26); chest infection (n = 13); logistics (n = 13); positive virology result (n = 10) and unsuitable past medical history (n = 7). Fifty-four donors were initially accepted and assessed by the retrieval team. Of them, 34 donors were declined due to abnormal bronchoscopy n = 11, poor function n = 10, consolidation n = 5, emphysema n = 4. Another four donors did not meet criteria for donation after cardiac death. Lungs were retrieved from 20 donors; one pair of lungs was discarded after assessment with EVLP, whereas 19 lungs were successfully implanted in the recipients.
A total of 302 LTxs performed in this period included 277 (92%) double- and 25 (8%) single-lung transplants. In 60 (20%) cases, lungs were retrieved from donation after circulatory death (DCD) donors. The donors from ‘cannabis’ and control groups had comparable baseline characteristics, except for significantly higher incidence of male gender, younger, taller donors in the ‘cannabis’ group compared with the control group. All the donors in the ‘cannabis’ group were smokers compared with 43% in the control group. Incidence of heavy smoking was also higher in the ‘cannabis’ group than in the control group (11 vs 3%); however, the number of pack-years smoked was significantly lesser in the ‘cannabis’ group—may be because of the higher incidence of younger donors in this group. There were no statistically significant differences in donor weight, percentage of DCD, abnormal chest X-ray, abnormal bronchoscopy, use of ex vivo lung perfusion, duration of the donor mechanical ventilation, last preretrieval PaO2/FiO2 ratio and the total ischaemic time (Table 1). Table 2 shows preoperative recipient demographics and distribution of recipients' diagnosis. There were no statistically significant differences in the recipients' age, gender, height, weight, predicted total lung capacity, preoperative saturation, preoperative hospitalization, preoperative dependence on long-term oxygen therapy (continuous and intermittent), preoperative use of extracorporeal life support and primary diagnosis.
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| Age (years) | 30 ± 10 | 43 ± 13 | <0.001 |
| Gender (female) | 6 (32%) | 176 (62%) | 0.008 |
| Height (cm) | 176 ± 9 | 169 ± 10 | 0.003 |
| Weight (kg) | 73 ± 15 | 73 ± 14 | 0.99 |
| PaO2 preretrieval (kPa) | 53 ± 13 | 56 ± 14 | 0.289 |
| Ventilation duration (days) | 1 (1;4) | 2 (1;3) | 0.32 |
| Total ischaemic time (min) | 380 ± 114 | 371 ± 145 | 0.87 |
| Use of EVLP | 0 | 14 (5%) | 1.00 |
| DCD | 4 (21%) | 56 (20%) | 1.00 |
| Cardiac arrest | 4 (21%) | 61 (22%) | 1.00 |
| Cardiac arrest time (min) | 22 ± 26 | 21 ± 16 | 0.97 |
| Abnormal bronchoscopy | 9 (47%) | 81 (29%) | 0.1 |
| Abnormal chest X-ray | 4 (21%) | 73 (26%) | 0.79 |
| Extended donor criteria | 5 (26%) | 98 (35%) | 0.46 |
| Smoking | 19 (100%) | 122 (43%) | <0.001 |
| Heavy smoking | 2 (11%) | 7 (2%) | 0.11 |
| Smoking pack-years | 10 ± 9 | 17 ± 11 | 0.01 |
| Cause of death | 0.53 | ||
| ICH | 11 (58%) | 188 (66%) | |
| HBI | 1 (5%) | 31 (11%) | |
| Trauma | 5 (24%) | 26 (9%) | |
| CVA | 2 (11%) | 22 (8%) | |
| Meningitis | 1 (5%) | 11 (4%) | |
| Other | 0 | 5 (2%) |
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| Age (years) | 30 ± 10 | 43 ± 13 | <0.001 |
| Gender (female) | 6 (32%) | 176 (62%) | 0.008 |
| Height (cm) | 176 ± 9 | 169 ± 10 | 0.003 |
| Weight (kg) | 73 ± 15 | 73 ± 14 | 0.99 |
| PaO2 preretrieval (kPa) | 53 ± 13 | 56 ± 14 | 0.289 |
| Ventilation duration (days) | 1 (1;4) | 2 (1;3) | 0.32 |
| Total ischaemic time (min) | 380 ± 114 | 371 ± 145 | 0.87 |
| Use of EVLP | 0 | 14 (5%) | 1.00 |
| DCD | 4 (21%) | 56 (20%) | 1.00 |
| Cardiac arrest | 4 (21%) | 61 (22%) | 1.00 |
| Cardiac arrest time (min) | 22 ± 26 | 21 ± 16 | 0.97 |
| Abnormal bronchoscopy | 9 (47%) | 81 (29%) | 0.1 |
| Abnormal chest X-ray | 4 (21%) | 73 (26%) | 0.79 |
| Extended donor criteria | 5 (26%) | 98 (35%) | 0.46 |
| Smoking | 19 (100%) | 122 (43%) | <0.001 |
| Heavy smoking | 2 (11%) | 7 (2%) | 0.11 |
| Smoking pack-years | 10 ± 9 | 17 ± 11 | 0.01 |
| Cause of death | 0.53 | ||
| ICH | 11 (58%) | 188 (66%) | |
| HBI | 1 (5%) | 31 (11%) | |
| Trauma | 5 (24%) | 26 (9%) | |
| CVA | 2 (11%) | 22 (8%) | |
| Meningitis | 1 (5%) | 11 (4%) | |
| Other | 0 | 5 (2%) |
EVLP: Ex-vivo lung perfusion; DCD: donation after circulatory death; ICH: intracranial haemorrhage; HBI: Hypoxic brain injury; CVA: Cerebrovascular accident.
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| Age (years) | 30 ± 10 | 43 ± 13 | <0.001 |
| Gender (female) | 6 (32%) | 176 (62%) | 0.008 |
| Height (cm) | 176 ± 9 | 169 ± 10 | 0.003 |
| Weight (kg) | 73 ± 15 | 73 ± 14 | 0.99 |
| PaO2 preretrieval (kPa) | 53 ± 13 | 56 ± 14 | 0.289 |
| Ventilation duration (days) | 1 (1;4) | 2 (1;3) | 0.32 |
| Total ischaemic time (min) | 380 ± 114 | 371 ± 145 | 0.87 |
| Use of EVLP | 0 | 14 (5%) | 1.00 |
| DCD | 4 (21%) | 56 (20%) | 1.00 |
| Cardiac arrest | 4 (21%) | 61 (22%) | 1.00 |
| Cardiac arrest time (min) | 22 ± 26 | 21 ± 16 | 0.97 |
| Abnormal bronchoscopy | 9 (47%) | 81 (29%) | 0.1 |
| Abnormal chest X-ray | 4 (21%) | 73 (26%) | 0.79 |
| Extended donor criteria | 5 (26%) | 98 (35%) | 0.46 |
| Smoking | 19 (100%) | 122 (43%) | <0.001 |
| Heavy smoking | 2 (11%) | 7 (2%) | 0.11 |
| Smoking pack-years | 10 ± 9 | 17 ± 11 | 0.01 |
| Cause of death | 0.53 | ||
| ICH | 11 (58%) | 188 (66%) | |
| HBI | 1 (5%) | 31 (11%) | |
| Trauma | 5 (24%) | 26 (9%) | |
| CVA | 2 (11%) | 22 (8%) | |
| Meningitis | 1 (5%) | 11 (4%) | |
| Other | 0 | 5 (2%) |
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| Age (years) | 30 ± 10 | 43 ± 13 | <0.001 |
| Gender (female) | 6 (32%) | 176 (62%) | 0.008 |
| Height (cm) | 176 ± 9 | 169 ± 10 | 0.003 |
| Weight (kg) | 73 ± 15 | 73 ± 14 | 0.99 |
| PaO2 preretrieval (kPa) | 53 ± 13 | 56 ± 14 | 0.289 |
| Ventilation duration (days) | 1 (1;4) | 2 (1;3) | 0.32 |
| Total ischaemic time (min) | 380 ± 114 | 371 ± 145 | 0.87 |
| Use of EVLP | 0 | 14 (5%) | 1.00 |
| DCD | 4 (21%) | 56 (20%) | 1.00 |
| Cardiac arrest | 4 (21%) | 61 (22%) | 1.00 |
| Cardiac arrest time (min) | 22 ± 26 | 21 ± 16 | 0.97 |
| Abnormal bronchoscopy | 9 (47%) | 81 (29%) | 0.1 |
| Abnormal chest X-ray | 4 (21%) | 73 (26%) | 0.79 |
| Extended donor criteria | 5 (26%) | 98 (35%) | 0.46 |
| Smoking | 19 (100%) | 122 (43%) | <0.001 |
| Heavy smoking | 2 (11%) | 7 (2%) | 0.11 |
| Smoking pack-years | 10 ± 9 | 17 ± 11 | 0.01 |
| Cause of death | 0.53 | ||
| ICH | 11 (58%) | 188 (66%) | |
| HBI | 1 (5%) | 31 (11%) | |
| Trauma | 5 (24%) | 26 (9%) | |
| CVA | 2 (11%) | 22 (8%) | |
| Meningitis | 1 (5%) | 11 (4%) | |
| Other | 0 | 5 (2%) |
EVLP: Ex-vivo lung perfusion; DCD: donation after circulatory death; ICH: intracranial haemorrhage; HBI: Hypoxic brain injury; CVA: Cerebrovascular accident.
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| Age (years) | 45 ± 14 | 43 ± 514 | 0.45 |
| Female | 7 (37%) | 138 (49%) | 0.31 |
| Height (cm) | 171 ± 8 | 167 ± 13 | 0.30 |
| Weight (kg) | 70 (64;84) | 62 (54;77) | 0.10 |
| Predicted TLC (L) | 6.2 ± 1.0 | 5.9 ± 1.1 | 0.20 |
| Preoperative saturation | 93 ± 5 | 94 ± 3 | 0.50 |
| Mech ventilation (days) | 1 (8%) | 9 (4%) | 0.44 |
| Preoperative hospitalization | 2 (15%) | 35 (16%) | 1.00 |
| LTOT continuous | 6 (50%) | 111 (54%) | 0.80 |
| LTOT intermittent | 4 (36%) | 40 (19%) | 0.24 |
| ECLS | 1 (5%) | 9 (3%) | 0.48 |
| Primary diagnosis | 0.61 | ||
| CF | 7 (37%) | 107 (38%) | |
| Emphysema | 5 (26%) | 83 (29%) | |
| α-1 antitrypsin deficiency | 5 (26%) | 34 (12%) | |
| PF | 1 (5%) | 21 (7%) | |
| PH | 0 | 15 (5%) | |
| LAM | 0 | 8 (3%) | |
| Sarcoidosis | 0 | 6 (2%) | |
| BO | 0 | 4 (1%) | |
| Bronchiectasis | 1 (5%) | 5 (2%) |
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| Age (years) | 45 ± 14 | 43 ± 514 | 0.45 |
| Female | 7 (37%) | 138 (49%) | 0.31 |
| Height (cm) | 171 ± 8 | 167 ± 13 | 0.30 |
| Weight (kg) | 70 (64;84) | 62 (54;77) | 0.10 |
| Predicted TLC (L) | 6.2 ± 1.0 | 5.9 ± 1.1 | 0.20 |
| Preoperative saturation | 93 ± 5 | 94 ± 3 | 0.50 |
| Mech ventilation (days) | 1 (8%) | 9 (4%) | 0.44 |
| Preoperative hospitalization | 2 (15%) | 35 (16%) | 1.00 |
| LTOT continuous | 6 (50%) | 111 (54%) | 0.80 |
| LTOT intermittent | 4 (36%) | 40 (19%) | 0.24 |
| ECLS | 1 (5%) | 9 (3%) | 0.48 |
| Primary diagnosis | 0.61 | ||
| CF | 7 (37%) | 107 (38%) | |
| Emphysema | 5 (26%) | 83 (29%) | |
| α-1 antitrypsin deficiency | 5 (26%) | 34 (12%) | |
| PF | 1 (5%) | 21 (7%) | |
| PH | 0 | 15 (5%) | |
| LAM | 0 | 8 (3%) | |
| Sarcoidosis | 0 | 6 (2%) | |
| BO | 0 | 4 (1%) | |
| Bronchiectasis | 1 (5%) | 5 (2%) |
TLC: total lung capacity; LTOT: long-term oxygen therapy; ECLS: extra-corporeal life support; CF: cystic fibrosis; PF: pulmonary fibrosis; PH: pulmonary hypertension; LAM: lymphangioleiomyomatosis.
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| Age (years) | 45 ± 14 | 43 ± 514 | 0.45 |
| Female | 7 (37%) | 138 (49%) | 0.31 |
| Height (cm) | 171 ± 8 | 167 ± 13 | 0.30 |
| Weight (kg) | 70 (64;84) | 62 (54;77) | 0.10 |
| Predicted TLC (L) | 6.2 ± 1.0 | 5.9 ± 1.1 | 0.20 |
| Preoperative saturation | 93 ± 5 | 94 ± 3 | 0.50 |
| Mech ventilation (days) | 1 (8%) | 9 (4%) | 0.44 |
| Preoperative hospitalization | 2 (15%) | 35 (16%) | 1.00 |
| LTOT continuous | 6 (50%) | 111 (54%) | 0.80 |
| LTOT intermittent | 4 (36%) | 40 (19%) | 0.24 |
| ECLS | 1 (5%) | 9 (3%) | 0.48 |
| Primary diagnosis | 0.61 | ||
| CF | 7 (37%) | 107 (38%) | |
| Emphysema | 5 (26%) | 83 (29%) | |
| α-1 antitrypsin deficiency | 5 (26%) | 34 (12%) | |
| PF | 1 (5%) | 21 (7%) | |
| PH | 0 | 15 (5%) | |
| LAM | 0 | 8 (3%) | |
| Sarcoidosis | 0 | 6 (2%) | |
| BO | 0 | 4 (1%) | |
| Bronchiectasis | 1 (5%) | 5 (2%) |
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| Age (years) | 45 ± 14 | 43 ± 514 | 0.45 |
| Female | 7 (37%) | 138 (49%) | 0.31 |
| Height (cm) | 171 ± 8 | 167 ± 13 | 0.30 |
| Weight (kg) | 70 (64;84) | 62 (54;77) | 0.10 |
| Predicted TLC (L) | 6.2 ± 1.0 | 5.9 ± 1.1 | 0.20 |
| Preoperative saturation | 93 ± 5 | 94 ± 3 | 0.50 |
| Mech ventilation (days) | 1 (8%) | 9 (4%) | 0.44 |
| Preoperative hospitalization | 2 (15%) | 35 (16%) | 1.00 |
| LTOT continuous | 6 (50%) | 111 (54%) | 0.80 |
| LTOT intermittent | 4 (36%) | 40 (19%) | 0.24 |
| ECLS | 1 (5%) | 9 (3%) | 0.48 |
| Primary diagnosis | 0.61 | ||
| CF | 7 (37%) | 107 (38%) | |
| Emphysema | 5 (26%) | 83 (29%) | |
| α-1 antitrypsin deficiency | 5 (26%) | 34 (12%) | |
| PF | 1 (5%) | 21 (7%) | |
| PH | 0 | 15 (5%) | |
| LAM | 0 | 8 (3%) | |
| Sarcoidosis | 0 | 6 (2%) | |
| BO | 0 | 4 (1%) | |
| Bronchiectasis | 1 (5%) | 5 (2%) |
TLC: total lung capacity; LTOT: long-term oxygen therapy; ECLS: extra-corporeal life support; CF: cystic fibrosis; PF: pulmonary fibrosis; PH: pulmonary hypertension; LAM: lymphangioleiomyomatosis.
Intraoperative variables and parameters of early postoperative outcomes are presented in Table 3. The mean follow-up in the ‘cannabis’ group was 809 ± 729 [median 672 (164;1137)], and the mean follow-up in the control group was 854 ± 691 [median 755 (220;1308)]. There were no statistically significant differences in terms of off-pump/on-pump strategy; cardiopulmonary bypass time; total ischaemia time; proportion of the patients receiving a single LTx; retransplantation; pO2/FiO2 ratios on arrival in ICU; at 24, 48 and 72 h after LTx and ICU and hospital stay. There were no statistically significant differences in the incidence of primary graft dysfunction Grade 3, rejection, prevalence of postoperative BOS and predicted forced expiratory volume in first second (%FEV1) over the follow-up. There were no significant differences in BOS-free survival between the control and ‘cannabis’ groups, log-rank (Mantel–Cox) P = 0.62: 92.6 vs 92.9% at 1 year, 78.4 vs 57.9% at 3 years and 52.9 vs 57.9% at 6 years. The estimated cumulative survival was also similar between both groups, log-rank (Mantel–Cox) P = 0.35: 84.0 vs 93.3% at 1 year, 69.7 vs 74.2% at 3 years and 57.4 vs 74.2% at 6 years in the control and ‘cannabis’ group, respectively.
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| Intraoperative data | |||
| Off pump | 8 (42%) | 78 (28%) | 0.39 |
| CPB time | 151 ± 36 | 164 ± 71 | 0.58 |
| Total ischaemic time | 394 ± 167 | 369 ± 142 | 0.47 |
| Retransplantation | 1 (5%) | 5 (2%) | 0.33 |
| SLTx | 1 (5%) | 24 (9%) | 1.00 |
| Postoperative outcome | |||
| PaO2/FiO2 ratio on arrival | 307 ± 110 | 315 ± 134 | 0.78 |
| PaO2/FiO2 ratio 24 h | 387 ± 138 | 340 ± 112 | 0.09 |
| PaO2/FiO2 ratio 48 h | 369 ± 139 | 350 ± 113 | 0.48 |
| PaO2/FiO2 ratio 72 h | 330 ± 84 | 346 ± 114 | 0.56 |
| Ventilation (h) | 32 (16;86) | 37 (18;327) | 0.37 |
| ICU stay (days) | 5 (3;13) | 6 (3;21) | 0.64 |
| Hospital stay (days) | 26 (22;51) | 33 (22;52) | 0.48 |
| Follow-up | 809 ± 729 | 854 ± 691 | 0.80 |
| PGD Grade 3 | |||
| PGD on arrival | 3 (16%) | 50 (19%) | 1.00 |
| PGD 24 h | 1 (5%) | 28 (10%) | 0.70 |
| PGD 48 h | 2 (11%) | 20 (8%) | 0.66 |
| PGD 72 h | 0 | 23 (10%) | 0.39 |
| Rejection | 0.43 | ||
| A0 | 16 (82%) | 211 (75%) | |
| A1 | 0 | 29 (10%) | |
| A2 | 2 (11%) | 36 (13%) | |
| A3 | 1 (5%) | 7 (3%) | |
| FEV1 (% of predicted) | |||
| 3 months | 82 ± 24 | 71 ± 29 | 0.16 |
| 6 months | 82 ± 25 | 75 ± 22 | 0.27 |
| 1 year | 81 ± 31 | 78 ± 24 | 0.62 |
| 3 years | 97 ± 24 | 75 ± 25 | 0.14 |
| 5 years | 82 ± 0 | 78 ± 24 | 0.88 |
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| Intraoperative data | |||
| Off pump | 8 (42%) | 78 (28%) | 0.39 |
| CPB time | 151 ± 36 | 164 ± 71 | 0.58 |
| Total ischaemic time | 394 ± 167 | 369 ± 142 | 0.47 |
| Retransplantation | 1 (5%) | 5 (2%) | 0.33 |
| SLTx | 1 (5%) | 24 (9%) | 1.00 |
| Postoperative outcome | |||
| PaO2/FiO2 ratio on arrival | 307 ± 110 | 315 ± 134 | 0.78 |
| PaO2/FiO2 ratio 24 h | 387 ± 138 | 340 ± 112 | 0.09 |
| PaO2/FiO2 ratio 48 h | 369 ± 139 | 350 ± 113 | 0.48 |
| PaO2/FiO2 ratio 72 h | 330 ± 84 | 346 ± 114 | 0.56 |
| Ventilation (h) | 32 (16;86) | 37 (18;327) | 0.37 |
| ICU stay (days) | 5 (3;13) | 6 (3;21) | 0.64 |
| Hospital stay (days) | 26 (22;51) | 33 (22;52) | 0.48 |
| Follow-up | 809 ± 729 | 854 ± 691 | 0.80 |
| PGD Grade 3 | |||
| PGD on arrival | 3 (16%) | 50 (19%) | 1.00 |
| PGD 24 h | 1 (5%) | 28 (10%) | 0.70 |
| PGD 48 h | 2 (11%) | 20 (8%) | 0.66 |
| PGD 72 h | 0 | 23 (10%) | 0.39 |
| Rejection | 0.43 | ||
| A0 | 16 (82%) | 211 (75%) | |
| A1 | 0 | 29 (10%) | |
| A2 | 2 (11%) | 36 (13%) | |
| A3 | 1 (5%) | 7 (3%) | |
| FEV1 (% of predicted) | |||
| 3 months | 82 ± 24 | 71 ± 29 | 0.16 |
| 6 months | 82 ± 25 | 75 ± 22 | 0.27 |
| 1 year | 81 ± 31 | 78 ± 24 | 0.62 |
| 3 years | 97 ± 24 | 75 ± 25 | 0.14 |
| 5 years | 82 ± 0 | 78 ± 24 | 0.88 |
ICU: intensive care unit; PGD: primary graft dysfuntion; FEV1: fraction of expired volume in 1 s.
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| Intraoperative data | |||
| Off pump | 8 (42%) | 78 (28%) | 0.39 |
| CPB time | 151 ± 36 | 164 ± 71 | 0.58 |
| Total ischaemic time | 394 ± 167 | 369 ± 142 | 0.47 |
| Retransplantation | 1 (5%) | 5 (2%) | 0.33 |
| SLTx | 1 (5%) | 24 (9%) | 1.00 |
| Postoperative outcome | |||
| PaO2/FiO2 ratio on arrival | 307 ± 110 | 315 ± 134 | 0.78 |
| PaO2/FiO2 ratio 24 h | 387 ± 138 | 340 ± 112 | 0.09 |
| PaO2/FiO2 ratio 48 h | 369 ± 139 | 350 ± 113 | 0.48 |
| PaO2/FiO2 ratio 72 h | 330 ± 84 | 346 ± 114 | 0.56 |
| Ventilation (h) | 32 (16;86) | 37 (18;327) | 0.37 |
| ICU stay (days) | 5 (3;13) | 6 (3;21) | 0.64 |
| Hospital stay (days) | 26 (22;51) | 33 (22;52) | 0.48 |
| Follow-up | 809 ± 729 | 854 ± 691 | 0.80 |
| PGD Grade 3 | |||
| PGD on arrival | 3 (16%) | 50 (19%) | 1.00 |
| PGD 24 h | 1 (5%) | 28 (10%) | 0.70 |
| PGD 48 h | 2 (11%) | 20 (8%) | 0.66 |
| PGD 72 h | 0 | 23 (10%) | 0.39 |
| Rejection | 0.43 | ||
| A0 | 16 (82%) | 211 (75%) | |
| A1 | 0 | 29 (10%) | |
| A2 | 2 (11%) | 36 (13%) | |
| A3 | 1 (5%) | 7 (3%) | |
| FEV1 (% of predicted) | |||
| 3 months | 82 ± 24 | 71 ± 29 | 0.16 |
| 6 months | 82 ± 25 | 75 ± 22 | 0.27 |
| 1 year | 81 ± 31 | 78 ± 24 | 0.62 |
| 3 years | 97 ± 24 | 75 ± 25 | 0.14 |
| 5 years | 82 ± 0 | 78 ± 24 | 0.88 |
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| Intraoperative data | |||
| Off pump | 8 (42%) | 78 (28%) | 0.39 |
| CPB time | 151 ± 36 | 164 ± 71 | 0.58 |
| Total ischaemic time | 394 ± 167 | 369 ± 142 | 0.47 |
| Retransplantation | 1 (5%) | 5 (2%) | 0.33 |
| SLTx | 1 (5%) | 24 (9%) | 1.00 |
| Postoperative outcome | |||
| PaO2/FiO2 ratio on arrival | 307 ± 110 | 315 ± 134 | 0.78 |
| PaO2/FiO2 ratio 24 h | 387 ± 138 | 340 ± 112 | 0.09 |
| PaO2/FiO2 ratio 48 h | 369 ± 139 | 350 ± 113 | 0.48 |
| PaO2/FiO2 ratio 72 h | 330 ± 84 | 346 ± 114 | 0.56 |
| Ventilation (h) | 32 (16;86) | 37 (18;327) | 0.37 |
| ICU stay (days) | 5 (3;13) | 6 (3;21) | 0.64 |
| Hospital stay (days) | 26 (22;51) | 33 (22;52) | 0.48 |
| Follow-up | 809 ± 729 | 854 ± 691 | 0.80 |
| PGD Grade 3 | |||
| PGD on arrival | 3 (16%) | 50 (19%) | 1.00 |
| PGD 24 h | 1 (5%) | 28 (10%) | 0.70 |
| PGD 48 h | 2 (11%) | 20 (8%) | 0.66 |
| PGD 72 h | 0 | 23 (10%) | 0.39 |
| Rejection | 0.43 | ||
| A0 | 16 (82%) | 211 (75%) | |
| A1 | 0 | 29 (10%) | |
| A2 | 2 (11%) | 36 (13%) | |
| A3 | 1 (5%) | 7 (3%) | |
| FEV1 (% of predicted) | |||
| 3 months | 82 ± 24 | 71 ± 29 | 0.16 |
| 6 months | 82 ± 25 | 75 ± 22 | 0.27 |
| 1 year | 81 ± 31 | 78 ± 24 | 0.62 |
| 3 years | 97 ± 24 | 75 ± 25 | 0.14 |
| 5 years | 82 ± 0 | 78 ± 24 | 0.88 |
ICU: intensive care unit; PGD: primary graft dysfuntion; FEV1: fraction of expired volume in 1 s.
Complications after LTx are mentioned in Table 4. There was no statistical significance between the groups in terms of post-LTx ECMO requirement, incidence of BOS, arrhythmia, arrhythmia requiring treatment, incidence of re-exploration, requirement of blood and blood products and incidence of overall, pulmonary and extrapulmonary infections.
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| ECMO | 0 | 26 (9%) | 0.39 |
| BOS (5 years) | 4 (24%) | 51 (22%) | 0.77 |
| Arrhythmia | 5 (26.3%) | 80 (28%) | 0.86 |
| Arrhythmia requiring treatment | 0 | 33 (12%) | 0.24 |
| RBC (units) | 1 (0;2) | 0 (0;4) | 0.68 |
| Platelets (units) | 0 (0;1) | 0 (0;1) | 0.30 |
| FFP (units) | 0 (0;2) | 0 (0;2) | 0.98 |
| Re-exploration | 0 | 19 (7%) | 0.62 |
| Infection | 7 (37%) | 19 (32%) | 0.65 |
| Pulmonary | 6 (32%) | 82 (29%) | 0.81 |
| Extrapulmonary | 2 (11%) | 28 (10%) | 0.70 |
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| ECMO | 0 | 26 (9%) | 0.39 |
| BOS (5 years) | 4 (24%) | 51 (22%) | 0.77 |
| Arrhythmia | 5 (26.3%) | 80 (28%) | 0.86 |
| Arrhythmia requiring treatment | 0 | 33 (12%) | 0.24 |
| RBC (units) | 1 (0;2) | 0 (0;4) | 0.68 |
| Platelets (units) | 0 (0;1) | 0 (0;1) | 0.30 |
| FFP (units) | 0 (0;2) | 0 (0;2) | 0.98 |
| Re-exploration | 0 | 19 (7%) | 0.62 |
| Infection | 7 (37%) | 19 (32%) | 0.65 |
| Pulmonary | 6 (32%) | 82 (29%) | 0.81 |
| Extrapulmonary | 2 (11%) | 28 (10%) | 0.70 |
ECMO: extracorporeal membrane oxygenation; BOS: bronchiolitis obliterans syndrome; RBC: red blood cell; FFP: fresh frozen plasma.
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| ECMO | 0 | 26 (9%) | 0.39 |
| BOS (5 years) | 4 (24%) | 51 (22%) | 0.77 |
| Arrhythmia | 5 (26.3%) | 80 (28%) | 0.86 |
| Arrhythmia requiring treatment | 0 | 33 (12%) | 0.24 |
| RBC (units) | 1 (0;2) | 0 (0;4) | 0.68 |
| Platelets (units) | 0 (0;1) | 0 (0;1) | 0.30 |
| FFP (units) | 0 (0;2) | 0 (0;2) | 0.98 |
| Re-exploration | 0 | 19 (7%) | 0.62 |
| Infection | 7 (37%) | 19 (32%) | 0.65 |
| Pulmonary | 6 (32%) | 82 (29%) | 0.81 |
| Extrapulmonary | 2 (11%) | 28 (10%) | 0.70 |
| . | Cannabis (n = 19) . | Control (n = 283) . | P-value . |
|---|---|---|---|
| ECMO | 0 | 26 (9%) | 0.39 |
| BOS (5 years) | 4 (24%) | 51 (22%) | 0.77 |
| Arrhythmia | 5 (26.3%) | 80 (28%) | 0.86 |
| Arrhythmia requiring treatment | 0 | 33 (12%) | 0.24 |
| RBC (units) | 1 (0;2) | 0 (0;4) | 0.68 |
| Platelets (units) | 0 (0;1) | 0 (0;1) | 0.30 |
| FFP (units) | 0 (0;2) | 0 (0;2) | 0.98 |
| Re-exploration | 0 | 19 (7%) | 0.62 |
| Infection | 7 (37%) | 19 (32%) | 0.65 |
| Pulmonary | 6 (32%) | 82 (29%) | 0.81 |
| Extrapulmonary | 2 (11%) | 28 (10%) | 0.70 |
ECMO: extracorporeal membrane oxygenation; BOS: bronchiolitis obliterans syndrome; RBC: red blood cell; FFP: fresh frozen plasma.
Results after propensity score matching
The direct comparison of the two groups revealed statistically significant differences in a number of preoperative variables that might have biased patient's outcome. To minimize the potential effects of selection bias on patient characteristics, we performed an additional analysis using one-to-three propensity score matching with 76 patients remaining for the analysis. The matching was performed based on donor and recipient characteristics that were statistically different between the two groups in the analysis of the entire cohort: donor age, gender and height as well as smoking history and extent of smoking. Unlike the variability in the baseline characteristics of both the groups in the entire population, these two propensity-matched groups were well balanced, and no significant differences were observed in donor and recipient baseline characteristics.
| After propensity matching (1:3) . | (Cannabis = 19) . | (Control = 57) . | P-value . |
|---|---|---|---|
| PaO2/FiO2 ratio on arrival | 307 ± 110 | 337 ± 147 | 0.40 |
| PaO2/FiO2 ratio 24 h | 387 ± 138 | 344 ± 115 | 0.18 |
| PaO2/FiO2 ratio 48 h | 369 ± 139 | 361 ± 109 | 0.78 |
| PaO2/FiO2 ratio 72 h | 330 ± 84 | 373 ± 130 | 0.15 |
| Ventilation (h) | 32 (16;86) | 33 (22;224) | 0.40 |
| ICU stay (days) | 5 (3;13) | 7 (3;20) | 0.73 |
| Hospital stay (days) | 26 (22;51) | 32 (23;54) | 0.32 |
| ECMO | 0 | 6 (11%) | 0.33 |
| BOS | 4 (24%) | 12 (23%) | 1.00 |
| PGD Grade 3 | |||
| PGD on arrival | 3 (16%) | 8 (14%) | 1.00 |
| PGD 24 h | 1 (5%) | 6 (11%) | 0.67 |
| PGD 48 h | 2 (11%) | 3 (5%) | 0.70 |
| PGD 72 h | 0 | 72 (6%) | 0.56 |
| Rejection | 0.12 | ||
| A0 | 16 (82%) | 41 (72%) | |
| A1 | 0 | 7 (12%) | |
| A2 | 2 (11%) | 9 (16%) | |
| A3 | 1 (5%) | 0 | |
| After propensity matching (1:3) . | (Cannabis = 19) . | (Control = 57) . | P-value . |
|---|---|---|---|
| PaO2/FiO2 ratio on arrival | 307 ± 110 | 337 ± 147 | 0.40 |
| PaO2/FiO2 ratio 24 h | 387 ± 138 | 344 ± 115 | 0.18 |
| PaO2/FiO2 ratio 48 h | 369 ± 139 | 361 ± 109 | 0.78 |
| PaO2/FiO2 ratio 72 h | 330 ± 84 | 373 ± 130 | 0.15 |
| Ventilation (h) | 32 (16;86) | 33 (22;224) | 0.40 |
| ICU stay (days) | 5 (3;13) | 7 (3;20) | 0.73 |
| Hospital stay (days) | 26 (22;51) | 32 (23;54) | 0.32 |
| ECMO | 0 | 6 (11%) | 0.33 |
| BOS | 4 (24%) | 12 (23%) | 1.00 |
| PGD Grade 3 | |||
| PGD on arrival | 3 (16%) | 8 (14%) | 1.00 |
| PGD 24 h | 1 (5%) | 6 (11%) | 0.67 |
| PGD 48 h | 2 (11%) | 3 (5%) | 0.70 |
| PGD 72 h | 0 | 72 (6%) | 0.56 |
| Rejection | 0.12 | ||
| A0 | 16 (82%) | 41 (72%) | |
| A1 | 0 | 7 (12%) | |
| A2 | 2 (11%) | 9 (16%) | |
| A3 | 1 (5%) | 0 | |
ICU: intensive care unit; ECMO: extracorporeal membrane oxygenation; BOS: bronchiolitis obliterans syndrome; PGD: primary graft dysfuntion.
| After propensity matching (1:3) . | (Cannabis = 19) . | (Control = 57) . | P-value . |
|---|---|---|---|
| PaO2/FiO2 ratio on arrival | 307 ± 110 | 337 ± 147 | 0.40 |
| PaO2/FiO2 ratio 24 h | 387 ± 138 | 344 ± 115 | 0.18 |
| PaO2/FiO2 ratio 48 h | 369 ± 139 | 361 ± 109 | 0.78 |
| PaO2/FiO2 ratio 72 h | 330 ± 84 | 373 ± 130 | 0.15 |
| Ventilation (h) | 32 (16;86) | 33 (22;224) | 0.40 |
| ICU stay (days) | 5 (3;13) | 7 (3;20) | 0.73 |
| Hospital stay (days) | 26 (22;51) | 32 (23;54) | 0.32 |
| ECMO | 0 | 6 (11%) | 0.33 |
| BOS | 4 (24%) | 12 (23%) | 1.00 |
| PGD Grade 3 | |||
| PGD on arrival | 3 (16%) | 8 (14%) | 1.00 |
| PGD 24 h | 1 (5%) | 6 (11%) | 0.67 |
| PGD 48 h | 2 (11%) | 3 (5%) | 0.70 |
| PGD 72 h | 0 | 72 (6%) | 0.56 |
| Rejection | 0.12 | ||
| A0 | 16 (82%) | 41 (72%) | |
| A1 | 0 | 7 (12%) | |
| A2 | 2 (11%) | 9 (16%) | |
| A3 | 1 (5%) | 0 | |
| After propensity matching (1:3) . | (Cannabis = 19) . | (Control = 57) . | P-value . |
|---|---|---|---|
| PaO2/FiO2 ratio on arrival | 307 ± 110 | 337 ± 147 | 0.40 |
| PaO2/FiO2 ratio 24 h | 387 ± 138 | 344 ± 115 | 0.18 |
| PaO2/FiO2 ratio 48 h | 369 ± 139 | 361 ± 109 | 0.78 |
| PaO2/FiO2 ratio 72 h | 330 ± 84 | 373 ± 130 | 0.15 |
| Ventilation (h) | 32 (16;86) | 33 (22;224) | 0.40 |
| ICU stay (days) | 5 (3;13) | 7 (3;20) | 0.73 |
| Hospital stay (days) | 26 (22;51) | 32 (23;54) | 0.32 |
| ECMO | 0 | 6 (11%) | 0.33 |
| BOS | 4 (24%) | 12 (23%) | 1.00 |
| PGD Grade 3 | |||
| PGD on arrival | 3 (16%) | 8 (14%) | 1.00 |
| PGD 24 h | 1 (5%) | 6 (11%) | 0.67 |
| PGD 48 h | 2 (11%) | 3 (5%) | 0.70 |
| PGD 72 h | 0 | 72 (6%) | 0.56 |
| Rejection | 0.12 | ||
| A0 | 16 (82%) | 41 (72%) | |
| A1 | 0 | 7 (12%) | |
| A2 | 2 (11%) | 9 (16%) | |
| A3 | 1 (5%) | 0 | |
ICU: intensive care unit; ECMO: extracorporeal membrane oxygenation; BOS: bronchiolitis obliterans syndrome; PGD: primary graft dysfuntion.
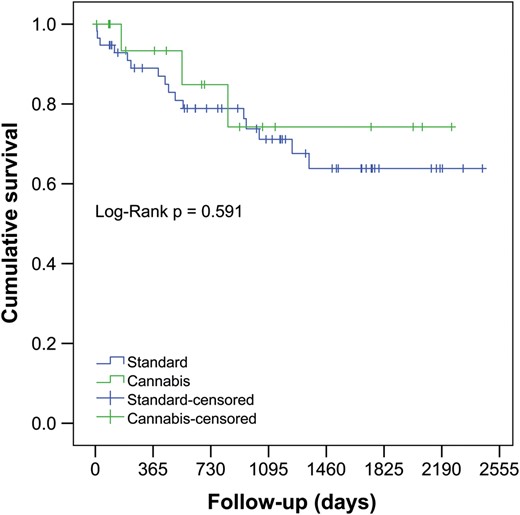
Kaplan–Meier estimate for overall cumulative survival in patients receiving lungs from cannabis smokers versus control donor group.
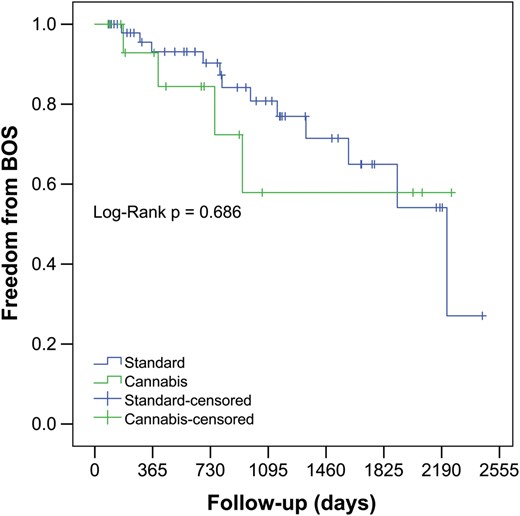
Kaplan–Meier estimate for freedom from bronchiolitis obliterans syndrome in patients receiving lungs from cannabis smokers versus control donor group. BOS: bronchiolitis obliterans syndrome.
Comment
The specific impact of smoking cannabis is difficult to assess precisely and to distinguish from the effect of tobacco as not only mostly they are smoked together but also due to the fact that cannabis smokers are usually tobacco smokers. Cannabis is considered potentially hazardous as its smoke contains many of the same chemicals as tobacco smoke in addition to 60 cannabinoid compounds [11]. In addition, Cannabis smoking results in greater delivery of tar to the lungs, because it is associated with prolonged and deeper inhalation and longer breath-holding times [4, 12]. Moreover, the cannabis cigarettes are unfiltered, smoked to a shorter butt length and at a higher combustion temperature [13]. The overall population incidence of cannabis is 2.5% worldwide, and the percentage of cannabis use in the donors in our study was 5.6. Donors in the ‘cannabis’ group were significantly younger compared with those in the ‘control’ group (30 vs 43 years). Additionally, the prevalence of cannabis smoking is generally higher in western population. A selection bias towards the young and the western European population which constituted majority of donor population of this study could be the reason for higher incidence of cannabis smoking in our donors.
Hazards of cannabis smoking
Cannabis smoking may be associated with airway inflammation and increased incidence of pulmonary infections. Cannabinoid acts as an immune modulator through endocannabinoid receptors present in many pathways of the immune system; affects the functioning of immune cells such as B and T lymphocytes and natural killer cells; can alter the expression of many cytokines such as interleukin (IL)-6, IL-8, IL-10, IL-12, tumour necrosis factor-alpha and interferon-gamma and impairs alveolar macrophage’s ability to ingest and destroy infectious organisms [14]. In some case reports, pulmonary infections are attributed to the contamination of cannabis by fungi and to the fact that cannabis is smoked without a filter [15].
Habitual cannabis smokers report a wide range of symptoms such as cough, dyspnoea, sputum production, wheezing, pharyngitis, hoarseness of voice and worsening of asthma symptoms [16]. Symptoms are comparable to the symptoms exhibited by tobacco smokers who have smoked for at least 10 years. Cannabis may be a risk factor for the development of bullous disease and pneumothorax [17, 18]. Although the exact mechanism of bulla formation in cannabis smokers is not known, it could be related to the customs like deep inhalation to hold smoke in the lung, performing a Valsalva manoeuvre [4].
Some major case–control studies have failed to demonstrate any association between cannabis smoking and cancer [19, 20]. Results from pooled analyses (2159 lung cancer cases and 2985 controls) from six case-control studies in the USA, Canada, UK and New Zealand within the International Lung Cancer Consortium provide little evidence for an increased risk of lung cancer among habitual or long-term cannabis smokers, although the possibility of potential adverse effect for heavy consumption cannot be excluded [21]. However, two large case–control studies with adjusted tobacco smoking found that the heavy cannabis use increased the risk of lung cancer more than 2- to 3-fold [22, 23]. In our previous work, we have demonstrated that the history and extent of donor smoking do not significantly affect early and mid-term outcomes after LTx, and the donor lungs from even heavy smokers may not per se contraindicate LTx [24]. The ill-effects of cannabis smoking might depend on the duration of exposure, the age, sex and ethnicity of the lung donors. The cannabis smoke-related lung injury is quite variable as mentioned above, and its extent varies significantly between the published studies.
History of cannabis smoking in donors, although, per se is not a contraindication of LTx, due to its potential hazard to the lung including emphysema, malignancy and poor survival; it seems the transplant programmes are sceptical about accepting such offers. History of cannabis smoking along with heavy tobacco smoking and high age further makes the offer unattractive. In the present study, the bias towards declining the offers with a history of cannabis smoking cannot be ruled out, as ‘logistical’ reason and ‘no suitable recipient’ reasons for declining these offers seemed fairly common. Usually, the donors with a history of cannabis smoking are young and moreover due to that the number of pack-years they have smoked is also less. The donors in the present study as well the donors from the ‘cannabis’ group were significantly younger and had significantly less number of pack-years in their smoking history compared with those in the control group. This makes the lung offer more lucrative as it assures better short- and long-term outcomes after LTx. Utilization of lungs from cannabis smoking donor constitutes a valuable source to expand the donor organ pool and decrease mortality on the waiting list; however, a caution is advised at the time of organ procurement. It is essential to determine the cannabis smoking pack-years depending on age, duration of smoking and duration of cannabis abuse. It is not always that the family members present at the time of organ donation know about drug abuse by the donor. Heavy cannabis use—more than two joint-years—may increase the risk of lung cancer more than 2- to 3-fold. Chest X-ray should be reviewed carefully for the signs of emphysema and tumour. A meticulous bronchoscopy should be performed to look for any evidence of tumour growth and airway infection. During direct examination of organs, attention should be paid particularly to the signs of bullous emphysema, blebs, anthracosis, deflation and hilar lymph node enlargement. These signs give a rough idea about the extent of lung damage due to smoking and cannabis exposure.
Limitations of the study
The study cohort was small. We cannot exclude bias and confounding in terms of patient selection as this was a non-blinded study of LTx at a single medical centre. As with most single-centre LTx analyses, study power was limited. Several variables in baseline characteristics and outcomes did not reach statistical significance due to the small study cohort.
CONCLUSIONS
Cannabis smoking can have a negative impact on respiratory health in terms of infection, obstructive airways disease, emphysema or the development of lung cancer. Its extent may vary significantly depending on the duration of exposure. The history of donor cannabis smoking does not appear to affect early and mid-term outcomes after LTx and potentially improve the donor pool. However, it is essential to assess the donor lungs for the signs of infection, emphysema and carcinoma. History of cannabis smoking in cadaveric lung donors should not be per se considered a contraindication for lung donation.
Conflict of interest: none disclosed.
REFERENCES
Author notes
The first two authors contributed equally to this study.




