-
PDF
- Split View
-
Views
-
Cite
Cite
Annabel J. Sharkey, Sara Tenconi, Apostolos Nakas, David A. Waller, The effects of an intentional transition from extrapleural pneumonectomy to extended pleurectomy/decortication , European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 6, June 2016, Pages 1632–1641, https://doi.org/10.1093/ejcts/ezv403
Close - Share Icon Share
Abstract
For many years, extrapleural pneumonectomy (EPP) was the operation of choice for the radical management of pleural mesothelioma in the UK. However, doubts surrounding the efficacy of EPP, and the change in demographics of the affected population, have prompted a transition in our practice towards extended pleurectomy/decortication (EPD). The aim of this study was to determine the effects an intentional transition from EPP to EPD has had on patient outcome.
Data from 362 patients undergoing radical surgery (229 EPD, 133 EPP) during 1999–2014 were included. Demographics and outcome were compared between the two groups; EPP versus EPD.
The median age of patients undergoing EPD was significantly higher than those undergoing EPP [57 years (range 14–70 years) vs 65 years (range 42–81 years), P < 0.001]. There was a significantly higher proportion of patients with performance status ≥1 in the EPD group (46.3 vs 35.4%, P = 0.047). There was no difference in the median length of hospital stay between the two groups [14 days (range 1–133 days) vs 13 days (range 0–93 days), P = 0.409]. There was also no difference between the groups in terms of in-hospital mortality (EPP 5.3% and EPD 6.6%, P = 0.389), 30-day mortality [EPP 8 (6.0%) and EPD 8 (3.5%), P = 0.294] or 90-day mortality [EPP 18 (13.5%) and EPD 21 (9.2%), P = 0.220]. There was a significantly higher early reoperation rate in the EPP group (15.0 vs 6.2%, P = 0.008) but a significantly higher late reoperation rate in the EPD group (0.8 vs 5.3%, P = 0.037). There was no significant difference in overall survival or disease-free interval between the two groups ( P = 0.899 and P = 0.399, respectively). However, overall survival was significantly greater in patients over the age of 65 undergoing EPD (12.5 vs 4.7 months, P = 0.001).
The transition from EPP to EPD in our standard practice has enabled us to operate on more elderly, frail patients with no significant increase in use of hospital resources, and without detriment to overall survival.
INTRODUCTION
The use of radical surgery in the management of malignant pleural mesothelioma (MPM) remains a topic of debate. The aim of radical surgery in this disease is to obtain complete macroscopic resection with removal of all visible tumour within the hemithorax. This can be achieved by either extrapleural pneumonectomy (EPP) or extended pleurectomy/decortication (EPD). EPP involves the en bloc resection of both the visceral and parietal pleura with the ipsilateral lung, the pericardium and hemi-diaphragm, while EPD has a similar extent but preserves the lung by visceral pleurectomy [ 1 ].
Prior to the publication of the results of the Mesothelioma and Radical Surgery (MARS) Feasibility study, which suggested that ‘radical surgery in the form of EPP within trimodal therapy offers no benefit and possibly harms patients’, we had already begun a transition from EPP to EPD within our institution [ 2 ]. This was due mainly to the changing demographics of the population of patients with MPM, and the strict selection criteria for EPP; older patients with more comorbidities would have been precluded from surgical treatment if only EPP were offered to them. Patient choice was also a major factor, with a lung-sparing operation seeming to be more acceptable to patients than a pneumonectomy, given the unknown benefits of EPP balanced against the morbidity and mortality associated with it.
In this study, we aimed to determine whether the transition from EPP to EPD has had any detrimental effects on the outcome of patients.
MATERIALS AND METHODS
Patients
From our retrospective institutional database, all patients who had undergone extrapleural pneumonectomy (EPP) or extended pleurectomy/decortication (EPD), for MPM between 1999 and 2014 were identified.
Preoperative assessment and selection
Prior to consideration for surgery, a histological diagnosis of malignant mesothelioma was made using percutaneous (image-guided in the majority of cases), thoracoscopic, either medical or surgical, or open biopsy. Where possible a histological subtype was attributed from the diagnostic biopsy sample. In cases where immunohistochemical analysis failed to provide a definitive histological subtype, despite the use of invasive biopsy techniques, the patients were counselled regarding the risk of a reduced survival if a non-epithelioid subtype was identified, and proceeded to surgery if they were happy to accept this risk.
All patients underwent thoracic computed tomography (CT) to determine clinical stage and resectability. Positron emission tomography (PET) is not performed routinely as part of the preoperative assessment or selection process for MPM surgery in our institution, or across the majority of the UK. The local selection criteria for EPP required exclusion of mediastinal N2 disease by cervical mediastinoscopy in all patients. The higher operative risk associated with EPP, and therefore the potentially reduced benefit of the procedure, led to patients not being offered surgery in cases of proven N2 disease. Pulmonary function tests, including forced expiratory volume in 1 s (FEV1) and forced vital capacity, alongside quantitative lung perfusion scintigraphy, were performed prior to selection for EPP. Prior to the transition, in patients whose pulmonary function precluded an EPP, an EPD was performed. These tests were also performed as a matter of routine in most cases prior to EPD to evaluate functional status. Patients routinely underwent cardiac function testing by echocardiogram prior to selection for EPP in order to exclude those with right ventricular dysfunction.
Operative techniques
Both procedures were performed using standardized techniques as defined by the International Association for the Study of Lung Cancer International Staging Committee and the International Mesothelioma Interest Group [ 1 ]. EPP was performed through either a thoracotomy or median sternotomy, resecting the lung, pericardium and diaphragm en bloc. EPD was performed via a posterolateral thoracotomy through the sixth intercostal space, with a second-level thoracotomy performed if required. In 2 cases, EPD was performed via median sternotomy to allow further access to the mediastinal, in particular the intrapericardial, structures. EPD comprised total visceral and parietal pleurectomy, including the fissures, and resection of the pericardium and hemi-diaphragm. In both procedures, the pericardium was replaced with an absorbable mesh patch, and the hemi-diaphragm with a synthetic prosthesis [ 3 ].
EPD included phrenectomy in all cases to avoid an R2 resection and the detrimental effects that has on survival [ 4 ]. However, in cases where the pericardium was not involved by tumour, this was left in situ .
Adjuvant therapy
The provision of adjuvant chemo- and/or radiotherapy was determined by the local referring oncologist. We received referrals from over 20 oncological centres around the UK and, thus, the neoadjuvant and adjuvant therapeutic decisions varied considerably. Our current local policy, and recommendation to referring centres, is not to give neoadjuvant therapy, but to consider adjuvant therapy following recovery from surgery. The decision regarding fitness for chemotherapy was made by the referring oncologist at the first postoperative visit. Patients were, in most cases, seen within 3 months of surgery. In the era of EPP, many patients were offered adjuvant whole-hemithorax radiation therapy, although this itself was not standardized throughout the country. At present, adjuvant radiotherapy is not available after EPD in the UK.
Data collection
Case notes and electronic hospital records were reviewed in order to determine demographic, pathological and immediate postoperative, and long-term, clinical outcome data. Some data fields including basic demographic data, preoperative test results and operative technique are collected prospectively into our institutional database. The remaining data for this study were collected retrospectively. Referral hospitals were contacted for missing information in cases where patients had been discharged from our routine follow-up. If adjuvant therapy was not given, a reason for this was sought directly from them.
Time to clinical disease progression was calculated from the date of surgery to first radiological finding of progression. Patients only underwent re-biopsy if clinically indicated, for example, if performing a contralateral surgical pleurodesis in cases of EPD, or if the presence of disease progression could not be confirmed with sufficient certainty by CT scanning. There was no protocol for routine CT surveillance following surgery with either EPP or EPD. Clinical and chest X-ray assessment were performed at each surgical follow-up appointment, with CT being reserved for clinical suspicion of disease progression. The majority of referring oncologists also performed 3–6 monthly CT scans. Surgical follow-up took place initially at 4–6 weeks following discharge, then every 3 months for the first year, then 6 monthly for the following year and annually thereafter. Overall survival was calculated from time of operation to death or to the date of censoring.
Statistical analysis
SPSS version 20 statistical software package was used for analysis. Continuous data were analysed using the Mann–Whitney U -test as all continuous dependent variables in this study were not normally distributed. A histogram was produced for all continuous variables and normality checked visually, with a test for normality also performed to ensure data were, or were not, normally distributed. Categorical data were analysed using the χ2 test, or in cases when one or more of the cells had an expected frequency of five or less, Fisher's exact test was used. A P -value of less than 0.05 was considered to be statistically significant.
Time to recurrence and survival analyses were estimated using the Kaplan–Meier method with log-rank test to compare for differences between the groups. The multivariable model was created using forward logistic regression within a Cox regression model. Variables with a P -value of less than 0.1 were included in the model.
Ethical approval was not required for this retrospective analysis of routinely collected data which are collected in a linked anonymized database.
RESULTS
Patients
Radical surgery was performed on 362 consecutive patients (133 EPP and 229 EPD) for MPM between 1999 and 2014. The median age in the EPP group was 57 years (range 14–70 years) and in the EPD group it was 65 years (range 42–81 years), P < 0.001. The majority of patients in each group were male (85.7% in the EPP group and 85.6% in the EPD group, P = 1.00). Likewise, the majority of patients had epithelioid histology (72.2% in the EPP group and 75.5% in the EPD group, P = 0.779). Most patients were stage pT3 (48.1% in the EPP group and 45.0% in the EPD group, P = 0.116) at operation. Nodal positivity was similar between the two operative groups (53.4 and 55.9% in the EPP and EPD groups, respectively, P = 0.166) although there were more patients with pN2 disease in the EPD group (54.6 vs 44.4%, P < 0.001) (Table 1 ).
| . | Total, n . | EPP ( n = 133) . | EPD ( n = 229) . | P . |
|---|---|---|---|---|
| Median age, years (range) | 362 | 57 (14–70) | 65 (42–81) | <0.001 |
| Male gender, n (%) | 362 | 114 (85.7) | 196 (85.6) | 1.00 |
| Performance Status ≥1, n (%) | 300 | 35 (35.4) | 93 (46.3) | 0.047 |
| Median % predicted FEV1 (range) | 179 | 71 (32–117) | 70 (44–123) | 0.317 |
| Cell type, n (%) | ||||
| Epithelioid | 269 | 96 (72.2) | 173 (75.5) | 0.779 |
| Biphasic | 83 | 33 (24.8) | 50 (21.8) | |
| Sarcomatoid | 10 | 4 (3.0) | 6 (2.6) | |
| T stage, n (%) | ||||
| 1 | 20 | 10 (7.5) | 10 (4.4) | 0.116 |
| 2 | 83 | 22 (16.5) | 61 (26.6) | |
| 3 | 167 | 64 (48.1) | 103 (45.0) | |
| 4 | 92 | 37 (27.8) | 55 (24.0) | |
| N stage, n (%) | ||||
| 0 | 145 | 62 (46.6) | 83 (36.2) | <0.001 |
| 1 | 15 | 12 (9.0) | 3 (1.3) | |
| 2 | 184 | 59 (44.4) | 125 (54.8) | |
| X | 18 | 0 | 18 (7.9) | |
| Nodal positivity, N1 or N2, n (%) | 199 | 71 (53.4) | 128 (55.9) | 0.166 |
| IMIG stage, n (%) | ||||
| I | 15 | 9 (6.8) | 6 (2.6) | 0.019 |
| II | 50 | 10 (7.5) | 40 (17.5) | |
| III | 206 | 78 (58.6) | 128 (55.9) | |
| IV | 91 | 36 (27.1) | 55 (24.0) | |
| . | Total, n . | EPP ( n = 133) . | EPD ( n = 229) . | P . |
|---|---|---|---|---|
| Median age, years (range) | 362 | 57 (14–70) | 65 (42–81) | <0.001 |
| Male gender, n (%) | 362 | 114 (85.7) | 196 (85.6) | 1.00 |
| Performance Status ≥1, n (%) | 300 | 35 (35.4) | 93 (46.3) | 0.047 |
| Median % predicted FEV1 (range) | 179 | 71 (32–117) | 70 (44–123) | 0.317 |
| Cell type, n (%) | ||||
| Epithelioid | 269 | 96 (72.2) | 173 (75.5) | 0.779 |
| Biphasic | 83 | 33 (24.8) | 50 (21.8) | |
| Sarcomatoid | 10 | 4 (3.0) | 6 (2.6) | |
| T stage, n (%) | ||||
| 1 | 20 | 10 (7.5) | 10 (4.4) | 0.116 |
| 2 | 83 | 22 (16.5) | 61 (26.6) | |
| 3 | 167 | 64 (48.1) | 103 (45.0) | |
| 4 | 92 | 37 (27.8) | 55 (24.0) | |
| N stage, n (%) | ||||
| 0 | 145 | 62 (46.6) | 83 (36.2) | <0.001 |
| 1 | 15 | 12 (9.0) | 3 (1.3) | |
| 2 | 184 | 59 (44.4) | 125 (54.8) | |
| X | 18 | 0 | 18 (7.9) | |
| Nodal positivity, N1 or N2, n (%) | 199 | 71 (53.4) | 128 (55.9) | 0.166 |
| IMIG stage, n (%) | ||||
| I | 15 | 9 (6.8) | 6 (2.6) | 0.019 |
| II | 50 | 10 (7.5) | 40 (17.5) | |
| III | 206 | 78 (58.6) | 128 (55.9) | |
| IV | 91 | 36 (27.1) | 55 (24.0) | |
EPP: extrapleural pneumonectomy; EPD: extended pleurectomy/decortication; FEV1: forced expiratory volume in 1 s.
| . | Total, n . | EPP ( n = 133) . | EPD ( n = 229) . | P . |
|---|---|---|---|---|
| Median age, years (range) | 362 | 57 (14–70) | 65 (42–81) | <0.001 |
| Male gender, n (%) | 362 | 114 (85.7) | 196 (85.6) | 1.00 |
| Performance Status ≥1, n (%) | 300 | 35 (35.4) | 93 (46.3) | 0.047 |
| Median % predicted FEV1 (range) | 179 | 71 (32–117) | 70 (44–123) | 0.317 |
| Cell type, n (%) | ||||
| Epithelioid | 269 | 96 (72.2) | 173 (75.5) | 0.779 |
| Biphasic | 83 | 33 (24.8) | 50 (21.8) | |
| Sarcomatoid | 10 | 4 (3.0) | 6 (2.6) | |
| T stage, n (%) | ||||
| 1 | 20 | 10 (7.5) | 10 (4.4) | 0.116 |
| 2 | 83 | 22 (16.5) | 61 (26.6) | |
| 3 | 167 | 64 (48.1) | 103 (45.0) | |
| 4 | 92 | 37 (27.8) | 55 (24.0) | |
| N stage, n (%) | ||||
| 0 | 145 | 62 (46.6) | 83 (36.2) | <0.001 |
| 1 | 15 | 12 (9.0) | 3 (1.3) | |
| 2 | 184 | 59 (44.4) | 125 (54.8) | |
| X | 18 | 0 | 18 (7.9) | |
| Nodal positivity, N1 or N2, n (%) | 199 | 71 (53.4) | 128 (55.9) | 0.166 |
| IMIG stage, n (%) | ||||
| I | 15 | 9 (6.8) | 6 (2.6) | 0.019 |
| II | 50 | 10 (7.5) | 40 (17.5) | |
| III | 206 | 78 (58.6) | 128 (55.9) | |
| IV | 91 | 36 (27.1) | 55 (24.0) | |
| . | Total, n . | EPP ( n = 133) . | EPD ( n = 229) . | P . |
|---|---|---|---|---|
| Median age, years (range) | 362 | 57 (14–70) | 65 (42–81) | <0.001 |
| Male gender, n (%) | 362 | 114 (85.7) | 196 (85.6) | 1.00 |
| Performance Status ≥1, n (%) | 300 | 35 (35.4) | 93 (46.3) | 0.047 |
| Median % predicted FEV1 (range) | 179 | 71 (32–117) | 70 (44–123) | 0.317 |
| Cell type, n (%) | ||||
| Epithelioid | 269 | 96 (72.2) | 173 (75.5) | 0.779 |
| Biphasic | 83 | 33 (24.8) | 50 (21.8) | |
| Sarcomatoid | 10 | 4 (3.0) | 6 (2.6) | |
| T stage, n (%) | ||||
| 1 | 20 | 10 (7.5) | 10 (4.4) | 0.116 |
| 2 | 83 | 22 (16.5) | 61 (26.6) | |
| 3 | 167 | 64 (48.1) | 103 (45.0) | |
| 4 | 92 | 37 (27.8) | 55 (24.0) | |
| N stage, n (%) | ||||
| 0 | 145 | 62 (46.6) | 83 (36.2) | <0.001 |
| 1 | 15 | 12 (9.0) | 3 (1.3) | |
| 2 | 184 | 59 (44.4) | 125 (54.8) | |
| X | 18 | 0 | 18 (7.9) | |
| Nodal positivity, N1 or N2, n (%) | 199 | 71 (53.4) | 128 (55.9) | 0.166 |
| IMIG stage, n (%) | ||||
| I | 15 | 9 (6.8) | 6 (2.6) | 0.019 |
| II | 50 | 10 (7.5) | 40 (17.5) | |
| III | 206 | 78 (58.6) | 128 (55.9) | |
| IV | 91 | 36 (27.1) | 55 (24.0) | |
EPP: extrapleural pneumonectomy; EPD: extended pleurectomy/decortication; FEV1: forced expiratory volume in 1 s.
More patients had a performance status ≥1 in the EPD group (46.3 vs 35.4%, P = 0.047). Pulmonary function test data were available for 179 patients; 52 patients undergoing EPP (39.1%) and 127 patients undergoing EPD (55.5%). For those for whom these data were available, there was no difference in preoperative percentage predicted FEV1 between the two operative groups (EPP median 71, range 32–117, and EPD median 70, range 44–123, P = 0.317).
Data regarding the type of preoperative biopsy were available for 346 patients (95.6%). The majority of patients underwent a thoracoscopic or open surgical biopsy [118/122 (96.7%) patients prior to EPP and 205/224 (91.5%) patients prior to EPD]. In the EPP group, 2 patients had their diagnosis obtained by pleural tap, and 4 by radiologically guided biopsy. In the EPD group, 1 patient underwent a blind needle biopsy and 18 patients a radiologically guided biopsy. In 3.2% of cases, a histological subtype was not established prior to surgery.
Operative procedures
Both EPP and EPD were performed until September 2009, where a decision was made to stop performing EPP. The transition to EPD began in 2005, where a steady decline in the use of EPP could be seen (Table 2 ). Patients who underwent EPD prior to 2009 did not undergo EPP because they did not reach the strict selection criteria required: poor FEV1, elderly patients and poor cardiac function, or because patients chose to undergo lung-sparing surgery after counselling preoperatively. All patients included in this study underwent successful macroscopic resection (R1) of their tumour. In no cases was a planned EPD converted to an EPP, or vice versa, due to intraoperative findings or difficulties.
| Date range . | EPP . | EPD . | Percentage EPP (%) . |
|---|---|---|---|
| August 1999–September 2000 | 11 | 4 | 73.3 |
| October 2000–September 2001 | 12 | 2 | 85.7 |
| October 2001–September 2002 | 10 | 1 | 90.9 |
| October 2002–September 2003 | 21 | 1 | 95.5 |
| October 2003–September 2004 | 23 | 3 | 88.5 |
| October 2004–September 2005 | 26 | 8 | 76.5 |
| October 2005–September 2006 | 9 | 17 | 34.6 |
| October 2006–September 2007 | 9 | 15 | 37.5 |
| October 2007–September 2008 | 4 | 9 | 30.8 |
| October 2008–September 2009 | 8 | 19 | 29.6 |
| October 2009–July 2014 | 0 | 138 | 0 |
| Date range . | EPP . | EPD . | Percentage EPP (%) . |
|---|---|---|---|
| August 1999–September 2000 | 11 | 4 | 73.3 |
| October 2000–September 2001 | 12 | 2 | 85.7 |
| October 2001–September 2002 | 10 | 1 | 90.9 |
| October 2002–September 2003 | 21 | 1 | 95.5 |
| October 2003–September 2004 | 23 | 3 | 88.5 |
| October 2004–September 2005 | 26 | 8 | 76.5 |
| October 2005–September 2006 | 9 | 17 | 34.6 |
| October 2006–September 2007 | 9 | 15 | 37.5 |
| October 2007–September 2008 | 4 | 9 | 30.8 |
| October 2008–September 2009 | 8 | 19 | 29.6 |
| October 2009–July 2014 | 0 | 138 | 0 |
EPP: extrapleural pneumonectomy; EPD: extended pleurectomy/decortication.
| Date range . | EPP . | EPD . | Percentage EPP (%) . |
|---|---|---|---|
| August 1999–September 2000 | 11 | 4 | 73.3 |
| October 2000–September 2001 | 12 | 2 | 85.7 |
| October 2001–September 2002 | 10 | 1 | 90.9 |
| October 2002–September 2003 | 21 | 1 | 95.5 |
| October 2003–September 2004 | 23 | 3 | 88.5 |
| October 2004–September 2005 | 26 | 8 | 76.5 |
| October 2005–September 2006 | 9 | 17 | 34.6 |
| October 2006–September 2007 | 9 | 15 | 37.5 |
| October 2007–September 2008 | 4 | 9 | 30.8 |
| October 2008–September 2009 | 8 | 19 | 29.6 |
| October 2009–July 2014 | 0 | 138 | 0 |
| Date range . | EPP . | EPD . | Percentage EPP (%) . |
|---|---|---|---|
| August 1999–September 2000 | 11 | 4 | 73.3 |
| October 2000–September 2001 | 12 | 2 | 85.7 |
| October 2001–September 2002 | 10 | 1 | 90.9 |
| October 2002–September 2003 | 21 | 1 | 95.5 |
| October 2003–September 2004 | 23 | 3 | 88.5 |
| October 2004–September 2005 | 26 | 8 | 76.5 |
| October 2005–September 2006 | 9 | 17 | 34.6 |
| October 2006–September 2007 | 9 | 15 | 37.5 |
| October 2007–September 2008 | 4 | 9 | 30.8 |
| October 2008–September 2009 | 8 | 19 | 29.6 |
| October 2009–July 2014 | 0 | 138 | 0 |
EPP: extrapleural pneumonectomy; EPD: extended pleurectomy/decortication.
Perioperative course
There was no difference in the median length of hospital stay between the two groups, 14 days (range 1–133 days) versus 13 days (range 0–93 days) ( P = 0.409). There was also no difference between the groups in terms of in-hospital mortality (EPP 5.3% and EPD 6.6%, P = 0.389), 30-day mortality [EPP 8 (6.0%) and EPD 8 (3.5%), P = 0.294] or 90-day mortality [EPP 18 (13.5%) and EPD 21 (9.2%), P = 0.220]. The most common cause of 30-day mortality in the EPP group was right heart failure (37.5%) and, in the EPD group, it was multiorgan failure secondary to sepsis (37.5%). Respiratory failure, myocardial infarction and pulmonary embolus were among the other causes of death in both groups. The most common cause of death within 90 days in both groups was sepsis and subsequent respiratory failure (EPP 22.2% and EPD 28.6%). Cancer-related death due to early disease progression was seen in 2 patients, (11.1%) in the EPP group, and 1 patient, (4.8%) in the EPD group.
Postoperative complications are displayed in Table 3 . Complications are termed ‘early’ if they occurred during the initial hospital stay. ‘Late’ complications are those occurring after initial discharge from hospital. There was a significantly higher rate of early reoperation in the EPP group (15.0 vs 6.2%, P = 0.008) and of late reoperation in the EPD group (0.8 vs 5.3%, P = 0.037). This is due to the higher number of early reoperations for bleeding and bronchopleural fistula (BPF) repair in the EPP group (bleeding 4.5 vs 0.4%, P = 0.007, BPF 4.5 vs 0%, P = 0.002), and the higher number of reoperations for late patch dehiscence or empyema in the EPD group (late patch dehiscence 0 vs 3.6%, P < 0.001, and late space infection 0 vs 3.1%, P = 0.040). There was a higher rate of late space infection in the EPD group (0.8 vs 4.9%, P = 0.037). The median length of intercostal drainage was significantly higher in the EPD group (EPP: 8 days, range 1–34 days; EPD: 12 days, range 0–70 days; P < 0.001). Prolonged air leak was defined as intercostal drainage for an air leak of longer than 7 days. A third of patients undergoing EPD experienced a prolonged air leak.
Postoperative complications in EPP and EPD groups (data available for 358 patients; EPP 133 patients, EPD 225 patients)
| Complication . | Total, n (%) . | EPP, n (%) . | EPD, n (%) . | P . |
|---|---|---|---|---|
| Lower respiratory tract infection | 28 (7.8) | 13 (9.8) | 15 (6.7) | 0.312 |
| Atrial fibrillation | 70 (19.6) | 29 (21.8) | 41 (18.2) | 0.412 |
| Reoperation | ||||
| Total | 47 (13.1) | 21 (15.8) | 26 (11.6) | 0.261 |
| Early | 34 (9.5) | 20 (15.0) | 14 (6.2) | 0.008 |
| Late | 13 (3.6) | 1 (0.8) | 12 (5.3) | 0.037 |
| Reasons for reoperation | ||||
| Bleeding | 7 (2.0) | 6 (4.5) | 1 (0.4) | 0.007 |
| Space infection (early) | 11 (3.1) | 4 (3.0) | 7 (3.1) | 0.956 |
| Space infection (late) | 8 (2.2) | 0 | 7 (3.1) | 0.040 |
| Patch dehiscence (early) | 16 (4.5) | 7 (5.3) | 9 (4.0) | 0.576 |
| Patch dehiscence (late) | 8 (2.2) | 0 | 8 (3.6) | <0.001 |
| Chylothorax | 8 (2.2) | 6 (4.5) | 2 (0.9) | 0.114 |
| Bronchopleural fistula repair | 10 (2.8) | 9 (6.8) | 1 (0.4) | <0.001 |
| Wound debridement | 1 (0.3) | 0 | 1 (0.4) | 0.441 |
| Patch dehiscence | ||||
| Total | 32 (8.9) | 11 (8.3) | 21 (9.3) | 0.849 |
| Mechanical (early) | 15 (4.2) | 9 (6.8) | 6 (2.7) | 0.098 |
| Space infection (early) | 8 (2.2) | 1 (0.8) | 7 (3.1) | 0.267 |
| Mechanical (late) | 3 (0.8) | 1 (0.8) | 2 (0.9) | 1.0 |
| Space infection (late) | 6 (1.7) | 0 | 6 (2.7) | 0.088 |
| Space infection (operated + non-operated) | ||||
| Total | 33 (9.2) | 10 (7.5) | 23 (10.2) | 0.099 |
| Early | 21 (5.9) | 9 (6.8) | 12 (5.3) | 0.644 |
| Late | 12 (3.4) | 1 (0.8) | 11 (4.9) | 0.037 |
| Chylothorax | 16 (4.5) | 8 (6.0) | 8 (3.6) | 0.298 |
| Bronchopleural fistula | ||||
| Early | 6 (1.7) | 6 (4.5) | 0 | 0.002 |
| Late | 4 (1.1) | 3 (2.3) | 1 (0.4) | 0.147 |
| Right heart failure | 3 (0.8) | 3 (2.3) | 0 | 0.051 |
| Ileus | 8 (2.2) | 7 (5.3) | 1 (0.4) | 0.005 |
| Pulmonary embolus | 11 (3.1) | 3 (2.3) | 8 (3.6) | 0.341 |
| Myocardial infarction | 3 (0.8) | 0 | 3 (1.3) | 0.298 |
| Wound infection requiring antibiotic treatment | ||||
| Early | 8 (2.2) | 4 (3.0) | 4 (1.8) | 0.476 |
| Late | 6 (1.7) | 3 (2.3) | 3 (1.3) | 0.674 |
| Rapid space filling requiring drainage (EPP only) | 12 (3.4) | 12 (9.0) | 0 | |
| Persistent air leak (EPD only) | 75 (20.9) | 0 | 75 (33.3) | |
| Complication . | Total, n (%) . | EPP, n (%) . | EPD, n (%) . | P . |
|---|---|---|---|---|
| Lower respiratory tract infection | 28 (7.8) | 13 (9.8) | 15 (6.7) | 0.312 |
| Atrial fibrillation | 70 (19.6) | 29 (21.8) | 41 (18.2) | 0.412 |
| Reoperation | ||||
| Total | 47 (13.1) | 21 (15.8) | 26 (11.6) | 0.261 |
| Early | 34 (9.5) | 20 (15.0) | 14 (6.2) | 0.008 |
| Late | 13 (3.6) | 1 (0.8) | 12 (5.3) | 0.037 |
| Reasons for reoperation | ||||
| Bleeding | 7 (2.0) | 6 (4.5) | 1 (0.4) | 0.007 |
| Space infection (early) | 11 (3.1) | 4 (3.0) | 7 (3.1) | 0.956 |
| Space infection (late) | 8 (2.2) | 0 | 7 (3.1) | 0.040 |
| Patch dehiscence (early) | 16 (4.5) | 7 (5.3) | 9 (4.0) | 0.576 |
| Patch dehiscence (late) | 8 (2.2) | 0 | 8 (3.6) | <0.001 |
| Chylothorax | 8 (2.2) | 6 (4.5) | 2 (0.9) | 0.114 |
| Bronchopleural fistula repair | 10 (2.8) | 9 (6.8) | 1 (0.4) | <0.001 |
| Wound debridement | 1 (0.3) | 0 | 1 (0.4) | 0.441 |
| Patch dehiscence | ||||
| Total | 32 (8.9) | 11 (8.3) | 21 (9.3) | 0.849 |
| Mechanical (early) | 15 (4.2) | 9 (6.8) | 6 (2.7) | 0.098 |
| Space infection (early) | 8 (2.2) | 1 (0.8) | 7 (3.1) | 0.267 |
| Mechanical (late) | 3 (0.8) | 1 (0.8) | 2 (0.9) | 1.0 |
| Space infection (late) | 6 (1.7) | 0 | 6 (2.7) | 0.088 |
| Space infection (operated + non-operated) | ||||
| Total | 33 (9.2) | 10 (7.5) | 23 (10.2) | 0.099 |
| Early | 21 (5.9) | 9 (6.8) | 12 (5.3) | 0.644 |
| Late | 12 (3.4) | 1 (0.8) | 11 (4.9) | 0.037 |
| Chylothorax | 16 (4.5) | 8 (6.0) | 8 (3.6) | 0.298 |
| Bronchopleural fistula | ||||
| Early | 6 (1.7) | 6 (4.5) | 0 | 0.002 |
| Late | 4 (1.1) | 3 (2.3) | 1 (0.4) | 0.147 |
| Right heart failure | 3 (0.8) | 3 (2.3) | 0 | 0.051 |
| Ileus | 8 (2.2) | 7 (5.3) | 1 (0.4) | 0.005 |
| Pulmonary embolus | 11 (3.1) | 3 (2.3) | 8 (3.6) | 0.341 |
| Myocardial infarction | 3 (0.8) | 0 | 3 (1.3) | 0.298 |
| Wound infection requiring antibiotic treatment | ||||
| Early | 8 (2.2) | 4 (3.0) | 4 (1.8) | 0.476 |
| Late | 6 (1.7) | 3 (2.3) | 3 (1.3) | 0.674 |
| Rapid space filling requiring drainage (EPP only) | 12 (3.4) | 12 (9.0) | 0 | |
| Persistent air leak (EPD only) | 75 (20.9) | 0 | 75 (33.3) | |
EPP: extrapleural pneumonectomy; EPD: extended pleurectomy/decortication.
Postoperative complications in EPP and EPD groups (data available for 358 patients; EPP 133 patients, EPD 225 patients)
| Complication . | Total, n (%) . | EPP, n (%) . | EPD, n (%) . | P . |
|---|---|---|---|---|
| Lower respiratory tract infection | 28 (7.8) | 13 (9.8) | 15 (6.7) | 0.312 |
| Atrial fibrillation | 70 (19.6) | 29 (21.8) | 41 (18.2) | 0.412 |
| Reoperation | ||||
| Total | 47 (13.1) | 21 (15.8) | 26 (11.6) | 0.261 |
| Early | 34 (9.5) | 20 (15.0) | 14 (6.2) | 0.008 |
| Late | 13 (3.6) | 1 (0.8) | 12 (5.3) | 0.037 |
| Reasons for reoperation | ||||
| Bleeding | 7 (2.0) | 6 (4.5) | 1 (0.4) | 0.007 |
| Space infection (early) | 11 (3.1) | 4 (3.0) | 7 (3.1) | 0.956 |
| Space infection (late) | 8 (2.2) | 0 | 7 (3.1) | 0.040 |
| Patch dehiscence (early) | 16 (4.5) | 7 (5.3) | 9 (4.0) | 0.576 |
| Patch dehiscence (late) | 8 (2.2) | 0 | 8 (3.6) | <0.001 |
| Chylothorax | 8 (2.2) | 6 (4.5) | 2 (0.9) | 0.114 |
| Bronchopleural fistula repair | 10 (2.8) | 9 (6.8) | 1 (0.4) | <0.001 |
| Wound debridement | 1 (0.3) | 0 | 1 (0.4) | 0.441 |
| Patch dehiscence | ||||
| Total | 32 (8.9) | 11 (8.3) | 21 (9.3) | 0.849 |
| Mechanical (early) | 15 (4.2) | 9 (6.8) | 6 (2.7) | 0.098 |
| Space infection (early) | 8 (2.2) | 1 (0.8) | 7 (3.1) | 0.267 |
| Mechanical (late) | 3 (0.8) | 1 (0.8) | 2 (0.9) | 1.0 |
| Space infection (late) | 6 (1.7) | 0 | 6 (2.7) | 0.088 |
| Space infection (operated + non-operated) | ||||
| Total | 33 (9.2) | 10 (7.5) | 23 (10.2) | 0.099 |
| Early | 21 (5.9) | 9 (6.8) | 12 (5.3) | 0.644 |
| Late | 12 (3.4) | 1 (0.8) | 11 (4.9) | 0.037 |
| Chylothorax | 16 (4.5) | 8 (6.0) | 8 (3.6) | 0.298 |
| Bronchopleural fistula | ||||
| Early | 6 (1.7) | 6 (4.5) | 0 | 0.002 |
| Late | 4 (1.1) | 3 (2.3) | 1 (0.4) | 0.147 |
| Right heart failure | 3 (0.8) | 3 (2.3) | 0 | 0.051 |
| Ileus | 8 (2.2) | 7 (5.3) | 1 (0.4) | 0.005 |
| Pulmonary embolus | 11 (3.1) | 3 (2.3) | 8 (3.6) | 0.341 |
| Myocardial infarction | 3 (0.8) | 0 | 3 (1.3) | 0.298 |
| Wound infection requiring antibiotic treatment | ||||
| Early | 8 (2.2) | 4 (3.0) | 4 (1.8) | 0.476 |
| Late | 6 (1.7) | 3 (2.3) | 3 (1.3) | 0.674 |
| Rapid space filling requiring drainage (EPP only) | 12 (3.4) | 12 (9.0) | 0 | |
| Persistent air leak (EPD only) | 75 (20.9) | 0 | 75 (33.3) | |
| Complication . | Total, n (%) . | EPP, n (%) . | EPD, n (%) . | P . |
|---|---|---|---|---|
| Lower respiratory tract infection | 28 (7.8) | 13 (9.8) | 15 (6.7) | 0.312 |
| Atrial fibrillation | 70 (19.6) | 29 (21.8) | 41 (18.2) | 0.412 |
| Reoperation | ||||
| Total | 47 (13.1) | 21 (15.8) | 26 (11.6) | 0.261 |
| Early | 34 (9.5) | 20 (15.0) | 14 (6.2) | 0.008 |
| Late | 13 (3.6) | 1 (0.8) | 12 (5.3) | 0.037 |
| Reasons for reoperation | ||||
| Bleeding | 7 (2.0) | 6 (4.5) | 1 (0.4) | 0.007 |
| Space infection (early) | 11 (3.1) | 4 (3.0) | 7 (3.1) | 0.956 |
| Space infection (late) | 8 (2.2) | 0 | 7 (3.1) | 0.040 |
| Patch dehiscence (early) | 16 (4.5) | 7 (5.3) | 9 (4.0) | 0.576 |
| Patch dehiscence (late) | 8 (2.2) | 0 | 8 (3.6) | <0.001 |
| Chylothorax | 8 (2.2) | 6 (4.5) | 2 (0.9) | 0.114 |
| Bronchopleural fistula repair | 10 (2.8) | 9 (6.8) | 1 (0.4) | <0.001 |
| Wound debridement | 1 (0.3) | 0 | 1 (0.4) | 0.441 |
| Patch dehiscence | ||||
| Total | 32 (8.9) | 11 (8.3) | 21 (9.3) | 0.849 |
| Mechanical (early) | 15 (4.2) | 9 (6.8) | 6 (2.7) | 0.098 |
| Space infection (early) | 8 (2.2) | 1 (0.8) | 7 (3.1) | 0.267 |
| Mechanical (late) | 3 (0.8) | 1 (0.8) | 2 (0.9) | 1.0 |
| Space infection (late) | 6 (1.7) | 0 | 6 (2.7) | 0.088 |
| Space infection (operated + non-operated) | ||||
| Total | 33 (9.2) | 10 (7.5) | 23 (10.2) | 0.099 |
| Early | 21 (5.9) | 9 (6.8) | 12 (5.3) | 0.644 |
| Late | 12 (3.4) | 1 (0.8) | 11 (4.9) | 0.037 |
| Chylothorax | 16 (4.5) | 8 (6.0) | 8 (3.6) | 0.298 |
| Bronchopleural fistula | ||||
| Early | 6 (1.7) | 6 (4.5) | 0 | 0.002 |
| Late | 4 (1.1) | 3 (2.3) | 1 (0.4) | 0.147 |
| Right heart failure | 3 (0.8) | 3 (2.3) | 0 | 0.051 |
| Ileus | 8 (2.2) | 7 (5.3) | 1 (0.4) | 0.005 |
| Pulmonary embolus | 11 (3.1) | 3 (2.3) | 8 (3.6) | 0.341 |
| Myocardial infarction | 3 (0.8) | 0 | 3 (1.3) | 0.298 |
| Wound infection requiring antibiotic treatment | ||||
| Early | 8 (2.2) | 4 (3.0) | 4 (1.8) | 0.476 |
| Late | 6 (1.7) | 3 (2.3) | 3 (1.3) | 0.674 |
| Rapid space filling requiring drainage (EPP only) | 12 (3.4) | 12 (9.0) | 0 | |
| Persistent air leak (EPD only) | 75 (20.9) | 0 | 75 (33.3) | |
EPP: extrapleural pneumonectomy; EPD: extended pleurectomy/decortication.
Additional treatment
The provision of neoadjuvant or adjuvant chemotherapy, and adjuvant radiotherapy, was determined by the local policy of the referring oncologist, and as such the regimes given were not standardized. Data regarding neoadjuvant therapy were available for 359 patients, and for adjuvant therapy, for 313 patients. There was a significantly higher proportion of patients receiving neoadjuvant chemotherapy in the EPP group (EPP 27.1% vs EPD 17.2%, P = 0.032). However, there was no difference between the groups in terms of the use of immediate adjuvant chemotherapy (EPP 33.9% vs EPD 36.8%, P = 0.625) (Fig. 1 ). Patients receiving radiotherapy in the EPD group were given port site radiotherapy only. As the regimes in both groups were incomparable, we have not included these data in further analyses.
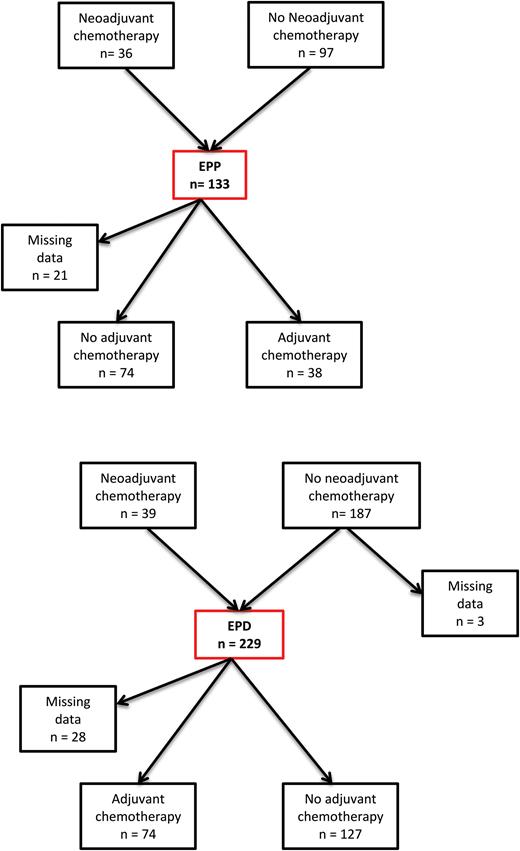
Chemotherapy treatment received. EPP: extrapleural pneumonectomy; EPD: extended pleurectomy/decortication.
There was no difference between the two groups in the proportion of patients deemed unfit to receive chemotherapy due to postoperative morbidity (EPP 8.3% and EPD 12.3%, P = 0.470).
Survival
There was no difference in median overall survival between the groups (EPP 12.9 months vs EPD 12.3 months, P = 0.899), or in disease-free interval (EPP 11.5 months vs EPD 10.6 months, P = 0.399) (Figs 2 and 3 ). Three patients were lost to follow-up for survival data in the EPD group, none in the EPP group. Seven cases were censored in the EPP group and 44 in the EPD group. Survival was calculated up to the last date at which these patients were known to be alive.
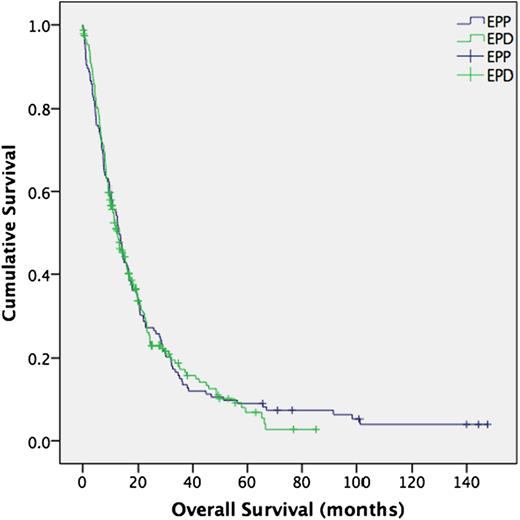
Overall survival (all patients). Median survival; EPP 12.9 months, EPD 12.3 months, P = 0.899. (95% confidence interval 0.779–1.234). EPP: extrapleural pneumonectomy; EPD: extended pleurectomy/decortication.
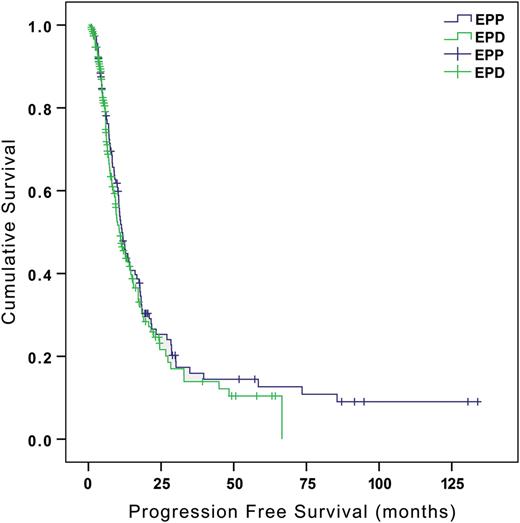
Progression-free survival (all patients). Median disease-free interval; EPP 11.5 months, EPD 10.6 months P = 0.399. (95% confidence interval 0.662–1.179). EPP: extrapleural pneumonectomy; EPD: extended pleurectomy/decortication.
Recurrence data were available for 113 (85.0%) patients in the EPP group and 181 (79.0%) patients in the EPD group. In the EPP group, 26 cases were censored, and in the EPD group 88. The first site of disease progression in the majority of cases was local, either the ipsilateral hemithorax or thoracotomy wound (EPP 42.5% and EPD 53.4%). The patterns of progression at other sites were as follows: contralateral chest (EPP 11.5%, EPD 13.8%), peritoneal (EPP 21.8%, EPD 9.5%) and widespread disease (EPP 24.1%, EPD 17.2%). Data regarding initial site of progression were missing for 7 patients in the EPD group.
In the group of patients with non-epithelioid, node-positive disease, there was a higher survival in those undergoing EPP [EPP, n = 19, 27 events; 9.9 months vs EPD, n = 22, 21 events; 5.8 months; hazard ratio (HR) 1.961, 95% confidence interval (CI) 0.993–3.870, P = 0.050]. In the patients with epithelioid, node-negative disease, there was a trend towards an improved survival with EPD although this was not statistically significant (EPP, n = 45, 42 events; 24.2 months vs EPD, n = 51, 35 events; 20.7 months; P = 0.714).
Being rendered unfit for chemotherapy following surgical resection had a detrimental effect on overall survival across both surgical groups (all patients; 14.8 vs 7.0 months, P < 0.001). Compared with those who received chemotherapy in the immediate postoperative setting, these patients had a particularly poor survival (20.7 vs 7.0 months, P < 0.001). We found no difference between the groups in overall survival (EPP, n = 11, 11 events; 10.5 months vs EPD, n = 25, 25 events; 5.8 months, P = 0.532) or disease-free interval (EPP, n = 10, 7 events; 11.5 months vs EPD, n = 23, 11 events; 10.6 months, P = 0.399) in those patients deemed unfit for chemotherapy following surgical resection, although the numbers were small.
For patients over the age of 65 years ( n = 139), overall survival was significantly higher in the group undergoing EPD, 12.5 versus 4.7 months in the EPP group (HR 2.314, 95% CI 1.352–3.959, P = 0.001) (Fig. 4 ). There was no difference in performance status, cell type, T stage and nodal stage between the two groups ( P = 0.743, P = 0.810, P = 0.472 and P = 0.242, respectively). There was a significantly higher 30- and 90-day mortality in the EPP group (30-day mortality: 33.0 vs 5.1%, P = 0.016; and 90-day mortality: 60 vs 16.0%, P = 0.028). On multivariable analysis, using a forward logistic regression within a Cox model, of factors known to affect survival in pleural mesothelioma [ 5 ], EPP remained a significantly poor prognostic factor for overall survival in patients over 65 years of age ( P = 0.001, HR = 2.698, 95% CI 1.534–4.747) (see Table 4 for univariable and multivariable results). Factors included in this analysis were: pathological stage (International Mesothelioma Interest Group [IMIG] stage), nodal positivity, histological cell type, gender, age, neoadjuvant and adjuvant chemotherapy, high white cell count, low haemoglobin and high platelet count, along with operation type. The standardized normal ranges for laboratory blood tests performed in our laboratory were used rather than those stated in the paper by Pass et al . [ 5 ], to ensure accuracy, as different laboratory systems, with corresponding different normal ranges, may be employed in our centre.
Univariable and multivariable analysis of prognostic factors for survival in patients over 65 years of age (139 patients unless otherwise stated)
| Factor . | n (%) . | Median survival (months) . | Number of events . | Univariable, P . | Multivariable, P . | HR . | 95% CI low . | 95% CI high . |
|---|---|---|---|---|---|---|---|---|
| Male gender | 122 (87.8) | 10.0 | 100 | 0.418 | 0.781 | 0.429 | 1.423 | |
| Cell type (epithelioid vs others) | ||||||||
| Epithelioid | 104 (74.8) | 13.1 | 79 | <0.001 | 0.062 | |||
| Biphasic | 32 (23.0) | 6.2 | 30 | 0.003 | 1.722 | 1.044 | 2.841 | |
| Sarcomatoid | 3 (2.2) | 2.8 | 3 | 0.107 | 3.456 | 0.765 | 15.623 | |
| Nodal positivity | 79 (56.8) | 8.3 | 65 | 0.013 | 0.011 | 1.817 | 1.148 | 2.876 |
| IMIG stage (IMIG stage I vs others) | ||||||||
| I | 5 (3.6) | All cases are censored | 0 | 0.017 | 0.362 | |||
| II | 25 (18.0) | 11.3 | 21 | 0.368 | ||||
| III | 74 (53.2) | 10.0 | 57 | 0.488 | 1.151 | 0.695 | 1.907 | |
| IV | 35 (25.2) | 6.5 | 33 | 0.516 | 1.465 | 0.845 | 2.539 | |
| Neoadjuvant therapy ( n = 138) | 19 (13.8) | 9.6 | 15 | 0.338 | 0.788 | 0.447 | 1.390 | |
| Performance status ≥1 ( n = 119) | 59 (49.6) | 10.1 | 47 | 0.747 | 0.935 | 0.622 | 1.407 | |
| High white cell count (>11.0 × 10 9 /l males, >6.0 × 10 9 /l females) | 21 (15.1) | 6.1 | 19 | 0.141 | 1.449 | 0.881 | 2.383 | |
| High platelet count (>400 × 10 9 /l) | 41 (29.5) | 8.1 | 36 | 0.190 | 1.305 | 0.875 | 1.944 | |
| Operation type (EPD) | 123 (88.5) | 11.2 | 96 | 0.002 | 0.001 | 2.698 | 1.534 | 4.747 |
| Adjuvant chemotherapy ( n = 117) | 44 (37.6) | 20.7 | 35 | 0.001 | 0.001 | 2.426 | 1.423 | 4.136 |
| Low haemoglobin (<13.0 g/dl males, <11.5 g/dl females) | 52 (37.4) | 6.7 | 45 | 0.004 | 0.006 | 1.917 | 1.209 | 3.037 |
| Factor . | n (%) . | Median survival (months) . | Number of events . | Univariable, P . | Multivariable, P . | HR . | 95% CI low . | 95% CI high . |
|---|---|---|---|---|---|---|---|---|
| Male gender | 122 (87.8) | 10.0 | 100 | 0.418 | 0.781 | 0.429 | 1.423 | |
| Cell type (epithelioid vs others) | ||||||||
| Epithelioid | 104 (74.8) | 13.1 | 79 | <0.001 | 0.062 | |||
| Biphasic | 32 (23.0) | 6.2 | 30 | 0.003 | 1.722 | 1.044 | 2.841 | |
| Sarcomatoid | 3 (2.2) | 2.8 | 3 | 0.107 | 3.456 | 0.765 | 15.623 | |
| Nodal positivity | 79 (56.8) | 8.3 | 65 | 0.013 | 0.011 | 1.817 | 1.148 | 2.876 |
| IMIG stage (IMIG stage I vs others) | ||||||||
| I | 5 (3.6) | All cases are censored | 0 | 0.017 | 0.362 | |||
| II | 25 (18.0) | 11.3 | 21 | 0.368 | ||||
| III | 74 (53.2) | 10.0 | 57 | 0.488 | 1.151 | 0.695 | 1.907 | |
| IV | 35 (25.2) | 6.5 | 33 | 0.516 | 1.465 | 0.845 | 2.539 | |
| Neoadjuvant therapy ( n = 138) | 19 (13.8) | 9.6 | 15 | 0.338 | 0.788 | 0.447 | 1.390 | |
| Performance status ≥1 ( n = 119) | 59 (49.6) | 10.1 | 47 | 0.747 | 0.935 | 0.622 | 1.407 | |
| High white cell count (>11.0 × 10 9 /l males, >6.0 × 10 9 /l females) | 21 (15.1) | 6.1 | 19 | 0.141 | 1.449 | 0.881 | 2.383 | |
| High platelet count (>400 × 10 9 /l) | 41 (29.5) | 8.1 | 36 | 0.190 | 1.305 | 0.875 | 1.944 | |
| Operation type (EPD) | 123 (88.5) | 11.2 | 96 | 0.002 | 0.001 | 2.698 | 1.534 | 4.747 |
| Adjuvant chemotherapy ( n = 117) | 44 (37.6) | 20.7 | 35 | 0.001 | 0.001 | 2.426 | 1.423 | 4.136 |
| Low haemoglobin (<13.0 g/dl males, <11.5 g/dl females) | 52 (37.4) | 6.7 | 45 | 0.004 | 0.006 | 1.917 | 1.209 | 3.037 |
EPD: extended pleurectomy/decortication; HR: hazard ratio, CI: confidence interval.
Univariable and multivariable analysis of prognostic factors for survival in patients over 65 years of age (139 patients unless otherwise stated)
| Factor . | n (%) . | Median survival (months) . | Number of events . | Univariable, P . | Multivariable, P . | HR . | 95% CI low . | 95% CI high . |
|---|---|---|---|---|---|---|---|---|
| Male gender | 122 (87.8) | 10.0 | 100 | 0.418 | 0.781 | 0.429 | 1.423 | |
| Cell type (epithelioid vs others) | ||||||||
| Epithelioid | 104 (74.8) | 13.1 | 79 | <0.001 | 0.062 | |||
| Biphasic | 32 (23.0) | 6.2 | 30 | 0.003 | 1.722 | 1.044 | 2.841 | |
| Sarcomatoid | 3 (2.2) | 2.8 | 3 | 0.107 | 3.456 | 0.765 | 15.623 | |
| Nodal positivity | 79 (56.8) | 8.3 | 65 | 0.013 | 0.011 | 1.817 | 1.148 | 2.876 |
| IMIG stage (IMIG stage I vs others) | ||||||||
| I | 5 (3.6) | All cases are censored | 0 | 0.017 | 0.362 | |||
| II | 25 (18.0) | 11.3 | 21 | 0.368 | ||||
| III | 74 (53.2) | 10.0 | 57 | 0.488 | 1.151 | 0.695 | 1.907 | |
| IV | 35 (25.2) | 6.5 | 33 | 0.516 | 1.465 | 0.845 | 2.539 | |
| Neoadjuvant therapy ( n = 138) | 19 (13.8) | 9.6 | 15 | 0.338 | 0.788 | 0.447 | 1.390 | |
| Performance status ≥1 ( n = 119) | 59 (49.6) | 10.1 | 47 | 0.747 | 0.935 | 0.622 | 1.407 | |
| High white cell count (>11.0 × 10 9 /l males, >6.0 × 10 9 /l females) | 21 (15.1) | 6.1 | 19 | 0.141 | 1.449 | 0.881 | 2.383 | |
| High platelet count (>400 × 10 9 /l) | 41 (29.5) | 8.1 | 36 | 0.190 | 1.305 | 0.875 | 1.944 | |
| Operation type (EPD) | 123 (88.5) | 11.2 | 96 | 0.002 | 0.001 | 2.698 | 1.534 | 4.747 |
| Adjuvant chemotherapy ( n = 117) | 44 (37.6) | 20.7 | 35 | 0.001 | 0.001 | 2.426 | 1.423 | 4.136 |
| Low haemoglobin (<13.0 g/dl males, <11.5 g/dl females) | 52 (37.4) | 6.7 | 45 | 0.004 | 0.006 | 1.917 | 1.209 | 3.037 |
| Factor . | n (%) . | Median survival (months) . | Number of events . | Univariable, P . | Multivariable, P . | HR . | 95% CI low . | 95% CI high . |
|---|---|---|---|---|---|---|---|---|
| Male gender | 122 (87.8) | 10.0 | 100 | 0.418 | 0.781 | 0.429 | 1.423 | |
| Cell type (epithelioid vs others) | ||||||||
| Epithelioid | 104 (74.8) | 13.1 | 79 | <0.001 | 0.062 | |||
| Biphasic | 32 (23.0) | 6.2 | 30 | 0.003 | 1.722 | 1.044 | 2.841 | |
| Sarcomatoid | 3 (2.2) | 2.8 | 3 | 0.107 | 3.456 | 0.765 | 15.623 | |
| Nodal positivity | 79 (56.8) | 8.3 | 65 | 0.013 | 0.011 | 1.817 | 1.148 | 2.876 |
| IMIG stage (IMIG stage I vs others) | ||||||||
| I | 5 (3.6) | All cases are censored | 0 | 0.017 | 0.362 | |||
| II | 25 (18.0) | 11.3 | 21 | 0.368 | ||||
| III | 74 (53.2) | 10.0 | 57 | 0.488 | 1.151 | 0.695 | 1.907 | |
| IV | 35 (25.2) | 6.5 | 33 | 0.516 | 1.465 | 0.845 | 2.539 | |
| Neoadjuvant therapy ( n = 138) | 19 (13.8) | 9.6 | 15 | 0.338 | 0.788 | 0.447 | 1.390 | |
| Performance status ≥1 ( n = 119) | 59 (49.6) | 10.1 | 47 | 0.747 | 0.935 | 0.622 | 1.407 | |
| High white cell count (>11.0 × 10 9 /l males, >6.0 × 10 9 /l females) | 21 (15.1) | 6.1 | 19 | 0.141 | 1.449 | 0.881 | 2.383 | |
| High platelet count (>400 × 10 9 /l) | 41 (29.5) | 8.1 | 36 | 0.190 | 1.305 | 0.875 | 1.944 | |
| Operation type (EPD) | 123 (88.5) | 11.2 | 96 | 0.002 | 0.001 | 2.698 | 1.534 | 4.747 |
| Adjuvant chemotherapy ( n = 117) | 44 (37.6) | 20.7 | 35 | 0.001 | 0.001 | 2.426 | 1.423 | 4.136 |
| Low haemoglobin (<13.0 g/dl males, <11.5 g/dl females) | 52 (37.4) | 6.7 | 45 | 0.004 | 0.006 | 1.917 | 1.209 | 3.037 |
EPD: extended pleurectomy/decortication; HR: hazard ratio, CI: confidence interval.

Overall survival of patients >65 years ( n = 139). Median overall survival; EPD, 12.5 months, EPP 4.7 months. (HR 2.314 95% CI 1.352–3.959 P = 0.001). EPP: extrapleural pneumonectomy; EPD: extended pleurectomy/decortication; HR: hazard ratio.
DISCUSSION
We have demonstrated the potential for operating on older patients with poorer performance status by performing EPD, in line with the current trend of the ageing population. More patients with N2 disease underwent EPD in this cohort, with no detriment to overall or progression-free survival. The higher proportion of N2 disease in the EPD groups could be explained by the use of preoperative cervical mediastinoscopy in the EPP group. We stopped using cervical mediastinoscopy as a preoperative screening tool when we moved to performing EPD, and therefore did not exclude patients from surgery based on clinical nodal staging.
Despite the use of preoperative cervical mediastinoscopy in the EPP group, there was still a high rate of pN2 disease. Many nodes designated as N2 are inaccessible by cervical mediastinoscopy, such as internal mammary, diaphragmatic and pericardial nodes, and as such there remains a high rate of pathological N2 disease, despite this preoperative screening. The inaccessibility of these nodes, which are often found to be involved at operation, is one of the reasons that led us to stop using this method as a selection tool prior to EPD. It is well documented that nodal positivity, either N1 or N2, is a poor prognostic factor for patients undergoing radical surgery for MPM, and we have also shown that in this series [ 5 , 6 ]. However, our group has previously shown that EPD can be performed in the context of N2 disease without detriment to overall survival outcomes and, therefore, we do not exclude patients from consideration for EPD based on clinical nodal staging [ 3 ].
We showed a comparable length of hospital stay and a lower early reoperation rate with EPD. Despite a third of patients having a prolonged air leak, and there being a much longer length of intercostal drainage in the EPD group, hospital stay was not prolonged when compared with the younger, fitter, EPP group. It appears that, by performing EPD rather than EPP, we are able to maintain efficient use of resources, in the context of a more elderly cohort of patients with reduced physiological reserve. However, there was a higher number of late reoperations for neodiaphragm replacement or decortication for empyema in the EPD group, with some patients requiring both neodiaphragm replacement and decortication for empyema. One-third of patients undergoing EPD had a prolonged air leak following surgery and were subsequently at an increased risk of developing an empyema, often requiring surgical intervention. Further technological advances are required to reduce the parenchymal air leak after extensive visceral pleurectomy.
While there was no difference in early mortality or overall survival between the EPP and EPD groups, in non-epithelioid, node-positive patients (the poorest pathological prognostic group), there appeared to be a possible survival benefit in EPP over EPD. Despite the selection bias of patients being younger, and having a better performance status in the EPP group, this did not translate to an improved short- or long-term survival as would be expected if the procedures were comparable, or if EPP was superior, in terms of mortality/morbidity and oncological outcome. This survival benefit was 4 months and it could be argued that such a marginal survival increase does not justify the morbidity and mortality risks associated with EPP, particularly in a group of patients with such a poor survival following diagnosis. However, as the median overall survival for patients with non-epithelioid node-positive disease in our cohort is only 7.6 months, a 50% increase in life expectancy may be perceived as significantly beneficial.
The patients in the EPD group were significantly older (mean age 65.0 vs 55.6 years, P < 0.001) and this may explain, in part, the poorer long-term outcome of patients in this group undergoing EPD. Also, the numbers in these groups were small, EPP group ( n = 19) and EPD group ( n = 22). We would not therefore recommend from these data that patients with non-epithelioid, clinical/pathological node-positive disease should undergo EPP as the operation of choice, and they may be better served with chemotherapy alone.
Given the potential inaccuracies in diagnostic biopsy histological subtyping, it is difficult to justify excluding patients from radical surgery following a diagnosis of biphasic disease, particularly as there is no randomized evidence to show a benefit, or lack thereof, in operating on these patients. MARS 2: A Feasibility Study Comparing (Extended) Pleurectomy Decortication Versus no Pleurectomy Decortication in Patients With Malignant Pleural Mesothelioma (MARS2) (NCT02040272), which recently opened in the UK, includes all histological subtypes and should address this question. At present, outside the MARS2 trial, we do not offer radical surgery to patients with biopsy-proven sarcomatoid disease, as these patients have very poor survival following radical surgery [ 7 ]. Even in the context of a potentially inaccurate diagnostic biopsy, we do not feel that the risks of EPD are outweighed by a survival benefit in this group.
The provision of adjuvant chemotherapy has been shown to be an important prognostic factor in patients undergoing radical, and non-radical surgery for MPM [ 5 , 6 ]. Therefore, we must not render our patients unfit for adjuvant therapy following either surgical procedure. Surprisingly, we showed no difference in the proportion of patients who were unable to receive adjuvant chemotherapy following EPP or EPD. The practice of reserving adjuvant treatment until the time at which disease progression is observed is becoming much more prevalent, and explains why many patients in the EPD group did not receive therapy in the immediate adjuvant setting. We found no survival advantage in the EPP group, despite slightly more patients having received neoadjuvant chemotherapy. Giving chemotherapy in the neoadjuvant setting has been thought to improve outcomes in radical surgery for MPM as some patients are selected out of undergoing EPP following chemotherapy, and as such, only the fittest patients are operated upon, improving the overall survival of the cohort [ 8 ]. As our patients are referred from over 20 oncological centres around the UK, we see differing practices with regard to provision of neo- or adjuvant therapy, and this is one limitation of this study.
The transition from EPP to EPD was driven mainly by the changing demographic profile of the patients referred to us for consideration of surgery. The aim of radical surgery is macroscopic complete resection, and we feel that can only be achieved by either EPP or EPD. A video-assisted pleurectomy/decortication is a debulking procedure that has not been shown to give a survival advantage. It can, however, be utilized to provide symptom control, and a possible improvement in quality of life, in patients who would be unsuitable for, or do not wish to undergo, radical surgery [ 9 , 10 ].
As EPP is associated with a high morbidity rate (62 vs 27.9% in EPD [ 11 , 12 ]), the selection criteria for this procedure are strict. It is well documented that the median age of those presenting with MPM is increasing, with the median age at diagnosis in England currently 73 years [ 13 , 14 ]. As the population of workers in industries leading to asbestos exposure has aged, the age at presentation has increased. Likewise the patterns and levels of exposure over the last 50 years have changed dramatically, and this is likely to have contributed to an older population now presenting with the disease. With a variable latency period following exposure, particularly in the case of the increasing usage of amosite (brown) asbestos in the latter years of asbestos use in the UK, it is not surprising that we are now seeing older patients presenting with MPM [ 15 ]. A longer overall survival following EPP has been associated with patients of a younger age, and the older patient is more likely to suffer from comorbidities precluding an EPP [ 16 ]. Pulmonary function must be sufficient to allow for a pneumonectomy to be performed, and so those with poorer lung function are able to undergo EPD with reduced impact on pulmonary reserve. N2 disease is often seen as a relative contraindication to performing EPP given the morbidity associated with pneumonectomy, balanced against an uncertain long-term survival benefit in this context. However, our group has previously shown that EPD can be performed in this cohort of patients without compromising overall survival, even in an older cohort of patients [ 3 ].
The majority of outcome data for both operative procedures comes from retrospective institutional reports with differing patient selection criteria and policies regarding neoadjuvant or adjuvant therapy provision, and as such are difficult to compare. Several retrospective studies have shown that EPD can be performed with good long-term oncological and overall survival outcomes [ 17 , 18 ]. One single-institution prospective study of multimodality therapy, with EPD as the surgical approach, showed an overall survival of 30 months [ 19 ]. In the recent systematic review of multimodality therapy in MPM from Cao et al ., outcomes of EPP and EPD were compared [ 20 ]. The median overall survival ranged 13–29 months for EPD and 12–22 months for EPP [ 20 ]. This study concluded that EPP and EPD can be performed safely with good long-term oncological outcomes, in selected patients, in specialized centres.
The IASLC staging project demonstrated no difference in outcome between EPP and EPD in all stages except for IMIG stage 1 disease, where patients had a 40-month survival with EPP and 23 months with EPD [ 21 ]. However, this may be partly explained by incomplete staging and the ‘Will Rogers phenomenon’ of stage migration, as patients undergoing EPD may not have lobar lymph nodes sampled at operation, and thus may be incorrectly under-staged as N0.
One major limitation of this study is its retrospective nature. We were unable to obtain data regarding chemotherapy and radiotherapy treatment for all of the patients (13.5%), which may have led to some inaccuracy in the reporting of outcomes with differing chemotherapy regimes. There was also a learning curve both technically, and in terms of postoperative and oncological management of patients with the introduction of EPD, which may have affected the early results. Another limitation of this study is that there was no standardized method of assessing disease progression, which could have overestimated the disease-free interval.
There is an inherent selection bias seen within this study; the EPP group were a highly selected group of younger patients with more favourable preoperative functional status. We would expect this bias to give improved long- and short-term outcomes in the EPP group but that has not been evident. Multivariable analysis of the prognostic factors affecting survival in the over-65 age group showed EPD to be an independent good prognostic factor. In the over-65 age group, there were far fewer patients undergoing EPP (16 vs 123), and this may have affected the outcome comparison between the two operation types in this group. The overall survival in the group undergoing EPD in those over 65 years of age is lower than the overall survival from radical surgery published in the literature [ 17–20 ]. However, these results are from groups of highly selected patients, and may be younger groups of patients, and are therefore are not comparable. Our future work includes comparing outcomes of those over 65 years treated non-surgically, a much more clinically relevant comparison to make. The conclusions of this study must be utilized with caution given the selection bias inherent in this retrospective analysis.
In conclusion, we have shown that the intentional transition to EPD from EPP within our institution has not been detrimental to patients, and has allowed us to operate on older patients, with poorer performance statuses, without increasing morbidity and mortality, or affecting long-term overall and progression-free survival. In the context of our ageing population, we feel that EPD offers selected patients with MPM the greatest chance of prolonged survival. The MARS2 study (NCT02040272) will address the lack of randomized evidence regarding the use of EPD as part of the multimodality management of MPM.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr S. Bölükbas (Wuppertal, Germany) : First of all, we have to acknowledge the ongoing contributions of Dr. Sharkey and the Leicester group to the field of malignant pleural mesothelioma. This dismal disease is still challenging with regard to diagnostic assessment and treatment options. I am sure that your article will help us to clarify some controversies. I have four questions for you.
Firstly, microscopic complete resection is the goal of surgery, as you mentioned before. In this study you aimed to determine whether the transition from EPP to EPD has been beneficial or detrimental to the outcome of radically operated patients. What was the rate of macroscopic complete resections within both groups and have you detected any statistical difference?
Secondly, the best supportive care provides a median survival of only several months, while chemotherapy alone is associated with a median survival of approximately 12 months. According to Dr. Utley et al , the survival advantage of any management that included surgical resection was estimated as being about nine months. In your study there was no difference in median survival between the groups: EPP 13 months, EPD 12 months. However, the survival rates were comparable to chemotherapy alone. Your data demonstrates no survival advantage for patients undergoing surgery unlike the other recent series around the world. How do you explain these discrepancies?
Thirdly, completion rates of surgery plus adjuvant chemotherapy therapy were 28% for EPP and 35% for EDP respectively. However, patients being rendered unfit for chemotherapy following radical surgery had a significantly inferior survival compared to those patients undergoing adjuvant chemotherapy, 7 months versus 20.7 months. Has this finding changed your daily clinical practice with regard to patient selection? I mean, should we offer surgery only to those patients who are fit for surgery and chemotherapy, rather than fit for surgery alone?
And my last question, in your study patients over the age of 65 had a very high mortality: 30-day mortality rates were 33% for EPP and 5% for EDP, and 90-day mortality was 60% for EPP and 16% for EDP respectively. Overall survival was 12.5 months for EPD and 4.7 months for the EPP group. How do you explain these high mortality rates in this age group? Should we stop offering surgery to those patients in general and should we apply different selection criteria for those patients?
Dr Sharkey : In terms of R2 resection, all of the patients in this cohort were complete macroscopic resections. We didn't include any patients who had an R2 resection, mainly because of the heterogeneity within the R2 resection itself. So leaving a 50 pence size piece versus leaving the entire hemidiaphragm obviously affects prognosis and the effect of the R2 resection on prognosis alone. We had about a 5% R2 resection rate overall for patients who were scheduled to undergo an EPD. So it is difficult to compare this with the EPP group because we had very, very few patients who had an R2 resection or an abandoned EPP in that group. All of the patients that I presented today were R1 resections.
In terms of our overall survival data as compared with chemotherapy data, in the chemotherapy group and in the surgical groups - that's all stages, all cell types, all pathological nodal diseases - we did show that with the epithelioid node negative patients they had a much higher survival but it is difficult to compare the two results.
But I am sure you, and others who operate on patients with mesothelioma, have seen in your series a stark difference in outcome between patients of the same cell type, same pathological stage, whom you would expect to do similarly well or similarly badly, but they don't. There are those who recur very early and those who have more indolent disease and recur very late. It is likely that that's due to a difference in the biology of the tumours themselves. So even if they are the same cell type, the genetic biology and the genetic makeup of those tumours must be different. And we are actually doing some work at the moment looking at the copy number and the mutational profile of those tumours from patients who have got this indolent disease versus those who have a very aggressive disease who were matched. The results that are coming out are quite interesting, but obviously I can't present them at this stage.
And as to your final question, the reason that we started the transition from EPP to EPD was because we noticed this very high early mortality in the older patients, and our patients are getting much, much older. The patients that are being referred to us are in their early 60s, 70s, and a few patients who are in their 80s, and so that is what has driven the transition to EPD. And that is also important in terms of the provision of adjuvant chemotherapy. So we have had to change the oncological practice of local oncologists at least, because if a patient is able to survive radical surgery, it is likely, if they don't have complications from that surgery, that they will be able to also undergo chemotherapy. So it is difficult to select them beforehand, because if they are suitable for surgery, they should be suitable for chemotherapy, and you can't predict which patients are going to have the morbidity associated with the operation. But lots of our patients didn't have adjuvant chemotherapy, not because they weren't fit enough but because the practice of our local oncologists is now to reserve chemotherapy until recurrence, and we have seen that those patients do equally as well as those who have upfront adjuvant chemotherapy. So it is difficult to make that part of the selection criteria, because technically if you are fit enough for an operation you should be fit enough as well to undergo chemotherapy.
Dr A. Turna (Istanbul, Turkey) : You pointed out the importance of lymph node positivity for these patients who underwent EPP or decortication. Did you change your strategy in terms of lymph node staging in this period? If you did change, why? What is the evidence behind that?
Dr Sharkey : The effect of lymph node metastasis is a controversial one in that we have also found that N1 and N2 disease patients do similarly badly as compared to those who have no node metastases. But we do a systematic lymph node dissection intraoperatively with an EPD. Actually most of our N2 disease is found in the internal mammary nodes, the diaphragmatic nodes and the pericardial nodes. So they come out anyway as part of the parietal pleurectomy. So we do do a lymph node dissection, and the prognostically important nodes that we found, at least in our patients, seemed to be those that would come out with a specimen anyway. But we do take, as with lung cancer resections, a systematic lymph node dissection as well.
Dr Turna : How many patients underwent mediastinoscopy in the two groups?
Dr Sharkey : None of the patients that we have in the EPD group have undergone mediastinoscopy unless there was evidence to suggest that they might have N2 disease, but mediastinoscopy was a requirement preoperatively for all our patients who underwent EPP. So that adds even more into the selection that we have in a highly selected group of EPP patients, because they have all been proven to be N2 negative, at least by mediastinoscopy, prior to surgery. And again, the difficulty comes then, because the patients who have N2 disease usually have N2 disease in the nodes that aren't accessible by mediastinoscopy. So we don't use that in our routine practice now.
Dr J. Kuzdzal (Krakow, Poland) : I have two questions. From your presentation it seems that some patients received their chemotherapy before the operation, some after that, and some also had adjuvant radiotherapy. Does this mean that you didn't use a unified protocol or are there some other reasons for this? Secondly, you provided survival as a mean value. Could you provide us also with the actual five-year survival rates for both groups of patients?
Dr Sharkey : Regarding chemotherapy, we receive referrals from over 20 centres around the UK, and the oncological practices of those referring centres apply; they make the decisions regarding the chemotherapy provisions. We are often referred patients who have already started chemotherapy, so they will have neoadjuvant chemotherapy. As a rule, we don't advise patients to have neoadjuvant chemotherapy, although obviously that has changed now because the MARS 2 trial has started. So all of those patients will receive neoadjuvant chemotherapy. And it is very much dependent on the oncologist. So the upfront adjuvant chemotherapy, there are some centres that give all patients that, and some patients receive chemotherapy only at recurrence, and it very much depends on the oncological practice of the referring centre. Therefore we don't have a standardized protocol because of where we receive our referrals from.
In terms of five-year survival, this is comparable between the two groups, and we do have, as in most series, and as Professor Treasure was saying earlier, that tail of long survivors at the end. So for the EPD patients the five-year survival was 6%, but we do have some patients who have survived 5, 10 years; our longest surviving patient is 12 years. So there are those patients who probably, whatever treatment they receive, would do well anyway, and so they are our long surviving group.
Dr W. Klepetko (Vienna, Austria) : My question is following on from the previous questions. I would be interested to know the actual number of your patients, or percentage of the patients, who completed trimodality therapy. The reason I ask this question is, as was mentioned before, the results that you showed us in EPP are somewhat lower than expected compared to studies from other groups that have been published. Maybe the low percentage of trimodality patients in your patient cohort might be a reason for that. When we looked at the results in our centre, there was a clear and significant difference between patients who were able to undergo complete trimodality therapy versus those who did not. Of course there is a bias of patient performance here. Could you give us some information how you did that in your study?
Dr Sharkey : As you have pointed out, the patients who receive trimodality therapy with EPP do best and that is what the best results come from. In terms of receiving trimodality therapy, the rate of radiotherapy was very low; I think it was about 12% of patients who received radiotherapy following EPP. Now, that may well be due to the fact that we had quite a high early mortality. So these patients who received neoadjuvant chemotherapy then went on to have an EPP were unable to complete that trimodality therapy, and that is one of the drivers towards moving to EPD, because the patients will need to complete their trimodality therapy if we are going to be as successful with EPD. And then our patient group that is getting older and is likely to have a high mortality, they are not going to be able to complete that, and having our patients with either early death or high morbidity rates, they are not going to be able to complete their trimodality therapy.
Dr I. Opitz (Zürich, Switzerland) : I just have a question about the rate of recurrences and the progression-free survival. Did you see any difference between the groups, and how did an added modality impact on this?
Dr Sharkey : We didn't see any difference between the EPP and EDP groups in terms of time to recurrence. When we looked at the chemotherapy data as a whole, and so not separated EPP and EDP, the recurrence rates were comparable between those who received neoadjuvant chemotherapy and those who received adjuvant chemotherapy, and at recurrence the patients who received adjuvant chemotherapy actually did much better than those who had received neoadjuvant chemotherapy. So we didn't see any difference in the time to recurrence if they received neoadjuvant or adjuvant chemotherapy.
Author notes
Presented at the 23rd European Conference on General Thoracic Surgery, Lisbon, Portugal, 31 May–3 June 2015.




