-
PDF
- Split View
-
Views
-
Cite
Cite
Alessandro Vivacqua, Jay J. Idrees, Douglas R. Johnston, Edward G. Soltesz, Lars G. Svensson, Eric E. Roselli, Thoracic endovascular repair first for extensive aortic disease: the staged hybrid approach , European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 3, March 2016, Pages 764–769, https://doi.org/10.1093/ejcts/ezv274
Close - Share Icon Share
Abstract
Repair of extensive aortic disease carries a significant risk of death and morbidity, the most feared complication being spinal cord ischaemia. Objectives of this study are to characterize patients, describe repair methods and assess feasibility and safety of hybrid staged repair for treatment of extensive aortic disease.
From to 2001 to 2013, 22 patients underwent extensive aortic repair that included a thoracic endovascular aortic repair (TEVAR) first followed by an open completion repair extending through the visceral and infrarenal aorta for degenerative aneurysm and dissection. At the time of initial repair, all patients were deemed to be at a high risk for conventional open repair and had extensive disease. Indications for open completion included emergency failure of TEVAR ( n = 3), early two-stage approach ( n = 6) and delayed disease progression after TEVAR ( n = 13). The median interval between stages was 6.5 months. The mean age was 56 ± 14 years, 5 patients had connective tissue disorder and the mean maximum aortic diameter was 58 ± 16 mm preoperatively.
There was no death or major complication after initial TEVAR, but the operative mortality rate was 9% ( n = 2) after the open procedure. One of these patients died from intraoperative myocardial infarction during emergency repair, and the other had disseminated intravascular coagulation during delayed repair for disease progression after TEVAR. Other complications included paralysis in 1 (4.5%), tracheostomy in 2 (9%) and dialysis in 1 (4.5%), and there was 1 reoperation for bleeding (4.5%). The median follow-up was 37 (range 3.3–93) months and there were no late deaths. There were four late reoperations for proximal disease progression leading to Type 1 endoleak ( n = 2), Type A dissection ( n = 1) and root aneurysm ( n = 1).
Use of a TEVAR-first approach in combination with a staged open repair is a safe and feasible treatment strategy for repair of extensive aortic disease. A staged hybrid approach to aortic repair in patients at high risk for total aortic replacement may limit morbidity.
INTRODUCTION
The treatment of extensive aortic aneurysms and aortic dissection carries a high risk of death and major morbidity including paralysis, renal and respiratory failure [ 1–3 ]. Open repair is currently the standard treatment, and several adjuncts have been implemented to minimize the risk of mortality and morbidity [ 4–7 ]. Alternative operative techniques have been proposed to further reduce the risk and decrease the surgical trauma [ 8–12 ]. Paraplegia remains the most devastating complication and is related to the extent of the aorta covered regardless of the repair technique [ 13 ]. Experimental animal models and previous observational studies have shown that a staged sacrifice of the spinal arteries (SAs) decreases the risk of paraplegia [ 14 , 15 ]. In an attempt to minimize major morbidity after repair of extensive aortic disease, we have developed a hybrid staged approach for treating these patients. Objectives of this study are to characterize patients, describe repair methods and assess feasibility and safety of hybrid staged repair for treatment of extensive aortic disease.
PATIENTS AND METHODS
Patients
Between January 2001 and December 2013, more than 1000 patients underwent thoraco-abdominal aortic repair at the Cleveland Clinic. Of these, 22 patients underwent a staged extensive aortic repair in which the first stage was an endovascular repair of the descending aorta (TEVAR) and the second stage was an open repair of the visceral and abdominal segments. In 4 patients, the hybrid staged approach was performed as an emergency for distal complications after descending thoracic endovascular aortic repair (TEVAR). In 8 patients, the second operation was planned as part of a hybrid strategy. These 8 patients were determined by our multidisciplinary aortic team to be too high risk for single-stage extensive repair. This determination was made based on a combination of multiple factors including comorbidities, anatomical limitations to safe cross-clamping and perceived risk for spinal cord injury if a single-stage repair was to be performed. In the other 10 cases, the second stage was to treat progressive enlargement of the distal aorta, mostly in the setting of chronic dissection. During this period of time, ∼400 patients had undergone TEVAR for the treatment of chronic distal dissection at out institution, and 17 of the 22 in the current series had their TEVAR performed here. All the patients had evidence of extensive aneurysmal degeneration with a mean diameter of the suprarenal aorta of 4.2 ± 0.9 mm. In the planned two-stage group, the suprarenal segment of the aorta was 4.7 ± 0.9 mm.
The median interval between stages was 6.5 months. The mean age was 56 ± 14, 17 patients were male and 5 (23%) had a confirmed connective tissue disorder. Aortic morphology at initial presentation included: degenerative aneurysm ( n = 2), chronic thoraco-abdominal dissection with aneurysm ( n = 18), pseudoaneurysm ( n = 1) and contained rupture of Type 2 thoraco-abdominal aneurysm ( n = 1). The mean maximum descending aortic diameter was 58 ± 16 mm. Table 1 summarizes preoperative patient characteristics.
| Characteristic . | n = 22 (%) . |
|---|---|
| Demographics | |
| Age (years) | 56 ± 14 |
| Male | 17 (77) |
| Comorbidities | |
| Hypertension | 18 (82) |
| Diabetes | 2 (9) |
| Coronary artery disease | 5 (23) |
| COPD | 2 (9) |
| Coagulopathy | 5 (23) |
| Renal failure | 4 (18) |
| Peripheral arterial disease | 2 (9) |
| Cancer | 5 (23) |
| Heart failure | 3 (14) |
| Myocardial infarction | 2 (9) |
| Cerebrovascular accident | 2 (9) |
| Prior cardiovascular procedure | 13 (59) |
| Aortic morphology and presentation | |
| Mean maximum descending aortic diameter (mm) | 58 ± 16 |
| Connective tissue disorder | 5 (23) |
| Contained rupture | 2 (9) |
| Degenerative aneurysm | 2 (9) |
| Aneurysm with dissection | 18 (82) |
| Chronic type B | 13 (59) |
| Residual type A | 3 (14) |
| Pseudoaneurysm, s/p acute type A repair | 2 (9) |
| Characteristic . | n = 22 (%) . |
|---|---|
| Demographics | |
| Age (years) | 56 ± 14 |
| Male | 17 (77) |
| Comorbidities | |
| Hypertension | 18 (82) |
| Diabetes | 2 (9) |
| Coronary artery disease | 5 (23) |
| COPD | 2 (9) |
| Coagulopathy | 5 (23) |
| Renal failure | 4 (18) |
| Peripheral arterial disease | 2 (9) |
| Cancer | 5 (23) |
| Heart failure | 3 (14) |
| Myocardial infarction | 2 (9) |
| Cerebrovascular accident | 2 (9) |
| Prior cardiovascular procedure | 13 (59) |
| Aortic morphology and presentation | |
| Mean maximum descending aortic diameter (mm) | 58 ± 16 |
| Connective tissue disorder | 5 (23) |
| Contained rupture | 2 (9) |
| Degenerative aneurysm | 2 (9) |
| Aneurysm with dissection | 18 (82) |
| Chronic type B | 13 (59) |
| Residual type A | 3 (14) |
| Pseudoaneurysm, s/p acute type A repair | 2 (9) |
| Characteristic . | n = 22 (%) . |
|---|---|
| Demographics | |
| Age (years) | 56 ± 14 |
| Male | 17 (77) |
| Comorbidities | |
| Hypertension | 18 (82) |
| Diabetes | 2 (9) |
| Coronary artery disease | 5 (23) |
| COPD | 2 (9) |
| Coagulopathy | 5 (23) |
| Renal failure | 4 (18) |
| Peripheral arterial disease | 2 (9) |
| Cancer | 5 (23) |
| Heart failure | 3 (14) |
| Myocardial infarction | 2 (9) |
| Cerebrovascular accident | 2 (9) |
| Prior cardiovascular procedure | 13 (59) |
| Aortic morphology and presentation | |
| Mean maximum descending aortic diameter (mm) | 58 ± 16 |
| Connective tissue disorder | 5 (23) |
| Contained rupture | 2 (9) |
| Degenerative aneurysm | 2 (9) |
| Aneurysm with dissection | 18 (82) |
| Chronic type B | 13 (59) |
| Residual type A | 3 (14) |
| Pseudoaneurysm, s/p acute type A repair | 2 (9) |
| Characteristic . | n = 22 (%) . |
|---|---|
| Demographics | |
| Age (years) | 56 ± 14 |
| Male | 17 (77) |
| Comorbidities | |
| Hypertension | 18 (82) |
| Diabetes | 2 (9) |
| Coronary artery disease | 5 (23) |
| COPD | 2 (9) |
| Coagulopathy | 5 (23) |
| Renal failure | 4 (18) |
| Peripheral arterial disease | 2 (9) |
| Cancer | 5 (23) |
| Heart failure | 3 (14) |
| Myocardial infarction | 2 (9) |
| Cerebrovascular accident | 2 (9) |
| Prior cardiovascular procedure | 13 (59) |
| Aortic morphology and presentation | |
| Mean maximum descending aortic diameter (mm) | 58 ± 16 |
| Connective tissue disorder | 5 (23) |
| Contained rupture | 2 (9) |
| Degenerative aneurysm | 2 (9) |
| Aneurysm with dissection | 18 (82) |
| Chronic type B | 13 (59) |
| Residual type A | 3 (14) |
| Pseudoaneurysm, s/p acute type A repair | 2 (9) |
Data were obtained from a retrospective chart review. The study was approved by the institutional review board at the Cleveland Clinic with patient consent waived.
Operative technique
First stage: TEVAR
Seventeen of the endovascular procedures were performed at our institution and five were done elsewhere. All procedures were done under general anaesthesia. A spinal drain was inserted in all patients and kept for at least 48 h postoperatively. Four patients underwent left carotid to left sub-clavian bypass 1 or 2 days before stent grafting due to planned left sub-clavian coverage and to optimize the proximal landing zone. Three patients had previously undergone first-stage elephant trunk for proximal aortic disease. The common femoral artery was accessed for device delivery, and percutaneous access of the opposite femoral or a brachial artery was obtained for angiography. In patients with chronic aortic dissection, intravascular ultrasound (IVUS) was used routinely to confirm the course of the wire within the true lumen and to evaluate the location of the branch vessels. The stent grafts were deployed to provide coverage down to the level of the diaphragm. A completion angiogram and IVUS were obtained to confirm good placement and patency of branch vessels, and to rule out endoleaks.
Second stage: open thoraco-abdominal completion repair
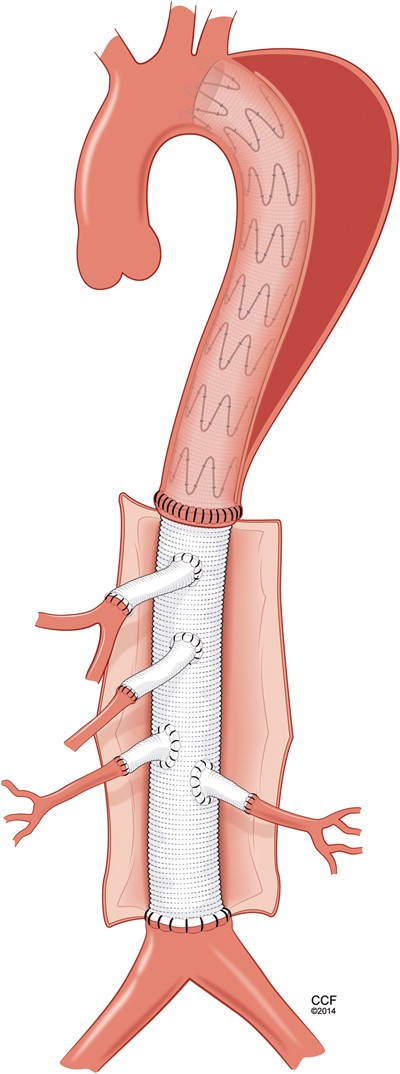
All patients received a spinal drain preoperatively. Under general anaesthesia, thoraco-abdominal incision was performed in the seventh or eighth intercostal space. The diaphragm was taken down and retroperitoneal dissection was extended to the level of the aortic bifurcation. All operations were performed with partial left atrial to left common femoral bypass and active cooling to a temperature of 31°C. Intrathecal papaverine was administered via the spinal drain prior to application of the aortic cross-clamp. Transoesophageal echocardiography helped in the location of the distal end of the stent graft within the aorta and a heavy-duty clamp was used to clamp the native descending aorta with the stent graft within it. The infrarenal aorta or iliac arteries were cross-clamped, and the aneurysm was opened. Cold renal perfusate solution was delivered into each of the renal arteries. The aorta was transected to create a clean edge where a multibranch graft was sewn end to end to the distal descending aorta, including the stent-graft in the anastomosis (Fig. 1 ). In cases of chronic dissection, the dissection membrane, or septum, was excised from around the stent graft to allow for full expansion of the last couple of stents. This anastomosis was reinforced with either a strip of felt or bovine pericardium. Once this was completed, the graft was clamped to allow for meticulous control of haemostasis around that anastomosis. Sequential reimplantation of the visceral and renal vessels was then performed. Additionally, segmental branch arteries were reimplanted in 5 (23%; intercostals in 4, lumbars in 1) patients. Then the graft was trimmed and sewn end to end to the infrarenal aorta (Fig. 2 ). Once all anastomoses were completed and rewarming was achieved, the patient was weaned from partial bypass. The aneurysm sac and a patch of bovine pericardium were used to cover the graft and to avoid direct contact with the lungs.
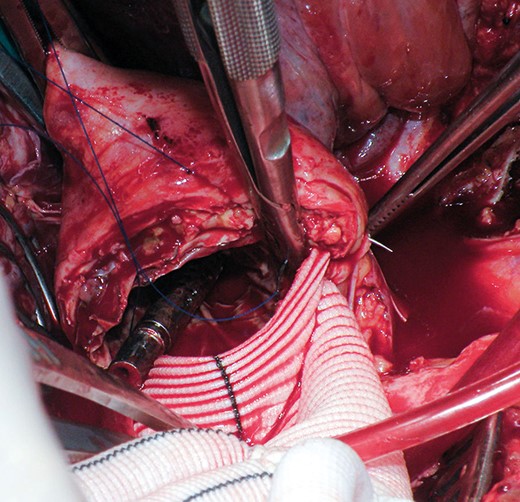
Intraoperative picture of the proximal anastomosis including the native aortic wall, the stent graft and the surgical graft.
Outcomes and statistics
Death and spinal cord injury were the primary end-points. Spinal cord injury was classified as immediate or delayed, and permanent or transient based on status at the time of hospital discharge. Renal failure was defined as the need for haemodialysis and respiratory failure was defined as the need for tracheostomy postoperatively. Follow-up was performed as scheduled outpatient visits at 3 months postoperatively and then annually, which included imaging with computed tomography angiography (Fig. 3 ) that was analysed using 3D reconstruction software (TeraRecon, San Mateo, CA, USA) to assess graft patency, device integrity, endoleaks and perfusion of the visceral vessels.
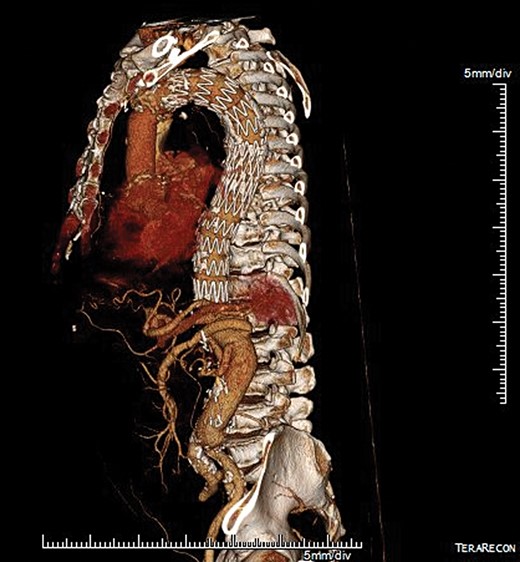
Postoperative 3D multiplanar reconstruction showing the hybrid extensive repair with an endovascular device in the descending aorta and the Dacron graft down to the aortic bifurcation.
Descriptive statistical analyses are used to present variables for the study. Continuous variables are presented as median or mean ± standard deviation, and categorical variables are presented as percentages. Survival analysis was performed using the Kaplan–Meier method.
RESULTS
First-stage TEVAR (endovascular elephant trunk)
There was no operative mortality or in-hospital death. There were no major complications after the first-stage TEVAR (no paraplegia, renal or respiratory failure and no reoperation for bleeding). In 2 patients, TEVAR was used as an emergency treatment for repair of a contained rupture as a bridge to definitive open completion repair during the same hospitalization, and 1 patient had urgent TEVAR for a large proximal pseudoaneurysm near a short graft used for previous descending repair prior to elective thoraco-abdominal completion repair. More than one device was used in 14 (63%) of the patients. The median hospital length of stay after TEVAR was 7 days (Table 2 ).
| Characteristic . | n = 22 (%) . |
|---|---|
| First-stage TEVAR (endovascular elephant trunk) | 22 |
| Urgent or emergency operation | 3 (14) |
| Mean suprarenal aortic diameter before TEVAR (mm, n = 17) | 42 ± 0.9 |
| Outcomes | |
| Mortality | 0 |
| Paralysis | 0 |
| Reoperation for bleeding | 0 |
| Tracheostomy | 0 |
| Renal dialysis | 0 |
| Median hospital length of stay (days) | 7 |
| Stent-graft details | |
| Two or more devices used | 14 (63) |
| Mean diameter (mm) | 34 |
| Device type | |
| Cook | 11 |
| Medtronic | 2 |
| Gore | 7 |
| Home made | 7 |
| Second-stage completion thoraco-abdominal repair | 22 |
| Median interval to completion (months) | 6.5 |
| Emergency repair | 3 (14) |
| Planned second stage | 9 (41) |
| Disease progression post TEVAR | 10 (45) |
| Outcomes | |
| Mortality | 2 (9) |
| Paralysis | 1 (4.5) |
| Reoperation for bleeding | 1 (4.5) |
| Tracheostomy | 2 (9) |
| Renal dialysis | 1 (4.5) |
| Median hospital length of stay (days) | 10 |
| Reintervention | 3 (14) |
| Emergency acute type A repair | 1 (4.5) |
| Frozen elephant trunk for ascending aortic aneurysm and Type 1 endoleak | 1 (4.5) |
| Valve-sparing root replacement for aneurysm growth | 1 (4.5) |
| Characteristic . | n = 22 (%) . |
|---|---|
| First-stage TEVAR (endovascular elephant trunk) | 22 |
| Urgent or emergency operation | 3 (14) |
| Mean suprarenal aortic diameter before TEVAR (mm, n = 17) | 42 ± 0.9 |
| Outcomes | |
| Mortality | 0 |
| Paralysis | 0 |
| Reoperation for bleeding | 0 |
| Tracheostomy | 0 |
| Renal dialysis | 0 |
| Median hospital length of stay (days) | 7 |
| Stent-graft details | |
| Two or more devices used | 14 (63) |
| Mean diameter (mm) | 34 |
| Device type | |
| Cook | 11 |
| Medtronic | 2 |
| Gore | 7 |
| Home made | 7 |
| Second-stage completion thoraco-abdominal repair | 22 |
| Median interval to completion (months) | 6.5 |
| Emergency repair | 3 (14) |
| Planned second stage | 9 (41) |
| Disease progression post TEVAR | 10 (45) |
| Outcomes | |
| Mortality | 2 (9) |
| Paralysis | 1 (4.5) |
| Reoperation for bleeding | 1 (4.5) |
| Tracheostomy | 2 (9) |
| Renal dialysis | 1 (4.5) |
| Median hospital length of stay (days) | 10 |
| Reintervention | 3 (14) |
| Emergency acute type A repair | 1 (4.5) |
| Frozen elephant trunk for ascending aortic aneurysm and Type 1 endoleak | 1 (4.5) |
| Valve-sparing root replacement for aneurysm growth | 1 (4.5) |
TEVAR: thoracic endovascular aortic repair.
| Characteristic . | n = 22 (%) . |
|---|---|
| First-stage TEVAR (endovascular elephant trunk) | 22 |
| Urgent or emergency operation | 3 (14) |
| Mean suprarenal aortic diameter before TEVAR (mm, n = 17) | 42 ± 0.9 |
| Outcomes | |
| Mortality | 0 |
| Paralysis | 0 |
| Reoperation for bleeding | 0 |
| Tracheostomy | 0 |
| Renal dialysis | 0 |
| Median hospital length of stay (days) | 7 |
| Stent-graft details | |
| Two or more devices used | 14 (63) |
| Mean diameter (mm) | 34 |
| Device type | |
| Cook | 11 |
| Medtronic | 2 |
| Gore | 7 |
| Home made | 7 |
| Second-stage completion thoraco-abdominal repair | 22 |
| Median interval to completion (months) | 6.5 |
| Emergency repair | 3 (14) |
| Planned second stage | 9 (41) |
| Disease progression post TEVAR | 10 (45) |
| Outcomes | |
| Mortality | 2 (9) |
| Paralysis | 1 (4.5) |
| Reoperation for bleeding | 1 (4.5) |
| Tracheostomy | 2 (9) |
| Renal dialysis | 1 (4.5) |
| Median hospital length of stay (days) | 10 |
| Reintervention | 3 (14) |
| Emergency acute type A repair | 1 (4.5) |
| Frozen elephant trunk for ascending aortic aneurysm and Type 1 endoleak | 1 (4.5) |
| Valve-sparing root replacement for aneurysm growth | 1 (4.5) |
| Characteristic . | n = 22 (%) . |
|---|---|
| First-stage TEVAR (endovascular elephant trunk) | 22 |
| Urgent or emergency operation | 3 (14) |
| Mean suprarenal aortic diameter before TEVAR (mm, n = 17) | 42 ± 0.9 |
| Outcomes | |
| Mortality | 0 |
| Paralysis | 0 |
| Reoperation for bleeding | 0 |
| Tracheostomy | 0 |
| Renal dialysis | 0 |
| Median hospital length of stay (days) | 7 |
| Stent-graft details | |
| Two or more devices used | 14 (63) |
| Mean diameter (mm) | 34 |
| Device type | |
| Cook | 11 |
| Medtronic | 2 |
| Gore | 7 |
| Home made | 7 |
| Second-stage completion thoraco-abdominal repair | 22 |
| Median interval to completion (months) | 6.5 |
| Emergency repair | 3 (14) |
| Planned second stage | 9 (41) |
| Disease progression post TEVAR | 10 (45) |
| Outcomes | |
| Mortality | 2 (9) |
| Paralysis | 1 (4.5) |
| Reoperation for bleeding | 1 (4.5) |
| Tracheostomy | 2 (9) |
| Renal dialysis | 1 (4.5) |
| Median hospital length of stay (days) | 10 |
| Reintervention | 3 (14) |
| Emergency acute type A repair | 1 (4.5) |
| Frozen elephant trunk for ascending aortic aneurysm and Type 1 endoleak | 1 (4.5) |
| Valve-sparing root replacement for aneurysm growth | 1 (4.5) |
TEVAR: thoracic endovascular aortic repair.
Second-stage thoraco-abdominal open completion
In 3 patients, the completion thoraco-abdominal repair was performed as an urgent or emergency indication. Two patients had contained rupture stabilized with stent grafting and required emergency open thoraco-abdominal completion as described above. Another patient developed Type 1b endoleak 5 years after TEVAR for degenerative aneurysm with rapid growth and a contained rupture. In the remaining patients, the median interval to the second-stage completion thoraco-abdominal repair was 6.5 months. Because many of these patients had chronic dissection, a patent false lumen and some evidence of distal dilatation, we often waited to see how their aorta would respond to endovascular treatment prior to moving towards open completion repair.
After second-stage open thoraco-abdominal completion repair, the operative mortality rate was 9% ( n = 2/22). One patient underwent emergency repair for contained rupture and died due to intraoperative myocardial infarction. She had a known right coronary stenosis and near the end of the emergency procedure sustained a cardiac arrest from which she could not be resuscitated. The other developed disseminated intravascular coagulation related to intraoperative bleeding from the proximal anastomosis. This case was performed early in the experience for progressive aortic growth after TEVAR for chronic aortic dissection. The anastomosis was performed between the surgical graft and the stent graft without including the native aortic wall.
Other complications after the second stage included paralysis in 1 (4.5%), tracheostomy in 2 (9%) and dialysis in 1 (4.5%) and 1 reoperation for bleeding (4.5%). The median hospital length of stay after open completion was 10 days (Table 2 ).
Late outcomes
The median follow-up was 37 (range 3.3–93) months after completion of the second-stage open completion repair. Four patients required additional interventions after the thoraco-abdominal completion repair, and all were related to proximal disease progression. Two patients developed a proximal Type 1 endoleak from growth of the aorta in the seal zone. In 1 patient, the endoleak was repaired by proximal extension of the previous TEVAR with additional stent grafting. This was done after performing a left carotid sub-clavian bypass and coil embolization of the left sub-clavian artery. The other 3 patients underwent open proximal aortic repair: one developed arch aneurysm and Type 1 endoleak, one presented with acute type A dissection and the third developed significant growth of the aortic root 2 years after thoraco-abdominal completion repair. The first 2 patients were treated with reverse frozen elephant trunk procedures performed urgently, and the third patient's surgery was performed electively. The patient with root growth underwent a modified valve-sparing root reimplantation procedure. There were no late deaths. Estimated survival rates at 1 month, 1 year, 3 years and 5 years after completion repair were 90, 81, 81 and 71%, respectively (Fig. 4 ).
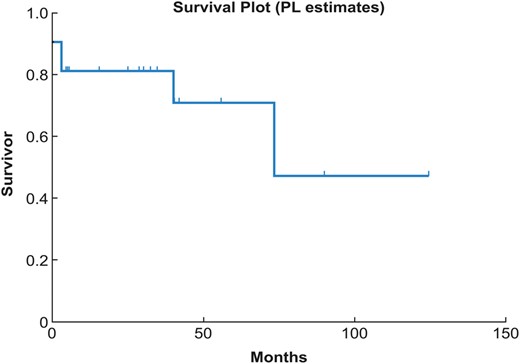
Estimated survival after completion of two-stage hybrid extensive repair using the Kaplan–Meier methodology. PL: product limit.
DISCUSSION
Extensive repair of thoraco-abdominal aneurysm and dissection carries a high risk of mortality and morbidity, especially in emergency indications and for those patients with multiple comorbidities. Furthermore, many patients are being treated with TEVAR for chronic dissection with failure to achieve false lumen remodelling in a significant proportion of patients [ 16 ]. This experience demonstrates that extensive aortic repair can be safely performed using a staged hybrid repair strategy that includes first-stage TEVAR of the descending aorta followed by a second-stage open thoraco-abdominal completion repair. This hybrid approach is not intended to replace open repair but may be considered when a patient with extensive disease is considered to be of too high risk for conventional repair. Also, this option needs to be discussed up front when choosing the endovascular treatment of chronic dissection since the intended reverse remodelling is not consistently achieved with that approach. This hybrid two-stage approach should be considered as an additional tool for the treatment of very complex extensive aortic disease.
This strategy may offer potential advantages over the conventional ‘all at once’ open approach for repair of the entire thoraco-abdominal aorta. The first endovascular stage eliminates the need for circulatory arrest, and this may be especially important in select patients with more complex disease encroaching on the arch. The risk of injuries to the phrenic and recurrent laryngeal nerves is also minimized by this approach. The ability to clamp the descending aorta during the second-stage repair may facilitate the conduct of surgery in those with previous thoracic or cardiovascular surgery and associated left pleural adhesions.
Patients with connective tissue disorders often require multiple aortic operations, and 5 patients in this series had confirmed connective tissue disorders. The numbers were too small to detect a difference, but these patients fared no different from the others. Nonetheless, caution should be used when placing stent grafts into the aorta of patients with a connective tissue disorder. Because of uncertainty about the durability of aortic integrity, attempts should be made to deploy devices within surgical graft material when plausible.
The open completion as a second-stage operation still carries significant risk but the potential benefit on the spinal cord circulation may justify this approach in select patients. Spinal cord ischaemia (SCI) may be the most devastating complication of TAAA repair. The risk correlates with the extent of the aorta repaired [ 13 ]. Several strategies have been implemented to protect the spinal cord through limiting the metabolic load or malperfusion [ 4–8 ]. It is also important to preserve the patency of the hypogastric and the sub-clavian arteries, which are important contributors to the collateral network supplying the spinal cord [ 17 ]. It is no longer believed that preservation of flow to a solitary critical artery is enough to protect the spinal cord. Rather, the SAs are part of a more extensive paraspinal and intraspinal collateral network which is fed predominantly from the sub-clavian artery branches from above, and from the hypogastric artery branches from below [ 18 ]. Experimental studies have demonstrated that the collateral network undergoes remodelling after extensive segmental artery sacrifice with an increase in the diameter of the spinal artery within 24 h and further enlargement by nearly 100% within 5 days [ 19 ]. Similarly, the pressure measured in the distal stump of the sacrificed segmental artery shows a nadir several hours postoperatively, increases to 60% of preoperative levels by 48 h and recovers to preoperative values at 5 days [ 20 ].
Some works in the literature suggest that staged repair of extensive aneurysm might reduce the risk of SCI. In an animal model, extensive sacrifice of segmental arteries in a staged fashion did not cause any paraplegia, while sacrificing the arteries in a single stage led to paraplegia in 40% of the animals [ 14 ]. The authors observed that the spinal cord perfusion pressure only dropped mildly in the staged approach when compared with those undergoing full sacrifice. Partial sacrifice also stimulated vascular remodelling that may minimize the impact of subsequent vessel sacrifice. Similar results are obtained by Bischoff et al. [ 14 ] who sacrificed the lumbar arteries first, and then covered the thoracic segmental arteries with an endovascular stent graft 7 days later. Retrospective studies in man suggest that the use of a staged approach is safe and may reduce the risk of paraplegia [ 15 , 21 , 22 ]. These initial data support the rationale to use a segmental approach to treat extensive disease in order to allow the spinal circulation to remodel and thereby decrease the lifetime risk of paraplegia. The risk of rupture between stages must be considered but, with one of the operations being endovascular, the staged repair strategy is easier for patients to handle and submit to than a two-staged open repair. Like any complex treatment strategy, careful patient selection and diligent surveillance are required.
Initially, this staged treatment modality came about from necessity. As TEVAR has been increasingly used for extended indications, an increasing number of patients are being seen with late complications. Many of the patients in this series underwent second-stage open repair after an attempt at TEVAR failed due to Type 1 endoleak or continued growth of the false lumen from retrograde perfusion after TEVAR [ 16 ]. As we gained experience with this approach, we began applying it to high-risk patients with more extensive disease. Now we will selectively choose this approach for patients with extensive disease who we believe may be at an elevated risk from a single-staged conventional repair.
The minimally invasive TEVAR during the first stage was shown to be very safe, but does not address the whole disease in many patients, particularly those with chronic aortic dissection [ 23 ]. Several authors have demonstrated that false lumen thrombosis only occurs in about two thirds of patients treated with TEVAR in the setting of a chronic dissection [ 24 ]. Because the endovascular approach is so much less invasive than the open repair, this unreliability of TEVAR has not dampened the enthusiasm for its use in this setting. In fact, we have recently seen two devices in the USA get expanded approval to include aortic dissection despite a limited amount of long-term data. We now know from the experience described here that conversion to open completion repair can be done safely and performing TEVAR first in the setting of chronic dissection does not necessarily interfere with later open repair.
CONCLUSIONS
Performing TEVAR first in combination with staged open repair as a staged hybrid approach is safe and feasible for repair of extensive thoraco-abdominal aneurysm. A staged hybrid approach may reduce morbidity in patients at high risk for extensive single-stage aortic repair.
Conflict of interest: Eric E. Roselli is a speaker and/or consultant for Medtronic, Cook, Bolton and Terumo.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr M. Schepens(Brugge, Belgium): You present the results of a so-called two-stage approach: First, the endovascular elephant trunk, a new term, followed about 12 months later by an open repair of the visceral and abdominal parts of the type 2 thoracoabdominal aortic aneurysm in a relatively small patient group.
The fact that you aim at the occlusion of the intercostal arteries in steps following the concept of the collateral network is probably without any doubt a strong argument to advocate this new technique.
Let me first ask you, suppose you yourself were a Marfan patient of 35 years old, having sustained a conservative treated type B aortic dissection and now present with a large type 2 thoracoabdominal aortic aneurysm, which kind of repair would you choose? Would you choose for your own presented technique, realizing that your overall mortality, 9%, and the paraplegia rate, 5%, is not lower than the conventional one-step open surgical repair? On top of that, you have about a 20% risk of developing an endoleak necessitating subsequent interventions including retrograde type A dissection, and I don't want to discuss the costs of the whole thing.
My second question is purely related to the technique. If I understood you very well, you put a clamp on the distal descending aorta and the distal clamp becomes below the renal arteries. Why not putting the two clamps close together, avoiding about 20 minutes of ischaemia of the renal arteries? I realize that the anastomosis between the new graft and the endoprosthesis, including the aortic wall, is difficult and I suppose you cannot do this safely in 10 minutes of time.
Dr Vivacqua: Regarding the first question, I think in a young patient with Marfan maybe the open treatment is still the gold standard because of the rate of complication and the progression of the disease that we can have in the future.
Regarding the second question, when we do the anastomosis, we actually like to sometimes take out one of the metal stents just to facilitate the suturing. We, of course, include the aortic wall just to stabilize and give haemostasis at the end.
Dr E. Roselli(Cleveland, OH, USA): I think that's right, just as Alessandro said, we will do sequential clamping if we can and where we clamp the descending aorta with the stent graft within it, depends on where along that tract it looks like a good place to clamp. So if we can, during the proximal anastomosis we'll put our lower clamp supraceliac, or sometimes right across the middle of the visceral segment, so we still maintain renal flow. But sometimes you just can't do that.
With regards to the Marfan question, I think what every Marfan's patient has to understand is that they are always going to be at risk for late operations no matter how you tackle the first segment of the aorta that you repair. Whether you do a type 2 thoracoabdominal or not, they're still at risk for having something done with their remaining root, arch, or their ascending aorta later on. The one late type A dissection we had in this series was not a guy that had a retrograde dissection related to the TEVAR device as much as he just had progression of disease with a de novo dissection.
Dr J. Bavaria(Philadelphia, PA, USA): I'd like to ask a question. Because the whole technique is going to depend on the robustness, so to speak, of the proximal landing zone, so that there are no type IA endoleaks, and also type II endoleaks. Otherwise you haven't really done a definitive procedure. I think it's an interesting idea, to be honest with you. But please give us some information on type II endoleaks and type IA endoleaks over the long term, do they exist? Because, obviously they are present, that's a very significant problem.
Dr Vivacqua: Yes, I know. We actually have a couple of operations for type I endoleak and we have to replace the arch, to fix it. So we use this one as an additional option to treat those patients. It is not really an alternative to any other, but it is just complementary to other techniques to use on those very complex patients.
For sure, what it does is it gives us time, to buy time, to allow the collateral network to remodel, and so we think to decrease in the long term the risk of spinal cord injury. We know that in some patients they will need a reoperation, so it might be in a few weeks, and we already know this when we plan in advance, or it may be little bit delayed and so we follow-up with a strict imaging and then we go back at some point.
Author notes
Presented at the 28th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Milan, Italy, 11–15 October 2014.
- aorta
- aortic diseases
- hemodialysis
- hemorrhage
- aneurysm
- connective tissue diseases
- disseminated intravascular coagulation
- disease progression
- tissue dissection
- follow-up
- objective (goal)
- paralysis
- repeat surgery
- safety
- spinal cord ischemia
- abdomen
- morbidity
- tracheostomy
- aortic surgery
- generalized illness
- infrarenal aorta
- perioperative myocardial infarction
- endoleak
- thoracic endovascular aortic repair
- surgical mortality
- aortic diameter




