-
PDF
- Split View
-
Views
-
Cite
Cite
Mohd Azhari Yakub, Paneer Selvam Krishna Moorthy, Sivakumar Sivalingam, Jeswant Dillon, Pau Kiew Kong, Contemporary long-term outcomes of an aggressive approach to mitral valve repair in children: is it effective and durable for both congenital and acquired mitral valve lesions?, European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 2, February 2016, Pages 553–560, https://doi.org/10.1093/ejcts/ezv099
Close - Share Icon Share
Abstract
We analysed the long-term outcomes of mitral valve (MV) repair in children and compared the repairs for both congenital and acquired lesions.
A review of 634 children (≤18 years) who underwent MV repair from 1992 to 2011 was conducted [excluding patients with complete atrioventricular septal defect (AVSD), single ventricle and atrioventricular (AV) discordance]. Associated cardiac anomalies were present in 473 patients (75%). Congenital mitral lesions were found in 270 (43%) patients compared with an acquired aetiology in 364 (57%) [mainly rheumatic: 329 patients (90%)]. Mitral regurgitation (MR) was predominant in 606 (96%) patients, and 544 (86%) of these showed ≥3+ MR. Modified techniques of MV reconstructions were used.
The early mortality rate was 2% (14 patients). The mean follow-up was 55 months (1–240 months; 85% complete). The late mortality rate was 4% (23 patients) and survival rates at 10 and 15 years were 91 and 86%, respectively. There was no significant difference in 10-year survival between repairing the congenital (98%) and acquired lesions (87%) (P = 0.17). The rate of freedom from reoperation after MV repair for the entire population was 79% at 10 years, with no significant difference between congenital (80%) and acquired lesions (79%) (P = 0.20). Fifty-six patients (9%) required reoperation. Mixed MV lesions, commissural fusions and residual MR (≥2+) were the predictors of valve failure and reoperation. All survivors remain in New York Heart Association class I and none had thromboembolism or pacemaker insertion.
MV repair can be successfully applied to both congenital and acquired MV disease in children. Aggressive repair techniques and avoidance of residual MR have improved durability and survival.
INTRODUCTION
Mitral valve (MV) disease in children remains a tough challenge because of the varied pathology, heterogeneous lesions and coexisting cardiac anomalies. The surgeon may have to repair or replace the diseased MV. Interestingly, MV repair has evolved with the introduction of standardized and reproducible techniques [1, 2]. Reconstruction and retention of the native MV and subvalvular apparatus conserves the ventricular geometry, preserving left ventricular function and resulting in better long-term survival. Moreover, MV replacement in this age group is significantly challenged by patient–prosthesis size mismatch, which is further complicated as the child grows [3, 4]. A further advantage of MV repair in comparison with replacement is avoidance of complications related to life-long anticoagulation therapy or systemic thromboembolism and greater freedom from endocarditis, which is particularly difficult to manage in the paediatric population [3–7].
Several groups have reported excellent results with MV repair for congenital lesions in children [8–11]. However, reconstruction of MV in children with acquired aetiologies, particularly rheumatic MV disease, remains difficult [12, 13]. The disease progression in such patients leads to early valve failure and reoperation. Nevertheless, the results in some selected rheumatic repair series have been encouraging and promising [14–17].
The aim of this study was to analyse our 20-year experience of MV repair in children to determine the long-term outcomes and compare the results of repairs for both congenital and acquired MV disease.
MATERIALS AND METHODS
Patients
Between January 1992 and December 2011, 634 children [271 boys and 363 girls; mean age: 10 years; range: 6 weeks to 18 years; median age: 11 years; with interquartile range: 5–14 years; of which 510 (80%) children were between 1–15 years, 40 (6%) children below 1 year and 84 (13%) children above 15 years of age] underwent MV repair for either congenital (n = 270; 43%) or acquired (n = 364; 57%) MV disease at the National Heart Institute, Kuala Lumpur, Malaysia. This accounted for 18% of the total 3458 patients who had reconstructive MV surgery at our institute during the same period. An aggressive approach has improved the feasibility of MV repair in children to almost 79% (see Supplementary material, Fig. S1). Patients who underwent concomitant cardiac surgery for complete AVSD, single ventricle and AV discordance were excluded. The data were collected by reviewing medical records and telephone interviews. The Institutional Ethical Committee approved this study. Haemodynamic assessment showed predominant mitral regurgitation (MR) was present in 606 patients (96%) and a combined stenotic and regurgitant lesion was found in 18 patients (3%). Isolated mitral stenosis (MS) was noted in ten patients (2%). Atrial fibrillation was present in 39 (6%) patients. Majority of patients presented with shortness of breath on exertion and 417 (66%) patients were in New York Heart Association (NYHA) functional class II or more. The characteristics of MV disease are listed in Table 1.
| Characteristics . | No. of cases (%) . |
|---|---|
| Aetiology of mitral valve disease | |
| Congenital lesions | 270 (43) |
| Acquired lesions | 364 (57) |
| Rheumatic | 329 (90) |
| Endocarditis | 25 (7) |
| Degenerative | 8 (2) |
| Undetermined | 2 (1) |
| Mechanism of MR (Carpentier's functional classification) | |
| Type I, normal leaflet motion | 295 (47) |
| Annular dilatation | 172 (58) |
| Cleft leaflet | 113 (38) |
| Leaflet defect | 20 (7) |
| Type II, leaflet prolapse | 230 (37) |
| Elongated chordate | 144 (63) |
| Ruptured chordate | 84 (36) |
| Absent chordate | 2 (1) |
| Type III, restricted leaflet | 99 (16) |
| Short chordae | 53 (54) |
| Fused commissures | 36 (36) |
| Others | 10 (10) |
| Mechanism of MS (Carpentier's functional classification) | |
| Type A, normal papillary muscle | 9 (90) |
| Supravalvular ring | 1 |
| Papillary muscle–commissural fusion | 8 |
| Type B, abnormal papillary muscle | 1 (10) |
| Parachute valve | 1 |
| Characteristics . | No. of cases (%) . |
|---|---|
| Aetiology of mitral valve disease | |
| Congenital lesions | 270 (43) |
| Acquired lesions | 364 (57) |
| Rheumatic | 329 (90) |
| Endocarditis | 25 (7) |
| Degenerative | 8 (2) |
| Undetermined | 2 (1) |
| Mechanism of MR (Carpentier's functional classification) | |
| Type I, normal leaflet motion | 295 (47) |
| Annular dilatation | 172 (58) |
| Cleft leaflet | 113 (38) |
| Leaflet defect | 20 (7) |
| Type II, leaflet prolapse | 230 (37) |
| Elongated chordate | 144 (63) |
| Ruptured chordate | 84 (36) |
| Absent chordate | 2 (1) |
| Type III, restricted leaflet | 99 (16) |
| Short chordae | 53 (54) |
| Fused commissures | 36 (36) |
| Others | 10 (10) |
| Mechanism of MS (Carpentier's functional classification) | |
| Type A, normal papillary muscle | 9 (90) |
| Supravalvular ring | 1 |
| Papillary muscle–commissural fusion | 8 |
| Type B, abnormal papillary muscle | 1 (10) |
| Parachute valve | 1 |
MR: mitral regurgitation; MS: mitral stenosis.
| Characteristics . | No. of cases (%) . |
|---|---|
| Aetiology of mitral valve disease | |
| Congenital lesions | 270 (43) |
| Acquired lesions | 364 (57) |
| Rheumatic | 329 (90) |
| Endocarditis | 25 (7) |
| Degenerative | 8 (2) |
| Undetermined | 2 (1) |
| Mechanism of MR (Carpentier's functional classification) | |
| Type I, normal leaflet motion | 295 (47) |
| Annular dilatation | 172 (58) |
| Cleft leaflet | 113 (38) |
| Leaflet defect | 20 (7) |
| Type II, leaflet prolapse | 230 (37) |
| Elongated chordate | 144 (63) |
| Ruptured chordate | 84 (36) |
| Absent chordate | 2 (1) |
| Type III, restricted leaflet | 99 (16) |
| Short chordae | 53 (54) |
| Fused commissures | 36 (36) |
| Others | 10 (10) |
| Mechanism of MS (Carpentier's functional classification) | |
| Type A, normal papillary muscle | 9 (90) |
| Supravalvular ring | 1 |
| Papillary muscle–commissural fusion | 8 |
| Type B, abnormal papillary muscle | 1 (10) |
| Parachute valve | 1 |
| Characteristics . | No. of cases (%) . |
|---|---|
| Aetiology of mitral valve disease | |
| Congenital lesions | 270 (43) |
| Acquired lesions | 364 (57) |
| Rheumatic | 329 (90) |
| Endocarditis | 25 (7) |
| Degenerative | 8 (2) |
| Undetermined | 2 (1) |
| Mechanism of MR (Carpentier's functional classification) | |
| Type I, normal leaflet motion | 295 (47) |
| Annular dilatation | 172 (58) |
| Cleft leaflet | 113 (38) |
| Leaflet defect | 20 (7) |
| Type II, leaflet prolapse | 230 (37) |
| Elongated chordate | 144 (63) |
| Ruptured chordate | 84 (36) |
| Absent chordate | 2 (1) |
| Type III, restricted leaflet | 99 (16) |
| Short chordae | 53 (54) |
| Fused commissures | 36 (36) |
| Others | 10 (10) |
| Mechanism of MS (Carpentier's functional classification) | |
| Type A, normal papillary muscle | 9 (90) |
| Supravalvular ring | 1 |
| Papillary muscle–commissural fusion | 8 |
| Type B, abnormal papillary muscle | 1 (10) |
| Parachute valve | 1 |
MR: mitral regurgitation; MS: mitral stenosis.
Preoperative evaluation
Preoperative valve analysis by transthoracic echocardiography (Hewlett-Packard Sonos 2500 or Philips IE 33 equipped with a 2.5–3.5-MHz transducer) was performed on all patients before surgery. The morphology of the valve was documented to classify the aetiology, pathology and mechanism of the MV disease. The severity of MR was estimated by semiquantitative grading [trivial or mild (1+ MR), moderate (2+ MR), moderate to severe (3+ MR) and severe (4+ MR)], according to the ratio of the regurgitation jet area over the left atrium area and pulmonary vein systolic flow reversal and vena contracta. Mitral stenosis (MS) was graded based on MV area, estimated using the planimetry and pressure half-time method and the mean pressure gradient (MPG). In some patients, several pathological findings coexisted, in which case the predominant one was used to classify the lesion according to Carpentier's functional classification [18] (Table 1). The spectrum of severity of MR in our study was grade 4+ in 324 patients; grade 3+ in 75; grade 2+ in 145 and grade 1+ in 90. MS was severe in 7 patients (MPG > 10 mmHg) and moderate in 3 patients (MPG 5–10 mmHg). All patients above 3 kg in weight had intraoperative transoesophageal echocardiography (TOE) to analyse the valve before and after the valve repair. For patients below 3 kg, intraoperative epicardial echocardiography was performed to assess the MV.
Timing of surgery
To justify surgical intervention, there were usually multiple indications for operating, depending on the patient's age, symptoms and MV lesions. MV disease in neonates and young infants was tackled only if they had severe symptoms despite maximum antifailure therapy. Older children were considered for surgery if they showed: increasing symptoms (lethargy, fatigue, noted diminution in exercise tolerance, failure to thrive); evidence of rising pulmonary pressures (half systemic or greater) or increasing left ventricular chamber size on surveillance echocardiography. MV repair was performed on patients with MR > 2+ secondary to annular dilatation due to volume loading associated with lesions. If, however, MR was mild (<2+), it was at the discretion of the surgeon whether or not to repair the MV concurrently with the correction of the associated cardiac anomalies.
Surgical techniques
MV repair was performed through median sternotomy. A conventional ascending aorta and bicaval cannulation was performed to establish cardiopulmonary bypass under moderate hypothermia (28–32°C). Antegrade intermittent cold crystalloid or blood cardioplegia was given every 20–30 min to protect the myocardium. The access to the MV was gained either through a left atrial incision parallel to the interatrial groove or through the interatrial septum from the right atrium. Following a careful analysis of the MV apparatus, a variety of reconstructive procedures were performed. Details of the MV repair techniques and concomitant associated procedures are summarized in Table 2. The repair techniques were based on Carpentier's reconstruction principles, with some modifications [24]. Annular dilatation was repaired by various annuloplasty techniques. In the early stages of our work, we commonly performed various non-ring annuloplasty techniques. However, in recent years, we have moved to artificial ring annuloplasty and biodegradable rings. The MV cleft was corrected by a direct suture technique, either completely or partially. Chordal elongation or rupture, particularly involving the anterior leaflet, was corrected by chordal shortening, transfer or even replacement. In patients with combined mitral stenosis and incompetence, a commissurotomy was performed to increase the orifice area. Papillary muscle splitting, excision of thickened and shortened secondary chordae (and sometimes even primary chords) and thinning of leaflet edges were performed to improve mobility and pliability of the valve leaflets. In the absence of adequate leaflet tissue or leaflet mobilization, leaflet extension or augmentation was performed. An autologous pericardial patch was harvested, trimmed and soaked in 0.6% gluteraldehyde-buffered solution at room temperature for 5–10 min and then rinsed with normal saline solution for 15 min in three separate baths. A liberal incision was made on the intended leaflet ∼3 mm from the annulus, parallel to it and stopping 5 mm away from the commissures. The thickened secondary chordae and occasionally primary chordae were excised. Following this, an oval-shaped treated pericardium was used to extend the leaflets. Leaflet extension increases their surface area and improves leaflet coaptation, plus allows the insertion of a larger annuloplasty ring, thereby reducing the risk of stenosis, which can influence the somatic growth of the annulus in children. After completing the repair, the competency of the MV was assessed by injecting cold saline solution with a bulb syringe into the left ventricle directly through the MV. The end result was further evaluated in detail by intraoperative TOE analysis after coming off the cardiopulmonary bypass. Part of the above-mentioned surgical methods were previously described [16].
Details of mitral valve repair techniques and concomitant procedures in 634 patients
| Procedure . | Value . |
|---|---|
| . | No. of cases (%) . |
| Mitral valve repair techniques | |
| Ring annuloplasty | 422 (67) |
| Rigid, complete ring | 137 (22) |
| Semi-rigid, complete ring | 308 (49) |
| Flexible, complete ring | 105 (17) |
| Flexible, partial ring | 83 (13) |
| Biodegradable | 75 (12) |
| Non-ring annuloplasty | 22 (4) |
| Leaflet procedure | 174 (27) |
| Leaflet/cleft suture | 113 (18) |
| Leaflet resection (thinning, peeling, shaving) | 89 (14) |
| Leaflet extension or augmentation | 53 (8) |
| (Anterior: posterior: both) | 12:37:4 |
| Leaflet plication | 32 (5) |
| Chordal procedure | 313 (49) |
| Chordal replacement (neochordae) | 103 (16) |
| Chordal shortening | 127 (20) |
| Chordal transfer | 64 (10) |
| Chordal resection | 20 (3) |
| Commissurotomy | 27 (4) |
| Commissuroplasty | 38 (6) |
| Papillary muscle splitting | 30 (5) |
| Concomitant cardiac procedures | |
| Tricuspid valve repair | 154 (24) |
| Repair of primum atrial septal defect | 113 (18) |
| Closure of secundum atrial defect | 61 (10) |
| Closure of ventricular septal defect | 70 (11) |
| Aortic valve repair | 32 (5) |
| Aortic valve replacement | 38 (6) |
| Maze procedure | 3 (1) |
| Others | 2 (0.3) |
| Procedure . | Value . |
|---|---|
| . | No. of cases (%) . |
| Mitral valve repair techniques | |
| Ring annuloplasty | 422 (67) |
| Rigid, complete ring | 137 (22) |
| Semi-rigid, complete ring | 308 (49) |
| Flexible, complete ring | 105 (17) |
| Flexible, partial ring | 83 (13) |
| Biodegradable | 75 (12) |
| Non-ring annuloplasty | 22 (4) |
| Leaflet procedure | 174 (27) |
| Leaflet/cleft suture | 113 (18) |
| Leaflet resection (thinning, peeling, shaving) | 89 (14) |
| Leaflet extension or augmentation | 53 (8) |
| (Anterior: posterior: both) | 12:37:4 |
| Leaflet plication | 32 (5) |
| Chordal procedure | 313 (49) |
| Chordal replacement (neochordae) | 103 (16) |
| Chordal shortening | 127 (20) |
| Chordal transfer | 64 (10) |
| Chordal resection | 20 (3) |
| Commissurotomy | 27 (4) |
| Commissuroplasty | 38 (6) |
| Papillary muscle splitting | 30 (5) |
| Concomitant cardiac procedures | |
| Tricuspid valve repair | 154 (24) |
| Repair of primum atrial septal defect | 113 (18) |
| Closure of secundum atrial defect | 61 (10) |
| Closure of ventricular septal defect | 70 (11) |
| Aortic valve repair | 32 (5) |
| Aortic valve replacement | 38 (6) |
| Maze procedure | 3 (1) |
| Others | 2 (0.3) |
Details of mitral valve repair techniques and concomitant procedures in 634 patients
| Procedure . | Value . |
|---|---|
| . | No. of cases (%) . |
| Mitral valve repair techniques | |
| Ring annuloplasty | 422 (67) |
| Rigid, complete ring | 137 (22) |
| Semi-rigid, complete ring | 308 (49) |
| Flexible, complete ring | 105 (17) |
| Flexible, partial ring | 83 (13) |
| Biodegradable | 75 (12) |
| Non-ring annuloplasty | 22 (4) |
| Leaflet procedure | 174 (27) |
| Leaflet/cleft suture | 113 (18) |
| Leaflet resection (thinning, peeling, shaving) | 89 (14) |
| Leaflet extension or augmentation | 53 (8) |
| (Anterior: posterior: both) | 12:37:4 |
| Leaflet plication | 32 (5) |
| Chordal procedure | 313 (49) |
| Chordal replacement (neochordae) | 103 (16) |
| Chordal shortening | 127 (20) |
| Chordal transfer | 64 (10) |
| Chordal resection | 20 (3) |
| Commissurotomy | 27 (4) |
| Commissuroplasty | 38 (6) |
| Papillary muscle splitting | 30 (5) |
| Concomitant cardiac procedures | |
| Tricuspid valve repair | 154 (24) |
| Repair of primum atrial septal defect | 113 (18) |
| Closure of secundum atrial defect | 61 (10) |
| Closure of ventricular septal defect | 70 (11) |
| Aortic valve repair | 32 (5) |
| Aortic valve replacement | 38 (6) |
| Maze procedure | 3 (1) |
| Others | 2 (0.3) |
| Procedure . | Value . |
|---|---|
| . | No. of cases (%) . |
| Mitral valve repair techniques | |
| Ring annuloplasty | 422 (67) |
| Rigid, complete ring | 137 (22) |
| Semi-rigid, complete ring | 308 (49) |
| Flexible, complete ring | 105 (17) |
| Flexible, partial ring | 83 (13) |
| Biodegradable | 75 (12) |
| Non-ring annuloplasty | 22 (4) |
| Leaflet procedure | 174 (27) |
| Leaflet/cleft suture | 113 (18) |
| Leaflet resection (thinning, peeling, shaving) | 89 (14) |
| Leaflet extension or augmentation | 53 (8) |
| (Anterior: posterior: both) | 12:37:4 |
| Leaflet plication | 32 (5) |
| Chordal procedure | 313 (49) |
| Chordal replacement (neochordae) | 103 (16) |
| Chordal shortening | 127 (20) |
| Chordal transfer | 64 (10) |
| Chordal resection | 20 (3) |
| Commissurotomy | 27 (4) |
| Commissuroplasty | 38 (6) |
| Papillary muscle splitting | 30 (5) |
| Concomitant cardiac procedures | |
| Tricuspid valve repair | 154 (24) |
| Repair of primum atrial septal defect | 113 (18) |
| Closure of secundum atrial defect | 61 (10) |
| Closure of ventricular septal defect | 70 (11) |
| Aortic valve repair | 32 (5) |
| Aortic valve replacement | 38 (6) |
| Maze procedure | 3 (1) |
| Others | 2 (0.3) |
Three patients (1%) underwent a concomitant modified Cox-Maze ШIII procedure using radiofrequency ablation for atrial fibrillation. We were selective in performing the surgery for atrial fibrillation only on those who had atrial fibrillation for less than 2 years and where the left atrial size was <6 cm. In addition, 473 (75%) patients underwent various associated procedures, as summarized in Table 2.
Postoperative management
All patients who had prosthetic ring annuloplasty were routinely given warfarin for anticoagulation for 6 weeks postoperatively, with a target International Normalized Ratio of 2.0–3.0, and continued indefinitely for those who had atrial fibrillation. Patients who had biodegradable ring annuloplasty were given aspirin (3–5 mg/kg daily) for 3 months. The patients with rheumatic valvular disease were given oral penicillin as secondary prophylaxis against rheumatic fever for 10 years or until 40 years of age (whichever was longer), as is currently recommended. These follow-up plans were similar to the description in our previous paper [16].
Follow-up
All patients underwent transthoracic echocardiography before discharge, at 3 months, 6 months and annually after surgery. The follow-up was 85% complete, 97 patients (15%) being lost to the follow-up. The mean follow-up was 55 ± 53 months (range: 8 days to 240 months). Early mortality was defined as death within 30 days of surgery or in-hospital death. Valve-related complications such as thromboembolic events, infective endocarditis, bleeding complications secondary to anticoagulation or the need for reoperation were recorded during the follow-up. Valve failure was defined as recurrent significant regurgitation of >2+ MR (moderate MR) or in those that required reoperation. The indications for reoperation were the same as the initial operation.
Statistical analysis
The data are presented as frequencies, or means with standard deviations. Univariate analysis of categorical data was carried out with χ2 or Fisher's exact tests. Univariate analysis of continuous variables was carried out with Student's t-test. Logistic regression was used to determine the risk factors for early death. Age, NYHA class IV, MR/MS, cardiopulmonary bypass time, emergency operation and postoperative sepsis were included in the logistic regression model to identify the risk factors for early mortality. The risk factors for reoperation and valve failure were determined with Cox regression analysis. Age, MR/MS, commissural fusion, ring annuloplasty, concomitant procedures and residual MR postoperation were included in the Cox regression model for reoperation and valve failure outcomes. Variables with P ≤ 0.1 in univariate analysis were subjected to the multivariate analysis. Analysis of survival and freedom from either reoperation or valve failure was performed with the Kaplan–Meier estimator. A P-value of <0.05 was considered to indicate significance. SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis.
RESULTS
General outcomes
There was no intraoperative death. The mean cross-clamp time was 79 ± 35 min (range: 25–283 min) and the mean cardiopulmonary bypass time was 112 ± 47 min (range: 38–478 min). The patients stayed in an intensive care unit for 1–44 days (median, 1 day) and the mean hospital stay was 10 ± 9 days (range: 3–168 days).
Early outcome
The early in-hospital mortality rate was 2% (14 patients). Nine deaths were due to persistent low cardiac output syndrome. Other causes of early death included severe cerebrovascular accident (n = 2), acute respiratory distress syndrome (n = 1), septicaemia (n = 1) and retroperitoneal haemorrhage (n = 1). Univariate and multivariate analysis identified advanced NYHA functional class, emergency surgery and mixed MV disease as significant predictors for early death (Table 3). The variance (R2) explained by the final model was 0.038, indicating the likelihood of other factors contributing to early mortality.
| Variables . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P–values . | OR . | 95% CI . | P-values . | |
| Age | 0.93 | 0.84–1.02 | 0.13 | |||
| NYHA IV | 9.23 | 1.84–46.22 | 0.007* | 11.60 | 2.13–63.28 | 0.005* |
| MR/MS | 7.41 | 1.93–28.47 | 0.004* | 6.50 | 1.42–29.72 | 0.016* |
| CPB | 1.01 | 0.10–1.01 | 0.15 | |||
| Emergency surgery | 42.00 | 8.38–210.39 | <0.001* | 46.74 | 8.49–257.52 | <0.001* |
| Postoperative sepsis | 0.60 | 0.08–4.69 | 0.63 | |||
| Variables . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P–values . | OR . | 95% CI . | P-values . | |
| Age | 0.93 | 0.84–1.02 | 0.13 | |||
| NYHA IV | 9.23 | 1.84–46.22 | 0.007* | 11.60 | 2.13–63.28 | 0.005* |
| MR/MS | 7.41 | 1.93–28.47 | 0.004* | 6.50 | 1.42–29.72 | 0.016* |
| CPB | 1.01 | 0.10–1.01 | 0.15 | |||
| Emergency surgery | 42.00 | 8.38–210.39 | <0.001* | 46.74 | 8.49–257.52 | <0.001* |
| Postoperative sepsis | 0.60 | 0.08–4.69 | 0.63 | |||
OR: odds ratio; CI: confidence interval; NYHA: New York Heart Association; MR: mitral regurgitation; MS: mitral stenosis.
*P < 0.05.
| Variables . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P–values . | OR . | 95% CI . | P-values . | |
| Age | 0.93 | 0.84–1.02 | 0.13 | |||
| NYHA IV | 9.23 | 1.84–46.22 | 0.007* | 11.60 | 2.13–63.28 | 0.005* |
| MR/MS | 7.41 | 1.93–28.47 | 0.004* | 6.50 | 1.42–29.72 | 0.016* |
| CPB | 1.01 | 0.10–1.01 | 0.15 | |||
| Emergency surgery | 42.00 | 8.38–210.39 | <0.001* | 46.74 | 8.49–257.52 | <0.001* |
| Postoperative sepsis | 0.60 | 0.08–4.69 | 0.63 | |||
| Variables . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P–values . | OR . | 95% CI . | P-values . | |
| Age | 0.93 | 0.84–1.02 | 0.13 | |||
| NYHA IV | 9.23 | 1.84–46.22 | 0.007* | 11.60 | 2.13–63.28 | 0.005* |
| MR/MS | 7.41 | 1.93–28.47 | 0.004* | 6.50 | 1.42–29.72 | 0.016* |
| CPB | 1.01 | 0.10–1.01 | 0.15 | |||
| Emergency surgery | 42.00 | 8.38–210.39 | <0.001* | 46.74 | 8.49–257.52 | <0.001* |
| Postoperative sepsis | 0.60 | 0.08–4.69 | 0.63 | |||
OR: odds ratio; CI: confidence interval; NYHA: New York Heart Association; MR: mitral regurgitation; MS: mitral stenosis.
*P < 0.05.
Late outcome and overall survival
There were 23 late deaths (4%) including 7 cardiac deaths from congestive cardiac failure and 8 non-cardiac deaths caused by pneumonia (n = 3), dengue fever (n = 3) and meningitis (n = 2). The causes of death for the remaining 8 patients were unknown. The overall 10- and 15-year survival rates were 91 ± 2 and 86 ± 5%, respectively. There was no significant difference in 10-year survival between the congenital (98 ± 1%) and acquired lesion groups (87 ± 4%) (P = 0.17) (Fig. 1). The majority of patients were in NYHA class I (94%). Majority of the survivors in our series either had no residual MR [282 patients (45%)] or 1+ MR (trivial or mild MR, 178 patients (28%)] recorded at their last follow-up visit. Additionally, many of those who had residual 2+ MR (n = 75 patients; 12%) were asymptomatic. All survivors were found to be in sinus rhythm except 25 (4%) patients, who had atrial fibrillation. There was no documentation of any thromboembolism, bleeding related to anticoagulation or endocarditis.
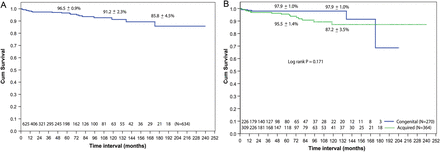
Kaplan–Meier curves showing survival: (A) overall (B) comparison between congenital and acquired groups.
Reoperation and valve failure
The rate of freedom from reoperation for the entire population over 10 and 15 years was 79 ± 3 and 68 ± 6%, respectively. There was no significant difference in freedom from reoperation at 10 years between MV repair for congenital and acquired aetiologies [80 ± 6 vs 78 ± 4%, respectively (P = 0.20)] (Fig. 2). Fifty-six patients (9%) had MV reoperation. The mean interval from the initial surgery was 50 months (range, 1 day to 233 months). Two patients required a second procedure during the same hospital stay. Technical failures with inadequate initial repair were responsible for earlier reoperation in 6 (11%) (4 had residual prolapse; 2 had ring dehiscence) including 2 patients who developed haemolysis and 1 with infective endocarditis. However, progression of disease with associated stenosis and recurrent insufficiency accounted for later reoperations in 47 patients (84%). During reoperation, MV replacement was performed in 39 patients (70%) and a second MV repair (re-repair) was done in 17 patients (30%).
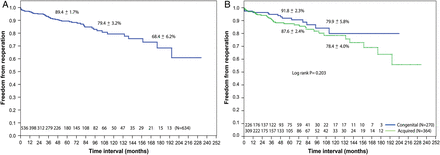
Kaplan–Meier curves showing freedom from MV reoperation: (A) overall, (B) comparison between congenital and acquired aetiology groups. MV: mitral valve.
The rate of actuarial freedom from valve failure for the entire population at 10 years was 73 ± 3% and there was no statistically significant difference in the failure rates at 10 years between the congenital and acquired groups [75 ± 6 vs 72 ± 4%, respectively (P = 0.21)] (Fig. 3). Significant independent predictors of reoperation (Table 4) were younger age, mixed mitral disease and commissural fusion, whereas for valve failure (Table 5), they were mixed mitral disease and residual MR > 2+.
Univariate and multivariate analysis of risk factors for mitral valve reoperation
| Variables . | Univariate analysis for reoperation . | Multivariate analysis for reoperation . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-values . | OR . | 95% CI . | P-values . | |
| Age | 0.93 | 0.87–0.99 | 0.030* | 0.93 | 0.86–0.99 | 0.033* |
| MR/MS | 5.50 | 2.43–12.4 | <0.001* | 2.97 | 1.08–8.19 | 0.035* |
| Commissural fusion | 4.33 | 2.00–9.38 | <0.001* | 2.77 | 1.06–7.21 | 0.037* |
| Ring annuloplasty | 0.52 | 0.23–1.16 | 0.11 | |||
| Concomitant procedure | 1.08 | 0.60–1.94 | 0.80 | |||
| Residual MR ≥ 2+ | 0.82 | 0.25–2.66 | 0.74 | |||
| Variables . | Univariate analysis for reoperation . | Multivariate analysis for reoperation . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-values . | OR . | 95% CI . | P-values . | |
| Age | 0.93 | 0.87–0.99 | 0.030* | 0.93 | 0.86–0.99 | 0.033* |
| MR/MS | 5.50 | 2.43–12.4 | <0.001* | 2.97 | 1.08–8.19 | 0.035* |
| Commissural fusion | 4.33 | 2.00–9.38 | <0.001* | 2.77 | 1.06–7.21 | 0.037* |
| Ring annuloplasty | 0.52 | 0.23–1.16 | 0.11 | |||
| Concomitant procedure | 1.08 | 0.60–1.94 | 0.80 | |||
| Residual MR ≥ 2+ | 0.82 | 0.25–2.66 | 0.74 | |||
OR: odds ratio; CI: confidence interval.
Univariate and multivariate analysis of risk factors for mitral valve reoperation
| Variables . | Univariate analysis for reoperation . | Multivariate analysis for reoperation . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-values . | OR . | 95% CI . | P-values . | |
| Age | 0.93 | 0.87–0.99 | 0.030* | 0.93 | 0.86–0.99 | 0.033* |
| MR/MS | 5.50 | 2.43–12.4 | <0.001* | 2.97 | 1.08–8.19 | 0.035* |
| Commissural fusion | 4.33 | 2.00–9.38 | <0.001* | 2.77 | 1.06–7.21 | 0.037* |
| Ring annuloplasty | 0.52 | 0.23–1.16 | 0.11 | |||
| Concomitant procedure | 1.08 | 0.60–1.94 | 0.80 | |||
| Residual MR ≥ 2+ | 0.82 | 0.25–2.66 | 0.74 | |||
| Variables . | Univariate analysis for reoperation . | Multivariate analysis for reoperation . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-values . | OR . | 95% CI . | P-values . | |
| Age | 0.93 | 0.87–0.99 | 0.030* | 0.93 | 0.86–0.99 | 0.033* |
| MR/MS | 5.50 | 2.43–12.4 | <0.001* | 2.97 | 1.08–8.19 | 0.035* |
| Commissural fusion | 4.33 | 2.00–9.38 | <0.001* | 2.77 | 1.06–7.21 | 0.037* |
| Ring annuloplasty | 0.52 | 0.23–1.16 | 0.11 | |||
| Concomitant procedure | 1.08 | 0.60–1.94 | 0.80 | |||
| Residual MR ≥ 2+ | 0.82 | 0.25–2.66 | 0.74 | |||
OR: odds ratio; CI: confidence interval.
Univariate and multivariate analysis of risk factors for mitral valve failure
| Variables . | Univariate analysis for valve failure . | Multivariate analysis for valve failure . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P–values . | OR . | 95% CI . | P-values . | |
| Age | 0.95 | 0.89–1.00 | 0.07 | |||
| MR/MS | 3.87 | 1.75–8.56 | 0.001* | 3.55 | 1.27–9.97 | 0.016* |
| Commissural fusion | 3.00 | 1.42–6.35 | 0.004* | |||
| Ring annuloplasty | 0.55 | 0.28–1.07 | 0.08 | |||
| Concomitant procedure | 1.17 | 0.71–1.94 | 0.53 | |||
| Residual MR ≥ 2+ | 7.26 | 4.41–12.0 | <0.001* | 8.77 | 5.22–14.7 | <0.001* |
| Variables . | Univariate analysis for valve failure . | Multivariate analysis for valve failure . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P–values . | OR . | 95% CI . | P-values . | |
| Age | 0.95 | 0.89–1.00 | 0.07 | |||
| MR/MS | 3.87 | 1.75–8.56 | 0.001* | 3.55 | 1.27–9.97 | 0.016* |
| Commissural fusion | 3.00 | 1.42–6.35 | 0.004* | |||
| Ring annuloplasty | 0.55 | 0.28–1.07 | 0.08 | |||
| Concomitant procedure | 1.17 | 0.71–1.94 | 0.53 | |||
| Residual MR ≥ 2+ | 7.26 | 4.41–12.0 | <0.001* | 8.77 | 5.22–14.7 | <0.001* |
OR: odds ratio; CI: confidence interval.
Univariate and multivariate analysis of risk factors for mitral valve failure
| Variables . | Univariate analysis for valve failure . | Multivariate analysis for valve failure . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P–values . | OR . | 95% CI . | P-values . | |
| Age | 0.95 | 0.89–1.00 | 0.07 | |||
| MR/MS | 3.87 | 1.75–8.56 | 0.001* | 3.55 | 1.27–9.97 | 0.016* |
| Commissural fusion | 3.00 | 1.42–6.35 | 0.004* | |||
| Ring annuloplasty | 0.55 | 0.28–1.07 | 0.08 | |||
| Concomitant procedure | 1.17 | 0.71–1.94 | 0.53 | |||
| Residual MR ≥ 2+ | 7.26 | 4.41–12.0 | <0.001* | 8.77 | 5.22–14.7 | <0.001* |
| Variables . | Univariate analysis for valve failure . | Multivariate analysis for valve failure . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P–values . | OR . | 95% CI . | P-values . | |
| Age | 0.95 | 0.89–1.00 | 0.07 | |||
| MR/MS | 3.87 | 1.75–8.56 | 0.001* | 3.55 | 1.27–9.97 | 0.016* |
| Commissural fusion | 3.00 | 1.42–6.35 | 0.004* | |||
| Ring annuloplasty | 0.55 | 0.28–1.07 | 0.08 | |||
| Concomitant procedure | 1.17 | 0.71–1.94 | 0.53 | |||
| Residual MR ≥ 2+ | 7.26 | 4.41–12.0 | <0.001* | 8.77 | 5.22–14.7 | <0.001* |
OR: odds ratio; CI: confidence interval.
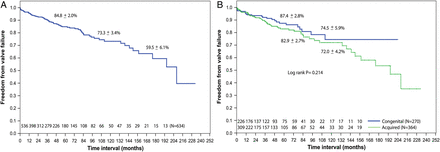
Kaplan–Meier curves showing freedom from MV failure: (A) overall (B) comparison between congenital and acquired aetiology groups. MV: mitral valve.
DISCUSSION
It is indeed challenging to repair MV in children, primarily because of the small and fragile nature of the MV tissue, in addition to the wide spectrum of pathology. Congenital malformations of the MV are not common and frequently associated with other cardiac malformations. Rheumatic valvular heart disease is the leading cause in the acquired group that affects the younger population in developing nations [12]. Many studies have reported mixed results from both repairing and replacing the MV in children [3, 4, 7–11]. Replacement of the diseased MV poses significant problems because of the limited availability of adequately sized prostheses for small children, suboptimal preservation of ventricular function, anticoagulation-related complications and reduced survival [3–7]. Issues such as poor compliance with anticoagulation, somatic growth and pregnancy remain as problems in younger patients, particularly in developing nations. Therefore, MV repair is recognized as the procedure of choice for children in all aetiologies. Several groups have reported satisfactory results with MV repair in children, with a mid- to long-term survival rate of 70–97% [7–11, 14–16].
Congenital malformations often included annular dilatation secondary to volume overloading of associated congenital lesions, cleft MV and dysplastic MV leaflets. Starting off by performing non-ring-type annuloplasty, we slowly progressed to artificial ring annuloplasty for older children. In recent years we have begun to use more biodegradable rings. Many other studies have popularized this technique, and shown a good result in managing children with small annuli, as it allows the annular growth [18, 19].
Leaflet prolapse was repaired by shortening the elongated chordae in the earlier part of our experience. Recently we have favoured using sutures made from artificial material, namely polytetrafluoroethylene (PTFE), as it is simple and has a good outcome in children [20, 21]. Minami et al. [20] have shown echocardiographic evidence of compensatory excessive growth of the MV leaflet and papillary muscle known as biological adaptation in children who had chordal replacement with PTFE.
An aggressive repair of the MV cleft by simple suture was done in all patients, even with mild or no MR, despite being controversial [22]. We strongly believe in repairing early as it is easier to do at an early stage with no risk and can prevent the secondary changes in the leaflets that would otherwise make the repair difficult later.
MS in children is rare and frequently associated with other obstructive lesions of the left heart structures. Repair techniques for MS are rather limited and carry higher mortality and morbidity. Commisurotomy and papillary muscle splitting were commonly used in our study to tackle this problem.
The majority of the acquired lesions were caused by rheumatic MV disease (90%). Repairing rheumatic MV is technically more difficult, challenging and complex [7, 14–17, 25]. This study on MV repair in rheumatic patients has shown encouraging results. We have attempted to repair most of the rheumatic valves that were non-calcified and had some pliable leaflet tissue. The feasibility of repairing rheumatic MV disease in children at our institute was almost 50%.
Leaflet procedures, which included extensive commisurotomy, thinning, shaving, plication and leaflet extension, were all done on severe and advanced forms of rheumatic disease, particularly mixed mitral disease. These methods were also used extensively in other series [14, 16, 17, 25]. Our experience has brought about the use of semirigid or rigid and complete prosthetic annuloplasty rings to stabilize the repair and restore the size and shape of the deformed rheumatic annulus, especially in older patients [16]. In addition to the aggressive surgical techniques, antibiotic prophylaxis in the form of oral penicillin plays an important role as a secondary prevention therapy against rheumatic fever for 10 years or until 40 years of age (whichever is longer) even after a successful valve repair for rheumatic heart disease [23]. Some of the above-mentioned reconstruction techniques particularly in rheumatic valvular disease were similar to the previous description [16].
The routine use of intraoperative TOE (>3 kg) or epicardial echocardiography (<3 kg) to assess the competency of the repaired MV has improved the outcome. In the last ten years, we have learnt not to accept residual MR of more than grade 1+, as it has been consistently shown to be the main predictor of valve failure and reoperation. Any amount of eccentric jet of residual MR can result in haemolysis, which often requires reoperation. Achieving a good leaflet coaptation length of >5 mm improves long-term durability of the repair.
The significant predictors of reoperation and valve failure were younger age, mixed MV disease, commissural fusion and early residual MR > 2+. Others have also found that younger age and mixed lesions are the important predictors for valve failure [14–17]. Early valve failure happened within the first 3 years of surgery and this was due to suboptimal repair or the inherent complexity of the disease process. On the other hand, late failures of repair were due to recurrence and progression of the inflammatory process particularly in rheumatic heart disease patients [13–17]. In most instances, we use the patient's own tissue to reconstruct the valve. These diseased tissues will continue to get worse if untreated. These explanations for the reoperation and valve failure have also been previously described [16].
These aggressive approaches have improved our long-term results for MV repair in children with both congenital and acquired mitral disease and the outcomes are effective and durable.
CONCLUSIONS
Aggressive MV repair can be successfully applied to children. Modifications of standard repair techniques, good leaflet coaptation and avoidance of residual MR have improved long-term results, with minimal valve-related complications. It is indeed an effective treatment to provide an acceptable durability and survival for both congenital and acquired MV disease in children. However, MV repair for younger patients, particularly with rheumatic valvular disease, remains a challenge.
LIMITATIONS
This study is limited by its non-randomized and retrospective design. The variations in the forms of unmeasured parameters and procedures may have affected the results, despite the statistical adjustments.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Funding
This work was supported by the National Heart Institute, Kuala Lumpur, Malaysia
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
The authors are grateful to the consultants and senior registrars in the Department of Paediatric Cardiology for the echocardiographic studies. We would also like to thank Faizal Ramli, Norfazlina Jaffar and Intan Fariza Gaaffar from the Research Department of the National Heart Institute, Kuala Lumpur, for their contribution to data collection and statistical analyses for this study, and also Regina David for her invaluable administrative and secretarial assistance.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr M. Hazekamp(Leiden, Netherlands): I have two comments. The first one is about the congenital type of anomalies. In the congenital group you have 270 patients, and in one of the tables you say that you closed the primum ASD in 113 of them, so I suppose those were partial or intermediate AVSDs, as you excluded complete AVSDs. Could you tell us a little bit more on the remainder, so the non-AVSD congenital anomalies, because this is not very clear from the paper.
Dr Krishna Moorthy: As we all know the atrioventricular septal defect is a spectrum of disease. The main objective in this study is to look at the primary disease of the mitral valve. The complete AV canal defect involves mitral and tricuspid as well. Therefore we have decided from the beginning to leave the complete AV canal defect out and just include the other forms. There were 113 patients diagnosed as ostium primum with mitral cleft as you have mentioned, and we also have seen isolated mitral clefts in 16 patients. We have actually classified congenital lesions based on the leaflet morphology of mitral valve, whether they are dysplastic or non-dysplastic. Dysplastic leaflet were found in 10 cases, whereas the other group was made up of non-dysplastic leaflets seen in 131 patients involving congenital conditions like VSD, ASD and others which have volume overload leading to the annular dilatation and later, elongation of chordae.
Dr Hazekamp: I have a second question. Could you expand or comment a little bit more on what gradient on postop TOE would be acceptable to you.
Dr Krishna Moorthy: Yes, we have the data on this as well. We have used the mean pressure a gradient of upon coming off bypass to assess the adequacy of the repair. We have documented a mean gradient of 5 and below in 505 patients (80%), which was the desired value. In 30 patients (5%) we came off bypass documenting a gradient of more than 10 mmHg, which was not desired at all. In fact, we went back on bypass in all these patients to re-repair the valve, and have improved this in 22 patients where we managed to bring down the mean pressure gradients. Unfortunately, we failed to do so in 8 patients, and ended up replacing the valves.
We were cautious in the intermediate group involving 99 patients (16%) with the gradient between 5 to 10 mmHg. The morphology of the valve was assessed in addition to the mean gradient. We accept the intermediate gradient if the valve is actually pliable and is moving well, with a good coaptation length. The pressure gradient did drop to 5 and below in 80% of the patients during the discharge echocardiography. You have to understand that these mean gradients were taken when the patients were on-pump, on inotropic supports and under different intravascular volume. In most cases, the pressure gradients do settle under the physiological conditions in the ward. However, it remained intermediate in 19 patients (20%). As they were clinically well, we decided to follow them up. During the course, 7 patients required reoperations due to the progression of the disease.
Dr H. Dave(Zurich, Switzerland): You mentioned that you have gone back from using suture angioplasty towards rings, and I see that the median age of the series was 9 years, so they're not yet fully grown. In view of this, can you expand on why you abandoned suture annuloplasty, and what sort of rings did you use? Furthermore, what was your experience with the complete rings?
Dr Krishna Moorthy: Yes. Again, we have to look at the aetiology. In rheumatic aetiology, the annulus are usually dilated and deformed. Rheumatic disease is very different from congenital disease. The usage of complete rings helps us to achieve a successful repair and prevent them from coming back early. This disease is progressive and we are looking at a younger age group.
We tend to use an incomplete ring or biodegradable ring in congenital lesions. My fellow colleague from my institution has actually presented a paper in the last STS meeting about biodegradable ring usage in our setup.
Dr M. Danton(Glasgow, UK): Do you prefer Gore-Tex chords or natural chords in your repair?
Dr Krishna Moorthy: Yes. We have done a lot of chordae shortenings and transfers. However in rheumatics, we tend to create neochords with Gore-Tex sutures. There are a couple of papers which have shown that patients who have had neo-Gore-Tex chords had significant excessive growth in the chords and papillary muscle during the follow-up echocardiography.
Author notes
Presented at the 28th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Milan, Italy, 11–15 October 2014.
- mitral valve insufficiency
- common ventricle
- mitral valve repair
- thromboembolism
- rheumatic disorders
- cardiac pacemaker implantation
- mitral valve
- atrioventricular septal defect
- child
- follow-up
- objective (goal)
- reconstructive surgical procedures
- repeat surgery
- surgical procedures, operative
- survival rate
- survivors
- heart
- mortality
- avoidance behavior
- causality




