-
PDF
- Split View
-
Views
-
Cite
Cite
Lay Ping Ong, Emily Thompson, Ashwin Sachdeva, B.C. Ramesh, Hazel Muse, Kirstie Wallace, Gareth Parry, Stephen Charles Clark, Allogeneic blood transfusion in bilateral lung transplantation: impact on early function and mortality, European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 2, February 2016, Pages 668–674, https://doi.org/10.1093/ejcts/ezv155
Close - Share Icon Share
Abstract
Blood transfusion is associated with higher morbidity and mortality after general cardiothoracic surgery but its impact within the transplant population is unclear. We investigated the profile of blood product transfusion in the bilateral lung transplant population and its impact on function and mortality.
Three hundred and eleven adult patients who underwent bilateral lung transplant between 2003 and 2013 were retrospectively reviewed. Patients were stratified according to pretransplant diagnoses and amount of blood products transfused within 24 h of transplant. All-cause mortality at the 1-year follow-up was analysed using a Cox proportional hazards regression model.
One hundred and seventy-four male patients and 137 female patients (mean age = 41.4 ± 14.0 years) underwent bilateral lung transplant using cardiopulmonary bypass for cystic fibrosis (48.9%), fibrotic lung disease (12.2%), emphysema (27.0%), bronchiectasis (5.8%), pulmonary hypertension (1.3%) and others (4.5%). The median number of red blood cells in the first 24 h was 3 (0–40) units, fresh frozen plasma (FFP) = 2 (0–26) units and platelets = 1 (0–7) units. The unadjusted all-cause mortality at the 1-year follow-up did not appear to be different between patient subgroups stratified by the median number of units of red blood cells (P = 0.827) or FFP transfused (P = 0.456). However, 1-year mortality was adversely affected when more than the median number of units of platelets was transfused (P = 0.010). Upon adjustment for confounding variables, 1-year mortality was noted to be greater among patients transfused more than the median unit of platelets (adjusted hazard ratios: 2.3, 95% confidence interval: 1.15–4.61, P = 0.019) and those with longer bypass times (P = 0.046). No significant difference in the number of units transfused was noted when patients were stratified by pretransplant diagnosis. Predicted lung function at 3 and 6 months was not significantly affected by greater blood product use.
Unlike general cardiothoracic surgery, blood transfusion had no effect on all-cause mortality, whereas a greater administration of platelets was observed to be associated with higher adjusted 1-year mortality. Transfusion rates were not significantly influenced by pretransplant diagnoses. Interestingly, lung function at 3 and 6 months was similar for patients who received more blood product transfusion.
INTRODUCTION
Survival in lung transplantation has greatly improved with better technology and more advanced cardiorespiratory support. With regards to lung transplantation, in particular, there is little within the literature reporting on the impact of allogeneic blood transfusion (ABT) on functional and survival outcomes [1–4]. ABT carries a significant morbidity and mortality within any surgical specialty, trauma setting or in critically ill patients [5–7]. ABT alone is associated with transfusion-related lung injury (TRALI), transfusion-associated circulatory overload (TACO), pulmonary infections, prolonged intensive care unit (ICU) stays and other morbidities [8–10]. Hence, we aimed to answer these questions: (i) is the transfusion of red blood cells (RBC), platelets or fresh frozen plasma (FFP) during the perioperative period a risk factor for mortality? (ii) Does RBC, platelet or FFP transfusion confer an increased morbidity such as prolonged ICU stay or reduced lung function? In the current climate of increasing transplant waiting lists and donor organ shortages, these answers may be invaluable in reducing further risk to the donated organ and thereby help improve outcomes for transplant patients.
MATERIALS AND METHODS
Patients and study design
We reviewed all patients who underwent bilateral lung transplantation in Freeman Hospital, Newcastle-upon-Tyne, UK from 2003 to 2013. All patients were under 18 years old, and retransplants were excluded. We examined clinical variables potentially related to bleeding or transfusion risk, such as patients' age, pretransplant disease, duration of cardiopulmonary bypass (CPB), gender and the following preoperative laboratory tests: haemoglobin (Hb), activated partial thromboplastin time, prothrombin time/international normalized ratio and platelet count. The Freeman Hospital Cardiothoracic Transplant Unit patient database was utilized for this study. Any variables being examined not contained within the database were collected retrospectively from patient's electronic or medical records. This retrospective study was approved by the Freeman Hospital and has no material ethical issues. A 10-year study period was chosen to ensure that we have a sufficient sample size to allow for robust analyses. Departmental blood transfusion protocols, ICU model of care or approach towards lung transplantation had not changed dramatically over this time period. However, aprotinin use had undergone stepwise changes over three time periods; (i) full aprotinin use (Era A: 2003–2007), (ii) aprotinin use stopped (Era B: 2008–2010) and (iii) off-licensed usage of aprotinin (Era C: 2011–2013). To address the time effect of a decade-long study and the stepwise changes in aprotinin use, these 3 separate eras were used as covariates in our analyses.
Operative methods, immunosuppression and prophylaxis
The general operative principles of bilateral lung transplantation were the same but patients were under the care of different consultant surgeons, each with their own preferred surgical techniques. All operations were started at the earliest possible opportunity on an urgent basis. All operations were undertaken using CPB. All patients undergoing lung transplantation were initially administered a standardized immunosuppressive regimen of methylprednisolone, azathioprine and ciclosporin.
Blood transfusion and lung function
The primary outcome measured was blood product transfusion RBCs, FFP and platelets) in units during operative day 0, defined as the start of surgery through to the first 24 h. The decision for blood product transfusion was based on joint clinical assessment of the patients' perioperative clinical status by the surgeon, anaesthetist and/or intensivist. Patients were subdivided into groups based on the median number of each blood product transfused within 24 h of transplantation for statistical analysis. Lung function at 3- and 6-month post-transplant was measured as predicted forced expiratory volume in 1 s (FEV1) (%) to account for the patient's body surface area.
Statistical analyses
Continuous data were reported as mean ± one standard deviation (SD) when appropriate. For continuous data, Student's t-test and analysis of variance were used to determine whether differences among groups were significant. For categorical variables, the χ2 test was used to determine whether differences among groups were significant. A P-value of <0.05 was considered significant. Patient mortality was calculated using the time from transplant to death. We were interested in investigating the immediate and short-term effects of perioperative blood transfusion on mortality, thereby using the 1-year follow-up data. No patients were lost to the follow-up within 1 year. Unadjusted all-cause mortality at the 1-year follow-up between groups was calculated using the χ2 test. Confounding variables such as sex, age, waiting time on transplant list, bypass time, ischaemic time, donor smoking status, length of ICU stay and 3 eras (Era A: 2003–2007, Era B: 2008–2010, Era C: 2011–2013), reflecting the stepwise changes in aprotinin use and the year of lung transplantation, were included in the multivariate Cox proportional hazards regression model with right-censoring at 1 year to estimate hazard ratios (HRs) with 95% confidence intervals (CIs), thus allowing a comparison of adjusted 1-year all-cause mortality between patient groups. Statistical analyses were performed using the GraphPad Prism 6 and Stata 12 software packages.
RESULTS
Patient characteristics, pattern of blood product transfusion
From 2003 to 2013, a total of 311 patients underwent bilateral lung transplantation under CPB. Preoperative and perioperative characteristics, and the pattern of blood product transfusion over 24 h from the start of surgery are summarized in Table 1. Of note, the distribution of perioperative blood product use within RBC, FFP and platelets groups were highly skewed to the left with a wide range and non-normal distribution. To investigate the effect of RBC, FFP and platelets transfusion on mortality and function, patients were subdivided into groups, based on whether they were transfused more or less than the median number of units transfused. Comparison of subgroup preoperative and perioperative characteristics are summarized in Table 2. Increased blood product transfusion was associated with significantly longer CPB times, as given in Table 2. Patients transfused with more than one unit of platelets were significantly older, as given in Table 2. Pretransplant diagnoses did not significantly affect the use of blood products during transplantation.
Preoperative, perioperative characteristics and mortality for the entire cohort
| Pretransplant diagnosis, n = 311 | n | % |
| Cystic fibrosis | 152 | 48.9 |
| Lung fibrosis | 38 | 12.2 |
| Emphysema | 85 | 27.0 |
| Bronchiectasis | 18 | 5.8 |
| Pulmonary hypertension | 4 | 1.3 |
| Others | 14 | 4.5 |
| Recipient characteristics | ||
| Mean recipient age (years) | 41.4 ± 14.0 | |
| Recipient gender | 174M:137F | |
| Median recipient waiting times (days) | 254.5 (2–2653) | |
| Donor characteristics | n | % |
| Donor comorbidity (smoking) | 139 | 44.7 |
| Cause of death (head trauma) | 48 | 15.4 |
| Perioperative characteristics | Median (Range) Units | |
| Red blood cells (RBCs) | 3 (0–40) | |
| Fresh frozen plasma (FFP) | 2 (0–26) | |
| Platelets | 1 (0–7) | |
| Mean bypass time (min) | 243.7 ± 57.9 | |
| Mean ischaemic time (min) | 368 ± 114 | |
| Median length of ICU stay (days) | 4 (1–155) | |
| Mortality | n | % |
| 30-day mortality | 21 | 6.8 |
| 90-day mortality | 35 | 11.3 |
| 1-year mortality | 55 | 17.7 |
| Pretransplant diagnosis, n = 311 | n | % |
| Cystic fibrosis | 152 | 48.9 |
| Lung fibrosis | 38 | 12.2 |
| Emphysema | 85 | 27.0 |
| Bronchiectasis | 18 | 5.8 |
| Pulmonary hypertension | 4 | 1.3 |
| Others | 14 | 4.5 |
| Recipient characteristics | ||
| Mean recipient age (years) | 41.4 ± 14.0 | |
| Recipient gender | 174M:137F | |
| Median recipient waiting times (days) | 254.5 (2–2653) | |
| Donor characteristics | n | % |
| Donor comorbidity (smoking) | 139 | 44.7 |
| Cause of death (head trauma) | 48 | 15.4 |
| Perioperative characteristics | Median (Range) Units | |
| Red blood cells (RBCs) | 3 (0–40) | |
| Fresh frozen plasma (FFP) | 2 (0–26) | |
| Platelets | 1 (0–7) | |
| Mean bypass time (min) | 243.7 ± 57.9 | |
| Mean ischaemic time (min) | 368 ± 114 | |
| Median length of ICU stay (days) | 4 (1–155) | |
| Mortality | n | % |
| 30-day mortality | 21 | 6.8 |
| 90-day mortality | 35 | 11.3 |
| 1-year mortality | 55 | 17.7 |
Preoperative, perioperative characteristics and mortality for the entire cohort
| Pretransplant diagnosis, n = 311 | n | % |
| Cystic fibrosis | 152 | 48.9 |
| Lung fibrosis | 38 | 12.2 |
| Emphysema | 85 | 27.0 |
| Bronchiectasis | 18 | 5.8 |
| Pulmonary hypertension | 4 | 1.3 |
| Others | 14 | 4.5 |
| Recipient characteristics | ||
| Mean recipient age (years) | 41.4 ± 14.0 | |
| Recipient gender | 174M:137F | |
| Median recipient waiting times (days) | 254.5 (2–2653) | |
| Donor characteristics | n | % |
| Donor comorbidity (smoking) | 139 | 44.7 |
| Cause of death (head trauma) | 48 | 15.4 |
| Perioperative characteristics | Median (Range) Units | |
| Red blood cells (RBCs) | 3 (0–40) | |
| Fresh frozen plasma (FFP) | 2 (0–26) | |
| Platelets | 1 (0–7) | |
| Mean bypass time (min) | 243.7 ± 57.9 | |
| Mean ischaemic time (min) | 368 ± 114 | |
| Median length of ICU stay (days) | 4 (1–155) | |
| Mortality | n | % |
| 30-day mortality | 21 | 6.8 |
| 90-day mortality | 35 | 11.3 |
| 1-year mortality | 55 | 17.7 |
| Pretransplant diagnosis, n = 311 | n | % |
| Cystic fibrosis | 152 | 48.9 |
| Lung fibrosis | 38 | 12.2 |
| Emphysema | 85 | 27.0 |
| Bronchiectasis | 18 | 5.8 |
| Pulmonary hypertension | 4 | 1.3 |
| Others | 14 | 4.5 |
| Recipient characteristics | ||
| Mean recipient age (years) | 41.4 ± 14.0 | |
| Recipient gender | 174M:137F | |
| Median recipient waiting times (days) | 254.5 (2–2653) | |
| Donor characteristics | n | % |
| Donor comorbidity (smoking) | 139 | 44.7 |
| Cause of death (head trauma) | 48 | 15.4 |
| Perioperative characteristics | Median (Range) Units | |
| Red blood cells (RBCs) | 3 (0–40) | |
| Fresh frozen plasma (FFP) | 2 (0–26) | |
| Platelets | 1 (0–7) | |
| Mean bypass time (min) | 243.7 ± 57.9 | |
| Mean ischaemic time (min) | 368 ± 114 | |
| Median length of ICU stay (days) | 4 (1–155) | |
| Mortality | n | % |
| 30-day mortality | 21 | 6.8 |
| 90-day mortality | 35 | 11.3 |
| 1-year mortality | 55 | 17.7 |
Comparison of perioperative and postoperative characteristics of patient subgroups divided based on the median units transfused for RBCs, FFP and platelets (Plts)
| . | RBC ≤ 3, n = 159 . | RBC > 3, n = 152 . | P-values . | FFP ≤ 2, n = 162 . | FFP > 2, n = 149 . | P-values . | Plts ≤ 1, n = 257 . | Plts > 1, n = 54 . | P-values . |
|---|---|---|---|---|---|---|---|---|---|
| Pretransplant diagnosis | |||||||||
| Cystic fibrosis | 65 | 87 | 76 | 76 | 134 | 18 | |||
| Lung fibrosis | 25 | 13 | 26 | 12 | 33 | 5 | |||
| Emphysema | 52 | 33 | 48 | 37 | 65 | 20 | |||
| Bronchiectasis | 9 | 9 | 8 | 10 | 15 | 3 | |||
| Pulmonary hypertension | 4 | 0 | 1 | 3 | 3 | 1 | |||
| Others | 4 | 10 | 3 | 11 | 7 | 7 | |||
| Recipient characteristics | |||||||||
| Recipient age (years) | 43 ± 14 | 40 ± 15 | ns | 42 ± 14 | 41 ± 14 | ns | 40 ± 14 | 47 ± 13 | <0.001 |
| Recipient gender (male) | 98 (62%) | 76 (50%) | 91 (56%) | 83 (56%) | 144 (56%) | 30 (56%) | |||
| Recipient median waiting times (days) | 251 (4–1953) | 267 (2–1653) | ns | 247.5 (4–2653) | 285.5 (2–2002) | ns | 258 (4–2653) | 242 (2–2002) | ns |
| Donor characteristics | |||||||||
| Donor comorbidity (smoking) | 67 (42%) | 72 (47%) | 68 (42%) | 71 (48%) | 106 (41%) | 33 (61%) | |||
| Donor cause of death (head trauma) | 22 (14%) | 26 (17%) | 26 (16%) | 22 (15%) | 41 (16%) | 7 (13%) | |||
| Perioperative characteristics | |||||||||
| Ischaemic time (min) | 362 ± 104 | 375 ± 124 | ns | 368 ± 120 | 369 ± 108 | ns | 366 ± 119 | 377 ± 86 | ns |
| Mean bypass time (min) | 228 ± 47 | 260 ± 63 | <0.001 | 234 ± 49 | 254 ± 64 | 0.004 | 235 ± 47 | 284 ± 81 | <0.001 |
| Median length of ICU stay (days) | 3 (1–155) | 4.5 (1–127) | ns | 4 (1–155) | 4 (1–127) | ns | 4 (1–155) | 8 (1–127) | <0.001 |
| Patients with PGD as cause of death | 6 (3.8%) | 14 (9.2%) | 0.051 | 7 (4.3%) | 13 (8.7%) | ns | 11 (4.3%) | 9 (16.7%) | <0.001 |
| Postoperative lung function | |||||||||
| Mean predicted FEV1% at 3 months | 74.7 ± 23 | 78.5 ± 22.4 | ns | 74.9 ± 22.1 | 77.8 ± 23.4 | ns | 76.5 ± 22.2 | 75.7 ± 26.7 | ns |
| Mean predicted FEV1% at 6 months | 80.9 ± 24.9 | 85.2 ± 24.2 | ns | 80.8 ± 24.7 | 85 ± 24.5 | ns | 83.3 ± 24 | 79.9 ± 28.5 | ns |
| . | RBC ≤ 3, n = 159 . | RBC > 3, n = 152 . | P-values . | FFP ≤ 2, n = 162 . | FFP > 2, n = 149 . | P-values . | Plts ≤ 1, n = 257 . | Plts > 1, n = 54 . | P-values . |
|---|---|---|---|---|---|---|---|---|---|
| Pretransplant diagnosis | |||||||||
| Cystic fibrosis | 65 | 87 | 76 | 76 | 134 | 18 | |||
| Lung fibrosis | 25 | 13 | 26 | 12 | 33 | 5 | |||
| Emphysema | 52 | 33 | 48 | 37 | 65 | 20 | |||
| Bronchiectasis | 9 | 9 | 8 | 10 | 15 | 3 | |||
| Pulmonary hypertension | 4 | 0 | 1 | 3 | 3 | 1 | |||
| Others | 4 | 10 | 3 | 11 | 7 | 7 | |||
| Recipient characteristics | |||||||||
| Recipient age (years) | 43 ± 14 | 40 ± 15 | ns | 42 ± 14 | 41 ± 14 | ns | 40 ± 14 | 47 ± 13 | <0.001 |
| Recipient gender (male) | 98 (62%) | 76 (50%) | 91 (56%) | 83 (56%) | 144 (56%) | 30 (56%) | |||
| Recipient median waiting times (days) | 251 (4–1953) | 267 (2–1653) | ns | 247.5 (4–2653) | 285.5 (2–2002) | ns | 258 (4–2653) | 242 (2–2002) | ns |
| Donor characteristics | |||||||||
| Donor comorbidity (smoking) | 67 (42%) | 72 (47%) | 68 (42%) | 71 (48%) | 106 (41%) | 33 (61%) | |||
| Donor cause of death (head trauma) | 22 (14%) | 26 (17%) | 26 (16%) | 22 (15%) | 41 (16%) | 7 (13%) | |||
| Perioperative characteristics | |||||||||
| Ischaemic time (min) | 362 ± 104 | 375 ± 124 | ns | 368 ± 120 | 369 ± 108 | ns | 366 ± 119 | 377 ± 86 | ns |
| Mean bypass time (min) | 228 ± 47 | 260 ± 63 | <0.001 | 234 ± 49 | 254 ± 64 | 0.004 | 235 ± 47 | 284 ± 81 | <0.001 |
| Median length of ICU stay (days) | 3 (1–155) | 4.5 (1–127) | ns | 4 (1–155) | 4 (1–127) | ns | 4 (1–155) | 8 (1–127) | <0.001 |
| Patients with PGD as cause of death | 6 (3.8%) | 14 (9.2%) | 0.051 | 7 (4.3%) | 13 (8.7%) | ns | 11 (4.3%) | 9 (16.7%) | <0.001 |
| Postoperative lung function | |||||||||
| Mean predicted FEV1% at 3 months | 74.7 ± 23 | 78.5 ± 22.4 | ns | 74.9 ± 22.1 | 77.8 ± 23.4 | ns | 76.5 ± 22.2 | 75.7 ± 26.7 | ns |
| Mean predicted FEV1% at 6 months | 80.9 ± 24.9 | 85.2 ± 24.2 | ns | 80.8 ± 24.7 | 85 ± 24.5 | ns | 83.3 ± 24 | 79.9 ± 28.5 | ns |
Comparison of perioperative and postoperative characteristics of patient subgroups divided based on the median units transfused for RBCs, FFP and platelets (Plts)
| . | RBC ≤ 3, n = 159 . | RBC > 3, n = 152 . | P-values . | FFP ≤ 2, n = 162 . | FFP > 2, n = 149 . | P-values . | Plts ≤ 1, n = 257 . | Plts > 1, n = 54 . | P-values . |
|---|---|---|---|---|---|---|---|---|---|
| Pretransplant diagnosis | |||||||||
| Cystic fibrosis | 65 | 87 | 76 | 76 | 134 | 18 | |||
| Lung fibrosis | 25 | 13 | 26 | 12 | 33 | 5 | |||
| Emphysema | 52 | 33 | 48 | 37 | 65 | 20 | |||
| Bronchiectasis | 9 | 9 | 8 | 10 | 15 | 3 | |||
| Pulmonary hypertension | 4 | 0 | 1 | 3 | 3 | 1 | |||
| Others | 4 | 10 | 3 | 11 | 7 | 7 | |||
| Recipient characteristics | |||||||||
| Recipient age (years) | 43 ± 14 | 40 ± 15 | ns | 42 ± 14 | 41 ± 14 | ns | 40 ± 14 | 47 ± 13 | <0.001 |
| Recipient gender (male) | 98 (62%) | 76 (50%) | 91 (56%) | 83 (56%) | 144 (56%) | 30 (56%) | |||
| Recipient median waiting times (days) | 251 (4–1953) | 267 (2–1653) | ns | 247.5 (4–2653) | 285.5 (2–2002) | ns | 258 (4–2653) | 242 (2–2002) | ns |
| Donor characteristics | |||||||||
| Donor comorbidity (smoking) | 67 (42%) | 72 (47%) | 68 (42%) | 71 (48%) | 106 (41%) | 33 (61%) | |||
| Donor cause of death (head trauma) | 22 (14%) | 26 (17%) | 26 (16%) | 22 (15%) | 41 (16%) | 7 (13%) | |||
| Perioperative characteristics | |||||||||
| Ischaemic time (min) | 362 ± 104 | 375 ± 124 | ns | 368 ± 120 | 369 ± 108 | ns | 366 ± 119 | 377 ± 86 | ns |
| Mean bypass time (min) | 228 ± 47 | 260 ± 63 | <0.001 | 234 ± 49 | 254 ± 64 | 0.004 | 235 ± 47 | 284 ± 81 | <0.001 |
| Median length of ICU stay (days) | 3 (1–155) | 4.5 (1–127) | ns | 4 (1–155) | 4 (1–127) | ns | 4 (1–155) | 8 (1–127) | <0.001 |
| Patients with PGD as cause of death | 6 (3.8%) | 14 (9.2%) | 0.051 | 7 (4.3%) | 13 (8.7%) | ns | 11 (4.3%) | 9 (16.7%) | <0.001 |
| Postoperative lung function | |||||||||
| Mean predicted FEV1% at 3 months | 74.7 ± 23 | 78.5 ± 22.4 | ns | 74.9 ± 22.1 | 77.8 ± 23.4 | ns | 76.5 ± 22.2 | 75.7 ± 26.7 | ns |
| Mean predicted FEV1% at 6 months | 80.9 ± 24.9 | 85.2 ± 24.2 | ns | 80.8 ± 24.7 | 85 ± 24.5 | ns | 83.3 ± 24 | 79.9 ± 28.5 | ns |
| . | RBC ≤ 3, n = 159 . | RBC > 3, n = 152 . | P-values . | FFP ≤ 2, n = 162 . | FFP > 2, n = 149 . | P-values . | Plts ≤ 1, n = 257 . | Plts > 1, n = 54 . | P-values . |
|---|---|---|---|---|---|---|---|---|---|
| Pretransplant diagnosis | |||||||||
| Cystic fibrosis | 65 | 87 | 76 | 76 | 134 | 18 | |||
| Lung fibrosis | 25 | 13 | 26 | 12 | 33 | 5 | |||
| Emphysema | 52 | 33 | 48 | 37 | 65 | 20 | |||
| Bronchiectasis | 9 | 9 | 8 | 10 | 15 | 3 | |||
| Pulmonary hypertension | 4 | 0 | 1 | 3 | 3 | 1 | |||
| Others | 4 | 10 | 3 | 11 | 7 | 7 | |||
| Recipient characteristics | |||||||||
| Recipient age (years) | 43 ± 14 | 40 ± 15 | ns | 42 ± 14 | 41 ± 14 | ns | 40 ± 14 | 47 ± 13 | <0.001 |
| Recipient gender (male) | 98 (62%) | 76 (50%) | 91 (56%) | 83 (56%) | 144 (56%) | 30 (56%) | |||
| Recipient median waiting times (days) | 251 (4–1953) | 267 (2–1653) | ns | 247.5 (4–2653) | 285.5 (2–2002) | ns | 258 (4–2653) | 242 (2–2002) | ns |
| Donor characteristics | |||||||||
| Donor comorbidity (smoking) | 67 (42%) | 72 (47%) | 68 (42%) | 71 (48%) | 106 (41%) | 33 (61%) | |||
| Donor cause of death (head trauma) | 22 (14%) | 26 (17%) | 26 (16%) | 22 (15%) | 41 (16%) | 7 (13%) | |||
| Perioperative characteristics | |||||||||
| Ischaemic time (min) | 362 ± 104 | 375 ± 124 | ns | 368 ± 120 | 369 ± 108 | ns | 366 ± 119 | 377 ± 86 | ns |
| Mean bypass time (min) | 228 ± 47 | 260 ± 63 | <0.001 | 234 ± 49 | 254 ± 64 | 0.004 | 235 ± 47 | 284 ± 81 | <0.001 |
| Median length of ICU stay (days) | 3 (1–155) | 4.5 (1–127) | ns | 4 (1–155) | 4 (1–127) | ns | 4 (1–155) | 8 (1–127) | <0.001 |
| Patients with PGD as cause of death | 6 (3.8%) | 14 (9.2%) | 0.051 | 7 (4.3%) | 13 (8.7%) | ns | 11 (4.3%) | 9 (16.7%) | <0.001 |
| Postoperative lung function | |||||||||
| Mean predicted FEV1% at 3 months | 74.7 ± 23 | 78.5 ± 22.4 | ns | 74.9 ± 22.1 | 77.8 ± 23.4 | ns | 76.5 ± 22.2 | 75.7 ± 26.7 | ns |
| Mean predicted FEV1% at 6 months | 80.9 ± 24.9 | 85.2 ± 24.2 | ns | 80.8 ± 24.7 | 85 ± 24.5 | ns | 83.3 ± 24 | 79.9 ± 28.5 | ns |
Effect of blood product transfusion on mortality
Unadjusted all-cause mortality at the 1-year follow-up did not appear to be different between patient groups stratified by the median number of units of RBCs (P = 0.827) or FFP transfused (P = 0.456) as shown in Figs 1 and 2. However, mortality outcome was adversely affected when more than the median number of units of platelets was transfused (P = 0.010), as shown in Fig. 3. For patients with primary graft dysfunction (PGD) as the main cause of death, there was a significantly higher proportion of PGD deaths associated with patients transfused with more than the median number of units of platelets, but not for RBCs or FFP; Table 2. Upon adjustment for confounding variables (sex, age, waiting time on transplant list, bypass time, ischaemic time, donor smoking status, length of ICU stay and 3 eras (Era A: 2003–2007, Era B: 2008–2010, Era C: 2011–2013), 1-year all-cause mortality was still noted to be greater among patients transfused with more than the median unit of platelets (adjusted HR: 2.3, 95% CI: 1.16–4.65, P = 0.017), as given in Table 3.
Comparison of unadjusted 1-year all-cause mortality and adjusted 1-year all-cause mortality outcomes for patient subgroups divided based on the median units transfused for RBCs, FFP and platelets
| . | Unadjusted 1-year all-cause mortality . | Cox model-adjusted 1-year mortalitya . | |||
|---|---|---|---|---|---|
| Below median unit . | Above median unit . | P-values . | Hazard ratio with 95% CI . | P-values . | |
| As dichotomized data | |||||
| RBC | 16.2% | 20.7% | ns | 0.92 (0.49, 1.71) | 0.788 |
| FFP | 16.6% | 20.3% | ns | 1.13 (0.69, 2.08) | 0.682 |
| Platelets | 15.1% | 34% | 0.010 | 2.33 (1.16, 4.65) | 0.017 |
| . | Unadjusted 1-year all-cause mortality . | Cox model-adjusted 1-year mortalitya . | |||
|---|---|---|---|---|---|
| Below median unit . | Above median unit . | P-values . | Hazard ratio with 95% CI . | P-values . | |
| As dichotomized data | |||||
| RBC | 16.2% | 20.7% | ns | 0.92 (0.49, 1.71) | 0.788 |
| FFP | 16.6% | 20.3% | ns | 1.13 (0.69, 2.08) | 0.682 |
| Platelets | 15.1% | 34% | 0.010 | 2.33 (1.16, 4.65) | 0.017 |
aCox model-adjusted 1-year mortality (include covariates, e.g. sex, age, gender, waiting times on transplant list, donor smoking status, donor ischaemic times, bypass times, pretransplant diagnosis, year of lung transplant in 3 eras [Era A: 2003-2007, Era B: 2008-2010, Era C: 2011-2013] reflecting changes in aprotinin use).
Comparison of unadjusted 1-year all-cause mortality and adjusted 1-year all-cause mortality outcomes for patient subgroups divided based on the median units transfused for RBCs, FFP and platelets
| . | Unadjusted 1-year all-cause mortality . | Cox model-adjusted 1-year mortalitya . | |||
|---|---|---|---|---|---|
| Below median unit . | Above median unit . | P-values . | Hazard ratio with 95% CI . | P-values . | |
| As dichotomized data | |||||
| RBC | 16.2% | 20.7% | ns | 0.92 (0.49, 1.71) | 0.788 |
| FFP | 16.6% | 20.3% | ns | 1.13 (0.69, 2.08) | 0.682 |
| Platelets | 15.1% | 34% | 0.010 | 2.33 (1.16, 4.65) | 0.017 |
| . | Unadjusted 1-year all-cause mortality . | Cox model-adjusted 1-year mortalitya . | |||
|---|---|---|---|---|---|
| Below median unit . | Above median unit . | P-values . | Hazard ratio with 95% CI . | P-values . | |
| As dichotomized data | |||||
| RBC | 16.2% | 20.7% | ns | 0.92 (0.49, 1.71) | 0.788 |
| FFP | 16.6% | 20.3% | ns | 1.13 (0.69, 2.08) | 0.682 |
| Platelets | 15.1% | 34% | 0.010 | 2.33 (1.16, 4.65) | 0.017 |
aCox model-adjusted 1-year mortality (include covariates, e.g. sex, age, gender, waiting times on transplant list, donor smoking status, donor ischaemic times, bypass times, pretransplant diagnosis, year of lung transplant in 3 eras [Era A: 2003-2007, Era B: 2008-2010, Era C: 2011-2013] reflecting changes in aprotinin use).
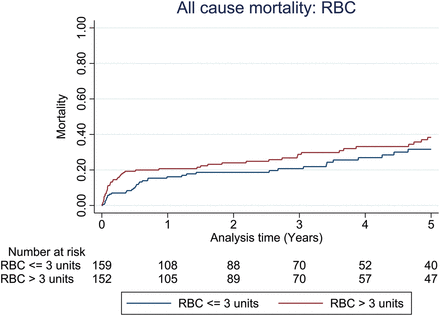
All-cause mortality for groups stratified by the median number of red blood cells (RBCs) transfused in the first 24 h. Unadjusted 1-year mortality for RBCs P-value 0.827, Cox model-adjusted 1-year mortality for RBCs = HR: 0.92, 95% CI: 0.49–1.71, P-value 0.788.
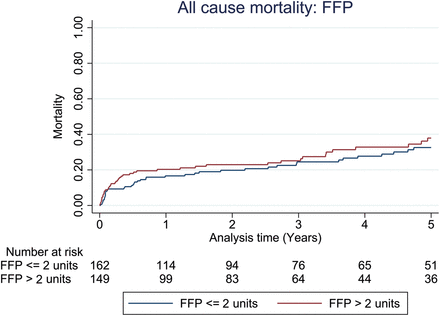
All-cause mortality for groups stratified by the median number of fresh frozen plasma (FFP) transfused in the first 24 h. Unadjusted 1-year mortality for FFP P-value 0.456, Cox model-adjusted 1-year mortality for FFP = HR: 1.13, 95% CI: 0.69–2.08, P-value 0.682.
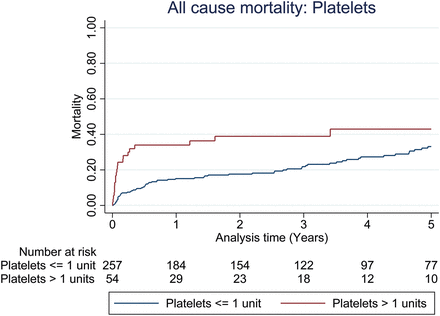
All-cause mortality for groups stratified by the median number of platelets transfused in the first 24 h. Unadjusted 1-year mortality for platelets P-value 0.010, Cox model-adjusted 1-year mortality for platelets = HR: 2.3, 95% CI: 1.16–4.65, P-value 0.0017.
Morbidity intensive care unit stay and lung function
The median length of ICU stay was comparable for patients transfused with more than the median number of units of RBC (3 vs 4.5 days) and FFP (4 vs 4 days) but significantly longer for patients transfused with more than the median number of units of platelets (4 vs 8 days, P < 0.001); Table 2. Mean predicted FEV1 (%) at 3 months was comparable for patients transfused with more than the median number of units of RBCs (74.7 vs 78.5%), FFP (74.9 vs 77.8%) and platelets (76.5 vs 75.7%). Similarly, there were no significant difference in predicted FEV1 (%) at 6 months for all blood products, as given in Table 2.
DISCUSSION
Mortality and morbidity
As we face greater donor organ shortages, any measures to reduce further risk to the donated organ are paramount and should be thoroughly investigated. Our study showed that more than 1 unit of platelets transfusion was significantly associated with worse early survival outcomes. Our patients who received more platelets are older and had longer CPB times. Similarly, Zalunardo et al. [3] reported that lung transplant patients who received platelets transfusion had poorer mortality outcomes compared with patients who did not receive platelets. Their group showed that CPB did not play a contributing role towards mortality outcomes [3]. Within cardiac surgery, there is conflicting evidence as Spiess et al. [6] showed that platelets have an adverse effect, while two other studies showed that platelets did not confer an adverse nor beneficial effect within cardiac surgery [6, 11, 12]. However, all these studies were discussed in the context of patients who may be on preoperative anti-platelet and/or aspirin therapy, which is largely dissimilar to the lung transplantation patient demographic. Within liver transplantation, platelets transfusion has shown to be associated with acute lung injury and has an adverse effect on mortality outcomes, independent of CPB use and anti-platelet therapy [7, 13].
Curiously, platelet use appeared to have an early adverse effect. A possible explanation for the significant association of greater platelet use with greater early mortality may be that one unit of platelet is obtained from 6 different donors, whereas one unit of RBC or FFP comes from just a single donor. Theoretically we could then expect a larger immediate systemic, inflammatory response to platelets compared with other blood products leading to further damage to the lung parenchyma and vasculature. Platelet transfusion had been shown to be associated with increased amount of soluble receptors for advanced glycation end products (sRAGE); a biomarker of lung epithelial injury [14]. High sRAGE plasma levels are significantly associated with PGD in lung transplant recipients [14] and mortality outcomes in critically ill patients [8, 9, 15]. Similarly, our study showed that the group who received more platelets had significantly longer ICU stay and, also, a significantly greater proportion of PGD-related deaths, Table 2. PGD grading was not available for the study duration from 2003 till 2013, as the ISHLT PGD grading was only introduced in 2005 [16]. The length of ICU stay is a useful surrogate marker for patients who developed PGD as they required longer mechanical ventilation times with advanced respiratory support [17]. Therefore, patients with greater platelets use may have a higher incidence of PGD.
Blood transfusions had been proposed as a possible recipient-related risk factor for PGD, although a clear, direct association has yet to be elucidated [16]. TRALI secondary to greater platelet use might contribute towards the development of PGD. Both PGD and TRALI present similarly, with progressive hypoxaemia, increasing oxygen requirements and radiographic evidence of pulmonary infiltrates. For TRALI, the onset of lung injury occurs within 1–2 h of transfusion and notably resolves by 72 h [16, 18]. PGD is diagnosed within 72 h post-transplantation, lasts 5–10 days and sometimes up to a few months [17]. Possible differentiating criteria between these two clinical entities are time to event, duration of the clinical syndrome and evidence of pathological blood donor antibodies reactivity with host or graft antigens [16, 18]. There might be a proportion of PGD attributed to TRALI that warrants future prospective investigations [18].
The causes of early death with lung transplantation are usually due to PGD or multiorgan failure, whereas the causes of late transplant-related death are usually due to chronic rejection or chronic graft failure (Obliterans Bronchiolitis syndrome). Despite the initial adverse effects on pulmonary morbidity and mortality, patients who survived to discharge from hospital had similar lung function (measured as predicted FEV1%) at 3 and 6 months for all blood product transfusions.
Perhaps, we do not see a long-term adverse effect of platelets due to an immuno-modulatory effect reducing the likelihood of graft failure secondary to chronic rejection. This immuno-modulatory effect has previously been documented with other solid-organ transplantation. Our results also suggest a dose-dependent adverse effect associated with platelet use. Therefore, we propose a multiple-hit hypothesis to explain the early adverse effect as a direct consequence of cumulative trauma sustained by the transplanted lung from the point of donor death, donor organ procurement, organ transportation, implantation, CPB and further dose-dependent inflammatory response invoked by platelets use. Hence, greater perioperative platelets use may be a potential ‘tipping point’ leading to PGD and early mortality.
For RBC and FFP transfusion, we found no statistical significance with mortality outcomes, unlike those widely reported within the literature [2, 14]. Weber et al. [2] reported that perioperative FFP transfusion had an adverse effect on survival within lung transplantation. Their overall FFP usage was greater than in our study, which may reflect the volume effects of TACO on their survival outcomes [2]. Similarly, Koch et al. [19] showed that FFP has an adverse effect on survival outcomes after cardiac surgery but also acknowledged the clinical difficulty in separating out TACO versus TRALI [2, 10].
Pattern and predictors of blood product use
The pattern of our blood product use in lung transplantation was similar to other studies in that the majority of blood product transfusion occurred perioperatively [4, 20]. The amount of RBCs, FFP and platelets transfused for double-lung transplantation were comparable with other studies [1, 2]. Our study showed that longer CPB duration was associated with higher amount of RBC, FFP and platelet transfusion. Double-lung transplantation using CPB has been widely reported to be correlated with higher blood product use, in particular RBC transfusion [4].
Time effect
Time effect is a likely confounding variable for survival analyses within any retrospective study, as subtle differences may contribute towards improved survival such as the effect of the learning curve, teamwork and improved workflow that would be difficult to quantify and analyse. First, Costache et al. [21] showed that lung transplantation survival had dramatically improved over the last decade due to better workflow patterns and moving up the learning curve. Secondly, the use of aprotinin, an anti-fibrinolytic, changed over the last decade. Aprotinin use had undergone stepwise changes over three time periods: (i) full aprotinin use (Era A: 2003–2007), (ii) aprotinin use stopped (Era B: 2008–2010) and (iii) off-licensed use of aprotinin (Era C: 2011–2013). With the accumulating evidence about the adverse effects of blood product use over time within the cardiac surgery population, our approach and behaviour towards blood product use may have become more restrictive and less liberal [22, 23]. To address the time effect and the dramatic changes in aprotinin use over this decade-long study, we analysed the year of lung transplantation under 3 different eras, within our multivariate Cox regression model. We found that the year of lung transplantation together with changes in aprotinin use did not significantly affect adjusted 1-year mortality.
Study limitations
There are several limitations with this study. As all of our patients underwent CPB during double-lung transplantation, we acknowledge that we cannot account for CPB as a confounding variable in our results. Secondly, the statistical analyses by dividing the group into more or less median units of blood transfused for statistical analyses carried limitations as there was balance of data within RBC and FFP groups but not within the platelets group. The median number of blood product units used, was chosen as the threshold as the units transfused were not normally distributed with a large variability in the range. Therefore, we controlled for other confounding variables and patients who received more than the median unit of platelets were still associated with worse 1-year mortality. For this single-unit, retrospective study, statistical analysis with the blood product as a continuous variable had more limitations, as the entire cohort was too small to allow for analyses of the HR with each incremental unit of blood product used. We had included the continuous data results and discussion within the supplementary material (see Supplementary material). As this was only a moderately sized study, our results should be interpreted very carefully within the clinical context.
CONCLUSIONS
A higher amount of perioperative platelet transfusion was associated with a significantly greater early mortality at the 1-year follow-up. However, RBC and FFP transfusion has no significant adverse effect on early mortality or length of ICU stay. Overall, patients with longer CPB times had more blood product transfusion. Lung function at 3 and 6 months was similar with more blood product transfusion. It is possible that the ICU or hospital survivors benefited from the immuno-modulatory effects of blood product transfusion, as documented in lung transplant and other solid-organ transplants [20, 24, 25].
Moving forwards, platelets use may be the missing link in studying the effects of blood products on mortality within lung transplantation. Using larger prospective registries, we could better investigate and elucidate the effects of RBCs, FFP and platelets in lung transplant morbidity and mortality.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
ACKNOWLEDGEMENTS
We acknowledge the statistical advisory support from Teng Yao Wang (Phd in Statistics, Department of Mathematics, University of Cambridge). We acknowledge the database and advisory support from Susan Whitehead (Department of Haematology and Blood Transfusion, Freeman Hospital), Zoe Tristram and the Department of Cardiothoracic Surgery and the Institute of Transplantation, Freeman Hospital.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr L. Voltolini(Florence, Italy): In your study, survival was adversely affected when more than the median numbers of units of platelets were transfused. Have you tried to figure out why only the transfusion of platelets and not also the red blood cells influenced the one-year survival?
Dr Ong: Unfortunately, I cannot show causation. I can just show an association effect between the platelets usage and the survival.
This is retrospective data, if there is more interest, we might be able to get more work done on this particular hypothesis.
Dr Voltolini: I didn't see the pulmonary artery pressure as a risk factor, as a variable in your statistical analysis. Do you think it's an important aspect?.
Dr Ong: In the Zalunardo et al. (2011) paper, they reported that preoperative right ventricular dysfunction was one of the prognostic factors for mortality. In this current cohort, we did not look at the pulmonary artery pressure, but it is something that we are interested in.
Dr Voltolini: Sometimes we use, not now, but before, aprotinin that has been shown to decrease the blood usage in lung transplantation. Did your patients receive aprotinin in this cohort?
Dr Ong: No. At the current time, we don't use aprotinin regularly anymore. It has fallen out of favour within this unit.
Dr Voltolini: Last question. Surprisingly, lung function at six months was similar or better for patients who received more blood products. Do you have any explanation for this? In other words, is it possible that transfusion is associated with an immunomodulatory effect and you observe less acute rejections?
Dr Ong: So that was very interesting and could be a possible explanation for it, but we did look at the rejection rates and we found that there was no difference in terms of acute rejection in relation to the amount of blood transfusion.
Time to first rejection and the incidence of acute rejection for all the blood products were not affected by it. It could be that the patient size was larger, that would be the other reasonable explanation for that.
Dr H. Ankersmit(Vienna, Austria): There is one very important contribution by Huppertz from the Heidelberg group who showed statistically that patients who received kidneys and also had blood transfusion had lower rejection rates, so that basically goes together.
Dr Ong: Yes, the immunomodulatory effect has been documented in other solid-organ transplantation but we are still investigating it within lung transplantation.
Author notes
Presented at the 28th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Milan, Italy, 11–15 October 2014.




