-
PDF
- Split View
-
Views
-
Cite
Cite
Jolanda Kluin, David R. Koolbergen, Vladimir Sojak, Mark G. Hazekamp, Valve-sparing root replacement in children , European Journal of Cardio-Thoracic Surgery, Volume 50, Issue 3, September 2016, Pages 476–481, https://doi.org/10.1093/ejcts/ezw096
Close - Share Icon Share
Abstract
In children, words of caution have been raised about valve-sparing procedures especially regarding the valve-remodelling technique. This study reviewed our experience with the valve-sparing reimplantation technique in children.
All consecutive paediatric (<18 years) patients who underwent valve-sparing root replacement in our centre in the past 12.5 years were retrospectively analysed.
Nineteen patients (median age 13.2 years, 10 months to 17.9 years) underwent valve-sparing root replacement using the reimplantation technique. Seventeen had connective tissue disease. An adult-sized vascular prosthesis could be implanted in all cases. Additional cusp repair was required in 3 patients. Follow-up was 4.4 ± 3.8 years. There was no perioperative mortality and one late death. Of the 3 patients that needed cusp repair, 1 developed Grade 3 aortic valve regurgitation (AR) and required aortic valve replacement and 2 had Grade 1 AR. Ninety-four percent of the other patients had Grade 0 AR at latest follow-up, 1 patient (6%) had Grade 1 AR.
Our data show that valve-sparing root surgery using the reimplantation technique can be performed safely in children. Mid-term follow-up yields stable and favourable results. When leaflet reconstruction is necessary on top of the reimplantation procedure, rate of recurrent AR seems to be higher.
INTRODUCTION
In the adult patient population, valve-sparing root replacement is gaining more and more attention due to the reported good results from expert centres [ 1–3 ]. In children, words of caution have been raised about valve-sparing procedures especially regarding the valve-remodelling technique. This technique yields high failure rates and should probably be abandoned in the paediatric population [ 4 ]. Implanting a composite graft therefore is still popular for aortic root dilatation in children. However, valve-sparing procedures promise excellent quality of life by avoiding anticoagulation [ 5 ]. This is of particular interest in the paediatric population.
In the present study, we present our retrospective single-centre experience with the valve-sparing reimplantation technique in children.
PATIENTS AND METHODS
In this report, we retrospectively analysed all consecutive paediatric (<18 years) patients who underwent valve-sparing root replacement in our centre in the past 12.5 years.
From September 2003 to March 2015, 19 patients under the age of 18 underwent valve-sparing root replacement at our centre for congenital heart disease Amsterdam–Leiden (CAHAL).
Perioperative and most recent outpatient visit data were compiled from chart review. On preoperative, perioperative and most recent echocardiograms, the aortic annulus and maximum root diameters were measured. The corresponding z -scores were calculated as previously described [ 6 ]. Aortic valve regurgitation (AR) was scored as 0–4 (none or trivial, mild, mild–moderate, moderate–severe or severe). Continuous variables are presented as mean ± standard deviation or median with ranges. The probability of freedom from greater than mild AR was estimated according to the Kaplan–Meier method.
RESULTS
Preoperative characteristics
Patient characteristics are given in Tables 1 and 2 . The median age at the time of operation was 13.2 years (range, 10 months to 17.9 years) and the median weight was 51.6 kg (range, 12–89 kg). There were 4 female and 15 male patients. All but 1 patient underwent an elective procedure. One patient (nr 16, 8 years) underwent an emergency procedure because of acute aortic dissection. Besides a bicuspid aortic valve, he had an undiagnosed coarctation and related severe (untreated) hypertension during years. In 1 patient (nr 9), the indication for surgery was severe AR of a bicuspid aortic valve. In all other patients, the indication for surgery was progressive aneurysmal dilatation of the aortic root; all these patients had a tricuspid aortic valve. Preoperative mean aortic annulus diameter was 24.4 ± 4.0 mm ( z -score, +2.4 ± 0.7). Preoperative mean maximum root diameter was 44.4 ± 8.4 mm ( z -score +4.9 ± 2.0). Only the patient with severe AR had a sinus Valsalva z -score of 1.2, and all other patients had severe dilatation of the aortic root (Fig. 1 ). Of these patients, 5 patients had Grade 1 AR preoperatively, 13 patients had no or trivial AR preoperatively. Seventeen of 19 patients had genetically proven associated connective tissue disease (12 Marfan syndrome, 3 Loeys–Dietz syndrome, 2 Shprintzen–Goldberg).
| Patient . | Sex . | Diagnosis . | Year of operation . | Age (years) . | Sinus (mm) . | Sinus ( z -score) . | Sinus (ASI) . | Graft size (mm) . | Leaflet repair . | FU (years) . | Late AR (grade) . | Reoperation . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Marfan | 2003 | 15.8 | NA | NA | NA | 28 a | – | 11.5 | 0 | Aneurysm b |
| 2 | M | Loeys–Dietz | 2004 | 11.4 | 40 | +5.1 | 3.39 | 26 a | – | 11.3 | 0 | – |
| 3 | M | Marfan | 2005 | 17.6 | 50 | +4.6 | 2.42 | 26 V | – | 9.9 | 0 | Coronary c |
| 4 | F | Marfan | 2005 | 16.3 | 47 | +5.8 | 2.80 | 28 a | – | 9.3 | 0 | – |
| 5 | M | Marfan | 2006 | 13.4 | 44 | +3.3 | 2.15 | 26 a | – | 1.3 d | 0 | – |
| 6 | M | Marfan | 2008 | 4.6 | 36 | +6.3 | 4.86 | 20 e | – | 6.6 | 0 | – |
| 7 | M | Marfan | 2009 | 13.6 | 41 | +2.9 | 2.11 | 28 V | NCC f | 5.6 | 3 | Bentall |
| 8 | M | Marfan | 2009 | 0.9 | 35 | NA | NA | 20 e | – | 5.5 | 0 | – |
| 9 | M | BAV | 2009 | 17.8 | 36 | +1.2 | 1.71 | 26 V | – | 5.5 | 0 | – |
| 10 | M | Loeys–Dietz | 2010 | 11.1 | 38 | +5.3 | 3.73 | 24 V | – | 4.4 | 0 | Aneurysm b |
| 11 | M | Marfan | 2012 | 14.4 | 46 | +5.0 | 2.89 | 28 V | RCC g | 3.0 | 1 | – |
| 12 | F | Marfan | 1012 | 17.9 | 44 | +5.1 | 2.63 | 28 V | – | 2.8 | 0 | – |
| 13 | M | Loeys–Dietz | 2012 | 5.7 | 35 | +5.1 | 3.89 | 26 V | – | 2.2 | 0 | – |
| 14 | M | Marfan | 2013 | 14.4 | 49 | +5.0 | 2.69 | 28 V | – | 1.9 | 1 | – |
| 15 | F | Marfan | 2014 | 16.7 | 46 | +4.4 | 2.15 | 28 V | – | 0.8 | 0 | – |
| 16 | M | Coarct + BAV h | 2014 | 8.7 | 68 | +11.1 | 6.67 | 22 e | – | 0.8 | 0 | – |
| 17 | M | Marfan | 2014 | 17.9 | 54 | +5.2 | 2.55 | 30 V | – | 0.6 | 0 | – |
| 18 | M | Shprintzen-G | 2014 | 16.9 | 52 | +5.5 | 2.83 | 28 V | NCC f | 0.3 | 1 | – |
| 19 | M | Shprintzen-G | 2015 | 15.4 | 38 | +3.2 | 2.45 | 30 V | – | 0.1 | 0 | – |
| Patient . | Sex . | Diagnosis . | Year of operation . | Age (years) . | Sinus (mm) . | Sinus ( z -score) . | Sinus (ASI) . | Graft size (mm) . | Leaflet repair . | FU (years) . | Late AR (grade) . | Reoperation . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Marfan | 2003 | 15.8 | NA | NA | NA | 28 a | – | 11.5 | 0 | Aneurysm b |
| 2 | M | Loeys–Dietz | 2004 | 11.4 | 40 | +5.1 | 3.39 | 26 a | – | 11.3 | 0 | – |
| 3 | M | Marfan | 2005 | 17.6 | 50 | +4.6 | 2.42 | 26 V | – | 9.9 | 0 | Coronary c |
| 4 | F | Marfan | 2005 | 16.3 | 47 | +5.8 | 2.80 | 28 a | – | 9.3 | 0 | – |
| 5 | M | Marfan | 2006 | 13.4 | 44 | +3.3 | 2.15 | 26 a | – | 1.3 d | 0 | – |
| 6 | M | Marfan | 2008 | 4.6 | 36 | +6.3 | 4.86 | 20 e | – | 6.6 | 0 | – |
| 7 | M | Marfan | 2009 | 13.6 | 41 | +2.9 | 2.11 | 28 V | NCC f | 5.6 | 3 | Bentall |
| 8 | M | Marfan | 2009 | 0.9 | 35 | NA | NA | 20 e | – | 5.5 | 0 | – |
| 9 | M | BAV | 2009 | 17.8 | 36 | +1.2 | 1.71 | 26 V | – | 5.5 | 0 | – |
| 10 | M | Loeys–Dietz | 2010 | 11.1 | 38 | +5.3 | 3.73 | 24 V | – | 4.4 | 0 | Aneurysm b |
| 11 | M | Marfan | 2012 | 14.4 | 46 | +5.0 | 2.89 | 28 V | RCC g | 3.0 | 1 | – |
| 12 | F | Marfan | 1012 | 17.9 | 44 | +5.1 | 2.63 | 28 V | – | 2.8 | 0 | – |
| 13 | M | Loeys–Dietz | 2012 | 5.7 | 35 | +5.1 | 3.89 | 26 V | – | 2.2 | 0 | – |
| 14 | M | Marfan | 2013 | 14.4 | 49 | +5.0 | 2.69 | 28 V | – | 1.9 | 1 | – |
| 15 | F | Marfan | 2014 | 16.7 | 46 | +4.4 | 2.15 | 28 V | – | 0.8 | 0 | – |
| 16 | M | Coarct + BAV h | 2014 | 8.7 | 68 | +11.1 | 6.67 | 22 e | – | 0.8 | 0 | – |
| 17 | M | Marfan | 2014 | 17.9 | 54 | +5.2 | 2.55 | 30 V | – | 0.6 | 0 | – |
| 18 | M | Shprintzen-G | 2014 | 16.9 | 52 | +5.5 | 2.83 | 28 V | NCC f | 0.3 | 1 | – |
| 19 | M | Shprintzen-G | 2015 | 15.4 | 38 | +3.2 | 2.45 | 30 V | – | 0.1 | 0 | – |
ASI: aortic size index (= aortic diameter divided by body surface area); FU: follow-up; BAV: bicuspid aortic valve; Shprintzen-G: Shprintzen–Goldberg; NA: not available; V: Valsalva; NCC: non-coronary cusp; RCC: right coronary cusp; AR: aortic valve regurgitation.
a A straight graft 24 mm was placed distally to fabricate sinuses.
b Aneurysm of the native distal ascending aorta and arch.
c Aneurysm of the right coronary artery.
d Patient 5 died 1.3 years after the operation presumably of ventricular arrhythmia.
e Sutures were used to tailor neosinuses in the straight graft.
f Central plication stitches of the non-coronary cusp.
g Closure of a fenestration in the right coronary cusp.
h Patient 16 presented with acute aortic dissection.
| Patient . | Sex . | Diagnosis . | Year of operation . | Age (years) . | Sinus (mm) . | Sinus ( z -score) . | Sinus (ASI) . | Graft size (mm) . | Leaflet repair . | FU (years) . | Late AR (grade) . | Reoperation . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Marfan | 2003 | 15.8 | NA | NA | NA | 28 a | – | 11.5 | 0 | Aneurysm b |
| 2 | M | Loeys–Dietz | 2004 | 11.4 | 40 | +5.1 | 3.39 | 26 a | – | 11.3 | 0 | – |
| 3 | M | Marfan | 2005 | 17.6 | 50 | +4.6 | 2.42 | 26 V | – | 9.9 | 0 | Coronary c |
| 4 | F | Marfan | 2005 | 16.3 | 47 | +5.8 | 2.80 | 28 a | – | 9.3 | 0 | – |
| 5 | M | Marfan | 2006 | 13.4 | 44 | +3.3 | 2.15 | 26 a | – | 1.3 d | 0 | – |
| 6 | M | Marfan | 2008 | 4.6 | 36 | +6.3 | 4.86 | 20 e | – | 6.6 | 0 | – |
| 7 | M | Marfan | 2009 | 13.6 | 41 | +2.9 | 2.11 | 28 V | NCC f | 5.6 | 3 | Bentall |
| 8 | M | Marfan | 2009 | 0.9 | 35 | NA | NA | 20 e | – | 5.5 | 0 | – |
| 9 | M | BAV | 2009 | 17.8 | 36 | +1.2 | 1.71 | 26 V | – | 5.5 | 0 | – |
| 10 | M | Loeys–Dietz | 2010 | 11.1 | 38 | +5.3 | 3.73 | 24 V | – | 4.4 | 0 | Aneurysm b |
| 11 | M | Marfan | 2012 | 14.4 | 46 | +5.0 | 2.89 | 28 V | RCC g | 3.0 | 1 | – |
| 12 | F | Marfan | 1012 | 17.9 | 44 | +5.1 | 2.63 | 28 V | – | 2.8 | 0 | – |
| 13 | M | Loeys–Dietz | 2012 | 5.7 | 35 | +5.1 | 3.89 | 26 V | – | 2.2 | 0 | – |
| 14 | M | Marfan | 2013 | 14.4 | 49 | +5.0 | 2.69 | 28 V | – | 1.9 | 1 | – |
| 15 | F | Marfan | 2014 | 16.7 | 46 | +4.4 | 2.15 | 28 V | – | 0.8 | 0 | – |
| 16 | M | Coarct + BAV h | 2014 | 8.7 | 68 | +11.1 | 6.67 | 22 e | – | 0.8 | 0 | – |
| 17 | M | Marfan | 2014 | 17.9 | 54 | +5.2 | 2.55 | 30 V | – | 0.6 | 0 | – |
| 18 | M | Shprintzen-G | 2014 | 16.9 | 52 | +5.5 | 2.83 | 28 V | NCC f | 0.3 | 1 | – |
| 19 | M | Shprintzen-G | 2015 | 15.4 | 38 | +3.2 | 2.45 | 30 V | – | 0.1 | 0 | – |
| Patient . | Sex . | Diagnosis . | Year of operation . | Age (years) . | Sinus (mm) . | Sinus ( z -score) . | Sinus (ASI) . | Graft size (mm) . | Leaflet repair . | FU (years) . | Late AR (grade) . | Reoperation . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Marfan | 2003 | 15.8 | NA | NA | NA | 28 a | – | 11.5 | 0 | Aneurysm b |
| 2 | M | Loeys–Dietz | 2004 | 11.4 | 40 | +5.1 | 3.39 | 26 a | – | 11.3 | 0 | – |
| 3 | M | Marfan | 2005 | 17.6 | 50 | +4.6 | 2.42 | 26 V | – | 9.9 | 0 | Coronary c |
| 4 | F | Marfan | 2005 | 16.3 | 47 | +5.8 | 2.80 | 28 a | – | 9.3 | 0 | – |
| 5 | M | Marfan | 2006 | 13.4 | 44 | +3.3 | 2.15 | 26 a | – | 1.3 d | 0 | – |
| 6 | M | Marfan | 2008 | 4.6 | 36 | +6.3 | 4.86 | 20 e | – | 6.6 | 0 | – |
| 7 | M | Marfan | 2009 | 13.6 | 41 | +2.9 | 2.11 | 28 V | NCC f | 5.6 | 3 | Bentall |
| 8 | M | Marfan | 2009 | 0.9 | 35 | NA | NA | 20 e | – | 5.5 | 0 | – |
| 9 | M | BAV | 2009 | 17.8 | 36 | +1.2 | 1.71 | 26 V | – | 5.5 | 0 | – |
| 10 | M | Loeys–Dietz | 2010 | 11.1 | 38 | +5.3 | 3.73 | 24 V | – | 4.4 | 0 | Aneurysm b |
| 11 | M | Marfan | 2012 | 14.4 | 46 | +5.0 | 2.89 | 28 V | RCC g | 3.0 | 1 | – |
| 12 | F | Marfan | 1012 | 17.9 | 44 | +5.1 | 2.63 | 28 V | – | 2.8 | 0 | – |
| 13 | M | Loeys–Dietz | 2012 | 5.7 | 35 | +5.1 | 3.89 | 26 V | – | 2.2 | 0 | – |
| 14 | M | Marfan | 2013 | 14.4 | 49 | +5.0 | 2.69 | 28 V | – | 1.9 | 1 | – |
| 15 | F | Marfan | 2014 | 16.7 | 46 | +4.4 | 2.15 | 28 V | – | 0.8 | 0 | – |
| 16 | M | Coarct + BAV h | 2014 | 8.7 | 68 | +11.1 | 6.67 | 22 e | – | 0.8 | 0 | – |
| 17 | M | Marfan | 2014 | 17.9 | 54 | +5.2 | 2.55 | 30 V | – | 0.6 | 0 | – |
| 18 | M | Shprintzen-G | 2014 | 16.9 | 52 | +5.5 | 2.83 | 28 V | NCC f | 0.3 | 1 | – |
| 19 | M | Shprintzen-G | 2015 | 15.4 | 38 | +3.2 | 2.45 | 30 V | – | 0.1 | 0 | – |
ASI: aortic size index (= aortic diameter divided by body surface area); FU: follow-up; BAV: bicuspid aortic valve; Shprintzen-G: Shprintzen–Goldberg; NA: not available; V: Valsalva; NCC: non-coronary cusp; RCC: right coronary cusp; AR: aortic valve regurgitation.
a A straight graft 24 mm was placed distally to fabricate sinuses.
b Aneurysm of the native distal ascending aorta and arch.
c Aneurysm of the right coronary artery.
d Patient 5 died 1.3 years after the operation presumably of ventricular arrhythmia.
e Sutures were used to tailor neosinuses in the straight graft.
f Central plication stitches of the non-coronary cusp.
g Closure of a fenestration in the right coronary cusp.
h Patient 16 presented with acute aortic dissection.
| n = 19 . | n (mean) . | % (SD) . |
|---|---|---|
| Diagnosis | ||
| Loeys–Dietz | 3 | 15.8% |
| Marfan | 12 | 63.2% |
| BAV | 1 | 5.3% |
| Coarctation + BAV | 1 | 5.3% |
| Shprintzen–Goldberg | 2 | 10.5% |
| Age | 13.2 | ±5.0 |
| Length (cm) | 171.6 | ±27.2 |
| Weight (kg) | 51.6 | ±23.6 |
| BSA (m 2 ) | 1.6 | ±0.5 |
| Annulus diameter (mm) | 24.4 | ±4.1 |
| Sinus of Valsalva diameter (mm) | 44.4 | ±8.4 |
| z -score annulus | +2.4 | ±0.7 |
| z -score sinus | +4.9 | ±2.0 |
| Aortic valve | ||
| Tricuspid | 17 | 89.5% |
| Bicuspid | 2 | 10.5% |
| n = 19 . | n (mean) . | % (SD) . |
|---|---|---|
| Diagnosis | ||
| Loeys–Dietz | 3 | 15.8% |
| Marfan | 12 | 63.2% |
| BAV | 1 | 5.3% |
| Coarctation + BAV | 1 | 5.3% |
| Shprintzen–Goldberg | 2 | 10.5% |
| Age | 13.2 | ±5.0 |
| Length (cm) | 171.6 | ±27.2 |
| Weight (kg) | 51.6 | ±23.6 |
| BSA (m 2 ) | 1.6 | ±0.5 |
| Annulus diameter (mm) | 24.4 | ±4.1 |
| Sinus of Valsalva diameter (mm) | 44.4 | ±8.4 |
| z -score annulus | +2.4 | ±0.7 |
| z -score sinus | +4.9 | ±2.0 |
| Aortic valve | ||
| Tricuspid | 17 | 89.5% |
| Bicuspid | 2 | 10.5% |
BAV: bicuspid aortic valve; BSA: body surface area; SD: standard deviation.
| n = 19 . | n (mean) . | % (SD) . |
|---|---|---|
| Diagnosis | ||
| Loeys–Dietz | 3 | 15.8% |
| Marfan | 12 | 63.2% |
| BAV | 1 | 5.3% |
| Coarctation + BAV | 1 | 5.3% |
| Shprintzen–Goldberg | 2 | 10.5% |
| Age | 13.2 | ±5.0 |
| Length (cm) | 171.6 | ±27.2 |
| Weight (kg) | 51.6 | ±23.6 |
| BSA (m 2 ) | 1.6 | ±0.5 |
| Annulus diameter (mm) | 24.4 | ±4.1 |
| Sinus of Valsalva diameter (mm) | 44.4 | ±8.4 |
| z -score annulus | +2.4 | ±0.7 |
| z -score sinus | +4.9 | ±2.0 |
| Aortic valve | ||
| Tricuspid | 17 | 89.5% |
| Bicuspid | 2 | 10.5% |
| n = 19 . | n (mean) . | % (SD) . |
|---|---|---|
| Diagnosis | ||
| Loeys–Dietz | 3 | 15.8% |
| Marfan | 12 | 63.2% |
| BAV | 1 | 5.3% |
| Coarctation + BAV | 1 | 5.3% |
| Shprintzen–Goldberg | 2 | 10.5% |
| Age | 13.2 | ±5.0 |
| Length (cm) | 171.6 | ±27.2 |
| Weight (kg) | 51.6 | ±23.6 |
| BSA (m 2 ) | 1.6 | ±0.5 |
| Annulus diameter (mm) | 24.4 | ±4.1 |
| Sinus of Valsalva diameter (mm) | 44.4 | ±8.4 |
| z -score annulus | +2.4 | ±0.7 |
| z -score sinus | +4.9 | ±2.0 |
| Aortic valve | ||
| Tricuspid | 17 | 89.5% |
| Bicuspid | 2 | 10.5% |
BAV: bicuspid aortic valve; BSA: body surface area; SD: standard deviation.
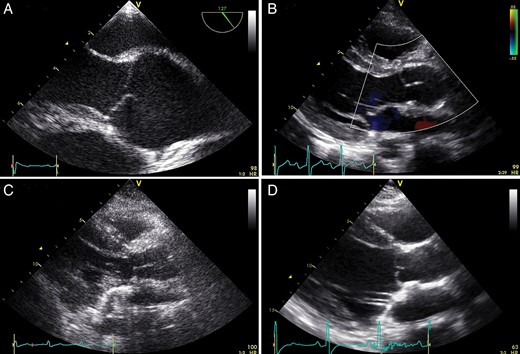
A + B : typical preoperative ( A ) and postoperative ( B ) echocardiographic image of a Marfan patient (14 years old, root diameter 46 mm, z -score +5, 28 mm Valsalva prosthesis, no AR). Note in echo B how root geometry is restored with the technique. There is a large coaptation height as well as effective height and no billowing or prolapse. C + D : echo images of the patient that developed Grade 3 AR, 4 years after the initial valve-sparing root replacement with plication of the non-coronary cusp (14 years old, root diameter 41 mm, z-score +2.9, 28 mm Valsalva prosthesis). ( C ) Postoperative echo after the initial operation with no prolapse of the cusps and no AR. ( D ) The echo image 4 years thereafter shows some billowing/prolapse of the cusps (and a Grade 3 excentric AR that cannot be seen on this echo still). Please note the smallish coaptation and effective height. Patient underwent a Bentall procedure. AR: aortic valve regurgitation.
Operative data
All patients underwent aortic valve-sparing root replacement using the reimplantation technique, generally as we described earlier for adolescent and adult patients [ 7 ] with some modifications for the very young. In the early years, a straight graft was used with a smaller diameter distally to it to fabricate sinuses as described by Demers and Miller [ 8 ]. In children who needed a smaller graft (20 or 22 mm), no neosinuses were created to avoid too small grafts in the ascending aorta. In more recent years, a Valsalva-type graft was used whenever possible with a modified reimplantation technique as described by Pacini et al (Table 1 ) [ 9 ]. In 3 patients, the required graft diameter was less than 24 (the smallest available Valsalva graft diameter). These 3 patients received a straight graft with a diameter of 20 mm ( n = 2) or 22 mm ( n = 1). In the 12 patients that received a Valsalva graft, diameters were 24 ( n = 1), 26 ( n = 3), 28 ( n = 6) and 30 mm ( n = 2). To anchor the prosthesis, a complete ring of braided polyester pledged mattress sutures was passed (between 9 and 13 sutures) under the annulus and commissures to the outside of the root in an almost horizontal plane. For younger patients with a graft size of <26 mm, 4–0 braided polyester sutures were used and for sizes >26 mm 2–0 sutures were used. For the internal proximal suture line, 4–0 or 5–0 polyprolene was used. In some cases, the distal anastomosis and/or the coronary buttons were reinforced with an autologous pericardial strip. Additional surgery was necessary in 6 patients (mitral valve reconstruction in all with additional tricuspid valve reconstruction in 3). The mean cardiopulmonary bypass time was 174 ± 33 min, the mean cross-clamp time was 135 ± 40 min. A second clamp session was necessary in 2 patients. In both patients, residual AR was the indication; in 1 patient (nr 14), all three central plication stitches were removed and in the other patient a fenestration of the right coronary cusp was closed (nr 11). In general, reconstruction of the leaflets was necessary in 3 patients. Two patients needed central plication of the non-coronary cusp (NCC) (nr 7 and 18), 1 the aforementioned closure of a fenestration. There were no operative deaths. AR at the end of the operation was Grade 0 in 18 patients and Grade 1 in 1 patient (nr 3).
Follow-up
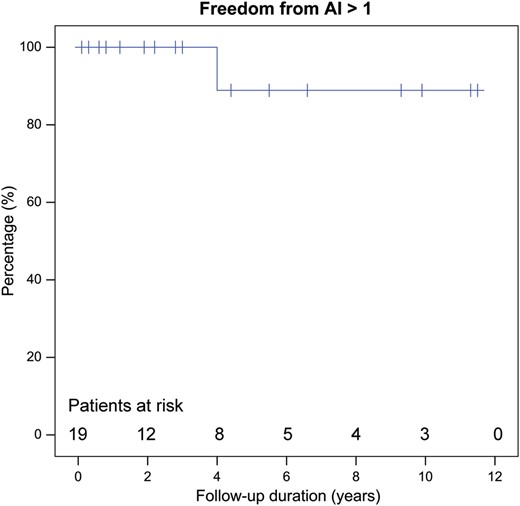
The mean follow-up time was 4.4 ± 3.8 years (Table 1 ). There was one late death (nr 5): this Marfan patient suddenly died 1.3 years after the operation at the age of 14. Autopsy showed no signs of AR or dissection. Most probably, the patient died from ventricular arrhythmia. There was only 1 patient that needed a reoperation for AR (Grade 3), 4 years after the valve-sparing root replacement (Tables 1 and 3 , Fig. 1 ). This was one of the 3 patients that needed leaflet reconstruction (central plication stitches of the NCC) at the initial operation. Three other patients were operated on a second time. Two patients developed aneurysm of the residual ascending aorta and the arch, the other patient developed an aneurysm of the right coronary artery. All were operated on uneventfully. Three patients have Grade 1 AR at latest follow-up. Two of them had additional leaflet reconstruction at the initial procedure. Of the patients that needed no leaflet reconstruction all but 1 have Grade 0 AR at latest follow-up. One patient is in Grade 1 AR. The actuarial freedom from greater than mild AR is depicted in Fig. 2 .
| n = 19 . | Preoperative . | Postoperative . | Follow-up . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| None or trivial | 13 | 68 | 18 | 95 | 15 | 79 |
| Mild (Grade 1) | 5 | 26 | 1 | 5 | 3 | 16 |
| Mild–moderate (Grade 2) | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate–severe (Grade 3) | 0 | 0 | 0 | 0 | 1 a | 5 |
| Severe (Grade 4) | 1 | 5 | 0 | 0 | 0 | 0 |
| n = 19 . | Preoperative . | Postoperative . | Follow-up . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| None or trivial | 13 | 68 | 18 | 95 | 15 | 79 |
| Mild (Grade 1) | 5 | 26 | 1 | 5 | 3 | 16 |
| Mild–moderate (Grade 2) | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate–severe (Grade 3) | 0 | 0 | 0 | 0 | 1 a | 5 |
| Severe (Grade 4) | 1 | 5 | 0 | 0 | 0 | 0 |
a This patient was reoperated.
| n = 19 . | Preoperative . | Postoperative . | Follow-up . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| None or trivial | 13 | 68 | 18 | 95 | 15 | 79 |
| Mild (Grade 1) | 5 | 26 | 1 | 5 | 3 | 16 |
| Mild–moderate (Grade 2) | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate–severe (Grade 3) | 0 | 0 | 0 | 0 | 1 a | 5 |
| Severe (Grade 4) | 1 | 5 | 0 | 0 | 0 | 0 |
| n = 19 . | Preoperative . | Postoperative . | Follow-up . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| None or trivial | 13 | 68 | 18 | 95 | 15 | 79 |
| Mild (Grade 1) | 5 | 26 | 1 | 5 | 3 | 16 |
| Mild–moderate (Grade 2) | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate–severe (Grade 3) | 0 | 0 | 0 | 0 | 1 a | 5 |
| Severe (Grade 4) | 1 | 5 | 0 | 0 | 0 | 0 |
a This patient was reoperated.
DISCUSSION
Our data show that valve-sparing root surgery using the reimplantation technique can be performed safely in children. Mid-term follow-up yields stable and favourable results.
There are no guidelines for the management of aortic aneurysms in children. In children, indications for root replacement in case of root aneurysm are based on extrapolations from older age groups as the reported incidence of catastrophic events below the age of 12 years is extremely low. At least, the absolute diameter thresholds for adults should be respected or progressive AR can be a reason to act [ 10 ]. In the adult guidelines, an absolute aortic diameter of >45 mm in patients with connective tissue disease and additional risk factors (family history of dissection or growth >2 mm/year according to the 2010 ESC guidelines for the management of grown-up congenital heart disease or >3 mm/year according to the 2014 ESC guidelines on the diagnosis and treatment of aortic disease) or 50 mm if there are no additional risk factors is an indication for surgery [ 11 , 12 ]. However, absolute diameters cannot be translated to the paediatric population. The indication of growth of the aneurysm by >2–3 mm/year is difficult in children as well, as there is a natural growth of the aorta in children. By novel measurements, Davies et al . [ 13 ] established that an aortic size index (ASI) of 2.75–4.25 cm/m 2 is associated with a moderate risk of rupture (8%/year), whereas an ASI of >4.25 cm/m 2 carries a high risk of rupture (20–25%/year). The study of Davies et al . included patients >6 years of age; however, it is not mentioned how many paediatric patients were included. Our study included 3 patients under the age of 6. Gaultier et al . [ 6 ] assessed the normograms for aortic root diameter in children using 2D echocardiography. We think that these z -scores are most useful in our study population and allow comparison of measurements for a given patient at different periods.
The indication for surgery in our series was a combination of absolute aortic diameter, z -score, rapid growth (rise in z -score rather than an absolute number of mm growth/year), type of connective tissue disorder and family history. We did not actually measure the ASI preoperatively in our population. In general, we can say that in Marfan patients we operated on whenever the aortic diameter exceeded 45 mm in case of additional risk factors (family history of dissection) or 50 mm if there were no additional risk factors. In most cases, this resulted in a z -score >4 or 5. In case of Loeys–Dietz syndrome, we operated when the diameter exceeded 35 mm in combination with a z -score of >3. When these thresholds are reached, an adult-sized prosthesis can be implanted in almost all cases, which makes valve-sparing root replacement an attractive and reasonable option in children. Our youngest patient was 10 months old and received a 20 mm graft. The single patient with a normal z -score (1.2), an ASI of 1.71 and an absolute aortic diameter of 36 mm, was operated on because of severe AR.
In recent years, four papers have emerged on valve-sparing root replacement in children. The largest series come from The John Hopkins Hospital in Baltimore [ 14 ]. In 2011, they reported on valve-sparing root replacement in 56 children during 12 years. In 2004, the Baltimore group reported on their earliest results [ 15 ]. They used the remodelling technique in all 14 children and they observed late AR in 3 patients. A year later, they reported on 19 children of which 14 underwent root remodelling and 5 a reimplantation procedure [ 16 ]. In their latest report form 2011, they report on 12 patients after remodelling and 44 after reimplantation procedure [ 14 ]. From 2002 on, they only performed the reimplantation procedure. There was one operative death and no reoperation in the reimplantation group.
Other reports came from the Sick Children Hospital in Paris (11 patients underwent root remodelling, 3 root implantation) [ 4 ], The German Heart Center in Munich (4 remodelling, 9 reimplantation) [ 7 ] and The Royal's Childrens Hospital in Melbourne (1 remodelling, 9 reimplantation) [ 17 ]. All concluded that root remodelling is not suitable in children due to too high failure rate and that the mid-term results of the reimplantation technique seem favourable.
Our study is in accordance with this conclusion. In our institution, we have not used the remodelling technique, neither in adults, nor in children [ 18 ]. The addition of an extra-aortic ring to the remodelling technique (as described by Lansac) might resolve the problem of redilatation of the annulus [ 2 ]. We are not aware of the use of an extra-aortic ring in paediatric patients. In theory though, it should be possible. The rationale of using reimplantation with extra-aortic ring rather than reimplantation is the (theoretical) advantage of less stress on the aortic valve leaflets. If the reimplantation technique with an extra-aortic ring results in better long-term outcome when compared with the reimplantation technique hopefully will be addressed by the results that will come out of the AVIATOR registry, an international registry of patients with potentially reparable aortic valves [ 19 ]. At present, paediatric patients cannot be included in the AVIATOR registry yet. Children with aortic root aneurysm probably have the most severe forms of connective tissue disease and should therefore be strictly followed up. This should include regular echocardiography and computerized tomography or magnetic resonance imaging. In our study group, there was 1 Marfan patient who developed an aneurysm of the right coronary button. In adult patients, we mostly add a bovine pericardial strip when sewing the coronary buttons unto the graft, in children we thus far did not do that. In these children with severe connective tissue defects, it seems important to make the buttons not too big. The other 2 patients (1 Marfan, 1 Loeys–Dietz) developed an arch aneurysm distal to the root. During the initial operation, the distal aorta and arch looked fine. We think that a ‘preventive’ arch replacement should not be advocated because of its risk of neurological damage. All but 1 of our patients with connective tissue defects are on medication (14/16 on β Blockers, 6/16 on Angiotensin II antagonists).
Regarding the diameter of the prosthesis, we follow the recommendations of Cameron and Vricella [ 20 ]. While holding the commissural stay sutures in upward position and creating low pressure in the left ventricle using the left vent, coaptation of the cusps was installed and facilitated. Now, while using valve sizers and moving the stay sutures in- and outwards, we determined the diameter of the sinotubular junction that provides optimal leaflet apposition. The selected graft was consistently 4 mm larger, as the prosthesis was fitted outside of the aortic valve complex.
In our series, only 3 patients needed leaflet repair on top of the reimplantation procedure. Interestingly, none of these patients were in Grade 0 AR at latest follow-up. Moreover, the single patient that required reoperation because of recurrent AR had central plication of the NCC at the initial operation. In contrast, of the 16 patients that did not need leaflet repair, all but 1 have Grade 0 AR at latest follow-up, 1 had Grade 1 AR. It is remarkable in itself that none of the patients with a dilated root had more than mild AR (5 Grade 1, 13 no or trivial AR) preoperatively. Apparently, the leaflets also grow proportionally and thus pathologically. Following the valve-sparing root replacement, you actually do not want them to grow. Due to the relative short follow-up of the present studies, we do not know if leaflets that are fixed in a (Valsalva) graft grow or not. Because these children have severe phenotypes of the connective tissue disease (especially the very young ones), it might well be that the leaflets still grow after the operation. This might result in renewed prolapse and AR as was observed in 1 patient who needed a Bentall after 4 years and in whom there was no prolapse after the initial operation (Fig. 1 C + D). In aortic valve repair surgery, the Schäfers’ group introduced the calliper to measure effective coaptation height, as an effective coaptation height of ≥9 mm seems to be important in achieving stable results [ 1 ]. Although we use the calliper now in our adult patients undergoing aortic valve repair surgery, we have not used it in paediatric patients so far. On the basis of our results now, it might be favourable to use effective height measurement in paediatric patients who need additional leaflet reconstruction on top of the implantation procedure. It is not clear if the inherent variability in size impedes the use of the calliper. Surgeon experience and patient selection are important factors and relate to outcome following valve-sparing root replacement. In our opinion, large central fenestrations or the need to repair more than one cusp is a contraindication for valve-sparing root replacement. Moreover, in our institution, we as congenital surgeons also operate on grown ups with congenital heart disease patients including a considerable amount of adult patients with dilated aortic roots and connective tissue disease [ 18 ]. We strongly feel that this experience is important in achieving good results in children.
We did not include quality-of-life data in our report. However, the reported favourable quality-of-life scores in adult patients after valve repair when compared with valve replacement with a mechanical valve [ 5 ] probably is even more true in the paediatric population.
Study limitations
The main limitations of this study are its retrospective design and its inherent selection bias and the small numbers of patients. Within the study period, there was 1 Marfan patient (17.5 years, no AR) in whom first the reimplantation technique was tried but who was converted to a Bentall procedure during initial surgery because too little coaptation was achieved. Furthermore, follow-up time is only long enough to report on mid-term results.
CONCLUSIONS
In conclusion, thresholds for root replacement in children are mostly reached when an adult-sized prosthesis can be implanted, which makes valve-sparing root replacement an attractive and reasonable option. Using the reimplantation technique, valve-sparing root replacement in our centre has stable and favourable intermediate outcome in children as young as 10 months. Surgical experience with valve-sparing root replacement in adult patients is helpful. When leaflet reconstruction is necessary on top of the reimplantation procedure, rate of recurrent AR seems to be higher.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr D. Barron(Birmingham, UK): I think you have shown very clearly that the valve sparing root is both safe and appropriate in children with these rare problems. The first thing I wanted to ask you, was about your technique, because the technique that Duke Cameron has described from Johns Hopkins is slightly different to maybe the Tirone David classical re-implantation technique. So which technique do you favour?
Dr Kluin: I do not know exactly what the technique is.
Dr Barron: Do you put a full reinforcement set of sutures below the annulus?
Dr Kluin: Yes.
Dr Barron: So do you feel that this is the right way to secure the region?
Dr Kluin: Yes. We never tried to put only 3.
Dr Barron: So the issue of the valve repair is interesting and so what lessons have you learned? You say, you found if you have to repair the leaflets they have not done quite so well. So are there some patterns of leaflet morphology you think that are not repairable, or that we should not try and repair?
Dr Kluin: Well, the one with fenestration of one of the cusps that is a leaflet thing. I think the other two, they neede a reconstruction because maybe the size of the graft was not appropriate, maybe a little bit too small so that you have a bit of a prolapse or the commissure was implanted a bit too low and then you have to plicate one of the cups to get them a bit higher. As you can see in our results, whenever you need it, there is a higher chance that you get recurrent AR.
Dr Barron: But you would still favour trying to repair all the valves?
Dr Kluin: Well, when there is one fenestration in one of the leaflets, we think we can; when there are more, we hesitate. But we have no experience with those valves, so maybe it is not very common in children.
Dr Barron: In this same time period, did you have any patients who you went directly to a Bentall and did not try and repair the valve?
Dr Kluin: No.
Dr Barron: You also had two patients who came back for reoperations because they developed further aneurysms of the ascending aorta. Do you think in retrospect, you may have been able to foresee these cases, should you maybe have had a more extensive repair the first time, or did the aorta look normal?
Dr Kluin: Yes, it is always a difficult decision, but the arch looked normal at the initial operation. So we decided not to do anything about it.
Dr Barron: Finally, there is a mixture of different underlying connective tissue disorders. Some of them are very aggressive like Loeys-Dietz, which have different recommended criteria as to when to intervene. So did you use different criteria according to the underlying pathology? Because I think several of your patients already had a root of sinus dimension of 45 mm.
Dr Kluin: Yes. Those were mainly the Marfan patients and what we also saw is that within the Marfan population, there are huge differences. One can have severe and rapid dilatation, as our youngest patient of 10 months old, but there were also Marfans that grow very slowly. So also within that group, there are differences.
Dr Barron: For Loeys-Dietz, would you go sooner?
Dr Kluin: Sooner, yes, yes.
Dr Barron: Do you have a particular message, you think, more than anything over what we have seen so far?
Dr Kluin: I myself have a lot of experience with valve sparing roots in adult patients, and I am also involved in the AVIATOR Registry. I do not know if you are familiar with that. It is an international registry, in which everyone can participate to include consecutive patients; and each year, there is echocardiographic follow up. Now, there is no congenital part of the AVIATOR. But I would very much love to introduce such a thing so that we can learn, not only from our own results, but from others, to see what patients are not suitable for repair, and in whom you have to implant a composite graft, and which patients you can repair. I think it is in the details. It is in the echocardiographic details, in the leaflets, and we can only come to a real good conclusion when we join efforts, I think.
Dr Barron: Thank you.
Dr Kluin: Also use of the caliper. I do not know if any one of you uses the calipers.
Dr Barron: For the height, yes.
Dr Kluin: Yes, for the effective height. I always use it in adults, but in children, we have not used it so far.
Dr D. Cameron(Baltimore, MA, USA): The patients who develop arch aneurysms later, were they Loeys-Dietz patients? Or was it just the one patient who had the dissection? It is unusual in Marfan syndrome to dilate the arch after a root replacement if the arch is not dissected.
Dr Kluin: It was not a dissection patient. It was a Marfan, and the other a Loeys-Dietz patient.
Dr R. Cesnjevar(Erlangen, Germany): Just a brief question about your management. I think there is one patient with a bicuspid aortic valve. Would you always repair the bicuspid aortic valve in a valve sparing root, or is there any consideration about thinking that the root may become too small, and like that, create stenosis? Because with a bicuspid valve opening, you need a larger root, in fact, to have no stenosis?
Dr Kluin: Yes, so that was the only patient in which AR was the indication for surgery. So the Z scores of the sinus of all the other patients were +4 or something, and in this particular patient, it was normal. So he had a normal root.
Dr Cesnjevar: Okay, but for a bicuspid aortic valve, what would you consider?
Dr Kluin: A valve sparing root also, yes.
Author notes
† Presented at the 29th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Amsterdam, Netherlands, 3–7 October 2015.




