-
PDF
- Split View
-
Views
-
Cite
Cite
François Laborde, Theodor Fischlein, Kavous Hakim-Meibodi, Martin Misfeld, Thierry Carrel, Marian Zembala, Francesco Madonna, Bart Meuris, Axel Haverich, Malakh Shrestha, on behalf of the Cavalier Trial Investigators, Thierry Folliguet, Kostantinos Zannis, Steffen Pfeiffer, Giuseppe Santarpino, Samir Sarikouch, Christoph Bara, Jan F. Gummert, Friedrich W. Mohr, Pascal Dohmen, Mario Stalder, Eva Roost, Krzysztof Filipiak, Tomasz Niklewski, Xavier Roques, Willem J. Flameng, Axel M.M. Laczkovics, Matthias Bechtel, Alain G. Prat, Carlo Banfi, Otto E. Dapunt, Harald C. Eichstaedt, Wolfang Harringer, Ulrike Carstens-Fitz, Tom J. Spyt, Jan Gerhard Wimmer-Greinecker, Matthias Machner, Erwin S.H. Tan, Filip P.A. Casselman, Alaaddin Yilmaz, Uday Sonker, Sabine Bleiziffer, Peter J. Oberwalder, Alfred A. Kocher, Rainald Seitelberger, Hendrik Treede, Leonard Conradi, Riccardo Cocchieri, Bas De Mol, Jean-Christian Roussel, Philippe Despins, Heinz G. Jakob, Daniel Wendt, on behalf of the Cavalier Trial Investigators, Clinical and haemodynamic outcomes in 658 patients receiving the Perceval sutureless aortic valve: early results from a prospective European multicentre study (the Cavalier Trial) , European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 3, March 2016, Pages 978–986, https://doi.org/10.1093/ejcts/ezv257
Close - Share Icon Share
Abstract
The aim of the Cavalier trial was to evaluate the safety and performance of the Perceval sutureless aortic valve in patients undergoing aortic valve replacement (AVR). We report the 30-day clinical and haemodynamic outcomes from the largest study cohort with a sutureless valve.
From February 2010 to September 2013, 658 consecutive patients (mean age 77.8 years; 64.4% females; mean logistic EuroSCORE 10.2%) underwent AVR in 25 European Centres. Isolated AVRs were performed in 451 (68.5%) patients with a less invasive approach in 219 (33.3%) cases. Of the total, 40.0% were octogenarians. Congenital bicuspid aortic valve was considered an exclusion criterion.
Implantation was successful in 628 patients (95.4%). In isolated AVR through sternotomy, the mean cross-clamp time and the cardiopulmonary bypass (CPB) time were 32.6 and 53.7 min, and with the less invasive approach 38.8 and 64.5 min, respectively. The 30-day overall and valve-related mortality rates were 3.7 and 0.5%, respectively. Valve explants, stroke and endocarditis occurred in 0.6, 2.1 and in 0.1% of cases, respectively. Preoperative mean and peak pressure gradients decreased from 44.8 and 73.24 mmHg to 10.24 and 19.27 mmHg at discharge, respectively. The mean effective orifice area improved from 0.72 to 1.46 cm 2 .
The current 30-day results show that the Perceval valve is safe (favourable haemodynamic effect and low complication rate), and can be implanted with a fast and reproducible technique after a short learning period. Short cross-clamp and CPB times were achieved in both isolated and combined procedures. The Perceval valve represents a promising alternative to biological AVR, especially with a less invasive approach and in older patients.
INTRODUCTION
Aortic valve replacement (AVR) is the treatment of choice for patients with severe aortic stenosis and valve replacement with a biological prosthesis is especially recommended for patients aged ≥65 years.
In Europe, it was recently suggested that approximately one-third of patients aged more than 75 years with valvular heart disease do not undergo surgical AVR because of risks arising from age and comorbidities [ 1 ].
This observation triggered the development and the broad adoption of the less invasive transcatheter aortic valve (TAV) procedures in a high-risk population or in patients deemed unsuitable to AVR. Even though it was believed that TAV procedures would have been associated with lower mortality and morbidity compared with surgical AVR in elderly patients, they showed instead the potential for serious complications related to the transcatheter positioning and/or the concept itself, such as significant paravalvular regurgitation, vascular complications, aortic dissection/perforation, stroke, myocardial infarction and major ventricular tachyarrhythmia [ 1 , 2 ].
Indeed, it has been shown that the overall 30-day rates of TAV-related major adverse cardiovascular and cerebral events range from 3% to 35% [ 1 ].
The challenges of removing the stenosed native valve, along with the lack of evidence on the relationship between the degree of calcification and the high incidence of paravalvular leakage after TAV placement has triggered the shift towards an alternative collapsible, stent-mounted aortic valve prosthesis that can be placed in a sutureless fashion with a conventional surgical technique [ 3 ].
This technology includes a classic extracorporeal circulation, cross-clamping of the aorta and an aortotomy, allowing for a complete removal of the diseased native valve. Furthermore, since no suturing is needed, it shortens the aortic cross-clamp and myocardial ischaemic time.
The Perceval valve (Sorin Group, Saluggia, Italy) is a sutureless aortic bioprosthesis that was developed to combine the advantages of the TAVI procedure, allowing for a fast implantation with no need for suturing, with the benefits of a conventional surgical approach owing to the possibility of removing the native valve along with the calcifications. Given the above features, the use of sutureless valves is not limited to high-risk or inoperable patients as per TAVI technology, but it could broadened to include all patients who are suitable for open heart surgery, regardless of their risk profile.
We report the early outcomes in terms of safety and performance of the Perceval valve in over 800 patients enrolled in the Cavalier trial requiring AVR either via conventional median sternotomy or a less invasive approach.
Even though we only report the short-term follow-up outcomes, the Cavalier trial is still ongoing with up to 5 years of follow-up. Clinical visits and echocardiographic examinations are planned for all patients at 3–6 months, 1 year and on annual basis up to 5 years post implant in order to provide full mid-term performance data of the Perceval valve.
MATERIALS AND METHODS
Patient selection and enrolment
The Cavalier study was designed as a European, multicentre, prospective, non-randomized, clinical trial and was registered at the US National Institutes of Health (NCT01368666, www.clinicaltrials.org ). The study was approved by the ethical committee of each study centre and each patient gave their signed informed consent before being enrolled in the trial.
From February 2010 to September 2013, a total of 815 consecutive patients were enrolled in 25 centres in eight European countries. To be included in the study, patients with diagnosed aortic valve stenosis or steno-insufficiency had to be at least 65 years of age and capable of providing written informed consent.
All patients eligible for isolated replacement of their aortic valve (native or previously implanted prosthesis) were evaluated for the Cavalier study.
In 157 of the 815 enrolled patients, the implant of the Perceval valve was not attempted due to the presence of intraoperative exclusion criteria. The most frequent reasons were the following:
lack of availability of valve size in 42% of cases (valve size XL/PVS 27 was not introduced in the CAVALIER study until April 2012);
congenital bicuspid valve (10%) that could not be estimated preoperatively by echocardiographic means;
ratio between the annulus and the sinotubular junction >1.3 (8%).
All the inclusion and exclusion criteria are listed in Table 1 . All types of congenital bicuspid aortic valves (Type 0 with no raphe; Type 1 with one raphe; Type 2 with two raphes) were considered as an exclusion criterion.
| Inclusion criteria . | Exclusion criteria . |
|---|---|
| Age ≥65 years; | Subjects involved in any other clinical study for drugs or devices |
| Subjects with aortic valve stenosis or steno-insufficiency | Subjects with a previously implanted Perceval prosthesis, within the clinical study, that requires replacement |
| Subjects in which preoperative evaluation indicated the need for native or prosthetic aortic valve replacement with a biological prosthesis | Subjects with previous implantation of valve prostheses or annuloplasty ring not being replaced by the study valve |
| Subjects willing to sign the informed consent | Subjects requiring simultaneous cardiac procedures, apart from septal myectomy and/or coronary bypass |
| Subjects willing to undergo all the medical follow-ups and echocardiographic examinations and laboratory tests that form part of this present protocol | Subjects who require double or multiple valve replacement or repair in whom the mitral, tricuspid or pulmonic valve would be replaced with a non-Perceval valve or repaired |
| Subjects with aneurysmal dilatation or dissection of the ascending aortic wall | |
| Subjects needing non-elective intervention | |
| Subjects with active endocarditis | |
| Subjects with active myocarditis | |
| Subjects with congenital bicuspid aortic valve | |
| Subjects with aortic root enlargement, where the ratio between the diameter of the sinotubular junction and the annulus diameter, assessed by TTE, is >1.3 | |
| Subjects with aortic root height (measured from the aortic annulus to the sinotubular junction) ≥21 mm for size S/21, ≥22.5 mm for size M/23, ≥24 mm for size L/25 and ≥25 mm for size XL/27 | |
| Subjects with myocardial infarction ≤90 days before the planned valve implant surgery | |
| Subjects with known hypersensitivity to nickel alloys. | |
| Subjects involved in any other clinical study for drugs or devices. | |
| The subject is a prison inmate, institutionalized or is unable to give informed consent. | |
| The subject has a major or progressive non-cardiac disease that, in the investigator's experience, results in a life expectancy of less than 1 year, or the implant of the device produces an unacceptable increased risk to the patient. | |
| The subject is undergoing renal dialysis for chronic renal failure or has hyperparathyroidism. | |
| The subject has an acute preoperative neurological deficit, myocardial infarction or cardiac event that has not returned to baseline or stabilized ≥30 days prior to the planned valve implant surgery. |
| Inclusion criteria . | Exclusion criteria . |
|---|---|
| Age ≥65 years; | Subjects involved in any other clinical study for drugs or devices |
| Subjects with aortic valve stenosis or steno-insufficiency | Subjects with a previously implanted Perceval prosthesis, within the clinical study, that requires replacement |
| Subjects in which preoperative evaluation indicated the need for native or prosthetic aortic valve replacement with a biological prosthesis | Subjects with previous implantation of valve prostheses or annuloplasty ring not being replaced by the study valve |
| Subjects willing to sign the informed consent | Subjects requiring simultaneous cardiac procedures, apart from septal myectomy and/or coronary bypass |
| Subjects willing to undergo all the medical follow-ups and echocardiographic examinations and laboratory tests that form part of this present protocol | Subjects who require double or multiple valve replacement or repair in whom the mitral, tricuspid or pulmonic valve would be replaced with a non-Perceval valve or repaired |
| Subjects with aneurysmal dilatation or dissection of the ascending aortic wall | |
| Subjects needing non-elective intervention | |
| Subjects with active endocarditis | |
| Subjects with active myocarditis | |
| Subjects with congenital bicuspid aortic valve | |
| Subjects with aortic root enlargement, where the ratio between the diameter of the sinotubular junction and the annulus diameter, assessed by TTE, is >1.3 | |
| Subjects with aortic root height (measured from the aortic annulus to the sinotubular junction) ≥21 mm for size S/21, ≥22.5 mm for size M/23, ≥24 mm for size L/25 and ≥25 mm for size XL/27 | |
| Subjects with myocardial infarction ≤90 days before the planned valve implant surgery | |
| Subjects with known hypersensitivity to nickel alloys. | |
| Subjects involved in any other clinical study for drugs or devices. | |
| The subject is a prison inmate, institutionalized or is unable to give informed consent. | |
| The subject has a major or progressive non-cardiac disease that, in the investigator's experience, results in a life expectancy of less than 1 year, or the implant of the device produces an unacceptable increased risk to the patient. | |
| The subject is undergoing renal dialysis for chronic renal failure or has hyperparathyroidism. | |
| The subject has an acute preoperative neurological deficit, myocardial infarction or cardiac event that has not returned to baseline or stabilized ≥30 days prior to the planned valve implant surgery. |
TTE: transthoracic echocardiography.
| Inclusion criteria . | Exclusion criteria . |
|---|---|
| Age ≥65 years; | Subjects involved in any other clinical study for drugs or devices |
| Subjects with aortic valve stenosis or steno-insufficiency | Subjects with a previously implanted Perceval prosthesis, within the clinical study, that requires replacement |
| Subjects in which preoperative evaluation indicated the need for native or prosthetic aortic valve replacement with a biological prosthesis | Subjects with previous implantation of valve prostheses or annuloplasty ring not being replaced by the study valve |
| Subjects willing to sign the informed consent | Subjects requiring simultaneous cardiac procedures, apart from septal myectomy and/or coronary bypass |
| Subjects willing to undergo all the medical follow-ups and echocardiographic examinations and laboratory tests that form part of this present protocol | Subjects who require double or multiple valve replacement or repair in whom the mitral, tricuspid or pulmonic valve would be replaced with a non-Perceval valve or repaired |
| Subjects with aneurysmal dilatation or dissection of the ascending aortic wall | |
| Subjects needing non-elective intervention | |
| Subjects with active endocarditis | |
| Subjects with active myocarditis | |
| Subjects with congenital bicuspid aortic valve | |
| Subjects with aortic root enlargement, where the ratio between the diameter of the sinotubular junction and the annulus diameter, assessed by TTE, is >1.3 | |
| Subjects with aortic root height (measured from the aortic annulus to the sinotubular junction) ≥21 mm for size S/21, ≥22.5 mm for size M/23, ≥24 mm for size L/25 and ≥25 mm for size XL/27 | |
| Subjects with myocardial infarction ≤90 days before the planned valve implant surgery | |
| Subjects with known hypersensitivity to nickel alloys. | |
| Subjects involved in any other clinical study for drugs or devices. | |
| The subject is a prison inmate, institutionalized or is unable to give informed consent. | |
| The subject has a major or progressive non-cardiac disease that, in the investigator's experience, results in a life expectancy of less than 1 year, or the implant of the device produces an unacceptable increased risk to the patient. | |
| The subject is undergoing renal dialysis for chronic renal failure or has hyperparathyroidism. | |
| The subject has an acute preoperative neurological deficit, myocardial infarction or cardiac event that has not returned to baseline or stabilized ≥30 days prior to the planned valve implant surgery. |
| Inclusion criteria . | Exclusion criteria . |
|---|---|
| Age ≥65 years; | Subjects involved in any other clinical study for drugs or devices |
| Subjects with aortic valve stenosis or steno-insufficiency | Subjects with a previously implanted Perceval prosthesis, within the clinical study, that requires replacement |
| Subjects in which preoperative evaluation indicated the need for native or prosthetic aortic valve replacement with a biological prosthesis | Subjects with previous implantation of valve prostheses or annuloplasty ring not being replaced by the study valve |
| Subjects willing to sign the informed consent | Subjects requiring simultaneous cardiac procedures, apart from septal myectomy and/or coronary bypass |
| Subjects willing to undergo all the medical follow-ups and echocardiographic examinations and laboratory tests that form part of this present protocol | Subjects who require double or multiple valve replacement or repair in whom the mitral, tricuspid or pulmonic valve would be replaced with a non-Perceval valve or repaired |
| Subjects with aneurysmal dilatation or dissection of the ascending aortic wall | |
| Subjects needing non-elective intervention | |
| Subjects with active endocarditis | |
| Subjects with active myocarditis | |
| Subjects with congenital bicuspid aortic valve | |
| Subjects with aortic root enlargement, where the ratio between the diameter of the sinotubular junction and the annulus diameter, assessed by TTE, is >1.3 | |
| Subjects with aortic root height (measured from the aortic annulus to the sinotubular junction) ≥21 mm for size S/21, ≥22.5 mm for size M/23, ≥24 mm for size L/25 and ≥25 mm for size XL/27 | |
| Subjects with myocardial infarction ≤90 days before the planned valve implant surgery | |
| Subjects with known hypersensitivity to nickel alloys. | |
| Subjects involved in any other clinical study for drugs or devices. | |
| The subject is a prison inmate, institutionalized or is unable to give informed consent. | |
| The subject has a major or progressive non-cardiac disease that, in the investigator's experience, results in a life expectancy of less than 1 year, or the implant of the device produces an unacceptable increased risk to the patient. | |
| The subject is undergoing renal dialysis for chronic renal failure or has hyperparathyroidism. | |
| The subject has an acute preoperative neurological deficit, myocardial infarction or cardiac event that has not returned to baseline or stabilized ≥30 days prior to the planned valve implant surgery. |
TTE: transthoracic echocardiography.
The Cavalier study cohort eventually accounted for 658 patients with aortic valve replacement.
The baseline preoperative data are reported in Table 2 .
| Patients . | n = 658 . | % . |
|---|---|---|
| Mean age ± SD (range) | 77.8 ± 5.6 (61–92) | |
| Age (years) | ||
| <65 | 7 | 1.1 |
| 65–69 | 41 | 6.2 |
| 70–74 | 138 | 21.0 |
| 75–79 | 209 | 31.8 |
| 80–84 | 186 | 28.3 |
| 85–89 | 70 | 10.6 |
| ≥90 | 7 | 1.1 |
| Sex | ||
| Female | 424 | 64.4% |
| Male | 234 | 35.6% |
| Mean BSA ± SD (range) | 1.8 ± 0.2 (1.3–2.4) | |
| NYHA | ||
| I | 22 | 3.3% |
| II | 198 | 30.1% |
| III | 386 | 58.7% |
| IV | 32 | 4.9% |
| Not available | 20 | 3.0% |
| Mean EuroSCORE ± SD (range) | 10.2 ± 7.8 (1.2–75.3) | |
| Mean STS Score ± SD (range) | 7.2 ± 7.4 (0.8–50.0) | |
| Cardiac rhythm a | ||
| Sinus | 559 | 85.5% |
| Atrial fibrillation | 52 | 8.0% |
| Other | 22 | 3.4% |
| Paced | 21 | 3.2% |
| Previous cardiovascular surgery b | ||
| None | 599 (91.0%) | |
| Previous surgery | 59 (9.0%) | |
| CABG | 13 | 22.0 |
| Pacemaker | 32 | 54.2 |
| Valve replacement | 8 | 13.5 |
| Other | 5 | 8.5 |
| Previous non-surgical procedures | 78 | 11.9 |
| PCI | 19 | 2.9 |
| PCI with stent | 67 | 10.2 |
| Risk factors | ||
| Systemic hypertension | 551 | 83.7 |
| Dyslipidaemia | 393 | 59.7 |
| Diabetes | 191 | 29 |
| Insulin-dependent diabetes | 63 | 34.2 |
| Smokers | 156 | 23.7 |
| Active smokers | 31 | 4.7 |
| Extracardiac arteriopathy | 112 | 17 |
| Chronic lung disease | 103 | 15.7 |
| Renal insufficiency | 97 | 14.8 |
| Cerebrovascular disease | 75 | 11.4 |
| Neurological dysfunction | 12 | 1.8 |
| Immunosuppressive treatment | 7 | 1.1 |
| Dialysis | 5 | 0.8 |
| Treated infective endocarditis | 3 | 0.5 |
| Patients . | n = 658 . | % . |
|---|---|---|
| Mean age ± SD (range) | 77.8 ± 5.6 (61–92) | |
| Age (years) | ||
| <65 | 7 | 1.1 |
| 65–69 | 41 | 6.2 |
| 70–74 | 138 | 21.0 |
| 75–79 | 209 | 31.8 |
| 80–84 | 186 | 28.3 |
| 85–89 | 70 | 10.6 |
| ≥90 | 7 | 1.1 |
| Sex | ||
| Female | 424 | 64.4% |
| Male | 234 | 35.6% |
| Mean BSA ± SD (range) | 1.8 ± 0.2 (1.3–2.4) | |
| NYHA | ||
| I | 22 | 3.3% |
| II | 198 | 30.1% |
| III | 386 | 58.7% |
| IV | 32 | 4.9% |
| Not available | 20 | 3.0% |
| Mean EuroSCORE ± SD (range) | 10.2 ± 7.8 (1.2–75.3) | |
| Mean STS Score ± SD (range) | 7.2 ± 7.4 (0.8–50.0) | |
| Cardiac rhythm a | ||
| Sinus | 559 | 85.5% |
| Atrial fibrillation | 52 | 8.0% |
| Other | 22 | 3.4% |
| Paced | 21 | 3.2% |
| Previous cardiovascular surgery b | ||
| None | 599 (91.0%) | |
| Previous surgery | 59 (9.0%) | |
| CABG | 13 | 22.0 |
| Pacemaker | 32 | 54.2 |
| Valve replacement | 8 | 13.5 |
| Other | 5 | 8.5 |
| Previous non-surgical procedures | 78 | 11.9 |
| PCI | 19 | 2.9 |
| PCI with stent | 67 | 10.2 |
| Risk factors | ||
| Systemic hypertension | 551 | 83.7 |
| Dyslipidaemia | 393 | 59.7 |
| Diabetes | 191 | 29 |
| Insulin-dependent diabetes | 63 | 34.2 |
| Smokers | 156 | 23.7 |
| Active smokers | 31 | 4.7 |
| Extracardiac arteriopathy | 112 | 17 |
| Chronic lung disease | 103 | 15.7 |
| Renal insufficiency | 97 | 14.8 |
| Cerebrovascular disease | 75 | 11.4 |
| Neurological dysfunction | 12 | 1.8 |
| Immunosuppressive treatment | 7 | 1.1 |
| Dialysis | 5 | 0.8 |
| Treated infective endocarditis | 3 | 0.5 |
Renal insufficiency: documented history of renal failure and/or a history of creatinine >200 µmol/l. Cerebrovascular disease: unresponsive coma, CVA, RIND, TIA; non-invasive/invasive carotid test with greater than 75% occlusion; prior carotid surgery.
BSA: body surface area; AVR: aortic valve replacement; CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; CVA: cerebrovascular accident; RIND: Reversible Ischaemic Neurologic Deficit; TIA: transient ischaemic attack; SD: standard deviation; NYHA: New York Heart Association; STS: society of thoracic surgeons.
a Missing data for 4 patients.
b Patient can have more than one previous surgery.
| Patients . | n = 658 . | % . |
|---|---|---|
| Mean age ± SD (range) | 77.8 ± 5.6 (61–92) | |
| Age (years) | ||
| <65 | 7 | 1.1 |
| 65–69 | 41 | 6.2 |
| 70–74 | 138 | 21.0 |
| 75–79 | 209 | 31.8 |
| 80–84 | 186 | 28.3 |
| 85–89 | 70 | 10.6 |
| ≥90 | 7 | 1.1 |
| Sex | ||
| Female | 424 | 64.4% |
| Male | 234 | 35.6% |
| Mean BSA ± SD (range) | 1.8 ± 0.2 (1.3–2.4) | |
| NYHA | ||
| I | 22 | 3.3% |
| II | 198 | 30.1% |
| III | 386 | 58.7% |
| IV | 32 | 4.9% |
| Not available | 20 | 3.0% |
| Mean EuroSCORE ± SD (range) | 10.2 ± 7.8 (1.2–75.3) | |
| Mean STS Score ± SD (range) | 7.2 ± 7.4 (0.8–50.0) | |
| Cardiac rhythm a | ||
| Sinus | 559 | 85.5% |
| Atrial fibrillation | 52 | 8.0% |
| Other | 22 | 3.4% |
| Paced | 21 | 3.2% |
| Previous cardiovascular surgery b | ||
| None | 599 (91.0%) | |
| Previous surgery | 59 (9.0%) | |
| CABG | 13 | 22.0 |
| Pacemaker | 32 | 54.2 |
| Valve replacement | 8 | 13.5 |
| Other | 5 | 8.5 |
| Previous non-surgical procedures | 78 | 11.9 |
| PCI | 19 | 2.9 |
| PCI with stent | 67 | 10.2 |
| Risk factors | ||
| Systemic hypertension | 551 | 83.7 |
| Dyslipidaemia | 393 | 59.7 |
| Diabetes | 191 | 29 |
| Insulin-dependent diabetes | 63 | 34.2 |
| Smokers | 156 | 23.7 |
| Active smokers | 31 | 4.7 |
| Extracardiac arteriopathy | 112 | 17 |
| Chronic lung disease | 103 | 15.7 |
| Renal insufficiency | 97 | 14.8 |
| Cerebrovascular disease | 75 | 11.4 |
| Neurological dysfunction | 12 | 1.8 |
| Immunosuppressive treatment | 7 | 1.1 |
| Dialysis | 5 | 0.8 |
| Treated infective endocarditis | 3 | 0.5 |
| Patients . | n = 658 . | % . |
|---|---|---|
| Mean age ± SD (range) | 77.8 ± 5.6 (61–92) | |
| Age (years) | ||
| <65 | 7 | 1.1 |
| 65–69 | 41 | 6.2 |
| 70–74 | 138 | 21.0 |
| 75–79 | 209 | 31.8 |
| 80–84 | 186 | 28.3 |
| 85–89 | 70 | 10.6 |
| ≥90 | 7 | 1.1 |
| Sex | ||
| Female | 424 | 64.4% |
| Male | 234 | 35.6% |
| Mean BSA ± SD (range) | 1.8 ± 0.2 (1.3–2.4) | |
| NYHA | ||
| I | 22 | 3.3% |
| II | 198 | 30.1% |
| III | 386 | 58.7% |
| IV | 32 | 4.9% |
| Not available | 20 | 3.0% |
| Mean EuroSCORE ± SD (range) | 10.2 ± 7.8 (1.2–75.3) | |
| Mean STS Score ± SD (range) | 7.2 ± 7.4 (0.8–50.0) | |
| Cardiac rhythm a | ||
| Sinus | 559 | 85.5% |
| Atrial fibrillation | 52 | 8.0% |
| Other | 22 | 3.4% |
| Paced | 21 | 3.2% |
| Previous cardiovascular surgery b | ||
| None | 599 (91.0%) | |
| Previous surgery | 59 (9.0%) | |
| CABG | 13 | 22.0 |
| Pacemaker | 32 | 54.2 |
| Valve replacement | 8 | 13.5 |
| Other | 5 | 8.5 |
| Previous non-surgical procedures | 78 | 11.9 |
| PCI | 19 | 2.9 |
| PCI with stent | 67 | 10.2 |
| Risk factors | ||
| Systemic hypertension | 551 | 83.7 |
| Dyslipidaemia | 393 | 59.7 |
| Diabetes | 191 | 29 |
| Insulin-dependent diabetes | 63 | 34.2 |
| Smokers | 156 | 23.7 |
| Active smokers | 31 | 4.7 |
| Extracardiac arteriopathy | 112 | 17 |
| Chronic lung disease | 103 | 15.7 |
| Renal insufficiency | 97 | 14.8 |
| Cerebrovascular disease | 75 | 11.4 |
| Neurological dysfunction | 12 | 1.8 |
| Immunosuppressive treatment | 7 | 1.1 |
| Dialysis | 5 | 0.8 |
| Treated infective endocarditis | 3 | 0.5 |
Renal insufficiency: documented history of renal failure and/or a history of creatinine >200 µmol/l. Cerebrovascular disease: unresponsive coma, CVA, RIND, TIA; non-invasive/invasive carotid test with greater than 75% occlusion; prior carotid surgery.
BSA: body surface area; AVR: aortic valve replacement; CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; CVA: cerebrovascular accident; RIND: Reversible Ischaemic Neurologic Deficit; TIA: transient ischaemic attack; SD: standard deviation; NYHA: New York Heart Association; STS: society of thoracic surgeons.
a Missing data for 4 patients.
b Patient can have more than one previous surgery.
An independent Clinical Events Committee (CEC) was formed to adjudicate the primary cause of death, the reason for valve explantation and specific adverse events according to the Akins [ 4 ] and ICH Guidelines [ 5 ].
An echo core laboratory was used for the Cavalier study. Each required echo was recorded digitally at the study site and sent to the core laboratory, which performed a full analysis of the images and relevant calculations. Whenever conflicting interpretations existed between the study site and the core laboratory, the interpretation of the core laboratory was recorded as the definitive result.
Device description
The Perceval valve is a bioprosthetic heart valve that gained European Conformity (CE) mark approval in 2011. The biological component consists of glutaraldehyde-fixed bovine pericardium treated with homocysteic acid in order remove the free aldehyde residues and prevent the calcification process.
The stent is made of an elastic Ni–Ti alloy featuring two rings and nine vertical struts covered by a thin coating of Carbofilm™ that improves biocompatibility (Fig. 1 ).
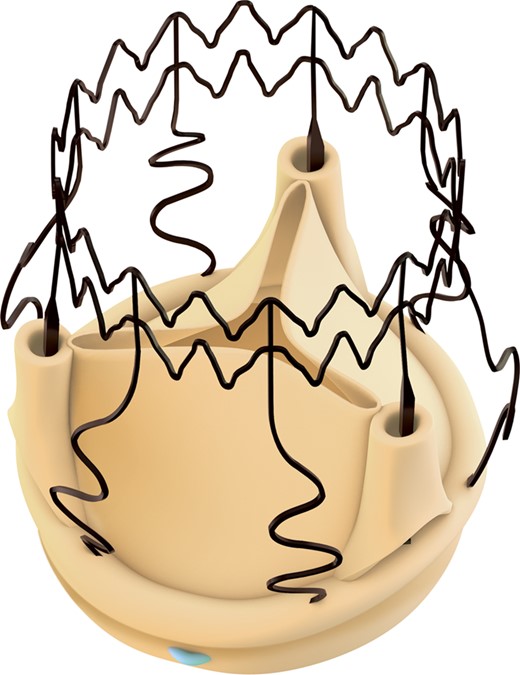
The stent has the dual task of supporting the valve and holding it in place without any permanent suture. The valve is collapsed with a proprietary device accessory provided by the Sponsor in order to reduce the diameter of the prosthesis without affecting the valve leaflets.
Valve implant procedure
Anaesthetic and surgical techniques were standardized according to the centre’s practice; full median sternotomy or less invasive approaches were adopted.
The exposure of the aorta is performed to provide optimum view of the native valve, allowing at the same time the Perceval prosthesis to be safely positioned. The aortic incision is performed distally from the sinotubular junction to preserve a rim of the ascending aorta above the prosthetic valve. The optimal approach consists of a transverse aortotomy performed ∼0.5 cm above the sinotubular junction.
The recommended implantation technique is to excise the aortic valve at the level of the insertion line of the native leaflets and to carefully decalcify the aortic annulus. A regular annular profile is beneficial to ensure the fixation of the prosthesis and optimal sealing, thus preventing the risk of a paraprosthetic leak.
The measurement of the aortic annular diameter was obtained by using dedicated sizers after the decalcification procedure. The implantation technique included several steps already described elsewhere [ 6–9 ].
Procedural success was defined as the patient leaving the operating theatre with a Perceval valve implanted.
Clinical assessment
According to the study protocol, all implanted patients underwent clinical evaluation [New York Heart Association (NYHA) class and cardiac status assessment], electrocardiography and blood exam [total blood count, creatinine phosphokinase, muscle band, international normalized ratio/activated clotting time, serum haptoglobin, plasma free haemoglobin, lactate dehydrogenase (LDH), reticulocytes] and transthoracic echocardiographic examination preoperatively and in the early postoperative period (at hospital discharge or within 30 days postoperatively).
Intraoperative transoesophageal echocardiography was routinely used for assessment of cardiac function, evaluation of surgical results and confirmation of the de-airing process. At the end of surgery, patients were transferred to the intensive care unit and managed according to the unit protocol.
Patients were considered at risk from the date of surgery to the date of death or to the date of an event (first occurrence). Follow-up was obtained for all study patients.
Echocardiography
According to the study protocol, transthoracic echocardiography was used to assess haemodynamic performance of the Perceval valve.
The left ventricle and aorta were recorded from 2D parasternal and four-chamber views. Aortic geometry and diameters were determined from parasternal long-axis views. Subaortic and aortic Doppler flow velocities were recorded from the apical four-chamber view using pulse wave and continuous wave Doppler mode. Peak and mean aortic valve pressure gradients, and effective orifice area (EOA) were determined.
Statistical analysis
Statistical analysis were performed on all patients and stratified by surgical approach and valve size. Categorical variables are reported as absolute and relative frequencies. For continuous data, means and standard deviations were calculated. Statistical analyses were performed using SAS software (Release 9.2, by SAS Institute, Inc., Cary, NC, USA).
RESULTS
Procedural outcomes
The majority of aortic valve replacement patients presented isolated valve stenosis ( n = 430; 65.3%) due to degenerative disease. Intraoperatively, the majority of patients ( n = 575; 87.4%) had calcified annuli and tricuspid aortic valves ( n = 637; 96.8%). Two hundred and seven ( n = 207; 31.5%) patients had concomitant procedures. In 154 (23.4%) patients, coronary artery bypass grafting (CABG) was performed. In some cases, Perceval was implanted in patients with congenital bicuspid valve, but no distinction based on the type of abnormality (Type 0, Type 1 or Type 2) was reported as this was not required per protocol.
In all instances of congenital bicuspid valve, the surgeon decided to proceed with the implantation as the best treatment option for the patient and duly reported it as protocol deviation. Furthermore, in all cases of a bicuspid aortic valve, the aortic annulus did not show anomalies. Operative data are summarized in Table 3 .
| Patients . | n = 658 . | % . |
|---|---|---|
| Aetiology | ||
| Degenerative | 568 | 86.3 |
| Rheumatic disease | 84 | 12.7 |
| Other | 6 | 0.9 |
| Surgical priority | ||
| Elective | 658 | 100 |
| Surgical approach | ||
| Median sternotomy | 439 | 66.7 |
| Less invasive approach | 219 | 33.3 |
| Aortic valve lesion | ||
| Stenosis | 430 | 65.3 |
| Stenosis and regurgitation | 226 | 34.3 |
| Regurgitation | 2 | 0.3 |
| Aortic valve condition | ||
| Tricuspid | 637 | 96.8 |
| Congenital bicuspid | 13 | 2.0 |
| Previous prosthesis | 8 | 1.2 |
| Valve size | ||
| S/21 | 84 | 12.8 |
| M/23 | 290 | 44.1 |
| L/25 | 255 | 38.8 |
| XL/27 | 29 | 4.4 |
| Concomitant procedures | ||
| None | 451 | 68.5 |
| Concomitant procedures | 207 | 31.5 |
| CABGs | 154 | 23.4 |
| Septal myectomy | 22 | 3.3 |
| Other concomitant procedures | 47 | 7.1 |
| Other cardiac concomitant procedures | 34 | 5.2 |
| Other non-cardiac concomitant procedures | 13 | 2.0 |
| Patients . | n = 658 . | % . |
|---|---|---|
| Aetiology | ||
| Degenerative | 568 | 86.3 |
| Rheumatic disease | 84 | 12.7 |
| Other | 6 | 0.9 |
| Surgical priority | ||
| Elective | 658 | 100 |
| Surgical approach | ||
| Median sternotomy | 439 | 66.7 |
| Less invasive approach | 219 | 33.3 |
| Aortic valve lesion | ||
| Stenosis | 430 | 65.3 |
| Stenosis and regurgitation | 226 | 34.3 |
| Regurgitation | 2 | 0.3 |
| Aortic valve condition | ||
| Tricuspid | 637 | 96.8 |
| Congenital bicuspid | 13 | 2.0 |
| Previous prosthesis | 8 | 1.2 |
| Valve size | ||
| S/21 | 84 | 12.8 |
| M/23 | 290 | 44.1 |
| L/25 | 255 | 38.8 |
| XL/27 | 29 | 4.4 |
| Concomitant procedures | ||
| None | 451 | 68.5 |
| Concomitant procedures | 207 | 31.5 |
| CABGs | 154 | 23.4 |
| Septal myectomy | 22 | 3.3 |
| Other concomitant procedures | 47 | 7.1 |
| Other cardiac concomitant procedures | 34 | 5.2 |
| Other non-cardiac concomitant procedures | 13 | 2.0 |
CABG: coronary artery bypass grafting.
| Patients . | n = 658 . | % . |
|---|---|---|
| Aetiology | ||
| Degenerative | 568 | 86.3 |
| Rheumatic disease | 84 | 12.7 |
| Other | 6 | 0.9 |
| Surgical priority | ||
| Elective | 658 | 100 |
| Surgical approach | ||
| Median sternotomy | 439 | 66.7 |
| Less invasive approach | 219 | 33.3 |
| Aortic valve lesion | ||
| Stenosis | 430 | 65.3 |
| Stenosis and regurgitation | 226 | 34.3 |
| Regurgitation | 2 | 0.3 |
| Aortic valve condition | ||
| Tricuspid | 637 | 96.8 |
| Congenital bicuspid | 13 | 2.0 |
| Previous prosthesis | 8 | 1.2 |
| Valve size | ||
| S/21 | 84 | 12.8 |
| M/23 | 290 | 44.1 |
| L/25 | 255 | 38.8 |
| XL/27 | 29 | 4.4 |
| Concomitant procedures | ||
| None | 451 | 68.5 |
| Concomitant procedures | 207 | 31.5 |
| CABGs | 154 | 23.4 |
| Septal myectomy | 22 | 3.3 |
| Other concomitant procedures | 47 | 7.1 |
| Other cardiac concomitant procedures | 34 | 5.2 |
| Other non-cardiac concomitant procedures | 13 | 2.0 |
| Patients . | n = 658 . | % . |
|---|---|---|
| Aetiology | ||
| Degenerative | 568 | 86.3 |
| Rheumatic disease | 84 | 12.7 |
| Other | 6 | 0.9 |
| Surgical priority | ||
| Elective | 658 | 100 |
| Surgical approach | ||
| Median sternotomy | 439 | 66.7 |
| Less invasive approach | 219 | 33.3 |
| Aortic valve lesion | ||
| Stenosis | 430 | 65.3 |
| Stenosis and regurgitation | 226 | 34.3 |
| Regurgitation | 2 | 0.3 |
| Aortic valve condition | ||
| Tricuspid | 637 | 96.8 |
| Congenital bicuspid | 13 | 2.0 |
| Previous prosthesis | 8 | 1.2 |
| Valve size | ||
| S/21 | 84 | 12.8 |
| M/23 | 290 | 44.1 |
| L/25 | 255 | 38.8 |
| XL/27 | 29 | 4.4 |
| Concomitant procedures | ||
| None | 451 | 68.5 |
| Concomitant procedures | 207 | 31.5 |
| CABGs | 154 | 23.4 |
| Septal myectomy | 22 | 3.3 |
| Other concomitant procedures | 47 | 7.1 |
| Other cardiac concomitant procedures | 34 | 5.2 |
| Other non-cardiac concomitant procedures | 13 | 2.0 |
CABG: coronary artery bypass grafting.
Of 658 patients, 439 patients (66.7%) underwent median sternotomy, whereas the remaining 219 patients (33.3%) underwent a less invasive surgical approach (216 mini-sternotomies, 3 right anterior mini-thoracotomies).
The procedure success rate was 95.4%. Thirty patients (4.6%) were classified as failure to implant, meaning that eligible enrolled patients underwent a cardiac surgical procedure, but the Perceval valve was not implanted mainly due to erroneous sizing or malpositioning. In each one of these cases, a different prosthetic valve was eventually implanted.
Size S (annulus range 19–21 mm) was implanted in 12.8% of patients, Size M (annulus range 21–23 mm) in 44.1%, Size L (annulus range 23–25 mm) in 38.8% and Size XL (annulus range 25–27 mm), which was available since July 2012, was implanted in 4.4% of the eligible patients.
The mean implant time defined as the period from the first guiding suture placement to the removal of the balloon catheter after dilatation was 9.7 ± 5.5 min (median 9 min). The aortic valve replacement could be successfully achieved with a mean cross-clamp time of 41.5 ± 20.3 min (min: 12.0; max: 126) and 39.0 ± 12.5 min (min: 18.0; max: 95.0) in median sternotomy and the less invasive approach, respectively. Surgical times including pump and cross-clamp time are reported in Table 4 .
Surgical times in patients with a Perceval valve successfully implanted (min)
| . | n . | Isolated AVR ( n = 424) . | n . | Combined AVR ( n = 204) . | n . | Overall ( n = 628) . |
|---|---|---|---|---|---|---|
| Mean (SD) . | Mean (SD) . | Mean (SD) . | ||||
| Median sternotomy | ||||||
| CPB time | 232 | 53.4 (20.5) | 185 | 79.1 (29.8) | 417 | 64.8 (28.1) |
| Cross-clamp time | 231 | 32.4 (10.9) | 186 | 52.9 (23.3) | 417 | 41.5 (20.3) |
| Less invasive | ||||||
| CPB time | 192 | 64.5 (18.0) | 18 | 68.9 (20.8) | 210 | 64.9 (18.2) |
| Cross-clamp time | 192 | 38.8 (12.5) | 18 | 41.9 (12.5) | 210 | 39.0 (12.5) |
| Overall | ||||||
| CPB time | 424 | 58.4 (20.2) | 203 | 78.2 (29.2) | 627 | 64.8 (25.2) |
| Cross-clamp time | 423 | 35.3 (12.1) | 204 | 51.9 (22.8) | 627 | 40.7 (18.1) |
| Implant time | NA | NA | 614 | 9.7 (5.5) | ||
| . | n . | Isolated AVR ( n = 424) . | n . | Combined AVR ( n = 204) . | n . | Overall ( n = 628) . |
|---|---|---|---|---|---|---|
| Mean (SD) . | Mean (SD) . | Mean (SD) . | ||||
| Median sternotomy | ||||||
| CPB time | 232 | 53.4 (20.5) | 185 | 79.1 (29.8) | 417 | 64.8 (28.1) |
| Cross-clamp time | 231 | 32.4 (10.9) | 186 | 52.9 (23.3) | 417 | 41.5 (20.3) |
| Less invasive | ||||||
| CPB time | 192 | 64.5 (18.0) | 18 | 68.9 (20.8) | 210 | 64.9 (18.2) |
| Cross-clamp time | 192 | 38.8 (12.5) | 18 | 41.9 (12.5) | 210 | 39.0 (12.5) |
| Overall | ||||||
| CPB time | 424 | 58.4 (20.2) | 203 | 78.2 (29.2) | 627 | 64.8 (25.2) |
| Cross-clamp time | 423 | 35.3 (12.1) | 204 | 51.9 (22.8) | 627 | 40.7 (18.1) |
| Implant time | NA | NA | 614 | 9.7 (5.5) | ||
AVR: aortic valve replacement; SD: standard deviation; CPB: cardiopulmonary bypass.
Surgical times in patients with a Perceval valve successfully implanted (min)
| . | n . | Isolated AVR ( n = 424) . | n . | Combined AVR ( n = 204) . | n . | Overall ( n = 628) . |
|---|---|---|---|---|---|---|
| Mean (SD) . | Mean (SD) . | Mean (SD) . | ||||
| Median sternotomy | ||||||
| CPB time | 232 | 53.4 (20.5) | 185 | 79.1 (29.8) | 417 | 64.8 (28.1) |
| Cross-clamp time | 231 | 32.4 (10.9) | 186 | 52.9 (23.3) | 417 | 41.5 (20.3) |
| Less invasive | ||||||
| CPB time | 192 | 64.5 (18.0) | 18 | 68.9 (20.8) | 210 | 64.9 (18.2) |
| Cross-clamp time | 192 | 38.8 (12.5) | 18 | 41.9 (12.5) | 210 | 39.0 (12.5) |
| Overall | ||||||
| CPB time | 424 | 58.4 (20.2) | 203 | 78.2 (29.2) | 627 | 64.8 (25.2) |
| Cross-clamp time | 423 | 35.3 (12.1) | 204 | 51.9 (22.8) | 627 | 40.7 (18.1) |
| Implant time | NA | NA | 614 | 9.7 (5.5) | ||
| . | n . | Isolated AVR ( n = 424) . | n . | Combined AVR ( n = 204) . | n . | Overall ( n = 628) . |
|---|---|---|---|---|---|---|
| Mean (SD) . | Mean (SD) . | Mean (SD) . | ||||
| Median sternotomy | ||||||
| CPB time | 232 | 53.4 (20.5) | 185 | 79.1 (29.8) | 417 | 64.8 (28.1) |
| Cross-clamp time | 231 | 32.4 (10.9) | 186 | 52.9 (23.3) | 417 | 41.5 (20.3) |
| Less invasive | ||||||
| CPB time | 192 | 64.5 (18.0) | 18 | 68.9 (20.8) | 210 | 64.9 (18.2) |
| Cross-clamp time | 192 | 38.8 (12.5) | 18 | 41.9 (12.5) | 210 | 39.0 (12.5) |
| Overall | ||||||
| CPB time | 424 | 58.4 (20.2) | 203 | 78.2 (29.2) | 627 | 64.8 (25.2) |
| Cross-clamp time | 423 | 35.3 (12.1) | 204 | 51.9 (22.8) | 627 | 40.7 (18.1) |
| Implant time | NA | NA | 614 | 9.7 (5.5) | ||
AVR: aortic valve replacement; SD: standard deviation; CPB: cardiopulmonary bypass.
The mean length of hospital stay was 12.0 ± 7.4 days (median 10, range 0–49, Q1:Q3 8–14), 12.4 days for the median sternotomy approach and 11.3 for the less invasive approach.
Haematological data
Changes in mean values of haematological parameters were observed and were consistent with those commonly observed early after cardiac surgery reflecting the response to blood loss [red blood cells (RBC) went from 4.2 to 3.6 × 10 12 /l], haemodilution (haematocrit decreased from 37.6 to 32.3%, haemoglobin decreased from 12.6 to 10.6 g/dl, reticulocyte count increased from 1.2 to 2.4%) and the effects of blood transfusion (LDH went from 245.4 to 471.3 IU7L, haptoglobin increased from 127.5 to 171.4 mg/dl) that was required in some of these patients.
Antithromboembolic therapy
Treatment with Coumadin was recommended for at least 3 months or, alternatively, administration of low molecular-weight heparin. However, the investigators could choose the most appropriate drug treatment at their discretion, considering the patients' clinical status.
At discharge, ∼53% of patients were prescribed aspirin therapy alone or in combination with anticoagulants or other antiplatelet agents after implant with the Perceval heart valve and ∼66% of the patients were taking oral anticoagulants.
Mortality and explants
There were 23 early deaths (3.7%): 3 (0.5%) were classified as cardiac valve-related, 5 (0.8%) as valve-related and procedure-related, 8 (1.3%) as cardiac death but not valve- nor procedure-related, and 7 (1.1%) as non-cardiac nor valve-related.
One (0.2%) perioperative explant at Day 0 and 5 (0.8%) early postoperative explants (from Day 1 to Day 30 after implant) occurred (Table 5 ). The single perioperative explant was due to significant bleeding. The patient was immediately returned to the operating room for repair of aortic bleeding and had the study valve explanted and replaced with a non-study prosthesis. The bleeding was caused by an aortic tear below the right coronary ostium, caused by extensive decalcification of the annulus.
Mortality, explants and postoperative valve-related and cardiovascular-related adverse events ( n = 628)
| . | Early events (≤30 days), n (%) . | Isolated AVR (424 patients) . | Combined AVR (204 patients) . |
|---|---|---|---|
| Death | |||
| Overall | 23 (3.7) | 10 (2.4) | 13 (6.4) |
| Valve-related death | 3 (0.5) | 2 (0.5) | 1 (0.5) |
| Valve-related and procedure-related | 5 (0.8) | 2 (0.5) | 3 (1.5) |
| Cardiac-related—non-valve-related | 8 (1.3) | 3 (0.7) | 5 (2.5) |
| Non-cardiac—not valve-related | 7 (1.1) | 3 (0.7) | 4 (2.0) |
| Reintervention | |||
| Reoperation for bleeding | 23 (3.7) | 13 (3.1) | 10 (4.9%) |
| Explant for intra- and/or paravalvular leakages | 5 (0.8) | 3 (0.7) | 2 (1.0) |
| Complications | |||
| Myocardial infarction | 2 (0.3) | 1 (0.2) | 1 (0.5) |
| AV block III without preoperative cardiac abnormalities | 42 (6.7) | 32 (7.5) | 10 (4.9) |
| Endocarditis | 1 (0.2) | 0 | 1 (0.5) |
| Tamponade | 3 (0.5) | 3 (0.7) | 2 (1.0) |
| Stroke | 13 (2.1) | 10 (2.4) | 3 (1.5) |
| . | Early events (≤30 days), n (%) . | Isolated AVR (424 patients) . | Combined AVR (204 patients) . |
|---|---|---|---|
| Death | |||
| Overall | 23 (3.7) | 10 (2.4) | 13 (6.4) |
| Valve-related death | 3 (0.5) | 2 (0.5) | 1 (0.5) |
| Valve-related and procedure-related | 5 (0.8) | 2 (0.5) | 3 (1.5) |
| Cardiac-related—non-valve-related | 8 (1.3) | 3 (0.7) | 5 (2.5) |
| Non-cardiac—not valve-related | 7 (1.1) | 3 (0.7) | 4 (2.0) |
| Reintervention | |||
| Reoperation for bleeding | 23 (3.7) | 13 (3.1) | 10 (4.9%) |
| Explant for intra- and/or paravalvular leakages | 5 (0.8) | 3 (0.7) | 2 (1.0) |
| Complications | |||
| Myocardial infarction | 2 (0.3) | 1 (0.2) | 1 (0.5) |
| AV block III without preoperative cardiac abnormalities | 42 (6.7) | 32 (7.5) | 10 (4.9) |
| Endocarditis | 1 (0.2) | 0 | 1 (0.5) |
| Tamponade | 3 (0.5) | 3 (0.7) | 2 (1.0) |
| Stroke | 13 (2.1) | 10 (2.4) | 3 (1.5) |
AVR: aortic valve replacement; AV: atrioventricular.
Mortality, explants and postoperative valve-related and cardiovascular-related adverse events ( n = 628)
| . | Early events (≤30 days), n (%) . | Isolated AVR (424 patients) . | Combined AVR (204 patients) . |
|---|---|---|---|
| Death | |||
| Overall | 23 (3.7) | 10 (2.4) | 13 (6.4) |
| Valve-related death | 3 (0.5) | 2 (0.5) | 1 (0.5) |
| Valve-related and procedure-related | 5 (0.8) | 2 (0.5) | 3 (1.5) |
| Cardiac-related—non-valve-related | 8 (1.3) | 3 (0.7) | 5 (2.5) |
| Non-cardiac—not valve-related | 7 (1.1) | 3 (0.7) | 4 (2.0) |
| Reintervention | |||
| Reoperation for bleeding | 23 (3.7) | 13 (3.1) | 10 (4.9%) |
| Explant for intra- and/or paravalvular leakages | 5 (0.8) | 3 (0.7) | 2 (1.0) |
| Complications | |||
| Myocardial infarction | 2 (0.3) | 1 (0.2) | 1 (0.5) |
| AV block III without preoperative cardiac abnormalities | 42 (6.7) | 32 (7.5) | 10 (4.9) |
| Endocarditis | 1 (0.2) | 0 | 1 (0.5) |
| Tamponade | 3 (0.5) | 3 (0.7) | 2 (1.0) |
| Stroke | 13 (2.1) | 10 (2.4) | 3 (1.5) |
| . | Early events (≤30 days), n (%) . | Isolated AVR (424 patients) . | Combined AVR (204 patients) . |
|---|---|---|---|
| Death | |||
| Overall | 23 (3.7) | 10 (2.4) | 13 (6.4) |
| Valve-related death | 3 (0.5) | 2 (0.5) | 1 (0.5) |
| Valve-related and procedure-related | 5 (0.8) | 2 (0.5) | 3 (1.5) |
| Cardiac-related—non-valve-related | 8 (1.3) | 3 (0.7) | 5 (2.5) |
| Non-cardiac—not valve-related | 7 (1.1) | 3 (0.7) | 4 (2.0) |
| Reintervention | |||
| Reoperation for bleeding | 23 (3.7) | 13 (3.1) | 10 (4.9%) |
| Explant for intra- and/or paravalvular leakages | 5 (0.8) | 3 (0.7) | 2 (1.0) |
| Complications | |||
| Myocardial infarction | 2 (0.3) | 1 (0.2) | 1 (0.5) |
| AV block III without preoperative cardiac abnormalities | 42 (6.7) | 32 (7.5) | 10 (4.9) |
| Endocarditis | 1 (0.2) | 0 | 1 (0.5) |
| Tamponade | 3 (0.5) | 3 (0.7) | 2 (1.0) |
| Stroke | 13 (2.1) | 10 (2.4) | 3 (1.5) |
AVR: aortic valve replacement; AV: atrioventricular.
Five (0.8%) early explants were performed at a mean of 13.8 days post implant (range 2–30 days). These explants were due to significant paravalvular leak discovered during follow-up echocardiography (in 1 case related to an inappropriate ratio between the annulus and sinotubular junction diameter, while in the other 4 due to malpositioning).
All patients survived the explant of the Perceval valve and were successfully discharged.
Morbidity results
Table 5 summarizes the early postoperative valve-related and cardiovascular-related adverse events. No cases of unanticipated adverse device effects, valve thrombosis, secondary valve dislodgement or valve-related haemolytic anaemia were reported. One case of prosthetic valve endocarditis occurred 8 days post implant due to a postoperative pneumonia. The patient was treated with antibiotics, but developed sepsis and disseminated intravascular coagulation and died 13 days postoperatively.
Four cases of haemolysis (0.6%) occurred in the early period. Three cases were adjudicated by the CEC as procedure-related and none of them required transfusion or additional therapy. The fourth case was secondary to a paravalvular leak and required the explant of the study valve.
A total of 13 (2.1%) postoperative strokes occurred (4 of them in patients with a history of previous cerebrovascular accident) and 23 cases (3.7%) of early bleeding led to reoperation.
The incidence rate of permanent pacemaker (PM) implantation was overall 11.6%, but only 6.7% in patients without preoperative cardiac rhythm disturbances (atrial fibrillation/flutter, heart block, paroxysmal atrial fibrillation, sustained ventricular tachycardia/ventricular fibrillation).
Haemodynamic results
At discharge, the echo core laboratory confirmed the presence of periprosthetic leak in 3.8% of the assessed cases (23/600). Twenty-two of 23 cases were rated as trace or mild and only in 1 case the severity could not be evaluated. Overall central trace or mild regurgitation was confirmed in 109/600 (18.2%), while only 3 cases (0.5%) were moderate.
Fourteen additional cases (2.3%) were confirmed having both central and perivalvular leak: 11 were rated as mild, 2 as moderate and 1 as severe.
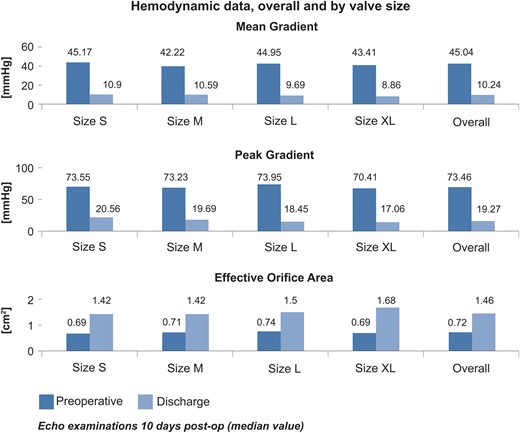
The mean and peak gradients were 10.24 and 19.27 mmHg, respectively.
Figure 2 provides paired comparisons of mean and peak pressure gradients and effective orifice area for each valve size.
DISCUSSION
Open surgery for AVR with the use of cardioplegic arrest under cardiopulmonary bypass (CPB) remains the golden standard for symptomatic patients with severe aortic valve stenosis [ 10 ].
The Perceval valve is a prosthesis meant for surgical patients eligible for AVR regardless of the surgical risk.
The concept of a sutureless valve was developed to facilitate the implantation of the prosthesis and shorten the procedure in order to reduce the adverse effects of an open heart procedure with aortic cross-clamping and cardiopulmonary bypass. In addition, this technology should facilitate the AVR regardless of the chosen surgical approach, potentially reducing the impact of surgery and trauma on the overall clinical outcomes. Such advantages are of paramount importance in patients with advanced age and coexisting morbidities.
The Perceval sutureless valve was designed to avoid passing the stitches through the annulus and knotting of the suture to minimize the surgical trauma to the aortic annulus and, consequently, reduce the ischaemic time. Following the preliminary experiences with the pilot and pivotal studies performed in a limited number of centres, the Cavalier represents the biggest multicentre prospective trial (25 selected European centres) reported to date with a sutureless valve including different surgical approaches.
Previous studies demonstrated that the duration of aortic cross-clamping and CPB are independent predictors of survival after valve replacement and combined valve operations with CABG [ 11 , 12 ]. Therefore, a technology focused on shortening aortic cross-clamp time and CPB time was mandatory.
The Cavalier study results show that it is possible to successfully implant a sutureless valve in the aortic position in less than 20 min of aortic cross-clamping, including the complete removal of the diseased native valve, without jeopardizing valve function. The study confirms the single-centre experience reported by Flameng et al. [ 9 ]. Remarkably, short procedural times were realized also in centres shortly after the initial experience with the Perceval valve, owing to the ease of implantability granted by the design of the prosthesis and the accessories.
In the Cavalier study, procedural times were shortened ∼40% compared with traditional AVR, with mean CPB and cross-clamp durations of 65 and 40 min, respectively, and were further shortened to 53 and 32 min for isolated AVR in the median sternotomy approach. These data compare favourably with most recent data from the Society of Thoracic Surgeons national database (the mean CPB and cross-clamp time for isolated AVR with full sternotomy was 78 and 106 min, respectively [ 13 ]). The decreased procedural times that were recorded in the Cavalier trial are a very positive achievement that is particularly amplified in a population of elderly patients (40% octogenarians) with comorbidities.
Moreover, a few studies have shown that less invasive AVR can reach excellent outcomes compared with traditional AVR in terms of postoperative complications and hospital stay [ 14 , 15 ], suggesting that it might be particularly beneficial to older and fragile patients. However, less invasive AVR has been shown to be associated with longer CPB and aortic cross-clamp times compared with traditional surgery [ 14 , 15 ]. The working space for the exposure and implantation of the prosthetic valves is significantly reduced, making the surgery more complex and technically challenging (especially in small aortic annuli). These are the principal reasons for the longer operative times and the slow learning curve. The drawback of the prolonged surgery times could be avoided by the adoption of sutureless technology, which can facilitate the less invasive approach in the AVR.
Despite the fact that some centres started their less invasive programme while this study was ongoing, there were no differences in terms of CPB and procedure-related times in the overall population undergoing either surgical approach. This is proof that implantation of the Perceval valve is easy and reproducible.
The low 30-day valve-related mortality and valve- and procedure-related mortality (1.3%) demonstrate that good short-term clinical outcomes can be achieved despite the increased risk profile (mean Logistic EuroSCORE score: 10.2 ± 7.8) and advanced age (40% were octogenarians) of the cohort.
Adverse valve-related events such as endocarditis and paravalvular leak (PVL) reported by the centres were also rare in this series.
The low incidence of reoperation for PVL (0.8%) leading to valve reoperation and the short operative times confirm the safety of this valve and also of the procedure even when performed through a less invasive approach.
The PM rate reported in the present study (11.6% overall and 6.7% in patients without preoperative cardiac rhythm disturbances) is within the rates reported in a literature review, showing that the incidence of PM implantation following conventional AVR varied from 3.0 to 11.8% (median 7.2%, mean 7%) [ 16 ]. Such a result may be due to the high-risk profile of the patients (40% over 80 years old and presence of comorbidities). An additional factor may be the learning curve effect in terms of procedural implanting steps and sizing. In addition, the high number of centres in this cohort and the variability of operators and protocols of rhythm disorder management may represent another factor, if we consider that in one of the biggest published experience the rate was lower (4.2%) [ 13 ]. Some centres prefer indeed the watchful waiting policy up to 7–10 days post surgery prior to implant of a permanent PM, while some others may be in favour of a PM implant as soon as the conversion to normal cardiac rhythm fails in the operating room.
A reduction in the mean gradient and peak pressure gradients was observed to an average of 10.2 and 19.3 mmHg at discharge.
Mean and peak pressure gradients were comparable with the values reported in literature for other biological prostheses. The mean EOA increased from 0.72 cm 2 preoperatively to 1.46 cm 2 at discharge 30 days, confirming the good haemodynamic outcomes in a patient population with predominantly small to medium-sized annular diameter (19–23 mm).
Study limitations
The main limitations of the present study are the lack of a control group of patients receiving conventional valves. Furthermore, EuroSCORE was used for the current trial even though it may overestimate the risk of mortality. The short follow-up period represents a temporary limitation, since the Cavalier trial is currently ongoing and data of up to 5 years will be provided in the future, to reflect the mid-term valve performance.
CONCLUSION
The Cavalier trial represents the biggest multicentre prospective study reported to date with a sutureless valve.
The early results showed short operative times and ease of implant in either surgical approach, despite the majority of the centres having their first experience with the Perceval valve. The low rates of mortality and complications support the concept that AVR with the Perceval valve is a safe and reproducible procedure, providing a good clinical outcome and very satisfactory haemodynamic performance, even in an older patient population. The Perceval valve represents a valuable alternative to traditional AVR for both isolated and concomitant procedures.
Funding
This work was supported by Sorin Group, Saluggia, Italy.
Conflict of interest: François Laborde, Theodor Fischlein, Bart Meuris, Martin Misfeld and Malakh Shrestha disclose a commercial/financial relationship with Sorin Group.
ACKNOWLEDGEMENTS
The authors express gratitude to the following Cavalier Trial investigators for their contribution to the study: Thierry Folliguet 1 , Kostantinos Zannis 1 , Steffen Pfeiffer 2 , Giuseppe Santarpino 2 , Samir Sarikouch 3 , Christoph Bara 3 , Jan F. Gummert 4 , Friedrich W. Mohr 5 , Pascal Dohmen 5 , Mario Stalder 6 , Eva Roost 6 , Krzysztof Filipiak 7 , Tomasz Niklewski 7 , Xavier Roques 8 , Willem J. Flameng 9 , Axel M.M. Laczkovics 10 , Matthias Bechtel 10 , Alain G. Prat 11 , Carlo Banfi 11 , Otto E. Dapunt 12 , Harald C. Eichstaedt 12 Wolfang Harringer 13 Ulrike Carstens-Fitz 13 Tom J. Spyt 14 , Jan Gerhard Wimmer-Greinecker 15 , Matthias Machner 15 , Erwin S.H. Tan 16 , Filip P.A. Casselman 17 Alaaddin Yilmaz 18 , Uday Sonker 18 , Sabine Bleiziffer 19 , Peter J. Oberwalder 20 , Alfred A. Kocher 21 , Rainald Seitelberger 21 , Hendrik Treede 22 , Leonard Conradi 22 , Riccardo Cocchieri 23 Bas De Mol 23 , Jean-Christian Roussel 24 , Philippe Despins 24 , Heinz G. Jakob 25 , Daniel Wendt 25 .
1 Cardiac Medico-Surgical Department, Institute Mutualiste Montsouris, Paris, France. 2 Cardiac Surgery, Paracelsus Medical University, Klinikum Nürnberg, Nuremberg, Germany. 3 Cardio-thoracic, Transplantation and Vascular Surgery, Hannover Medical School, Germany. 4 Klinik, Herz- und Diabeteszentrum, Bad Oeynhausen, Germany. 5 Herzzentrum der Universität Leipzig, Klinik fur Herzchirurgie, Leipzig, Germany. 6 Inselspital, University of Bern, Bern, Switzerland. 7 Silesian University Center for Heart Disease, Zabrze, Poland. 8 Hopital Cardiologique Du Haut- Lévêque, Pessac, France. 9 Cardiac Surgery, U.Z. Gasthuisberg, Leuven, Belgium. 10 Ruhr University of Bochum, Department of Cardiothoracic Surgery, Bochum, Germany. 11 Centre Hospitalier Regional Universitaire, Lille, France. 12 Klinikum Oldenburg, Department of Cardiovascular Surgery, Oldenburg, Germany. 13 Department of Thoracic and Cardiovascular Surgery, Klinikum Braunschweig, Braunschweig, Germany. 14 Regional Cardiothoracic Unit, Genfield General Hospital, Leicester, United Kingdom. 15 Herz und Gefäßzentrum, Bad Bevensen, Germany. 16 Catharina Ziekenhuis, Eindhoven, Netherlands. 17 OLV Clinic - Department of Cardiovascular and Thoracic Surgery, Aalst, Belgium. 18 Saint Antonius Hospital, Nieuwegein, Netherlands. 19 German Heart Center, Munich, Germany. 20 Medical School Graz, Department of Cardiac Surgery, Graz, Austria. 21 Department Cardiothoracic Surgery, Medical University, Vienna, Austria. 22 Universitäres Herzzentrum Hamburg GmbH, Hamburg, Germany. 23 Amsterdam Medical Centre - AMC, Amsterdam, Netherland. 24 Centre Hospitalier Universitaire, Nantes, France. 25 Department of Thoracic & Cardiovascular Surgery, University of Essen, West German Heart Center, Essen, Germany.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr A. Repossini(Brescia, Italy): This is probably one of the most important studies with a new device.
Of course, you denounced a very critical learning curve, because most of the centres implanted probably less than 10 valves, and this is probably a limitation to the study.
My concern regarding this particular device, which is very promising and very competitive regarding the TAVI technology, is with removing the calcified valves. So, the critical point today is extracorporeal circulation, which you demonstrated very well, which is reduced by more than half of the time. This should be very important, especially for high-risk patients.
The EuroSCORE of your population was not very high. It is not comparable to the TAVI population. And the range of ages, you had some patients who were 61. So my first question is, is there already a rationale for employing this kind of prosthesis even in medium and low-risk patients, or should it be reserved for the moment to, let's say, the grey zone, I mean patients who are not eligible for TAVI?
Dr Laborde: With the experience we have now, I think that this device has to be considered as a bioprosthesis, and for that, if the anatomical conditions are present, I would say that it is a logical way to implant this type of device.
Dr Repossini: So you will implant this valve with the same indications as the standard bioprosthesis?
Dr Laborde: Yes, I think so, for the reason of saving time and the haemodynamic profile.
Dr Repossini: The second question is concerning the AV block. You reported about a 7% block, 6.7, which is in line with the literature in aortic valve replacement, and for sure more limited than for a TAVI procedure. My question is, do you think that with a little bit more attention in dilating the valve or applying less pressure in the balloon or doing some manoeuvre that you could diminish this rate a little bit lower?
Dr Laborde: To answer your question, I have checked these different centres and made sure when you have an AV block immediately after the surgery, or you wait some days until you are sure that this does not recover, because sometimes it recovers and you can explore the conduction tissue, and other centres implant the pacemaker, I would say, the day after the surgery. So this can change finally the reality of the need for implanting a pacemaker.
Dr Repossini: So you are telling us that if you are not in a rush with implanting a pacemaker, the rate could be lower?
Dr Laborde: Yes, but concerning the technique, when you remove the calcium, you decide the size of the device, you implant this device, and the technique includes dilating the device with the balloon which corresponds to the size of the device, but this dilatation is not made to impact, although you do not overdilate the device, but it is not aggressive, from my point of view, to the conducting tissue. This may be important because the alloy of this device is thermal-sensitive. If you use cold cardioplegia, imagine you are around 10 degrees, and the diameter of the device is at 38 degrees. So this balloon ensures that your device will have the right diameter you choose before you remove the clamp and rewarm the blood. For me, this is one of the reasons to dilate this. The forces exerted on the aortic annulus, if you have the correct sizing, is probably not important.
Dr Repossini: Is there a rationale not to balloon the valve?
Dr Laborde: Why not? But you have to be sure that when you deploy the valve, if you choose M, you will have a 23. That's the reason why.
Dr M. Amrani(London, UK): In your department, am I right to believe that the only biological valve you use is the sutureless valve, or do you use others?
Dr Laborde: We use this.
Dr Amrani: That's all you use?
Dr Laborde: Yes.
Dr J. Dillon(Kuala Lumpur, Malaysia): In your initial selection criteria, anatomical selection, you excluded the bicuspid valve and you excluded a large sinotubular junction with a ratio of more than 1.3. After you have overcome your learning curve, does this still remain your contraindications, or do you include some of these patients?
Dr Laborde: You have to keep in mind that this was a clinical study, so you have to respect the inclusion criteria. But after the experience, make sure that you can adjust some off-label cases, and that means that what you have to respect is the size and the shape of the ascending aorta. This device is maintained at the aortic level within the sinus of Valsalva with the stent and at the sinotubular junction. If you don't have those three criteria with this ratio, the valve will probably not be as stable as it should be. It is a real contraindication. With the true bicuspid, congenital bicuspid valve, which the surgeons who have experience have already implanted, and in those cases you need to organize the three temporary stitches and the shape of the annulus to be sure that this will be correctly implanted, but usually in those true bicuspid valves, the sinotubular junction is frequently dilated, so you have to be sure of this.
Dr Dillon: The main anchoring point of the valve is at the radial force at the annulus; is that right?
Dr Laborde: Not only. The device is designed to be lodged in the aortic root and the shape of the device fits with the shape of the aortic root.
Dr H. Cullen(Adelaide, Australia): I think you said that 1% of the patients were reoperated on for severe paravalvular leak. How many patients had mild or moderate paravalvular leak and how are they being followed up?
Dr Laborde: When you correctly decalcify the orifice, when you remove all the calcium bulging into the orifice, you will not have paravalvular leak.
Dr Cullen: Were there no patients at all with mild or moderate paravalvular leak?
Dr Laborde: We can accept level 1, but you have to be sure when you check it by echo that you don't underestimate the paravalvular leak.
Dr G. Dellgren(Gothenburg, Sweden): Interesting data; however, in giving a word of caution, I must say that it seems to be that we as a surgical community have forgotten the history of valve surgery. Giving the very strong statement that you do and that this is the only bioprosthesis that you use in your institution, with only one year of follow-up, is to me unbelievable, and I would just like to ask you what is the rationale for not reporting the data according to the current guidelines?
Dr Laborde: I implanted the first valve 7 years ago, so I finally have a bigger experience that I presented to you. This is the global result after one year with this multicentre study and this is what I presented. I agree that we are a surgical community, but, as you know, the TAVI process, and in the near future the percutaneous mitral valve process, will be something which will be more and more used and more and more important, and I think that for the surgeons, we have to realise this, accept this, and try to organise the tools we have to perform surgery in this way, and if we continue to use our classic valves, which are 30 years old, and more for some of them, I think we probably will miss the train. It is important for us to have this in mind and change our way to continue to be competitive, otherwise I'm sure that things will change.
Dr Dellgren : But by pursuing it in this way, you will end up with having a 5-year outcome of structural valve degeneration and non-SVD of between 5% and 10%. So what we are pursuing is a poor outcome.
Dr Laborde : Yes, I agree, but we don't have a long follow-up with regard to this.
Dr Dellgren : Extrapolating the data you have shown and what has been previously shown today, are not great results.
Dr M. Amrani(London, UK) : There is probably a misunderstanding between what you have presented, which is multicentric, and your own experience. I know you started many years ago. Perhaps you could answer in light of what you have experienced.
Dr Laborde : We don't have more results that we can have from the beginning of this experience. We have shown you what you have noticed one year after with this complete multicentre trial. I cannot predict the future no more than you, but when we will have 15 years of follow-up of these 8,000 patients now implanted, I'm sure that I will be able to answer your question, but today I cannot.
Author notes
Presented at the 28th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Milan, Italy, 11–15 October 2014.
- aortic valve
- cardiopulmonary bypass
- endocarditis
- hemodynamics
- congenital aortic valve insufficiency
- cerebrovascular accident
- aortic valve replacement
- objective (goal)
- multicenter studies
- preoperative care
- safety
- surgical procedures, operative
- mortality
- older adult
- exclusion criteria
- octogenarians
- implants
- european system for cardiac operative risk evaluation




