-
PDF
- Split View
-
Views
-
Cite
Cite
Tomoyuki Hishida, Shogo Nomura, Motoki Yano, Hisao Asamura, Motohiro Yamashita, Yasuhisa Ohde, Keishi Kondo, Hiroshi Date, Meinoshin Okumura, Kanji Nagai, on behalf of the Japanese Association for Research on the Thymus (JART), Long-term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese Nationwide Database Study, European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 3, March 2016, Pages 835–841, https://doi.org/10.1093/ejcts/ezv239
Close - Share Icon Share
Abstract
Thymic carcinoma is a rare thymic malignancy. The purpose of this study was to evaluate the prognostic impact of clinicopathological variables and perioperative therapy for surgically treated thymic carcinoma using a nationwide database.
Of 2835 patients with surgically treated thymic epithelial tumours collected from 32 Japanese institutions, a total of 306 patients with thymic carcinomas, excluding neuroendocrine tumours, were enrolled in this retrospective study. Multivariable Cox regression analyses were performed for overall (OS) and recurrence-free survival (RFS) after R0 resection.
Of 306 patients, 228 (75%) patients presented with Masaoka stage III–IV. Squamous cell carcinoma was the most common histological type ( n = 216, 71%). R0 resection was performed in 181 (61%) patients, R1 in 46 (16%), R2 sub-total (≥80% tumour resection) in 43 (14%) and R2 non-resection in 27 (9%). The 5-year OS rate was 61%. Prognostic factors for OS were Masaoka stage and resection status. R0 resection was associated with most improved OS; however, both R1 and R2 sub-total resection resulted in superior OS compared with R2 non-resection [hazard ratio (95% confidence interval) for R0, R1 and R2 sub-total, 0.27 (0.15–0.48), 0.40 (0.22–0.74) and 0.38 (0.20–0.72), respectively]. Histological type and perioperative therapy did not affect OS, whereas tumour size and postoperative radiotherapy were associated with improved RFS after R0 resection.
R0 resection is essential for prolonged OS for surgically treated thymic carcinoma, but maximal debulking surgery might be beneficial and worth evaluating for advanced disease deemed difficult for R0 resection. The benefit of postoperative radiotherapy after R0 resection should also be evaluated prospectively.
INTRODUCTION
Thymic carcinoma is a rare thymic neoplasm that accounts for 10–15% of all thymic epithelial tumours [ 1–3 ]. Previous studies have shown that surgical resection is the mainstream treatment for thymic carcinoma [ 4–10 ]. However, due to the rarity of the disease, most of these studies have been derived from small retrospective series. Thus, multicentre studies have been needed globally to determine the modern surgical outcome and prognostic factors of thymic carcinoma.
The Japanese Association for Research of the Thymus (JART) developed a nationwide retrospective database to clarify disease profiles of thymic tumours, including thymic carcinoma. The purpose of this study was to clarify the clinical, pathological and treatment factors associated with survival of surgically treated thymic carcinoma using the information from the nationwide database.
PATIENTS AND METHODS
Study cohort
JART developed a nationwide database in 2012, and retrospectively collected the data of 2835 thymic epithelial tumour patients who underwent surgical management between 1991 and 2010 at 32 Japanese institutions. The JART database included patient characteristics, preoperative and final pathological Masaoka stage, preoperative and pathological tumour size, histological type, type of resection, resection status, perioperative therapies including chemotherapeutic regimens and radiation dose, pattern and treatment of recurrence and survival. Patients who were mainly treated by modalities other than surgery were essentially not included in the database. The study was approved by the respective institutional review boards, and the need for obtaining informed consent from each patient was waived.
From this database, a total of 306 (10.8%) patients with a pathological diagnosis of thymic carcinoma were identified and enrolled into the current study. Type B3 thymomas (well-differentiated thymic carcinomas, n = 321) were strictly excluded from the study cohort. We also excluded neuroendocrine tumours (NETs, n = 64), since, although the current WHO classification defines them as the same entity as thymic carcinoma, NETs have distinct features from those of typical thymic carcinomas [ 11 ].
Clinicopathological analysis
The preoperative and final pathological stage classification was based on the Masaoka staging system [ 12 ]. Histological type was diagnosed at each institution according to the latest WHO classification [ 11 ], collected by a questionnaire survey and classified into the following three groups by the previously proposed histological grade [ 4 ]: (i) low-grade thymic carcinoma (LG-TC; squamous cell carcinoma, mucoepidermoid carcinoma and basaloid carcinoma), (ii) high-grade thymic carcinoma (HG-TC; lymphoepithelioma-like carcinoma, clear-cell carcinoma, sarcomatoid carcinoma and adenocarcinoma) and (iii) thymic carcinoma with unclassified/unspecified histology (NOS-TC; thymic carcinoma, not otherwise specified).
The resection status was classified into four groups: R0 (complete resection as determined macroscopically and microscopically), R1 (microscopically incomplete resection), R2 sub-total [macroscopically incomplete but sub-total (≥80%) tumour resection] and R2 non-resection (<80% tumour resection, biopsy alone or exploratory thoracotomy). In this study, when pleural or pericardial disseminated disease (Masaoka stage IVa) was macroscopically completely removed together with the primary tumour, it was defined as R1 resection according to the JART criteria. For mediastinal/cervical lymph nodes or pulmonary metastases (Masaoka stage IVb), we assigned a classification of R0 if these metastatic spreads were completely resected with the main tumour. The pattern of recurrence after R0 resection was classified according to the proposal of the International Thymic Malignancy Interest Group (ITMIG) [ 13 ].
The survival outcome included overall survival (OS), recurrence-free survival (RFS) and post-recurrence survival (PRS). OS was calculated from the time of surgery to the time of death from any cause or the last follow-up for living patients. RFS was calculated from the time of R0 resection to the time of recurrence, death or the last follow-up if the patient remained recurrence-free. PRS was calculated from the date of recurrence to the date of any death or the last follow-up.
Statistical analysis
The main objective of this study was to identify independent prognostic factors by performing Cox regression analyses for OS and RFS. The candidate baseline factors included age, gender, histological type (LG-TC, HG-TC or NOS-TC), pathological tumour size, pathological Masaoka stage, resection status (R0, R1, R2 sub-total or R2 non-resection) and major perioperative therapeutic modalities (preoperative chemotherapy and postoperative radiotherapy). Age was included as a categorical variable (age ≥70 or not) because the effect of the geriatric subgroup was of interest. Preliminary analyses of this study revealed a lack of survival difference between Masaoka stages I and II and stages IVa and IVb. Therefore, Masaoka stages were categorized into three groups (I–II/III/IV). The patients whose baseline factors were missing were excluded from survival analyses. The baseline prognostic factors for OS and RFS were evaluated using univariable and multivariable stepwise Cox regression analyses with entry and removal significance level of 0.10 and 0.05. In stepwise multivariable analyses, preoperative chemotherapy and postoperative radiotherapy were forcedly included. Pathological tumour size, which was unavailable for non-resection cases, was added only for RFS analysis. In multivariable analyses, pathological tumour size was modelled through natural cubic spline functions. The Kaplan–Meier method was used to estimate survival curves, and the log-rank test was used to evaluate the differences among subgroups. Categorical measures between two groups were analysed utilizing cross-tables and Pearson's χ2 test. All statistical analyses were performed using JMP software version 8.02 and SAS Release 9.3 (SAS Institute, Inc., Cary, NC, USA). All P values reported were two-sided.
RESULTS
Patient characteristics
Table 1 shows the patient characteristics of the study cohort ( n = 306). Preoperative histological diagnosis was obtained in 135 (44%) patients, 7% (5/73) in preoperative stage I–II and 56% (106/189) in stage III–IV, mainly by needle biopsy. As a final pathology, 75% of the patients had Masaoka stage III or IV disease. In preoperative stage I and II disease, 15 and 53% were upstaged to pathological stage III or higher, respectively. The most common type of resection was total thymectomy (74%), and 85% of surgeries were performed via trans-sternal approach. There was no postoperative mortality. Squamous cell carcinoma was the most common histological type (71%). The proportion of R0 resection was 61% of all patients and was decreased according to pathological Masaoka stage progression (100% in Stage I, 68% in Stage III and 47% in Stage IVb). R0 resection in Stage IVb disease ( n = 30) was achieved for metastases in anterior (perithymic) nodes ( n = 17), deep intrathoracic/cervical nodes ( n = 9) and lung ( n = 4). Although R0 resection was equally achieved among three histological types [LG-TC: 60% (134/222), HG-TC: 63% (15/24), NOS-TC: 63% (32/51)], R2 sub-total resection was frequent in the HG-TC group [LG-TC: 15% (33/222), HG-TC: 25% (6/24), NOS-TC: 8% (4/51)]. R2 non-resection group contained more NOS-TC than R2 sub-total group [44% (12/27) vs 9% (4/43), P = 0.001].
| Variables . | No. . | % . |
|---|---|---|
| Age (years), median (range) | 61 (23–88) | |
| Gender | ||
| Male | 189 | 62 |
| Female | 117 | 38 |
| Past history of malignant disease | 28 | 9 |
| Preoperative tumour size (cm), median (range) | 5.6 (1.6–17) | |
| Preoperative histological diagnosis | ||
| Yes | 135 | 44 |
| Masaoka stage, preoperative ( n = 262) | ||
| I | 34 | 13 |
| II | 39 | 15 |
| III | 120 | 45 |
| IVa | 28 | 11 |
| IVb | 41 | 16 |
| Masaoka stage, final pathological ( n = 304) | ||
| I | 17 | 6 |
| II | 59 | 19 |
| III | 120 | 40 |
| IVa | 40 | 13 |
| IVb | 68 | 22 |
| Surgical approach ( n = 296) | ||
| Sternotomy (± thoracotomy) | 253 | 85 |
| Thoracotomy | 16 | 5 |
| VATS | 27 | 10 |
| Completeness of resection ( n = 297) | ||
| R0 | 181 | 61 |
| R1 | 46 | 16 |
| R2 sub-total | 43 | 14 |
| R2 non-resection | 27 | 9 |
| Pathological tumour size (cm), median (range) | 6.0 (1.5–15) | |
| Histology | ||
| Low-grade thymic carcinoma | 224 | 73 |
| Squamous cell carcinoma | 216 | 71 |
| Mucoepidermoid carcinoma | 6 | |
| Basaloid carcinoma | 2 | |
| High-grade thymic carcinoma | 25 | 8 |
| Adenocarcinoma | 16 | |
| Lymphoepithelioma-like carcinoma | 4 | |
| Sarcomatoid carcinoma | 3 | |
| Clear-cell carcinoma | 2 | |
| Thymic carcinoma, not otherwise specified | 57 | 19 |
| Variables . | No. . | % . |
|---|---|---|
| Age (years), median (range) | 61 (23–88) | |
| Gender | ||
| Male | 189 | 62 |
| Female | 117 | 38 |
| Past history of malignant disease | 28 | 9 |
| Preoperative tumour size (cm), median (range) | 5.6 (1.6–17) | |
| Preoperative histological diagnosis | ||
| Yes | 135 | 44 |
| Masaoka stage, preoperative ( n = 262) | ||
| I | 34 | 13 |
| II | 39 | 15 |
| III | 120 | 45 |
| IVa | 28 | 11 |
| IVb | 41 | 16 |
| Masaoka stage, final pathological ( n = 304) | ||
| I | 17 | 6 |
| II | 59 | 19 |
| III | 120 | 40 |
| IVa | 40 | 13 |
| IVb | 68 | 22 |
| Surgical approach ( n = 296) | ||
| Sternotomy (± thoracotomy) | 253 | 85 |
| Thoracotomy | 16 | 5 |
| VATS | 27 | 10 |
| Completeness of resection ( n = 297) | ||
| R0 | 181 | 61 |
| R1 | 46 | 16 |
| R2 sub-total | 43 | 14 |
| R2 non-resection | 27 | 9 |
| Pathological tumour size (cm), median (range) | 6.0 (1.5–15) | |
| Histology | ||
| Low-grade thymic carcinoma | 224 | 73 |
| Squamous cell carcinoma | 216 | 71 |
| Mucoepidermoid carcinoma | 6 | |
| Basaloid carcinoma | 2 | |
| High-grade thymic carcinoma | 25 | 8 |
| Adenocarcinoma | 16 | |
| Lymphoepithelioma-like carcinoma | 4 | |
| Sarcomatoid carcinoma | 3 | |
| Clear-cell carcinoma | 2 | |
| Thymic carcinoma, not otherwise specified | 57 | 19 |
VATS: video-assisted thoracic surgery.
| Variables . | No. . | % . |
|---|---|---|
| Age (years), median (range) | 61 (23–88) | |
| Gender | ||
| Male | 189 | 62 |
| Female | 117 | 38 |
| Past history of malignant disease | 28 | 9 |
| Preoperative tumour size (cm), median (range) | 5.6 (1.6–17) | |
| Preoperative histological diagnosis | ||
| Yes | 135 | 44 |
| Masaoka stage, preoperative ( n = 262) | ||
| I | 34 | 13 |
| II | 39 | 15 |
| III | 120 | 45 |
| IVa | 28 | 11 |
| IVb | 41 | 16 |
| Masaoka stage, final pathological ( n = 304) | ||
| I | 17 | 6 |
| II | 59 | 19 |
| III | 120 | 40 |
| IVa | 40 | 13 |
| IVb | 68 | 22 |
| Surgical approach ( n = 296) | ||
| Sternotomy (± thoracotomy) | 253 | 85 |
| Thoracotomy | 16 | 5 |
| VATS | 27 | 10 |
| Completeness of resection ( n = 297) | ||
| R0 | 181 | 61 |
| R1 | 46 | 16 |
| R2 sub-total | 43 | 14 |
| R2 non-resection | 27 | 9 |
| Pathological tumour size (cm), median (range) | 6.0 (1.5–15) | |
| Histology | ||
| Low-grade thymic carcinoma | 224 | 73 |
| Squamous cell carcinoma | 216 | 71 |
| Mucoepidermoid carcinoma | 6 | |
| Basaloid carcinoma | 2 | |
| High-grade thymic carcinoma | 25 | 8 |
| Adenocarcinoma | 16 | |
| Lymphoepithelioma-like carcinoma | 4 | |
| Sarcomatoid carcinoma | 3 | |
| Clear-cell carcinoma | 2 | |
| Thymic carcinoma, not otherwise specified | 57 | 19 |
| Variables . | No. . | % . |
|---|---|---|
| Age (years), median (range) | 61 (23–88) | |
| Gender | ||
| Male | 189 | 62 |
| Female | 117 | 38 |
| Past history of malignant disease | 28 | 9 |
| Preoperative tumour size (cm), median (range) | 5.6 (1.6–17) | |
| Preoperative histological diagnosis | ||
| Yes | 135 | 44 |
| Masaoka stage, preoperative ( n = 262) | ||
| I | 34 | 13 |
| II | 39 | 15 |
| III | 120 | 45 |
| IVa | 28 | 11 |
| IVb | 41 | 16 |
| Masaoka stage, final pathological ( n = 304) | ||
| I | 17 | 6 |
| II | 59 | 19 |
| III | 120 | 40 |
| IVa | 40 | 13 |
| IVb | 68 | 22 |
| Surgical approach ( n = 296) | ||
| Sternotomy (± thoracotomy) | 253 | 85 |
| Thoracotomy | 16 | 5 |
| VATS | 27 | 10 |
| Completeness of resection ( n = 297) | ||
| R0 | 181 | 61 |
| R1 | 46 | 16 |
| R2 sub-total | 43 | 14 |
| R2 non-resection | 27 | 9 |
| Pathological tumour size (cm), median (range) | 6.0 (1.5–15) | |
| Histology | ||
| Low-grade thymic carcinoma | 224 | 73 |
| Squamous cell carcinoma | 216 | 71 |
| Mucoepidermoid carcinoma | 6 | |
| Basaloid carcinoma | 2 | |
| High-grade thymic carcinoma | 25 | 8 |
| Adenocarcinoma | 16 | |
| Lymphoepithelioma-like carcinoma | 4 | |
| Sarcomatoid carcinoma | 3 | |
| Clear-cell carcinoma | 2 | |
| Thymic carcinoma, not otherwise specified | 57 | 19 |
VATS: video-assisted thoracic surgery.
Perioperative therapy
Perioperative therapy according to pathological Masaoka stage is shown in Table 2 . Preoperative therapy was totally administered in 22%. Chemotherapy was mainly administered especially for patients with Stage III–IV. The commonly used chemotherapeutic regimens included cisplatin-based regimens (mostly adriamycin/cisplatin/vincristine/cyclophosphamide) and carboplatin-based regimens (mostly carboplatin/paclitaxel). Postoperative therapy was administered in 59%. Radiotherapy with a median radiation dose of 50 Gy (range, 20–70 Gy) was mostly administered even for Stage I–II disease.
| . | Total ( n = 294) . | Stage I ( n = 17) . | Stage II ( n = 58) . | Stage III ( n = 114) . | Stage IVa ( n = 40) . | Stage IVb ( n = 65) . |
|---|---|---|---|---|---|---|
| Preoperative therapy | 67 (22) | |||||
| CT | 42 (14) | 1 (6) | 2 (3) | 12 (11) | 13 (33) | 14 (22) |
| RT | 4 (1) | 0 | 0 | 3 (3) | 0 | 1 (2) |
| CT + RT | 21 (7) | 0 | 1 (2) | 9 (8) | 5 (13) | 6 (9) |
| Postoperative therapy | 173 (59) | |||||
| CT | 28 (10) | 1 (6) | 1 (2) | 4 (4) | 11 (28) | 11 (17) |
| RT | 101 (34) | 4 (24) | 23 (40) | 42 (37) | 9 (23) | 23 (35) |
| CT + RT | 44 (15) | 1 (6) | 2 (3) | 21 (18) | 10 (25) | 10 (15) |
| . | Total ( n = 294) . | Stage I ( n = 17) . | Stage II ( n = 58) . | Stage III ( n = 114) . | Stage IVa ( n = 40) . | Stage IVb ( n = 65) . |
|---|---|---|---|---|---|---|
| Preoperative therapy | 67 (22) | |||||
| CT | 42 (14) | 1 (6) | 2 (3) | 12 (11) | 13 (33) | 14 (22) |
| RT | 4 (1) | 0 | 0 | 3 (3) | 0 | 1 (2) |
| CT + RT | 21 (7) | 0 | 1 (2) | 9 (8) | 5 (13) | 6 (9) |
| Postoperative therapy | 173 (59) | |||||
| CT | 28 (10) | 1 (6) | 1 (2) | 4 (4) | 11 (28) | 11 (17) |
| RT | 101 (34) | 4 (24) | 23 (40) | 42 (37) | 9 (23) | 23 (35) |
| CT + RT | 44 (15) | 1 (6) | 2 (3) | 21 (18) | 10 (25) | 10 (15) |
Numbers in parentheses are percentages.
CT: chemotherapy; RT: radiotherapy.
| . | Total ( n = 294) . | Stage I ( n = 17) . | Stage II ( n = 58) . | Stage III ( n = 114) . | Stage IVa ( n = 40) . | Stage IVb ( n = 65) . |
|---|---|---|---|---|---|---|
| Preoperative therapy | 67 (22) | |||||
| CT | 42 (14) | 1 (6) | 2 (3) | 12 (11) | 13 (33) | 14 (22) |
| RT | 4 (1) | 0 | 0 | 3 (3) | 0 | 1 (2) |
| CT + RT | 21 (7) | 0 | 1 (2) | 9 (8) | 5 (13) | 6 (9) |
| Postoperative therapy | 173 (59) | |||||
| CT | 28 (10) | 1 (6) | 1 (2) | 4 (4) | 11 (28) | 11 (17) |
| RT | 101 (34) | 4 (24) | 23 (40) | 42 (37) | 9 (23) | 23 (35) |
| CT + RT | 44 (15) | 1 (6) | 2 (3) | 21 (18) | 10 (25) | 10 (15) |
| . | Total ( n = 294) . | Stage I ( n = 17) . | Stage II ( n = 58) . | Stage III ( n = 114) . | Stage IVa ( n = 40) . | Stage IVb ( n = 65) . |
|---|---|---|---|---|---|---|
| Preoperative therapy | 67 (22) | |||||
| CT | 42 (14) | 1 (6) | 2 (3) | 12 (11) | 13 (33) | 14 (22) |
| RT | 4 (1) | 0 | 0 | 3 (3) | 0 | 1 (2) |
| CT + RT | 21 (7) | 0 | 1 (2) | 9 (8) | 5 (13) | 6 (9) |
| Postoperative therapy | 173 (59) | |||||
| CT | 28 (10) | 1 (6) | 1 (2) | 4 (4) | 11 (28) | 11 (17) |
| RT | 101 (34) | 4 (24) | 23 (40) | 42 (37) | 9 (23) | 23 (35) |
| CT + RT | 44 (15) | 1 (6) | 2 (3) | 21 (18) | 10 (25) | 10 (15) |
Numbers in parentheses are percentages.
CT: chemotherapy; RT: radiotherapy.
Survivals and prognostic factors
Figure 1 shows the scheme of the study population. A total of 294 and 169 patients were available for the analysis of OS and RFS after R0 resection, respectively. The median follow-up period was 8.7 years, during which 113 (38%) of 294 patients had died. Ninety (80%) deaths were associated with progression of thymic carcinoma. The 5-year OS rate for all the 294 patients was 61%. Pathological Masaoka stage clearly stratified the patients by OS, although differences were not observed between Stages I and II ( P = 0.562) or Stages IVa and IVb ( P = 0.250) (Fig. 2 A). Figure 2 B shows OS curves according to resection status. R0 resection group had the best survival; however, R1 and R2 sub-total resection groups showed similar OS curves ( P = 0.955), which were superior to that of R2 non-resection group. For Masaoka stage IVa disease presenting with dissemination, the 5-year OS rates after R1 ( n = 16, 50%) and R2 sub-total ( n = 18, 45%) resection were better than after R2 non-resection ( n = 6, 0%). Figure 2 C shows the OS curves according to three histological groups, and there was no significant difference between the groups ( P = 0.545).
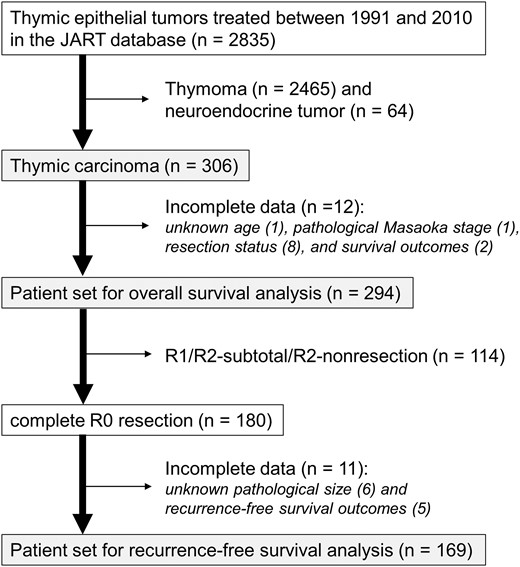
The scheme of the study population. JART: Japanese Association for Research on the Thymus.
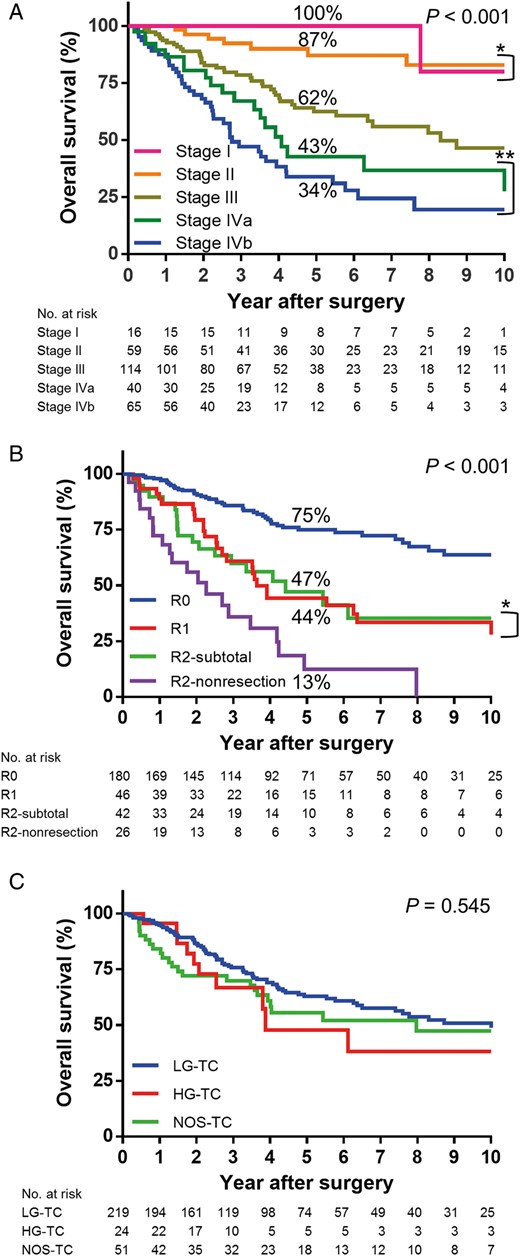
( A ) OS curves according to the pathological Masaoka stage: while there is a significant difference between each stage, there is no difference between Stages I and II (* P = 0.562) or Stages IVa and IVb (** P = 0.250). ( B ) OS curves according to resection status: while R0 resection group shows the best OS, OS curves between R1 and R2 sub-total groups are not different (* P = 0.955), which are superior to that of R2 non-resection group. ( C ) OS curves according to three histological groups: there is no significant difference between the groups ( P = 0.545). OS: overall survival; LG-TC: low-grade thymic carcinoma; HG-TC: high-grade thymic carcinoma; NOS-TC: thymic carcinoma, not otherwise specified.
The stepwise multivariable Cox regression analysis for OS returned the resection status and pathological Masaoka stage as prognostic factors (Table 3 ). The histological type, preoperative chemotherapy and postoperative radiotherapy were not significantly associated with OS. Although R0 resection resulted in most favourable OS, both R1 and R2 sub-total resection groups showed an equally favourable hazard ratio when compared with R2 non-resection group. In multivariable Cox analysis for RFS after R0 resection, early Masaoka stage (I–II) was still a variable conferring a protective effect on survival, but the hazard ratio of Stage IVb was similar to that of Stage III (Table 4 ). Patients with completely resected stage IVb disease including loco-regional nodal and pulmonary metastases showed relatively favourable RFS and OS (Fig. 3 A and B). Pathological tumour size, which was modelled thorough natural cubic spline function in the Cox model, affected RFS (Table 4 ). Postoperative radiotherapy also affected RFS favourably (Table 4 ) but not OS (Table 3 ). Survival curves according to postoperative radiotherapy status are shown in Fig. 4 A and B.
| Parameters . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age (ref. <70 years) | ||||||
| ≥70 | 1.14 | 0.71–1.76 | 0.563 | |||
| Gender (ref. male) | ||||||
| Female | 0.82 | 0.54–1.20 | 0.300 | |||
| Histological type (ref. LG-TC) | ||||||
| HG-TC | 1.35 | 0.65–2.49 | 0.391 | |||
| NOS-TC | 1.20 | 0.73–1.88 | 0.446 | |||
| Resection status (ref. R2 non-resection) | ||||||
| R0 | 0.16 | 0.09–0.28 | <0.001 | 0.27 | 0.15–0.48 | <0.001 |
| R1 | 0.42 | 0.23–0.77 | <0.001 | 0.40 | 0.22–0.74 | 0.004 |
| R2 sub-total | 0.43 | 0.22–0.81 | <0.001 | 0.38 | 0.20–0.72 | 0.003 |
| Pathological Masaoka stage (ref. I–II) | ||||||
| II | 3.72 | 1.94–7.88 | <0.001 | 2.97 | 1.45–6.10 | 0.003 |
| IV | 7.81 | 4.16–16.3 | <0.001 | 4.96 | 2.33–10.5 | <0.001 |
| Preoperative CT (ref. no) | ||||||
| Yes | 1.96 | 1.28–2.92 | 0.002 | 1.27 | 0.81–1.98 | 0.295 |
| Postoperative RT (ref. no) | ||||||
| Yes | 1.18 | 0.81–1.72 | 0.377 | 1.05 | 0.72–1.54 | 0.793 |
| Parameters . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age (ref. <70 years) | ||||||
| ≥70 | 1.14 | 0.71–1.76 | 0.563 | |||
| Gender (ref. male) | ||||||
| Female | 0.82 | 0.54–1.20 | 0.300 | |||
| Histological type (ref. LG-TC) | ||||||
| HG-TC | 1.35 | 0.65–2.49 | 0.391 | |||
| NOS-TC | 1.20 | 0.73–1.88 | 0.446 | |||
| Resection status (ref. R2 non-resection) | ||||||
| R0 | 0.16 | 0.09–0.28 | <0.001 | 0.27 | 0.15–0.48 | <0.001 |
| R1 | 0.42 | 0.23–0.77 | <0.001 | 0.40 | 0.22–0.74 | 0.004 |
| R2 sub-total | 0.43 | 0.22–0.81 | <0.001 | 0.38 | 0.20–0.72 | 0.003 |
| Pathological Masaoka stage (ref. I–II) | ||||||
| II | 3.72 | 1.94–7.88 | <0.001 | 2.97 | 1.45–6.10 | 0.003 |
| IV | 7.81 | 4.16–16.3 | <0.001 | 4.96 | 2.33–10.5 | <0.001 |
| Preoperative CT (ref. no) | ||||||
| Yes | 1.96 | 1.28–2.92 | 0.002 | 1.27 | 0.81–1.98 | 0.295 |
| Postoperative RT (ref. no) | ||||||
| Yes | 1.18 | 0.81–1.72 | 0.377 | 1.05 | 0.72–1.54 | 0.793 |
OS: overall survival; HR: hazard ratio; CI: confidence interval; LG-TC: low-grade thymic carcinoma; HG-TC: high-grade thymic carcinoma; NOS-TC: thymic carcinoma, not otherwise specified; CT: chemotherapy; RT: radiotherapy.
| Parameters . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age (ref. <70 years) | ||||||
| ≥70 | 1.14 | 0.71–1.76 | 0.563 | |||
| Gender (ref. male) | ||||||
| Female | 0.82 | 0.54–1.20 | 0.300 | |||
| Histological type (ref. LG-TC) | ||||||
| HG-TC | 1.35 | 0.65–2.49 | 0.391 | |||
| NOS-TC | 1.20 | 0.73–1.88 | 0.446 | |||
| Resection status (ref. R2 non-resection) | ||||||
| R0 | 0.16 | 0.09–0.28 | <0.001 | 0.27 | 0.15–0.48 | <0.001 |
| R1 | 0.42 | 0.23–0.77 | <0.001 | 0.40 | 0.22–0.74 | 0.004 |
| R2 sub-total | 0.43 | 0.22–0.81 | <0.001 | 0.38 | 0.20–0.72 | 0.003 |
| Pathological Masaoka stage (ref. I–II) | ||||||
| II | 3.72 | 1.94–7.88 | <0.001 | 2.97 | 1.45–6.10 | 0.003 |
| IV | 7.81 | 4.16–16.3 | <0.001 | 4.96 | 2.33–10.5 | <0.001 |
| Preoperative CT (ref. no) | ||||||
| Yes | 1.96 | 1.28–2.92 | 0.002 | 1.27 | 0.81–1.98 | 0.295 |
| Postoperative RT (ref. no) | ||||||
| Yes | 1.18 | 0.81–1.72 | 0.377 | 1.05 | 0.72–1.54 | 0.793 |
| Parameters . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age (ref. <70 years) | ||||||
| ≥70 | 1.14 | 0.71–1.76 | 0.563 | |||
| Gender (ref. male) | ||||||
| Female | 0.82 | 0.54–1.20 | 0.300 | |||
| Histological type (ref. LG-TC) | ||||||
| HG-TC | 1.35 | 0.65–2.49 | 0.391 | |||
| NOS-TC | 1.20 | 0.73–1.88 | 0.446 | |||
| Resection status (ref. R2 non-resection) | ||||||
| R0 | 0.16 | 0.09–0.28 | <0.001 | 0.27 | 0.15–0.48 | <0.001 |
| R1 | 0.42 | 0.23–0.77 | <0.001 | 0.40 | 0.22–0.74 | 0.004 |
| R2 sub-total | 0.43 | 0.22–0.81 | <0.001 | 0.38 | 0.20–0.72 | 0.003 |
| Pathological Masaoka stage (ref. I–II) | ||||||
| II | 3.72 | 1.94–7.88 | <0.001 | 2.97 | 1.45–6.10 | 0.003 |
| IV | 7.81 | 4.16–16.3 | <0.001 | 4.96 | 2.33–10.5 | <0.001 |
| Preoperative CT (ref. no) | ||||||
| Yes | 1.96 | 1.28–2.92 | 0.002 | 1.27 | 0.81–1.98 | 0.295 |
| Postoperative RT (ref. no) | ||||||
| Yes | 1.18 | 0.81–1.72 | 0.377 | 1.05 | 0.72–1.54 | 0.793 |
OS: overall survival; HR: hazard ratio; CI: confidence interval; LG-TC: low-grade thymic carcinoma; HG-TC: high-grade thymic carcinoma; NOS-TC: thymic carcinoma, not otherwise specified; CT: chemotherapy; RT: radiotherapy.
Univariable and multivariable analyses of parameters influencing RFS after R0 resection
| Parameters . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age (ref. <70 years) | ||||||
| ≥70 | 1.05 | 0.58–1.79 | 0.861 | |||
| Gender (ref. male) | ||||||
| Female | 0.72 | 0.43–1.16 | 0.177 | |||
| Histological type (ref. LG-TC) | ||||||
| HG-TC | 0.53 | 0.16–1.29 | 0.181 | |||
| NOS-TC | 0.66 | 0.31–1.23 | 0.198 | |||
| Pathological tumour size (1 cm up) | 1.15 | 1.05–1.26 | 0.002 | – | 0.049 a | |
| Pathological Masaoka stage (ref. I–II) | ||||||
| III | 3.01 | 1.75–5.39 | <0.001 | 2.58 | 1.43–4.66 | 0.002 |
| IV(b) | 3.67 | 1.83–7.25 | <0.001 | 2.74 | 1.30–5.77 | 0.008 |
| Preoperative CT (ref. no) | ||||||
| Yes | 2.54 | 1.36–4.44 | 0.005 | 1.72 | 0.91–3.25 | 0.094 |
| Postoperative RT (ref. no) | ||||||
| Yes | 0.59 | 0.36–0.95 | 0.027 | 0.54 | 0.34–0.88 | 0.013 |
| Parameters . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age (ref. <70 years) | ||||||
| ≥70 | 1.05 | 0.58–1.79 | 0.861 | |||
| Gender (ref. male) | ||||||
| Female | 0.72 | 0.43–1.16 | 0.177 | |||
| Histological type (ref. LG-TC) | ||||||
| HG-TC | 0.53 | 0.16–1.29 | 0.181 | |||
| NOS-TC | 0.66 | 0.31–1.23 | 0.198 | |||
| Pathological tumour size (1 cm up) | 1.15 | 1.05–1.26 | 0.002 | – | 0.049 a | |
| Pathological Masaoka stage (ref. I–II) | ||||||
| III | 3.01 | 1.75–5.39 | <0.001 | 2.58 | 1.43–4.66 | 0.002 |
| IV(b) | 3.67 | 1.83–7.25 | <0.001 | 2.74 | 1.30–5.77 | 0.008 |
| Preoperative CT (ref. no) | ||||||
| Yes | 2.54 | 1.36–4.44 | 0.005 | 1.72 | 0.91–3.25 | 0.094 |
| Postoperative RT (ref. no) | ||||||
| Yes | 0.59 | 0.36–0.95 | 0.027 | 0.54 | 0.34–0.88 | 0.013 |
RFS: recurrence-free survival; HR: hazard ratio; CI: confidence interval; LG-TC: low-grade thymic carcinoma; HG-TC: high-grade thymic carcinoma; NOS-TC: thymic carcinoma, not otherwise specified; CT: chemotherapy; RT: radiotherapy.
a Based on Type 3 test, which was a Wald test comparing the multivariable model including the pathological tumour size with that not including it.
Univariable and multivariable analyses of parameters influencing RFS after R0 resection
| Parameters . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age (ref. <70 years) | ||||||
| ≥70 | 1.05 | 0.58–1.79 | 0.861 | |||
| Gender (ref. male) | ||||||
| Female | 0.72 | 0.43–1.16 | 0.177 | |||
| Histological type (ref. LG-TC) | ||||||
| HG-TC | 0.53 | 0.16–1.29 | 0.181 | |||
| NOS-TC | 0.66 | 0.31–1.23 | 0.198 | |||
| Pathological tumour size (1 cm up) | 1.15 | 1.05–1.26 | 0.002 | – | 0.049 a | |
| Pathological Masaoka stage (ref. I–II) | ||||||
| III | 3.01 | 1.75–5.39 | <0.001 | 2.58 | 1.43–4.66 | 0.002 |
| IV(b) | 3.67 | 1.83–7.25 | <0.001 | 2.74 | 1.30–5.77 | 0.008 |
| Preoperative CT (ref. no) | ||||||
| Yes | 2.54 | 1.36–4.44 | 0.005 | 1.72 | 0.91–3.25 | 0.094 |
| Postoperative RT (ref. no) | ||||||
| Yes | 0.59 | 0.36–0.95 | 0.027 | 0.54 | 0.34–0.88 | 0.013 |
| Parameters . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age (ref. <70 years) | ||||||
| ≥70 | 1.05 | 0.58–1.79 | 0.861 | |||
| Gender (ref. male) | ||||||
| Female | 0.72 | 0.43–1.16 | 0.177 | |||
| Histological type (ref. LG-TC) | ||||||
| HG-TC | 0.53 | 0.16–1.29 | 0.181 | |||
| NOS-TC | 0.66 | 0.31–1.23 | 0.198 | |||
| Pathological tumour size (1 cm up) | 1.15 | 1.05–1.26 | 0.002 | – | 0.049 a | |
| Pathological Masaoka stage (ref. I–II) | ||||||
| III | 3.01 | 1.75–5.39 | <0.001 | 2.58 | 1.43–4.66 | 0.002 |
| IV(b) | 3.67 | 1.83–7.25 | <0.001 | 2.74 | 1.30–5.77 | 0.008 |
| Preoperative CT (ref. no) | ||||||
| Yes | 2.54 | 1.36–4.44 | 0.005 | 1.72 | 0.91–3.25 | 0.094 |
| Postoperative RT (ref. no) | ||||||
| Yes | 0.59 | 0.36–0.95 | 0.027 | 0.54 | 0.34–0.88 | 0.013 |
RFS: recurrence-free survival; HR: hazard ratio; CI: confidence interval; LG-TC: low-grade thymic carcinoma; HG-TC: high-grade thymic carcinoma; NOS-TC: thymic carcinoma, not otherwise specified; CT: chemotherapy; RT: radiotherapy.
a Based on Type 3 test, which was a Wald test comparing the multivariable model including the pathological tumour size with that not including it.
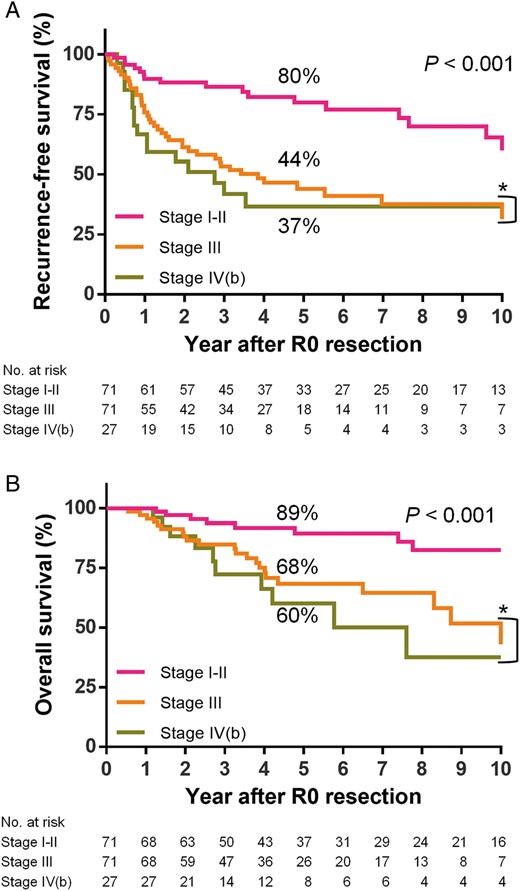
( A ) RFS curves after R0 resection according to the pathological Masaoka stage: while there is a significant difference among the three groups, no significant difference is observed between Stage III and IV(b) (* P = 0.527). ( B ) OS curves after R0 resection according to the Masaoka stage: no significant difference is observed between Stage III and IV(b) (* P = 0.545). RFS: recurrence-free survival; OS: overall survival.
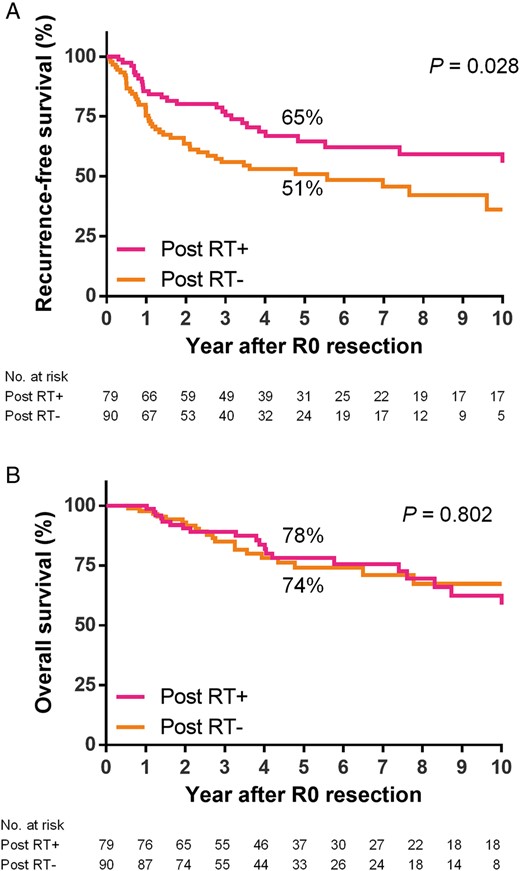
( A ) RFS and ( B ) OS curves after R0 resection according to the status of postoperative radiotherapy status: while patients who received postoperative radiotherapy had a favourable RFS ( P = 0.028), there was no significant difference in OS between the two groups ( P = 0.802). Post: postoperative; RT: radiotherapy; RFS: recurrence-free survival; OS: overall survival.
Recurrence patterns and post-recurrence survival
A total of 66 patients developed tumour recurrence after R0 resection. The initial recurrence was primarily detected in loco-regional sites ( n = 27, 41%) or as pulmonary metastases ( n = 22, 33%). Treatments for recurrence included chemotherapy with or without radiotherapy ( n = 28, 42%), repeat surgery ( n = 22, 33%), radiotherapy ( n = 5, 8%) and supportive care ( n = 11, 17%). The 1- and 3-year PRS rates were 80 and 44%, respectively.
DISCUSSION
In the present study, significant prognostic factors associated with OS were pathological Masaoka stage and resection status. Histological type and perioperative therapy did not affect OS, whereas pathological tumour size and the use of postoperative adjuvant radiotherapy were associated with an improved RFS after R0 resection.
Prognostic impact of Masaoka stage has been described in the previous studies [ 1 , 7 , 8 , 14 ], and validated in the recent European Society of Thoracic Surgeons (ESTS) database study and the joint ITMIG/ESTS global database study [ 3 , 15 ]. Although a new TNM staging system by the joint International Association for the Study of Lung Cancer/ITMIG thymic epithelial tumours staging project is under development [ 16 ], Masaoka staging system seems to be still the most consistent prognostic predictor across studies.
Complete R0 resection has also been reported as an essential prognostic factor for OS [ 1 , 3 , 7 , 8 , 14 , 15 ]. In this study, even Stage IVb patients had a relatively favourable survival if they underwent R0 resection for loco-regional nodal and pulmonary metastases (5-year RFS: 37%, OS: 60%), which was close to those of Stage III patients (5-year RFS: 44%, OS: 68%). R0 resection is essential for prolonged survival.
Unlike in thymoma cases, incomplete resection has not been considered to provide survival benefit for thymic carcinomas [ 1 , 8 ]. However, the present study implied the potential survival benefit of incomplete but sub-total resection. The R1 and R2 sub-total resection groups had median survival times (MST) of 3.6 and 4.4 years, respectively, which were longer than that of the R2 non-resection group (2.3 years) and reported MST after systemic chemotherapy for inoperable thymic carcinoma (20.0–27.3 months) [ 17–19 ]. Complete R0 resection is essentially impossible for disseminated disease even if there are no distant metastases. In this study, patients with dissemination (Masaoka stage IVa) had acceptable 5-year OS rates of 50% after R1 and 45% after R2 sub-total resection, respectively. Maximal debulking surgery by near total or sub-total resection might be useful not only for local control, but also for determining optimal additional treatments based on the detailed histological and molecular profiles available from resected specimens. In our series, R2 non-resection group included more NOS-TC sub-types than R2 sub-total resection group did, suggesting that adequate amount of tumour specimens available in maximum resection is necessary for precise tumour profiling. Clinical and survival benefit of maximal debulking surgery would be worth investigating in future prospective studies for advanced thymic carcinoma deemed difficult to achieve R0 resection, like those with pleural dissemination.
It has been a controversial issue as to whether histological types influence the survival outcome [ 3 , 4 , 20 ]. In the present study, frequent R2 sub-total resection in the high-grade thymic carcinoma (HG-TC) group initially implied an aggressive biological behaviour with poor outcome of this group. However, the histological type did not affect OS, as reported in the recent database studies [ 3 , 15 ]. HG-TCs in surgical database might be a selected population from entire HG-TCs. These indicate that aggressive surgical intervention is indicated for operable thymic carcinoma regardless of the histological type. In contrast to the recent database studies, this study showed that pathological tumour size was a prognostic factor for RFS. In thymoma, tumour size has been reported as a significant prognostic factor [ 21 ]. Useful size thresholds should be elucidated to predict exact patient outcome of thymic carcinoma.
Most thymic carcinomas present at an advanced stage and have a high frequency of relapse even after R0 resection, which indicates the need for a multidisciplinary therapeutic approach. However, we found no survival benefit of preoperative therapy, as reported in the ESTS database studies [ 3 ]. Although a neoadjuvant multimodality approach might be beneficial for marginally resectable disease to improve R0 rate [ 22 ], initially resectable disease is a candidate for primary surgery. On the other hand, we found that postoperative adjuvant radiotherapy after R0 resection was associated with favourable RFS, although it did not improve OS, as shown in our separate study targeted for Stage II–III disease with any resection status [ 23 ]. This is slightly different from the recent database studies. In the ESTS study, postoperative radiotherapy after any resection status improved OS but not RFS [ 3 ]. The ITMIG/ESTS study showed positive result for both OS and RFS [ 15 ]. Thymic malignancies are known to be radiosensitive [ 24 ], and residual and recurrent thymic carcinomas are usually indicated for radiotherapy in clinical practice. Postoperative adjuvant radiotherapy might be beneficial for improved survival; however, prophylactic mediastinal irradiation is associated with an increased risk of radiation pericarditis or esophagitis [ 25 ]. The usefulness of postoperative adjuvant radiotherapy after R0 resection should be evaluated by further prospective studies.
The present study has some limitations. The histological classification was collected by a questionnaire survey and not centrally reviewed. However, thymic carcinoma, mainly consisting of squamous cell carcinoma, has a morphology apparently different from thymoma, and its histological characteristics are clearly described in the WHO classification. Secondly, this study was retrospective and therefore may contain bias in patient and management selection. However, this study, the second largest series next to the ITMIG/ESTS study, has its novelty in showing new insight concerning clinical implication of maximal debulking surgery and postoperative radiotherapy for thymic carcinomas. It is also remarkable that the number of patients with missing data was very small and 96% of cases (294/306) were available for OS analyses.
In conclusion, Masaoka stage and resection status were significantly associated with OS of surgically treated thymic carcinoma. R0 resection is essential for prolonged OS; however, maximal debulking surgery might be beneficial and worth evaluating prospectively for advanced disease deemed difficult for R0 resection. The benefit of postoperative radiotherapy after R0 resection (shown in positive for RFS but not for OS) should also be evaluated in the prospective setting.
ACKNOWLEDGEMENTS
We thank all institutions that have joined the Japanese Association for Research on the Thymus (JART) database project to develop nationwide data for thymic epithelial tumours: Chiba University, Chiba; Shinsyu University, Nagano; Nagoya University, Nagoya; University of Tokyo, Tokyo; Okayama University, Okayama; Osaka Medical Centre for Cancer and Cardiovascular Diseases, Osaka; Juntendo University, Tokyo; Niigata University, Niigata; Nippon Medical University, Tokyo; Seirei Mikatagahara Hospital, Shizuoka; Tokushima University, Tokushima; Tokyo Metropolitan Cancer and Infectious Diseases Centre Komagome Hospital, Tokyo; National Hospital Organizatin Kinki-chuo Chest Medical Centre, Osaka; Kyusyu University, Fukuoka; Tokyo Medical University, Tokyo; University of Occupational and Environmental Health, Fukuoka; Kobe University, Hyogo; Tokyo Women's Medical University, Tokyo; Kumamoto University, Kumamoto; Nagasaki University, Nagasaki; Fukuoka University, Fukuoka; Tsuchiura Kyodo Hospital, Ibaraki; Aichi Medical University, Aichi; and Ehime University, Ehime, Japan. We also thank Junji Yoshida, National Cancer Centre Hospital East and Masayoshi Inoue, Osaka University Graduate School of Medicine for their helpful suggestions.
Conflict of interest: none declared.
REFERENCES
- squamous cell carcinoma
- debulking
- neuroendocrine tumors
- surgical procedures, operative
- patient prognosis
- surgery specialty
- thymus gland
- neoplasms, epithelial
- thymic carcinoma
- postoperative radiotherapy
- prognostic factors
- cox proportional hazards models
- tumor size
- tumor excision
- longitudinal relaxation rate
- malignant neoplasm of thymus
- japanese




