-
PDF
- Split View
-
Views
-
Cite
Cite
Regina Bokenkamp, Elizabeth Aguilar, Roel L.F. van der Palen, Vladimir Sojak, Eline F. Bruggemans, Jaroslav Hruda, Irene M. Kuipers, Mark G. Hazekamp, Reoperation for right ventricular outflow tract obstruction after arterial switch operation for transposition of the great arteries and aortic arch obstruction , European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 5, May 2016, Pages e91–e96, https://doi.org/10.1093/ejcts/ezw026
Close - Share Icon Share
Abstract
Right ventricular outflow tract obstruction (RVOTO) is one of the reasons for late reinterventions after repair of transposition of the great arteries (TGA) with aortic arch obstruction (AAO). The aim of the present study was to identify predictors of reoperation for RVOTO in patients who underwent arterial switch operation (ASO) and arch repair for TGA or Taussig–Bing anomaly with AAO.
Between 1977 and 2015, 45 patients [TGA/intact ventricular septum (IVS) 5, TGA/ventricular septal defect (VSD) 13, Taussig–Bing 27] with coarctation (21), arch hypoplasia (5), coarctation and hypoplasia (12) and aortic arch interruption (7) underwent ASO and arch repair. The median age at the ASO was 19 days (range, 1 day to 12.7 years). AAO was repaired concomitantly with ASO in 36 patients. Operation reports and 2D-echocardiographic data were retrospectively reviewed to determine the following parameters: position of the great arteries, coronary artery anatomy, and diameters of RVOT, aortic annulus, aortic sinotubular-junction, pulmonary annulus and transverse aortic arch previous to ASO. The median follow-up time was 6 years (range, 0–30 years). Four patients were lost to follow-up; reliable echo data were available in 24 subjects. Cox proportional hazard models were performed to examine predictors of reoperation for RVOTO.
Thirty-day mortality rate after ASO was 13% ( n = 6), and late mortality rate 9% ( n = 4). Ten patients (TGA/VSD 2, Taussig–Bing 8) had 14 reoperations for RVOTO. One patient died after reoperation. Taussig–Bing anomaly was a significant predictor of reoperation for RVOTO [hazard ratio (HR) = 5.5, 95% confidence interval (CI) = 1.15–26.38, P = 0.033]. Higher preoperative aortic annulus Z -score significantly decreased the reoperation risk (HR = 0.6, 95% CI = 0.42–0.93, P = 0.020). In reoperated patients, the mean gradient across the RVOT reduced from 84 ± 12.2 mmHg prior to reoperation to 15.29 ± 13.70 mmHg at latest follow-up.
Taussig–Bing anomaly and smaller preoperative aortic annulus diameter ( Z -score) were significant predictors of reoperation for RVOTO in patients after ASO for TGA or Taussig–Bing anomaly with AAO. In Taussig–Bing hearts, the more complex anatomy often necessitates modifications of the operation technique, sometimes precluding RVOT relief at primary ASO. During follow-up, the possibility of recurrent RVOTO should always be considered in this specific patient population. Yet, in case of a reoperation for RVOTO, the surgical relief is in general effective.
INTRODUCTION
Transposition of the great arteries (TGA) with VSD and also Taussig–Bing anomaly associated with aortic arch obstruction (AAO) can be repaired with good results in neonates [ 1–6 ]. Unfortunately, a substantial amount of patients will need reinterventions and reoperations in the future. Right ventricular outflow tract obstruction (RVOTO) is reported to be one of the main reasons for reoperations in these patients [ 3 , 4 ]. This is not surprising as anatomical narrowing of the RVOT is found in 25% of hearts with TGA and VSD [ 7 ]. The RVOTO is most commonly caused by rightward deviation of the outlet septum [ 7 , 8 ]. Abnormal muscular structures in the RVOT potentially limit the flow to the aorta during development and are often accompanied by AAO [ 8 ].
The aim of this study was to investigate potential predictors for reintervention at the RVOT level in a cohort of TGA and Taussig–Bing patients after arterial switch operation (ASO) and aortic arch repair since 1977.
MATERIALS AND METHODS
Patients
Patients with the diagnoses TGA/AAO, TGA/VSD/AAO and Taussig–Bing/AAO who underwent an ASO between May 1977 and September 2015 at the Leiden University Medical Center were included in the study. AAO was defined as an extended or local narrowing or interruption of the aortic arch that necessitated surgical intervention. From the cases with TGA/VSD/AAO, only patients in whom the VSD needed surgical closure were included in this study. For Taussig–Bing anomaly, a broad definition was used, including all cases with overriding of the pulmonary orifice. The need for written informed consent was waived by the local medical ethical committee.
Forty-five patients were identified, 5 with TGA/IVS, 13 with TGA/VSD and 27 with Taussig–Bing anomaly. Demographic and preoperative data are summarized in Table 1 . Most patients ( n = 36) had concurrent ASO and aortic arch repair. Four patients had a coarctectomy at a median of 38 days (range, 9–42 days) before the ASO. In 2 of these patients, a two-stage ASO repair was done at the age of 8 and 17 months after prior pulmonary banding and coarctectomy. Five patients developed coarctation after the ASO and underwent aortic arch repair subsequently at a median of 605 days (range, 15–1093 days) after ASO. Details on surgical management and perioperative data are summarized in Table 2 .
Demographic and preoperative characteristics of the total study population ( n = 45)
| Characteristics . | Number . | % . |
|---|---|---|
| Sex | ||
| Male | 29 | 64 |
| Female | 16 | 36 |
| Arterial switch operation | ||
| Age, median | 19 (1 day to 12.7 years) | |
| Weight, median, kg | 3.5 (1.7–33) | |
| VSD-type | ||
| Perimembranous | 11 | 24 |
| Muscular | 4 | 9 |
| Doubly committed | 3 | 7 |
| Sub-pulmonary | 21 | 47 |
| Multiple | 2 | 4 |
| Unknown | 4 | |
| Great vessel position | ||
| Ao anterior to PA | 5 | 11 |
| Ao right anterior to PA | 27 | 60 |
| Ao side-by-side to PA | 9 | 20 |
| Ao right posterior to PA | 1 | 2 |
| Ao left anterior to PA | 3 | 7 |
| Coronary pattern | ||
| 1LCx-2R | 29 | 64 |
| 1L-2CxR | 6 | 13 |
| 1LCxR | 2 | 4 |
| 1R-2LCx | 3 | 7 |
| 2RLCx | 1 | 2 |
| Other | 4 | 4 |
| Aortic arch obstruction type | ||
| Coarctation | 21 | 47 |
| Arch hypoplasia | 5 | 11 |
| Coarctation and arch hypoplasia | 12 | 27 |
| Aortic arch interruption | 7 | 16 |
| Characteristics . | Number . | % . |
|---|---|---|
| Sex | ||
| Male | 29 | 64 |
| Female | 16 | 36 |
| Arterial switch operation | ||
| Age, median | 19 (1 day to 12.7 years) | |
| Weight, median, kg | 3.5 (1.7–33) | |
| VSD-type | ||
| Perimembranous | 11 | 24 |
| Muscular | 4 | 9 |
| Doubly committed | 3 | 7 |
| Sub-pulmonary | 21 | 47 |
| Multiple | 2 | 4 |
| Unknown | 4 | |
| Great vessel position | ||
| Ao anterior to PA | 5 | 11 |
| Ao right anterior to PA | 27 | 60 |
| Ao side-by-side to PA | 9 | 20 |
| Ao right posterior to PA | 1 | 2 |
| Ao left anterior to PA | 3 | 7 |
| Coronary pattern | ||
| 1LCx-2R | 29 | 64 |
| 1L-2CxR | 6 | 13 |
| 1LCxR | 2 | 4 |
| 1R-2LCx | 3 | 7 |
| 2RLCx | 1 | 2 |
| Other | 4 | 4 |
| Aortic arch obstruction type | ||
| Coarctation | 21 | 47 |
| Arch hypoplasia | 5 | 11 |
| Coarctation and arch hypoplasia | 12 | 27 |
| Aortic arch interruption | 7 | 16 |
Ao: aorta; PA: pulmonary artery.
Demographic and preoperative characteristics of the total study population ( n = 45)
| Characteristics . | Number . | % . |
|---|---|---|
| Sex | ||
| Male | 29 | 64 |
| Female | 16 | 36 |
| Arterial switch operation | ||
| Age, median | 19 (1 day to 12.7 years) | |
| Weight, median, kg | 3.5 (1.7–33) | |
| VSD-type | ||
| Perimembranous | 11 | 24 |
| Muscular | 4 | 9 |
| Doubly committed | 3 | 7 |
| Sub-pulmonary | 21 | 47 |
| Multiple | 2 | 4 |
| Unknown | 4 | |
| Great vessel position | ||
| Ao anterior to PA | 5 | 11 |
| Ao right anterior to PA | 27 | 60 |
| Ao side-by-side to PA | 9 | 20 |
| Ao right posterior to PA | 1 | 2 |
| Ao left anterior to PA | 3 | 7 |
| Coronary pattern | ||
| 1LCx-2R | 29 | 64 |
| 1L-2CxR | 6 | 13 |
| 1LCxR | 2 | 4 |
| 1R-2LCx | 3 | 7 |
| 2RLCx | 1 | 2 |
| Other | 4 | 4 |
| Aortic arch obstruction type | ||
| Coarctation | 21 | 47 |
| Arch hypoplasia | 5 | 11 |
| Coarctation and arch hypoplasia | 12 | 27 |
| Aortic arch interruption | 7 | 16 |
| Characteristics . | Number . | % . |
|---|---|---|
| Sex | ||
| Male | 29 | 64 |
| Female | 16 | 36 |
| Arterial switch operation | ||
| Age, median | 19 (1 day to 12.7 years) | |
| Weight, median, kg | 3.5 (1.7–33) | |
| VSD-type | ||
| Perimembranous | 11 | 24 |
| Muscular | 4 | 9 |
| Doubly committed | 3 | 7 |
| Sub-pulmonary | 21 | 47 |
| Multiple | 2 | 4 |
| Unknown | 4 | |
| Great vessel position | ||
| Ao anterior to PA | 5 | 11 |
| Ao right anterior to PA | 27 | 60 |
| Ao side-by-side to PA | 9 | 20 |
| Ao right posterior to PA | 1 | 2 |
| Ao left anterior to PA | 3 | 7 |
| Coronary pattern | ||
| 1LCx-2R | 29 | 64 |
| 1L-2CxR | 6 | 13 |
| 1LCxR | 2 | 4 |
| 1R-2LCx | 3 | 7 |
| 2RLCx | 1 | 2 |
| Other | 4 | 4 |
| Aortic arch obstruction type | ||
| Coarctation | 21 | 47 |
| Arch hypoplasia | 5 | 11 |
| Coarctation and arch hypoplasia | 12 | 27 |
| Aortic arch interruption | 7 | 16 |
Ao: aorta; PA: pulmonary artery.
| Characteristics . | Number of patients . | % . |
|---|---|---|
| Lecompte | ||
| Yes | 23 | 51 |
| No | 20 | 44 |
| Unknown | 2 | 4 |
| Aortic arch repair | ||
| Concurrent | 36 | 80 |
| Before ASO | 4 | 9 |
| After ASO | 5 | 11 |
| Mode of arch repair | ||
| Coarctectomy and end-to-end anastomosis | 26 | 58 |
| Arch repair with patch | 15 | 33 |
| Unknown | 4 | 9 |
| Sternal closure | ||
| Primary | 15 | 33 |
| Secondary | 16 | 36 |
| Unknown | 14 | 31 |
| Postoperative mechanical support a | ||
| No | 42 | 98 |
| Yes | 1 | 2 |
| Mortality | ||
| Overall | 10 | 22 |
| Early | 4 | 9 |
| Late | 6 | 15 |
| Reoperations b | ||
| RVOTO | 10 | 22 |
| Other indications | 5 | 11 |
| RVOTO and other indication | 2 | NA |
| Indications for other reoperations | ||
| Aortic root dilatation | 1 | NA |
| Recoarctation | 4 | NA |
| Coronary obstruction | 2 | NA |
| Residual septal defects | 1 | NA |
| AV valve leakage | 2 | NA |
| MPA or/and MPAB stenosis | 1 | NA |
| Endocarditis | 1 | NA |
| Mode of RVOT reoperation c | ||
| Muscle resection | 2 | NA |
| Transannular patch | 7 | NA |
| Conduit RV to PA bypass | 4 | NA |
| Characteristics . | Number of patients . | % . |
|---|---|---|
| Lecompte | ||
| Yes | 23 | 51 |
| No | 20 | 44 |
| Unknown | 2 | 4 |
| Aortic arch repair | ||
| Concurrent | 36 | 80 |
| Before ASO | 4 | 9 |
| After ASO | 5 | 11 |
| Mode of arch repair | ||
| Coarctectomy and end-to-end anastomosis | 26 | 58 |
| Arch repair with patch | 15 | 33 |
| Unknown | 4 | 9 |
| Sternal closure | ||
| Primary | 15 | 33 |
| Secondary | 16 | 36 |
| Unknown | 14 | 31 |
| Postoperative mechanical support a | ||
| No | 42 | 98 |
| Yes | 1 | 2 |
| Mortality | ||
| Overall | 10 | 22 |
| Early | 4 | 9 |
| Late | 6 | 15 |
| Reoperations b | ||
| RVOTO | 10 | 22 |
| Other indications | 5 | 11 |
| RVOTO and other indication | 2 | NA |
| Indications for other reoperations | ||
| Aortic root dilatation | 1 | NA |
| Recoarctation | 4 | NA |
| Coronary obstruction | 2 | NA |
| Residual septal defects | 1 | NA |
| AV valve leakage | 2 | NA |
| MPA or/and MPAB stenosis | 1 | NA |
| Endocarditis | 1 | NA |
| Mode of RVOT reoperation c | ||
| Muscle resection | 2 | NA |
| Transannular patch | 7 | NA |
| Conduit RV to PA bypass | 4 | NA |
ASO: arterial switch operation; RVOTO: right ventricular outflow tract obstruction; AV: atrioventricular; MPA: main pulmonary artery; MPAB: main pulmonary artery branch; RV: right ventricle; PA: pulmonary artery; NA: not applicable.
a Two patients died intraoperatively.
b Two patients had more than one indication for reoperation. Some patients had more indications for reoperations at the same time or subsequently.
c Two patients had a second and one had a third reoperation for RVOTO, muscle resection and transannular patch was combined in 1 patient.
| Characteristics . | Number of patients . | % . |
|---|---|---|
| Lecompte | ||
| Yes | 23 | 51 |
| No | 20 | 44 |
| Unknown | 2 | 4 |
| Aortic arch repair | ||
| Concurrent | 36 | 80 |
| Before ASO | 4 | 9 |
| After ASO | 5 | 11 |
| Mode of arch repair | ||
| Coarctectomy and end-to-end anastomosis | 26 | 58 |
| Arch repair with patch | 15 | 33 |
| Unknown | 4 | 9 |
| Sternal closure | ||
| Primary | 15 | 33 |
| Secondary | 16 | 36 |
| Unknown | 14 | 31 |
| Postoperative mechanical support a | ||
| No | 42 | 98 |
| Yes | 1 | 2 |
| Mortality | ||
| Overall | 10 | 22 |
| Early | 4 | 9 |
| Late | 6 | 15 |
| Reoperations b | ||
| RVOTO | 10 | 22 |
| Other indications | 5 | 11 |
| RVOTO and other indication | 2 | NA |
| Indications for other reoperations | ||
| Aortic root dilatation | 1 | NA |
| Recoarctation | 4 | NA |
| Coronary obstruction | 2 | NA |
| Residual septal defects | 1 | NA |
| AV valve leakage | 2 | NA |
| MPA or/and MPAB stenosis | 1 | NA |
| Endocarditis | 1 | NA |
| Mode of RVOT reoperation c | ||
| Muscle resection | 2 | NA |
| Transannular patch | 7 | NA |
| Conduit RV to PA bypass | 4 | NA |
| Characteristics . | Number of patients . | % . |
|---|---|---|
| Lecompte | ||
| Yes | 23 | 51 |
| No | 20 | 44 |
| Unknown | 2 | 4 |
| Aortic arch repair | ||
| Concurrent | 36 | 80 |
| Before ASO | 4 | 9 |
| After ASO | 5 | 11 |
| Mode of arch repair | ||
| Coarctectomy and end-to-end anastomosis | 26 | 58 |
| Arch repair with patch | 15 | 33 |
| Unknown | 4 | 9 |
| Sternal closure | ||
| Primary | 15 | 33 |
| Secondary | 16 | 36 |
| Unknown | 14 | 31 |
| Postoperative mechanical support a | ||
| No | 42 | 98 |
| Yes | 1 | 2 |
| Mortality | ||
| Overall | 10 | 22 |
| Early | 4 | 9 |
| Late | 6 | 15 |
| Reoperations b | ||
| RVOTO | 10 | 22 |
| Other indications | 5 | 11 |
| RVOTO and other indication | 2 | NA |
| Indications for other reoperations | ||
| Aortic root dilatation | 1 | NA |
| Recoarctation | 4 | NA |
| Coronary obstruction | 2 | NA |
| Residual septal defects | 1 | NA |
| AV valve leakage | 2 | NA |
| MPA or/and MPAB stenosis | 1 | NA |
| Endocarditis | 1 | NA |
| Mode of RVOT reoperation c | ||
| Muscle resection | 2 | NA |
| Transannular patch | 7 | NA |
| Conduit RV to PA bypass | 4 | NA |
ASO: arterial switch operation; RVOTO: right ventricular outflow tract obstruction; AV: atrioventricular; MPA: main pulmonary artery; MPAB: main pulmonary artery branch; RV: right ventricle; PA: pulmonary artery; NA: not applicable.
a Two patients died intraoperatively.
b Two patients had more than one indication for reoperation. Some patients had more indications for reoperations at the same time or subsequently.
c Two patients had a second and one had a third reoperation for RVOTO, muscle resection and transannular patch was combined in 1 patient.
Technique for surgical right ventricular outflow tract obstruction relief
Management on RVOTO or potential RVOTO during primary arch repair was to resect all muscle bundles between the septum and RVOT anterior wall as much as possible. Primary transannular RVOT patching was not performed. Only in reoperations, transannular patches and conduits from the right ventricle to the pulmonary artery (RV to PA) were applied wherever necessary [e.g. when the right coronary artery (RCA) was in the way]. Posterior transannular enlargement was not performed in our institution.
Data collection
The hospital records were retrospectively reviewed for the following patient characteristics: diagnosis, especially position of the great arteries, coronary anatomy, morphology of the aortic arch and location of the VSD. Details of the surgical anatomy and technique were collected from the surgical notes. Information on postoperative course and follow-up was extracted from the hospital records and from letters/information provided by the referring cardiologist if the patient had follow-up in another hospital. Operative mortality (i.e. early mortality) was defined as mortality within 30 days from operation or later if the patient was still hospitalized. Late mortality was defined as mortality after 30 days from operation or after discharge from hospital after primary ASO.
Echocardiographic measurements
All preoperative echocardiograms and the echocardiograms at discharge and at the latest follow-up were reviewed. Echocardiographic measurements (2D) for dimensions of cardiac structures included: RVOT, aortic annulus (neopulmonary annulus), neoaortic annulus, sinotubular-junction (ST-junction), transverse and distal aortic arch. Dimensions for the valves were measured from hinge-point to hinge-point, for intracardiac and vessel diameter from inner-edge to inner-egde, according to the international guidelines [ 9 ]. To account for the range of patient age and body size, Z -scores for 2D anatomical structures were calculated for each patient from the measurements and body surface area using reference values of Pettersen et al. [ 10 ]. For the calculation of Z -scores, the function of the semilunar valve was leading for choosing the appropriate reference values. Furthermore, continuous-wave Doppler echocardiographic assessment across the RVOT and tricuspid valve were recorded. Since especially echocardiographic data of the earlier days were hard to obtain, reliable echocardiographic images and Doppler data were available for re-evaluation in 24 patients of the cohort. Preoperative echocardiographic measurements are presented in Table 3 .
| Parameter . | Mean . | SD . |
|---|---|---|
| Total study population ( n = 24) | ||
| Preoperative | ||
| RVOT diameter (mm) | 8.72 | 2.27 |
| Z -score aortic annulus | 0.53 | 1.99 |
| Z -score ST-junction | −1.10 | 1.53 |
| Z -score transverse arch | −3.24 | 1.92 |
| Z -score pulmonary annulus | 1.37 | 2.27 |
| At latest follow-up | ||
| Z -score pulmonary annulus | −0.91 | 1.16 |
| RVOT Doppler (CW in m/s) | 15.05 | 13.55 |
| Patients reoperated for RVOTO ( n = 9) | ||
| Prereoperation for RVOTO | ||
| RVOT diameter (mm) | 9.17 | 3.80 |
| Neopulmonary annulus diameter (mm) | 10.16 | 2.66 |
| Z -score neopulmonary annulus | −1.72 | 2.01 |
| RVOT Doppler (CW in m/s) | 84.25 | 12.20 |
| At latest follow-up | ||
| RVOT diameter (mm) | 15.82 | 5.64 |
| Neopulmonary annulus diameter (mm) | 13.00 | 3.92 |
| Z -score neopulmonary annulus | −0.97 | 1.63 |
| RVOT Doppler (CW in m/s) | 15.29 | 13.70 |
| Parameter . | Mean . | SD . |
|---|---|---|
| Total study population ( n = 24) | ||
| Preoperative | ||
| RVOT diameter (mm) | 8.72 | 2.27 |
| Z -score aortic annulus | 0.53 | 1.99 |
| Z -score ST-junction | −1.10 | 1.53 |
| Z -score transverse arch | −3.24 | 1.92 |
| Z -score pulmonary annulus | 1.37 | 2.27 |
| At latest follow-up | ||
| Z -score pulmonary annulus | −0.91 | 1.16 |
| RVOT Doppler (CW in m/s) | 15.05 | 13.55 |
| Patients reoperated for RVOTO ( n = 9) | ||
| Prereoperation for RVOTO | ||
| RVOT diameter (mm) | 9.17 | 3.80 |
| Neopulmonary annulus diameter (mm) | 10.16 | 2.66 |
| Z -score neopulmonary annulus | −1.72 | 2.01 |
| RVOT Doppler (CW in m/s) | 84.25 | 12.20 |
| At latest follow-up | ||
| RVOT diameter (mm) | 15.82 | 5.64 |
| Neopulmonary annulus diameter (mm) | 13.00 | 3.92 |
| Z -score neopulmonary annulus | −0.97 | 1.63 |
| RVOT Doppler (CW in m/s) | 15.29 | 13.70 |
RVOT: right ventricular outflow tract; RVOTO: right ventricular outflow tract obstruction; SD: standard deviation; ST-junction: sinotubular-junction.
| Parameter . | Mean . | SD . |
|---|---|---|
| Total study population ( n = 24) | ||
| Preoperative | ||
| RVOT diameter (mm) | 8.72 | 2.27 |
| Z -score aortic annulus | 0.53 | 1.99 |
| Z -score ST-junction | −1.10 | 1.53 |
| Z -score transverse arch | −3.24 | 1.92 |
| Z -score pulmonary annulus | 1.37 | 2.27 |
| At latest follow-up | ||
| Z -score pulmonary annulus | −0.91 | 1.16 |
| RVOT Doppler (CW in m/s) | 15.05 | 13.55 |
| Patients reoperated for RVOTO ( n = 9) | ||
| Prereoperation for RVOTO | ||
| RVOT diameter (mm) | 9.17 | 3.80 |
| Neopulmonary annulus diameter (mm) | 10.16 | 2.66 |
| Z -score neopulmonary annulus | −1.72 | 2.01 |
| RVOT Doppler (CW in m/s) | 84.25 | 12.20 |
| At latest follow-up | ||
| RVOT diameter (mm) | 15.82 | 5.64 |
| Neopulmonary annulus diameter (mm) | 13.00 | 3.92 |
| Z -score neopulmonary annulus | −0.97 | 1.63 |
| RVOT Doppler (CW in m/s) | 15.29 | 13.70 |
| Parameter . | Mean . | SD . |
|---|---|---|
| Total study population ( n = 24) | ||
| Preoperative | ||
| RVOT diameter (mm) | 8.72 | 2.27 |
| Z -score aortic annulus | 0.53 | 1.99 |
| Z -score ST-junction | −1.10 | 1.53 |
| Z -score transverse arch | −3.24 | 1.92 |
| Z -score pulmonary annulus | 1.37 | 2.27 |
| At latest follow-up | ||
| Z -score pulmonary annulus | −0.91 | 1.16 |
| RVOT Doppler (CW in m/s) | 15.05 | 13.55 |
| Patients reoperated for RVOTO ( n = 9) | ||
| Prereoperation for RVOTO | ||
| RVOT diameter (mm) | 9.17 | 3.80 |
| Neopulmonary annulus diameter (mm) | 10.16 | 2.66 |
| Z -score neopulmonary annulus | −1.72 | 2.01 |
| RVOT Doppler (CW in m/s) | 84.25 | 12.20 |
| At latest follow-up | ||
| RVOT diameter (mm) | 15.82 | 5.64 |
| Neopulmonary annulus diameter (mm) | 13.00 | 3.92 |
| Z -score neopulmonary annulus | −0.97 | 1.63 |
| RVOT Doppler (CW in m/s) | 15.29 | 13.70 |
RVOT: right ventricular outflow tract; RVOTO: right ventricular outflow tract obstruction; SD: standard deviation; ST-junction: sinotubular-junction.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median and range, as appropriate. Kaplan–Meier analysis was used to estimate probabilities of overall survival and freedom from reoperations. Risk factors for overall mortality and reoperation for RVOTO were explored by using univariable Cox regression analysis. The predictor variables considered were Taussig–Bing anomaly, left coronary artery originating from sinus 2, deviant great vessel arrangement, simple versus complex arch pathology and Z -scores of the aortic annulus, ST-junction, transverse arch and PA annulus. Deviant great vessel arrangement was defined as all arrangements in which the aorta was not located right anterior to the PA. Complex arch pathology included arch hypoplasia combined with coarctation and aortic arch interruption. A P -value of less than 0.05 was considered statistically significant. All analyses were performed with SPSS version 20.0.0.1 (SPSS, Chicago, IL, USA).
RESULTS
Follow-up
The median follow-up time was 6 years (range, 0–30 years). The follow-up rate was 91%; 4 patients were lost to follow-up because their families live abroad.
Mortality
Operative mortality rate was 13% ( n = 6). Five out of these 6 patients were operated before the year 2000. Two patients could not be weaned from cardiopulmonary bypass, 1 patient developed a ventricular tachycardia which did not respond to any therapeutic manoeuvre during transfer to the intensive care unit (ICU). The residual 3 patients died while staying on the ICU; 2 of them due to biventricular dysfunction after 2 and 14 days, respectively. One patient died after 29 days. This patient had a postoperative infarction and was on a left ventricle assist device, weaned 3 days later but finally suffered a cardiac arrest without response.
There were 4 late deaths (9%). One patient died 2 days after hospital discharge and experienced a sudden cardiac arrest at home. A second patient died during the reoperation for RVOTO with simultaneous mitral valve plasty and right coronary button reimplantation because of coronary problems 5 months after his corrective operation. Two patients died at the age of 5 and 30 years after the switch operation, respectively; the details surrounding the deaths were not documented in these cases.
The estimated 1-, 5-, 10- and 15-year overall survival rates for the 45 patients were 81.8, 78.5, 78.5 and 78.5%, respectively (Fig. 1 ). Univariable Cox regression analysis revealed a higher risk for overall mortality in patients with Taussig–Bing anomaly [hazard ratio (HR) = 54.2]. However, because of the small patient numbers, the 95% confidence interval (CI) was very large (0.24–12366.46) and the P -value was not statistically significant ( P = 0.150).

Estimated overall survival rates at 1, 5, 10 and 15 years were 81.8, 78.5, 78.5 and 78.5%, respectively. The patient with the longest follow-up had died at the age of 30 years.
Late reinterventions
There were 14 reoperations for RVOTO in 10 patients. Indications for reoperation for RVOTO were RVOTO with or without supravalvular obstruction based on echocardiographic measurements of a Doppler-gradient (cw) ≥60 mmHg (4 m/s) or RV pressures (as measured by tricuspid insufficiency) ≥70 mmHg or greater than two-third of systolic blood pressure. To relieve the RVOTO, eight RVOT transannular patches and four conduit RV to PA bypass grafts were implanted. Twice the RVOTO was treated with solely infundibular muscle resection. Combinations of these operation techniques/modalities to relieve the RVOTO were necessary for some patients. In 1 patient with an RCA running over the RVOT (coronary pattern: 1RL-2Cx), a valveless Goretex conduit was placed from RV to PA during the first reoperation. In a second reoperation, this conduit was replaced by a Hemashield prosthesis and a Contegra conduit was implanted during a third reoperation. In 2 patients, Contegra conduits were implanted as second reintervention after transannular patching was done at a first attempt to relieve the RVOTO.
The estimated 1-, 5- and 10-year freedom from reoperation for RVOTO rates were 87.5, 71.2 and 66.5%, respectively (Fig. 2 ). Univariable Cox regression analysis revealed the Taussig–Bing anomaly as a significant predictor of reoperation for RVOTO (HR = 5.5, 95% CI = 1.15–26.38, P = 0.033). A lower preoperative aortic annulus Z -score (HR = 0.6, 95% CI = 0.42–0.93, P = 0.020) and deviant great vessel arrangement (HR = 4.4, 95% CI = 1.22–15.88, P = 0.023) were also found to be significant predictors of reoperation at the RVOT (Table 4 ).
Univariable Cox regression analysis of potential predictors of reoperation for RVOTO
| Predictor . | HR . | 95% CI . | P -value . |
|---|---|---|---|
| Diagnosis | |||
| Taussig–Bing anomaly | 5.5 | 1.15–26.38 | 0.033 |
| LCA from sinus 2 | 1.6 | 0.42–6.26 | 0.49 |
| Deviant great vessel arrangement | 4.4 | 1.22–15.88 | 0.023 |
| Complex arch pathology | 3.0 | 0.84–10.68 | 0.091 |
| Preoperative echo data | |||
| Z -score aortic annulus | 0.6 | 0.42–0.93 | 0.020 |
| Z -score ST-junction | 0.9 | 0.52–1.40 | 0.53 |
| Z -score transverse arch | 0.6 | 0.32–1.12 | 0.11 |
| Z -score pulmonary annulus | 1.3 | 0.81–2.06 | 0.29 |
| Predictor . | HR . | 95% CI . | P -value . |
|---|---|---|---|
| Diagnosis | |||
| Taussig–Bing anomaly | 5.5 | 1.15–26.38 | 0.033 |
| LCA from sinus 2 | 1.6 | 0.42–6.26 | 0.49 |
| Deviant great vessel arrangement | 4.4 | 1.22–15.88 | 0.023 |
| Complex arch pathology | 3.0 | 0.84–10.68 | 0.091 |
| Preoperative echo data | |||
| Z -score aortic annulus | 0.6 | 0.42–0.93 | 0.020 |
| Z -score ST-junction | 0.9 | 0.52–1.40 | 0.53 |
| Z -score transverse arch | 0.6 | 0.32–1.12 | 0.11 |
| Z -score pulmonary annulus | 1.3 | 0.81–2.06 | 0.29 |
HR: hazard ratio; CI: confidence interval; LCA; left coronary artery , ST-junction: sinotubular-junction; RVOTO: right ventricular outflow tract obstruction.
Univariable Cox regression analysis of potential predictors of reoperation for RVOTO
| Predictor . | HR . | 95% CI . | P -value . |
|---|---|---|---|
| Diagnosis | |||
| Taussig–Bing anomaly | 5.5 | 1.15–26.38 | 0.033 |
| LCA from sinus 2 | 1.6 | 0.42–6.26 | 0.49 |
| Deviant great vessel arrangement | 4.4 | 1.22–15.88 | 0.023 |
| Complex arch pathology | 3.0 | 0.84–10.68 | 0.091 |
| Preoperative echo data | |||
| Z -score aortic annulus | 0.6 | 0.42–0.93 | 0.020 |
| Z -score ST-junction | 0.9 | 0.52–1.40 | 0.53 |
| Z -score transverse arch | 0.6 | 0.32–1.12 | 0.11 |
| Z -score pulmonary annulus | 1.3 | 0.81–2.06 | 0.29 |
| Predictor . | HR . | 95% CI . | P -value . |
|---|---|---|---|
| Diagnosis | |||
| Taussig–Bing anomaly | 5.5 | 1.15–26.38 | 0.033 |
| LCA from sinus 2 | 1.6 | 0.42–6.26 | 0.49 |
| Deviant great vessel arrangement | 4.4 | 1.22–15.88 | 0.023 |
| Complex arch pathology | 3.0 | 0.84–10.68 | 0.091 |
| Preoperative echo data | |||
| Z -score aortic annulus | 0.6 | 0.42–0.93 | 0.020 |
| Z -score ST-junction | 0.9 | 0.52–1.40 | 0.53 |
| Z -score transverse arch | 0.6 | 0.32–1.12 | 0.11 |
| Z -score pulmonary annulus | 1.3 | 0.81–2.06 | 0.29 |
HR: hazard ratio; CI: confidence interval; LCA; left coronary artery , ST-junction: sinotubular-junction; RVOTO: right ventricular outflow tract obstruction.
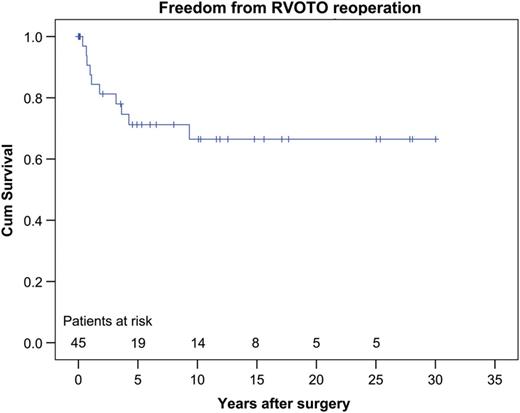
Estimated freedom from RVOTO reoperation rates at 1, 5 and 10 years was 87.5, 71.2 and 66.5%, respectively. RVOTO: right ventricular outflow tract obstruction.
Reoperations were predominantly but not only directed at relief of the RVOTO. Twenty-six all-cause reoperations occurred in 16 patients. The causes observed included recoarctation, coronary obstruction, residual septal defects, leakage of one of the atrioventricular valves, main PA and/or main PA branch stenosis and finally there was 1 patient who developed endocarditis in the tricuspid valve with a need for vegetation resection and plasty of this valve. The estimated 1-, 5- and 10-year freedom from any reoperation rates was 81.3, 54.9 and 49.9%, respectively (Fig. 3 ).
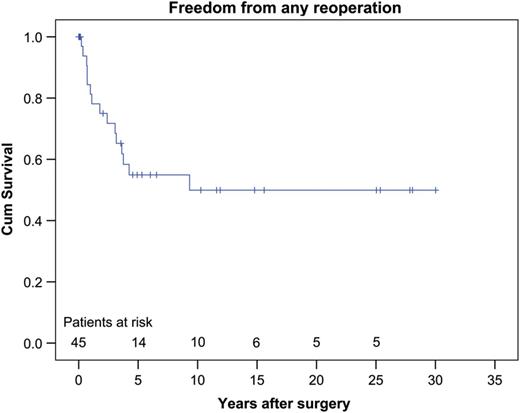
Estimated freedom from any reoperation rates at 1, 5 and 10 years was 81.3, 54.9 and 49.9%, respectively.
Eleven catheter interventions were performed in 7 patients. Interventions were done to treat recoarctation, supravalvular RVOTO or branch PA stenosis and/or valvular RVOTO. Three patients finally underwent surgical treatment because intervention was of insufficient success (Table 5 ).
| Indication . | Intervention ( n ) . | Number of patients ( n ) . |
|---|---|---|
| Stenosis PA and branches | 5 | 3 |
| Pulmonary valve stenosis | 1 | 1 |
| Recoarctation | 5 | 3 |
| Total | 11 | 7 |
| Indication . | Intervention ( n ) . | Number of patients ( n ) . |
|---|---|---|
| Stenosis PA and branches | 5 | 3 |
| Pulmonary valve stenosis | 1 | 1 |
| Recoarctation | 5 | 3 |
| Total | 11 | 7 |
Three patients finally underwent surgical treatment.
PA: pulmonary artery.
| Indication . | Intervention ( n ) . | Number of patients ( n ) . |
|---|---|---|
| Stenosis PA and branches | 5 | 3 |
| Pulmonary valve stenosis | 1 | 1 |
| Recoarctation | 5 | 3 |
| Total | 11 | 7 |
| Indication . | Intervention ( n ) . | Number of patients ( n ) . |
|---|---|---|
| Stenosis PA and branches | 5 | 3 |
| Pulmonary valve stenosis | 1 | 1 |
| Recoarctation | 5 | 3 |
| Total | 11 | 7 |
Three patients finally underwent surgical treatment.
PA: pulmonary artery.
Echocardiographic measurements
An overview of the most relevant echocardiographic measurements is presented in Table 3 . Preoperatively, the aortic annulus in the total study population was, on average, 70% of the diameter of the PA with a mean Z -score of 0.53 ± 1.99. For patients who had to be reoperated for RVOTO, the mean Z -score for the annulus of this valve had decreased to −1.72 ± 2.01 at the time of the reoperation. These patients had a mild-to-moderate echocardiographic Doppler-gradient across the RVOT at discharge after the ASO with a median of 36 mmHg (range, 16–57 mmHg). In patients without reoperation for RVOTO, there was no gradient across the RVOT present at discharge (in case of laminar colour-flow Doppler, quantitative measurements were not always performed). In reoperated patients, the mean gradient across the RVOT reduced from 84 ± 12.2 mmHg prior to reoperation to 15.29 ± 13.70 mmHg at latest follow-up. In non-reoperated patients, the mean gradient across the RVOT at latest follow-up was 14.50 ± 15.10 mmHg. The neopulmonary annulus Z -score in reoperated patients increased to a mean of −0.97 ± 1.63 at latest follow-up; in non-reoperated patients the mean neopulmonary annulus Z -score at latest follow-up was −0.95 ± 1.10.
DISCUSSION
One-stage repair for TGA and Taussig–Bing patients with AAO can be performed in almost all neonates with good results and therefore deserves to be the treatment of choice for these complex lesions [ 1–5 ]. The superiority of this approach has already been suggested more than 20 years ago [ 11 ]. However, despite good clinical outcome for most patients, a high proportion of them need various reinterventions and reoperations during the follow-up period [ 3 , 4 , 12 ]. The present study focused on the reoperations for RVOTO in TGA and Taussig–Bing patients with combined aortic arch anomalies for which ASO with VSD closure and aortic arch repair was required.
The prevalence of reoperation for RVOTO in the present series was 23% for all patients and 31% for those with Taussig–Bing anomaly. Univariable Cox regression analysis identified Taussig–Bing anomaly as a significant predictor of reoperation for RVOTO. This complicating effect of the Taussig–Bing morphology has also been found in previous studies [ 2–6 ]. The underlying reasons for the less favourable prognosis in this sub-group of patients have an anatomical and technical origin. Rightward deviation of the outlet septum, the ventricular infundibular fold and hypertrophied and aberrant muscle bundles may all contribute individually or collectively to the subaortic obstruction in TGA [ 7 ]. In Taussig–Bing hearts, the rightward deviation is more pronounced and the position of the aorta is more often side-by-side instead of (right) anterior. The side-by-side and other abnormal positions of the great vessels make the Lecompte manoeuvre, as an integral part of the ASO, not always possible. In less than half ( n = 11) of our Taussig–Bing patients, a Lecompte manoeuvre was performed. Five of these patients needed reoperations at the RVOT during follow-up. Doppler data were available in three of them. In all, a residual gradient was present at discharge potentially indicating the future problem.
In addition to Taussig–Bing anomaly, the univariable Cox regression analysis identified an aorta that is not located right anterior to the PA as a significant predictor of reoperation at the RVOT. Another factor that precludes RVOT repair at the primary ASO is abnormal coronary arteries, especially when an artery is crossing the RVOT. A right coronary artery that runs over the RVOT (e.g. when coronary anatomy is 1RL-2Cx) will make transannular patch augmentation more difficult and in some cases even impossible. Then the only solution to relieve RVOTO is by creation of a conduit from RV to PA. As these conduits can easily become compressed between the heart and sternum, recurrence of obstruction is not uncommon. The patient, who received two conduit replacements at a follow-up period of 20 years after the first conduit was implanted, is an example of this problem.
The analysis of our echocardiographic data, mainly in the 24 most recently operated patients of the study cohort demonstrated that a greater aortic annulus Z -score prior to ASO significantly decreased the chance of reoperation for RVOTO. Patients who had to be reoperated for RVOTO had a mild-to-moderate echocardiographic Doppler-gradient across the RVOT at discharge after the ASO, whereas there was no gradient present in patients without reoperation. Due to the lack of quantitative measurements for all echo studies (quantitative measurements were not always performed in case of laminar colour-flow Doppler), we were not able to explore whether the residual Doppler-gradient across the RVOT at discharge was a risk factor for reoperation using Cox regression analysis. However, on the basis of our observation, we certainly believe that a gradient across the RVOT at discharge might be indicative of a potential future problem. In case of a reoperation for RVOTO, the surgical relief is in general effective. In reoperated patients, this might be concluded from the reduction in mean gradient across the RVOT to a level that was comparable with the mean of the non-reoperated patients at latest follow-up. Also, the mean neopulmonary annulus Z -score at latest follow-up in reoperated patients as similar to the mean of the non-reoperated patients.
Limitations
This study is retrospective and includes patients with a follow-up period up to 30 years. Although this could be seen as an advantage for insights in the long-term clinical outcome in this patient population, this explains why reliable preoperative echocardiographic data were not available in all patients. In the survival analyses, the numbers of patients at risk after 10 years of follow-up were too small to allow for reliable estimates. Small patient numbers might also have hampered the analyses for potential risk factors for mortality and reoperation.
CONCLUSIONS
The Taussig–Bing anomaly, an aorta that is not located right anterior to the PA and smaller preoperative aortic annulus diameter ( Z -score) were significant predictors of reoperation for RVOTO in patients after ASO for TGA or Taussig–Bing anomaly with AAO. In the Taussig–Bing anomaly, the more complex anatomy often necessitates modifications of the operation technique, sometimes precluding RVOT relief at primary ASO. During follow-up, the possibility of recurrent RVOT obstruction should always be considered in this specific patient population. Yet, in case of a reoperation for RVOTO, the surgical relief is in general effective.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr R. Mair(Linz, Austria): I have two questions. The first question is: did you examine the immediate postoperative results after the arterial switch operation in your group of patients, and were there any minor obstructive lesions which gradually increased, or was everything perfect?
Dr Bokenkamp: For sure, we examined our patients postoperatively, but it was difficult to collect enough reliable echocardiographic data to have this follow-up. So as you can see, we have just about half of the patients with echocardiographic follow-up, among those, there were with some but there was some minor obstruction after repair.
Dr Mair: Because this would make our understanding of this problem easier, because the aortic valve Z-score that you showed on your presentation was almost normal. It was +0.06.
Dr Bokenkamp: Yes I agree.
Dr Mair: So it was almost exclusively a valvular problem? Because 11 patients of this series had either a trans annular patch or an RV-PA conduit. Was this valve just too small, or was it to some other extent dysplastic?
Dr Bokenkamp: In most of the patients it was indeed a valcular problem. Particularly the valve annulus was too small. In this case the subvalvular musculature can add obstruction with increasing hypertrophy over the years.
Dr Mair: Okay, thank you.
Author notes
Presented at the 29th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Amsterdam, Netherlands, 3–7 October 2015.
- aorta
- aortic arch
- echocardiography
- transposition of great vessels
- arterial switch operation
- lung
- ventricular septal defect
- follow-up
- preoperative care
- repeat surgery
- surgical procedures, operative
- mortality
- right ventricular outflow obstruction
- anulus fibrosus of aorta
- right ventricular outflow tract




