-
PDF
- Split View
-
Views
-
Cite
Cite
Per Hostrup Nielsen, Vibeke Hjortdal, Ivy Susanne Modrau, Henrik Jensen, Hans-Henrik Kimose, Kim Terp, Steen Hvitfelt Poulsen, Morten Smerup, Sten Lyager Nielsen, Durability after aortic valve replacement with the Mitroflow versus the Perimount pericardial bioprosthesis: a single-centre experience in 2393 patients , European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 6, June 2016, Pages 1705–1710, https://doi.org/10.1093/ejcts/ezv432
Close - Share Icon Share
Abstract
This study compares the durability and risk of reoperation in patients undergoing aortic valve replacement (AVR) with either a Mitroflow or a Carpentier-Edwards (CE) pericardial bioprosthesis. Since AVR with bioprosthetic valves has increased progressively in recent years as compared to mechanical valves, especially in patients aged 60–70 years, there has been renewed interest in the long-term durability of current pericardial bioprostheses.
We compared 440 AVR with Mitroflow valves with 1953 AVR with CE pericardial valves implanted from 1999 to 2014 with regard to reoperation, reoperation for structural valve deterioration (SVD) and all-cause mortality.
Ten-year freedom from explant of any cause was higher for CE Perimount (98 ± 0.7%) than for Mitroflow (95 ± 1.4%, P < 0.01). Reasons for explant for CE Perimount were SVD ( n = 2), endocarditis ( n = 8) and paraprosthetic leak ( n = 10). The reasons for explant for Mitroflow were SVD ( n = 11), endocarditis ( n = 3) SVD and pericarditis ( n = 1) and paraprosthetic leak ( n = 2). Ten-year freedom from explant due to SVD was higher for CE Perimount (100%) than for Mitroflow (96%) ( P < 0.01). In small aortic annuli (bioprosthesis size 19–21 mm), freedom from SVD at 10 years for CE Perimount and Mitroflow was 100 versus 96%, respectively. By multivariate analysis, it was found that bioprosthesis size was not a risk factor for SVD. The choice of valve type could not be demonstrated to influence long-term survival.
The Mitroflow pericardial bioprosthesis provides less than optimal mid- and long-term durability compared with the CE Perimount pericardial valve, especially for small aortic diameter implants (19 and 21 mm). This study hereby confirms the existence of a real risk of valvular deterioration of the Mitroflow valve that might compromise the prognosis of the patients.
INTRODUCTION
Aortic valve replacement (AVR) with a bioprosthesis is the standard treatment for older patients with severe aortic valve stenosis. According to the international guidelines, a bioprosthesis is recommended in patients above 65 years old and should be considered in younger patients depending on patient risk profile and patient preference [ 1 ]. Since the use of bioprosthetic valves relative to mechanical valves has increased progressively in recent years—especially in patients aged 60–70 years, there has been renewed interest in the long-term durability of current pericardial bioprostheses [ 2 ].
The Carpentier-Edwards (CE) Perimount (Edwards Lifesciences, Irvine, CA, USA) and the Mitroflow (Sorin, Milan, Italy) pericardial aortic bioprosthesis are commonly used worldwide [ 3–12 ]. Both bioprostheses have been reported to have satisfactory clinical outcomes in long-term follow-up studies. However, in recent years, a number of reports of early Mitroflow bioprosthetic aortic valve failure caused by structural valve deterioration (SVD) have been reported [ 13–17 ], suggesting a need for clinical vigilance.
Therefore, we conducted a study including all patients who underwent AVR with a Mitroflow or a CE Perimount bioprosthesis at our institution between 1999 and 2014. The primary objective was to compare rate of reoperation after AVR and late mortality with a Mitroflow and a CE Perimount bioprosthesis. The secondary objective was to assess the influence of bioprosthetic valve size on the occurrence of SVD.
PATIENTS AND METHODS
The study was approved by the Regional Ethical Review Board, Aarhus, Denmark. Methods for data analysis and reporting were according to the guidelines [ 18 ].
Study design and study population
We conducted a population-based cohort study including all patients who underwent AVR with a Mitroflow and a CE Perimount at Aarhus University Hospital, Aarhus, Denmark between 1999 and 2014. The Department of Cardiothoracic Surgery at the Aarhus University Hospital is serving ∼25% of the Danish population. Patients with concomitant procedures such as coronary artery bypass grafts and repair or replacement of another valve were included in this cohort.
Valves were implanted according to the standard surgical techniques. Biological valves were usually implanted in patients older than 65 years of age, but patient preference was the primary determinant of valve selection.
Because of institutional directives, the Mitroflow 12A bioprosthesis (Sorin Group, Inc., Milan, Italy) was predominantly used between 2002 and 2007 and was according to the institutional regulations the preferred bioprosthesis in small aortic annuli defined as 19 and 21 mm. The Mitroflow was therefore over-represented in these labelled valve sizes ( N = 435). The CE Perimount was used throughout the period in all valve sizes (CE Perimount Magna from 2002) and became the standard bioprosthesis from 2009. In the study period, a total of 609 CE Perimount valves in size 19–21 were implanted. In order to correct the influence of such institutional selection bias, subgroup analyses for patients having either a 19- or 21-mm bioprosthesis were conducted.
Outcome measures
The primary outcome measures were reoperation, reoperation for SVD and all-cause mortality. Additional outcomes were bioprosthetic valve endocarditis and other causes of reoperation. In general, our perioperative mortality has changed for the better throughout the years since 1999 and as a result the criteria for reoperation might have changed in a more liberal direction. The reoperations for SVD all were performed in the years 2007–2012 evenly distributed with two or three reoperations per year. There is no indication that a change in the criteria has occurred.
The secondary outcome measures included reoperation for SVD with reference to aortic bioprosthetic valve size. Pathological data of the explanted bioprostheses were not available. Survival status and date of death were determined in March 2014 by using the Danish Civil Registration System [ 19 ]. All patients were entered into our database Western Danish Heart Registry [ 20 ] at the time of the operation and we regularly perform analyses upon short- and long-term survival for the different classes of procedures and specified specialized procedures. The total (mean) follow-up was 2658 (6.04) years for the Mitroflow group and 7903 (4.05) years for the CE Perimount group. Follow-up was 100% complete.
All valve-related deaths and complications were defined according to the ‘Guidelines for reporting morbidity and mortality after cardiac valvular operations' [ 18 ]. The primary outcome of interest in this analysis was reoperation due to SVD. The cause of reoperation was established from hospital records. SVD was defined as any clinically relevant valvular stenosis or insufficiency of the bioprosthesis documented at the time of reoperation. Operative death was defined as any death in the hospital or within 30 days after operation.
Statistical analysis
Baseline characteristics were described using means and standard deviations for continuous variables and proportions for categorical variables. Time-to-event was calculated as the time in years from the date of operation to the time of death from any cause. Survival curves and event-free curves were obtained using Kaplan–Meier method and compared by log-rank test. Cox regression was used to detect the risk factors for reoperation of any cause and reoperation due to SVD. Only the first event for each patient during the study was used for event-free analysis. The statistical analysis was done by STATA v. 12.1. The log-rank test used for statistical comparison considered the experience for both valves only out to the follow-up time of the shortest series. Logistic regression was used to analyse perioperative death reporting odds ratios (ORs) and 95% confidence intervals (CIs).
RESULTS
We included 2393 patients who received AVR with either a Mitroflow [ n = 440 (18%)] or a CE Perimount [ n = 1953 (82%)] bioprosthesis between 1999 and 2014. The number of implantations per year of Mitroflow and CE Perimount is shown in Fig. 1 . The distribution of the manufacturer labelled valve size for the two bioprostheses is shown in Fig. 2 .
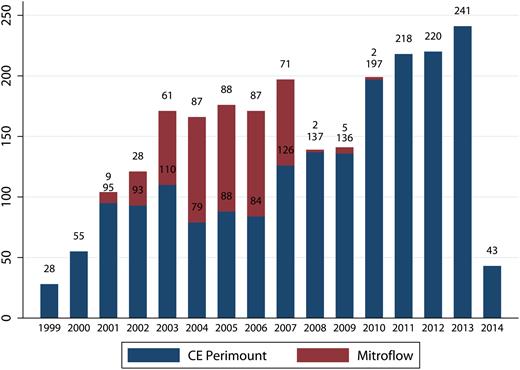
Number of aortic valve implantations over time. CE: Carpentier-Edwards.
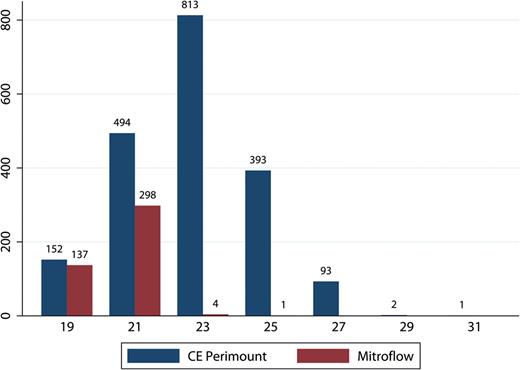
Number of valves according to valve size. CE: Carpentier-Edwards.
Baseline and operative characteristics
Table 1 summarizes the baseline patient characteristics. The mean age was 74.7 years, 40% of patients were women; 63% had a normal left ventricular ejection fraction; 3 % had undergone previous cardiac operations; and 50% had a concomitant cardiac procedure in addition to AVR (most commonly coronary artery bypass grafting). Since the Mitroflow was more selectively used in small aortic annuli, there was a higher proportion of females in the Mitroflow cohort. However, in the subgroup analysis of patients having bioprosthesis in size 19–21 mm, the Mitroflow and CE Perimount groups had similar baseline characteristics (Table 1 , right panels).
Baseline and operative characteristics in 2393 patients who underwent AVR with a CE Perimount or a Mitroflow bioprosthesis from 1999 to 2014
| . | . | All valve sizes . | . | Valve size 19 and 21 . | . | ||
|---|---|---|---|---|---|---|---|
| . | All . | CE Perimount . | Mitroflow . | . | CE Perimount . | Mitroflow . | . |
| N | 2393 | 1953 | 440 | 647 | 434 | ||
| Age | 74.7 ± 6.8 | 74.4 ± 7.1 | 76.2 ± 5.5 | 0.00 | 76.0 ± 6.5 | 76.2 ± 5.5 | NS |
| Women | 947 (39.6%) | 622 (31.9%) | 325 (73.9%) | 0.00 | 471 (72.8%) | 323 (74.4%) | NS |
| Renal insufficiency | 73 (3.1%) | 63 (3.9%) | 10 (3.2%) | NS | 10 (1.9%) | 10 (3.2%) | NS |
| Diabetes | 342 (14.3%) | 285 (14.6%) | 57 (12.9%) | NS | 96 (14.8%) | 56 (12.9%) | NS |
| COPD | 399 (16.7%) | 334 (17.1%) | 65 (14.8%) | NS | 103 (15.9%) | 65 (15.0%) | NS |
| Previous surgery | 73 (3.1%) | 53 (2.7%) | 20 (4.6%) | 0.04 | 12 (1.9%) | 20 (4.6%) | 0.009 |
| AVR + concomitant | 1187 (49.6%) | 968 (49.6%) | 219 (49.8%) | NS | 294 (45.4%) | 218 (50.2%) | NS |
| Endocarditis | 70 (2.9%) | 62 (3.2%) | 8 (1.8%) | NS | 13 (2.1%) | 8 (1.8%) | NS |
| Previous stroke | 211 (8.8%) | 165 (8.5%) | 46 (10.5%) | NS | 51 (7.9%) | 46 (10.6%) | NS |
| Peripheral artery disease | 177 (7.4%) | 149 (7.6) | 28 (6.4%) | NS | 42 (6.5%) | 28 (6.5%) | NS |
| Eject. Fract. 30–50% | 769 (32.8%) | 591 (30.3%) | 177 (40.3%) | 0.00 | 169 (26.2%) | 175 (40.3%) | 0.00 |
| Eject. Fract. <30% | 147 (6.5%) | 130 (6.7%) | 17 (3.9%) | 0.03 | 37 (5.7%) | 17 (3.9%) | NS |
| Emergency | 74(3.1%) | 64 (3.3%) | 10 (2.3%) | NS | 18 (2.8%) | 10 (2.3%) | NS |
| EuroSCORE logistic | 11.7 ± 12.5 | 11.4 ± 12.5 | 12.9 ± 12.3 | 0.01 | 12.1 ± 12.2 | 13.1 ± 12.4 | 0.05 |
| . | . | All valve sizes . | . | Valve size 19 and 21 . | . | ||
|---|---|---|---|---|---|---|---|
| . | All . | CE Perimount . | Mitroflow . | . | CE Perimount . | Mitroflow . | . |
| N | 2393 | 1953 | 440 | 647 | 434 | ||
| Age | 74.7 ± 6.8 | 74.4 ± 7.1 | 76.2 ± 5.5 | 0.00 | 76.0 ± 6.5 | 76.2 ± 5.5 | NS |
| Women | 947 (39.6%) | 622 (31.9%) | 325 (73.9%) | 0.00 | 471 (72.8%) | 323 (74.4%) | NS |
| Renal insufficiency | 73 (3.1%) | 63 (3.9%) | 10 (3.2%) | NS | 10 (1.9%) | 10 (3.2%) | NS |
| Diabetes | 342 (14.3%) | 285 (14.6%) | 57 (12.9%) | NS | 96 (14.8%) | 56 (12.9%) | NS |
| COPD | 399 (16.7%) | 334 (17.1%) | 65 (14.8%) | NS | 103 (15.9%) | 65 (15.0%) | NS |
| Previous surgery | 73 (3.1%) | 53 (2.7%) | 20 (4.6%) | 0.04 | 12 (1.9%) | 20 (4.6%) | 0.009 |
| AVR + concomitant | 1187 (49.6%) | 968 (49.6%) | 219 (49.8%) | NS | 294 (45.4%) | 218 (50.2%) | NS |
| Endocarditis | 70 (2.9%) | 62 (3.2%) | 8 (1.8%) | NS | 13 (2.1%) | 8 (1.8%) | NS |
| Previous stroke | 211 (8.8%) | 165 (8.5%) | 46 (10.5%) | NS | 51 (7.9%) | 46 (10.6%) | NS |
| Peripheral artery disease | 177 (7.4%) | 149 (7.6) | 28 (6.4%) | NS | 42 (6.5%) | 28 (6.5%) | NS |
| Eject. Fract. 30–50% | 769 (32.8%) | 591 (30.3%) | 177 (40.3%) | 0.00 | 169 (26.2%) | 175 (40.3%) | 0.00 |
| Eject. Fract. <30% | 147 (6.5%) | 130 (6.7%) | 17 (3.9%) | 0.03 | 37 (5.7%) | 17 (3.9%) | NS |
| Emergency | 74(3.1%) | 64 (3.3%) | 10 (2.3%) | NS | 18 (2.8%) | 10 (2.3%) | NS |
| EuroSCORE logistic | 11.7 ± 12.5 | 11.4 ± 12.5 | 12.9 ± 12.3 | 0.01 | 12.1 ± 12.2 | 13.1 ± 12.4 | 0.05 |
CE: Carpentier-Edwards; Eject. Fract.: ejection fraction; AVR: aortic valve replacement; COPD: chronic obstructive pulmonary disease; NS: non significant.
Baseline and operative characteristics in 2393 patients who underwent AVR with a CE Perimount or a Mitroflow bioprosthesis from 1999 to 2014
| . | . | All valve sizes . | . | Valve size 19 and 21 . | . | ||
|---|---|---|---|---|---|---|---|
| . | All . | CE Perimount . | Mitroflow . | . | CE Perimount . | Mitroflow . | . |
| N | 2393 | 1953 | 440 | 647 | 434 | ||
| Age | 74.7 ± 6.8 | 74.4 ± 7.1 | 76.2 ± 5.5 | 0.00 | 76.0 ± 6.5 | 76.2 ± 5.5 | NS |
| Women | 947 (39.6%) | 622 (31.9%) | 325 (73.9%) | 0.00 | 471 (72.8%) | 323 (74.4%) | NS |
| Renal insufficiency | 73 (3.1%) | 63 (3.9%) | 10 (3.2%) | NS | 10 (1.9%) | 10 (3.2%) | NS |
| Diabetes | 342 (14.3%) | 285 (14.6%) | 57 (12.9%) | NS | 96 (14.8%) | 56 (12.9%) | NS |
| COPD | 399 (16.7%) | 334 (17.1%) | 65 (14.8%) | NS | 103 (15.9%) | 65 (15.0%) | NS |
| Previous surgery | 73 (3.1%) | 53 (2.7%) | 20 (4.6%) | 0.04 | 12 (1.9%) | 20 (4.6%) | 0.009 |
| AVR + concomitant | 1187 (49.6%) | 968 (49.6%) | 219 (49.8%) | NS | 294 (45.4%) | 218 (50.2%) | NS |
| Endocarditis | 70 (2.9%) | 62 (3.2%) | 8 (1.8%) | NS | 13 (2.1%) | 8 (1.8%) | NS |
| Previous stroke | 211 (8.8%) | 165 (8.5%) | 46 (10.5%) | NS | 51 (7.9%) | 46 (10.6%) | NS |
| Peripheral artery disease | 177 (7.4%) | 149 (7.6) | 28 (6.4%) | NS | 42 (6.5%) | 28 (6.5%) | NS |
| Eject. Fract. 30–50% | 769 (32.8%) | 591 (30.3%) | 177 (40.3%) | 0.00 | 169 (26.2%) | 175 (40.3%) | 0.00 |
| Eject. Fract. <30% | 147 (6.5%) | 130 (6.7%) | 17 (3.9%) | 0.03 | 37 (5.7%) | 17 (3.9%) | NS |
| Emergency | 74(3.1%) | 64 (3.3%) | 10 (2.3%) | NS | 18 (2.8%) | 10 (2.3%) | NS |
| EuroSCORE logistic | 11.7 ± 12.5 | 11.4 ± 12.5 | 12.9 ± 12.3 | 0.01 | 12.1 ± 12.2 | 13.1 ± 12.4 | 0.05 |
| . | . | All valve sizes . | . | Valve size 19 and 21 . | . | ||
|---|---|---|---|---|---|---|---|
| . | All . | CE Perimount . | Mitroflow . | . | CE Perimount . | Mitroflow . | . |
| N | 2393 | 1953 | 440 | 647 | 434 | ||
| Age | 74.7 ± 6.8 | 74.4 ± 7.1 | 76.2 ± 5.5 | 0.00 | 76.0 ± 6.5 | 76.2 ± 5.5 | NS |
| Women | 947 (39.6%) | 622 (31.9%) | 325 (73.9%) | 0.00 | 471 (72.8%) | 323 (74.4%) | NS |
| Renal insufficiency | 73 (3.1%) | 63 (3.9%) | 10 (3.2%) | NS | 10 (1.9%) | 10 (3.2%) | NS |
| Diabetes | 342 (14.3%) | 285 (14.6%) | 57 (12.9%) | NS | 96 (14.8%) | 56 (12.9%) | NS |
| COPD | 399 (16.7%) | 334 (17.1%) | 65 (14.8%) | NS | 103 (15.9%) | 65 (15.0%) | NS |
| Previous surgery | 73 (3.1%) | 53 (2.7%) | 20 (4.6%) | 0.04 | 12 (1.9%) | 20 (4.6%) | 0.009 |
| AVR + concomitant | 1187 (49.6%) | 968 (49.6%) | 219 (49.8%) | NS | 294 (45.4%) | 218 (50.2%) | NS |
| Endocarditis | 70 (2.9%) | 62 (3.2%) | 8 (1.8%) | NS | 13 (2.1%) | 8 (1.8%) | NS |
| Previous stroke | 211 (8.8%) | 165 (8.5%) | 46 (10.5%) | NS | 51 (7.9%) | 46 (10.6%) | NS |
| Peripheral artery disease | 177 (7.4%) | 149 (7.6) | 28 (6.4%) | NS | 42 (6.5%) | 28 (6.5%) | NS |
| Eject. Fract. 30–50% | 769 (32.8%) | 591 (30.3%) | 177 (40.3%) | 0.00 | 169 (26.2%) | 175 (40.3%) | 0.00 |
| Eject. Fract. <30% | 147 (6.5%) | 130 (6.7%) | 17 (3.9%) | 0.03 | 37 (5.7%) | 17 (3.9%) | NS |
| Emergency | 74(3.1%) | 64 (3.3%) | 10 (2.3%) | NS | 18 (2.8%) | 10 (2.3%) | NS |
| EuroSCORE logistic | 11.7 ± 12.5 | 11.4 ± 12.5 | 12.9 ± 12.3 | 0.01 | 12.1 ± 12.2 | 13.1 ± 12.4 | 0.05 |
CE: Carpentier-Edwards; Eject. Fract.: ejection fraction; AVR: aortic valve replacement; COPD: chronic obstructive pulmonary disease; NS: non significant.
Survival
There were 34 operative deaths (8%) in the Mitroflow group and 73 (4%) in the CE Perimount group. Using logistic regression analysis, the operative mortality was dependent on logistic EuroSCORE [OR = 1.04 (95% CI: 1.03–1.05, P = 0.000)]. The ORs for operative mortality based on valve size 19 as reference were as follows: for valve size 21 OR 0.70 (95% CI: 0.40–1.20, P = 0.19), for valve size 23 OR 0.67 (95% CI: 0.67–1.26, P = 0.22), for valve size 25 OR 0.29 (95% CI: 0.11–0.72, P = 0.008) and for valve size 27 OR 0.19 (95% CI: 0.02–1.45, P = 0.109). This is not indicating a relation between valve size and operative mortality. Operative mortality was not related to the type of bioprosthesis OR 1.28 (95% CI: 0.99–1.66, P = 0.057).
There were 229 late deaths in the Mitroflow group and 557 late deaths in the CE Perimount group. In small aortic annuli, there were 226 late deaths in the Mitroflow group and 186 late deaths in the Perimount group. Figure 3 shows Kaplan–Meier survival plot for the Mitroflow and CE Perimount. Ten-year survival was 36% in the CE Perimount group versus 26% in the Mitroflow group. Using Cox logistic regression model on the long-term survival, we observed several statistically significant factors, but valve type was not included, see Table 2 .
Cox regression analysis on long-term survival in 2393 patients who underwent AVR with a CE Perimount or a Mitroflow bioprosthesis from 1999 to 2014
| . | Hazard ratio . | 95% CI . | . | P -value . |
|---|---|---|---|---|
| Age | 1.053 | 1.039 | 1.067 | <0.001 |
| Creatinine >200 µmol/l | 1.717 | 1.167 | 2.525 | 0.006 |
| Valve type Mitroflow | 1.130 | 0.914 | 1.398 | 0.259 |
| Diabetes | 1.267 | 1.039 | 1.545 | 0.019 |
| COPD | 1.646 | 1.383 | 1.959 | <0.001 |
| Peripheral artery disease | 1.329 | 1.043 | 1.693 | 0.021 |
| Neurological dysfunction | 1.367 | 1.096 | 1.705 | 0.005 |
| Active endocarditis | 3.575 | 2.230 | 5.733 | <0.001 |
| Recent myocardial infarct | 1.414 | 1.063 | 1.880 | 0.017 |
| Concommitant CABG | 1.199 | 1.039 | 1.384 | 0.013 |
| Valve size | ||||
| 19 | 1 | – | ||
| 21 | 0.965 | 0.785 | 1.187 | 0.738 |
| 23 | 0.938 | 0.734 | 1.199 | 0.610 |
| 25 | 0.781 | 0.580 | 1.052 | 0.104 |
| 27 | 0.798 | 0.447 | 1.424 | 0.445 |
| Smoking | ||||
| Active smoker | 1 | – | ||
| Non-smoker | 0.681 | 0.551 | 0.840 | <0.001 |
| Previous smoker | 0.823 | 0.679 | 0.997 | 0.047 |
| . | Hazard ratio . | 95% CI . | . | P -value . |
|---|---|---|---|---|
| Age | 1.053 | 1.039 | 1.067 | <0.001 |
| Creatinine >200 µmol/l | 1.717 | 1.167 | 2.525 | 0.006 |
| Valve type Mitroflow | 1.130 | 0.914 | 1.398 | 0.259 |
| Diabetes | 1.267 | 1.039 | 1.545 | 0.019 |
| COPD | 1.646 | 1.383 | 1.959 | <0.001 |
| Peripheral artery disease | 1.329 | 1.043 | 1.693 | 0.021 |
| Neurological dysfunction | 1.367 | 1.096 | 1.705 | 0.005 |
| Active endocarditis | 3.575 | 2.230 | 5.733 | <0.001 |
| Recent myocardial infarct | 1.414 | 1.063 | 1.880 | 0.017 |
| Concommitant CABG | 1.199 | 1.039 | 1.384 | 0.013 |
| Valve size | ||||
| 19 | 1 | – | ||
| 21 | 0.965 | 0.785 | 1.187 | 0.738 |
| 23 | 0.938 | 0.734 | 1.199 | 0.610 |
| 25 | 0.781 | 0.580 | 1.052 | 0.104 |
| 27 | 0.798 | 0.447 | 1.424 | 0.445 |
| Smoking | ||||
| Active smoker | 1 | – | ||
| Non-smoker | 0.681 | 0.551 | 0.840 | <0.001 |
| Previous smoker | 0.823 | 0.679 | 0.997 | 0.047 |
CE: Carpentier-Edwards; AVR: aortic valve replacement; CI: confidence interval; CABG: coronary artery bypass grafting.
Cox regression analysis on long-term survival in 2393 patients who underwent AVR with a CE Perimount or a Mitroflow bioprosthesis from 1999 to 2014
| . | Hazard ratio . | 95% CI . | . | P -value . |
|---|---|---|---|---|
| Age | 1.053 | 1.039 | 1.067 | <0.001 |
| Creatinine >200 µmol/l | 1.717 | 1.167 | 2.525 | 0.006 |
| Valve type Mitroflow | 1.130 | 0.914 | 1.398 | 0.259 |
| Diabetes | 1.267 | 1.039 | 1.545 | 0.019 |
| COPD | 1.646 | 1.383 | 1.959 | <0.001 |
| Peripheral artery disease | 1.329 | 1.043 | 1.693 | 0.021 |
| Neurological dysfunction | 1.367 | 1.096 | 1.705 | 0.005 |
| Active endocarditis | 3.575 | 2.230 | 5.733 | <0.001 |
| Recent myocardial infarct | 1.414 | 1.063 | 1.880 | 0.017 |
| Concommitant CABG | 1.199 | 1.039 | 1.384 | 0.013 |
| Valve size | ||||
| 19 | 1 | – | ||
| 21 | 0.965 | 0.785 | 1.187 | 0.738 |
| 23 | 0.938 | 0.734 | 1.199 | 0.610 |
| 25 | 0.781 | 0.580 | 1.052 | 0.104 |
| 27 | 0.798 | 0.447 | 1.424 | 0.445 |
| Smoking | ||||
| Active smoker | 1 | – | ||
| Non-smoker | 0.681 | 0.551 | 0.840 | <0.001 |
| Previous smoker | 0.823 | 0.679 | 0.997 | 0.047 |
| . | Hazard ratio . | 95% CI . | . | P -value . |
|---|---|---|---|---|
| Age | 1.053 | 1.039 | 1.067 | <0.001 |
| Creatinine >200 µmol/l | 1.717 | 1.167 | 2.525 | 0.006 |
| Valve type Mitroflow | 1.130 | 0.914 | 1.398 | 0.259 |
| Diabetes | 1.267 | 1.039 | 1.545 | 0.019 |
| COPD | 1.646 | 1.383 | 1.959 | <0.001 |
| Peripheral artery disease | 1.329 | 1.043 | 1.693 | 0.021 |
| Neurological dysfunction | 1.367 | 1.096 | 1.705 | 0.005 |
| Active endocarditis | 3.575 | 2.230 | 5.733 | <0.001 |
| Recent myocardial infarct | 1.414 | 1.063 | 1.880 | 0.017 |
| Concommitant CABG | 1.199 | 1.039 | 1.384 | 0.013 |
| Valve size | ||||
| 19 | 1 | – | ||
| 21 | 0.965 | 0.785 | 1.187 | 0.738 |
| 23 | 0.938 | 0.734 | 1.199 | 0.610 |
| 25 | 0.781 | 0.580 | 1.052 | 0.104 |
| 27 | 0.798 | 0.447 | 1.424 | 0.445 |
| Smoking | ||||
| Active smoker | 1 | – | ||
| Non-smoker | 0.681 | 0.551 | 0.840 | <0.001 |
| Previous smoker | 0.823 | 0.679 | 0.997 | 0.047 |
CE: Carpentier-Edwards; AVR: aortic valve replacement; CI: confidence interval; CABG: coronary artery bypass grafting.
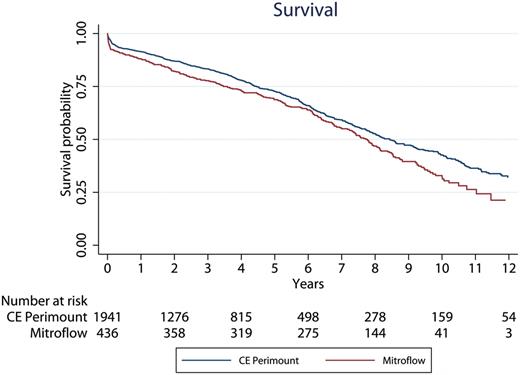
Patient survival after aortic valve implantation according to valve type. CE: Carpentier-Edwards.
Reoperation
During follow-up, the risk of reoperation of any cause in the Mitroflow group was 4% (17 of 440 patients). The risk of reoperation in the CE Perimount group was 1% (20 of 1953 patients). Causes of reoperations for the Mitroflow bioprosthesis were SVD ( n = 11), endocarditis ( n = 3), both SVD and endocarditis ( n = 1) and paraprosthetic leak ( n = 2). The causes of explant for the CE Perimount were SVD ( n = 2), endocarditis ( n = 8) and paraprosthetic leak ( n = 10). As depicted in Fig. 4 , the 10-year Kaplan–Meier freedom from explant of any cause was significantly lower for the Mitroflow group 0.949 (95% CI: 0.909–0.972) than for the CE Perimount group 0.979 (95% CI: 0.960–0.989). By Cox regression analysis, Mitroflow bioprosthesis was found to be a statistically significant risk factor for reoperation of any cause (Mitroflow hazard ratio 4.3, 95% CI: 1.5–12.2, P = 0.006). Younger age was also a significant risk factor (hazard ratio 0.91, CI: 0.87–0.95, P < 0.001), meaning that there was higher risk for reoperation in the young patient. There were 6 operative deaths in the 37 reoperations.
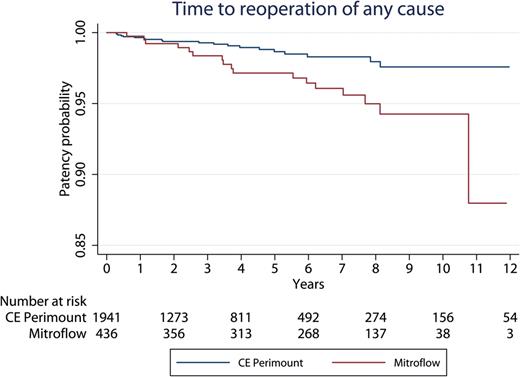
Time in years to reoperation of any cause. CE: Carpentier-Edwards.
Reoperation due to structural valve deterioration
The 10-year Kaplan–Meier freedom from reoperation due to SVD was significantly lower in the Mitroflow group (0.956; 95% CI: 0.918–0.976) than in the CE Perimount group (0.995; 95% CI: 0.978–0.999) (Fig. 5 ). By Cox regression analysis, Mitroflow bioprosthesis and younger age were found to be significant risk factors for reoperation due to SVD (Mitroflow hazard ratio: 28.0, CI: 5.4–144.0; P < 0.001 and hazard ratio age 0.84, CI: 0.76–0.92 P < 0.001). Of the 15 patients having a 19-mm CE Perimount, there was no reoperation due to SVD. Of the 134 patients having a 19-mm Mitroflow, there were three reoperations due to SVD (2%). Of the 495 patients having a 21-mm CE Perimount, there was no reoperation due to SVD. Of the 288 patients having a 21-mm Mitroflow, there were nine reoperations due to SVD. Accordingly, for patients with a 19-mm bioprosthesis 10-year freedom from reoperation for SVD was 100% in the CE Perimount group and 97% in the Mitroflow group. For patients with a 21-mm bioprosthesis, 10-year freedom from reoperation for SVD was 100% in the CE Perimount group and 94% in the Mitroflow group. The combined patency curve in relation to SVD for valve sizes19 and 21 mm is shown in Fig. 6 .
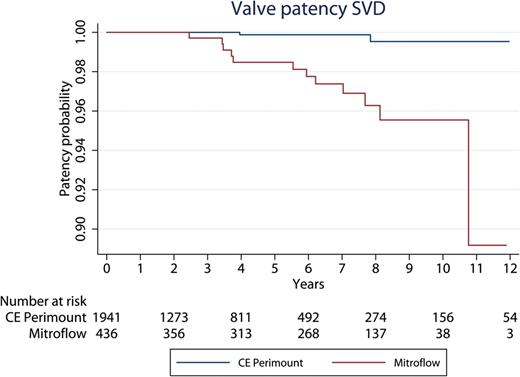
Time in years to reoperation due to SVD. SVD: structural valve deterioration; CE: Carpentier-Edwards.
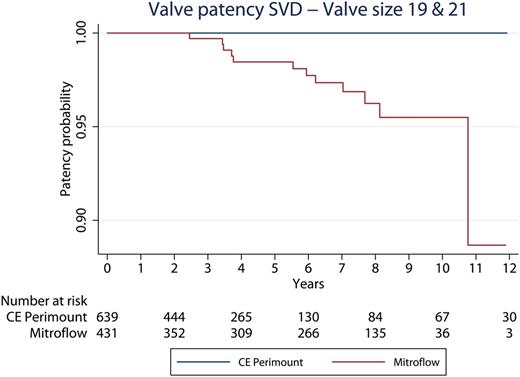
Time in years to reoperation due to SVD in valve sizes 19 and 21. SVD: structural valve deterioration; CE: Carpentier-Edwards.
The operative risk at reoperation due to SVD was 0%.
Reoperation due to bioprosthetic valve endocarditis
There was no early (<90 days) reoperation due to valve endocarditis in either group. There were four late reoperations due to valve endocarditis in the Mitroflow group and eight late reoperations in the CE Perimount group. Freedom from reoperation due to valve endocarditis after 10 years was 99% in the Mitroflow group and 99% in the CE Perimount group. The difference was not statistically significant, log-rank test, P = 0.33.
DISCUSSION
In this single-centre observational study with up to 15-year follow-up in 2393 patients having AVR with two currently used pericardial bioprostheses, we observed a high incidence of reoperation in patients having a Mitroflow compared with patients having a CE Perimount aortic valve bioprosthesis. The difference seemed to be principally driven by a significantly higher rate of SVD in the Mitroflow group, as only two CE Perimount valves have been explanted because of SVD. Notably, the operative risk at reoperation due to SVD was 0%. We found no difference in long-term survival after AVR with a CE Perimount compared with a Mitroflow bioprosthesis after more than 10 years of follow-up.
Although the treatment allocation (i.e. valve choice) was not randomized in our study, the risk of selection bias was reduced because valve selection was primarily regulated by institutional policy rather than surgeon preference. The Mitroflow bioprosthesis was preferentially used in small aortic annuli from 2002 to 2007. The CE Perimount was used throughout the study period in all bioprosthetic valve sizes. The groups were comparable regarding most baseline characteristics despite valve size, and it is noteworthy that the difference in the rate of reoperation for SVD was even more significant in the subgroup analysis of patients having small aortic bioprostheses (sizes 19 and 21 mm).
According to the international guidelines, an aortic valve bioprosthesis is recommended in patients older than 65 years and should also be considered in patients over 60 years of age or younger, depending on life expectancy, comorbidity, compliance and patient preference. In this decision-making, it is crucial to acknowledge long-term durability of the heart valve prosthesis and freedom from SVD in relation to the patient's expected remaining life [ 1 ].
Life expectancy for patients after AVR in the age group 60–65 years is 12–14 years [ 21 ]. According to the Euro Heart Survey on valvular heart disease, the use of bioprosthetic valves relative to mechanical valves has increased progressively in recent years, especially in patients aged 60–70 years, and 70% of these patients prefer an aortic valve bioprosthesis [ 22 ]. According to the current clinical practice, ∼65% of all patients who are receiving an aortic valve bioprosthesis are less than 75 years and during the last 5 years, 9% of patients are below 65 years versus previous below 3%.
Recently, several clinical studies have consistently demonstrated an early and high incidence of SVD after the insertion of Mitroflow aortic valve bioprostheses. Thus, these patients regardless of age cannot reasonably expect to avoid SVD and related complications in their remaining life expectancy. In a large cohort study including 1513 patients, the incidence of SVD within 15 years with patients aged 60–69 years was 40% [ 6 ]. Several studies have shown that SVD occurs a few years after valve implantation. In a large cohort study comprising 1516 patients having a Mitroflow bioprosthesis, SVD appeared increasingly already 5 years after implantation. The incidence of SVD after 15 years in the age group 70–75 years was 37 ± 5.8% and, in this study, it was stipulated that the Mitroflow bioprosthesis was not suitable for use in patients below 75 years of age [ 4 ]. However, even in elderly patients, early and high incidence of SVD in up to 44% of patients after 10 years has been observed [ 5 , 17 ]. The latter study compared SVD of the Mitroflow valve with the CE Perimount valve; at 10 years, SVD of the Mitroflow valve was 44 ± 6.8 versus 13 ± 4.7% for the CE Perimount valve [ 15 ].
Finally, in a recent prospective study from the University Hospital of Nantes, Sénage et al. have demonstrated a very high incidence of SVD among 617 consecutive patients who underwent implantation of a Mitroflow aortic valve bioprosthesis during the period of 2002–07 [ 13 ]. The prospective design of this study was unique since all patients after primary surgery were followed by regular echocardiographic controls. Only 8% of these patients developed haemodynamically significant SVD within 5 years after surgery and the onset of SVD was associated with an increased risk of death within 5 years (hazard ratio 7.7; P < 0.001). Only 10% of the patients, who developed haemodynamic SVD, were considered eligible for reoperation, and this proportion seems to be consistent with other studies [ 14 ].
In our study, the risk of reoperation due to SVD was higher in the Mitroflow group (3%) compared with the CE Perimount group (0.1%). We may expect a 10-fold higher incidence of SVD that may compromise clinical outcome for these patients [ 13 ]. Of the entire Mitroflow cohort of 440 patients, 263 patients died during follow-up. We may assume that a number of these patients may have died due to SVD without reoperation. We are now conducting a systematic and thorough echocardiographic and cardiac CT assessment of the 177 patients, who are still alive with a Mitroflow bioprosthesis, in order to anticipate the occurrence of serious adverse cardiac events.
Study limitations
Although the main limitation of our study was the relatively short follow-up time, specific strengths of our study include that it is a single-centre study with a comparatively large number of patients from the high-quality database of the Danish national registers. Additionally, patients were included consecutively from a single centre, which might minimize differences in pre-, peri- and postoperative care. The bioprosthesis selection was regulated by institutional policy rather than surgeon preference. The study was limited by what information was attainable from patient charts and institutional and national registers. Additionally, we did not have follow-up information on clinical and echocardiographic occurrence of SVD and left ventricular mass regression or postoperative haemodynamic parameters.
CONCLUSIONS
The Mitroflow pericardial bioprosthesis provides less mid- and long-term durability compared with the CE Perimount pericardial valve, especially for small aortic diameter implants (19 and 21 mm). This study hereby confirms the existence of a real risk of deleterious valvular deterioration of the Mitroflow bioprosthesis that might compromise the prognosis of the patients. Accordingly, all patients, who are still alive after AVR with a Mitroflow bioprosthesis in our institution, are now revisited for a cardiologic and echocardiographic follow-up in order to anticipate the occurrence of serious adverse cardiac events.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 28th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Milan, Italy, 11–15 October 2014.




