-
PDF
- Split View
-
Views
-
Cite
Cite
Yusuke Irisawa, Arudo Hiraoka, Toshinori Totsugawa, Genta Chikazawa, Kosuke Nakajima, Kentaro Tamura, Hidenori Yoshitaka, Taichi Sakaguchi, Re-expansion pulmonary oedema after minimally invasive cardiac surgery with right mini-thoracotomy , European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 2, February 2016, Pages 500–505, https://doi.org/10.1093/ejcts/ezv089
Close - Share Icon Share
Abstract
Re-expansion pulmonary oedema (RPO) sometimes occurs after minimally invasive cardiac surgery (MICS) with single-lung ventilation. However, it has not been widely recognized as a serious complication. The aim of this study is to evaluate the occurrence rate and risk factors of RPO.
A total of 381 consecutive patients who underwent MICS with right mini-thoracotomy from March 2005 to October 2013 were retrospectively reviewed.
RPO was observed in 8 (2.1%) patients. In the preoperative data, greater percentages of preoperative use of steroid or immunosuppressant were found in patients with RPO (25% [2/8] vs 1% [4/373]; P = 0.0056). In the operative data, significantly longer operation, cardiopulmonary bypass (CPB) and aortic cross-clamping (ACC) times as well as greater percentages of second CPB run were found in patients with RPO (388 ± 80 vs 272 ± 61 min; P < 0.0002, 253 ± 79 vs 158 ± 50 min; P = 0.0009, 162 ± 65 vs 108 ± 38 min; P = 0.020 and 38% [3/8] vs 1.3% [5/373]; P < 0.0003). The overall 30-day mortality rate was 0.8% (3/381) and the 30-day mortality rate of patients with RPO was 12.5% (1/8). Significantly prolonged initial ventilation time, intensive care unit and postoperative hospital stay were observed in patients with RPO ( P = 0.0022, <0.0001 and 0.0003, respectively). Multivariate logistic analysis detected preoperative use of steroid or immunosuppressant and prolonged ACC time (≥156 min) as independent risk factors for RPO after MICS (odds ratio [OR]: 87.6 [95% confidence interval, CI: 4.1–2463.8]; P = 0.006 and OR: 36.0 [95% CI: 4.8–731.4]; P < 0.001).
RPO should be recognized as one of the most serious complications after MICS with right mini-thoracotomy. More accurate risk factors of prolonged lung malperfusion and steroid use on RPO after MICS should be investigated.
INTRODUCTION
Pulmonary oedema sometimes occurs in the re-expanded lung following rapid expansion of the collapsed lung, and this is generally defined as re-expansion pulmonary oedema (RPO). The occurrence of RPO was reported unlikely when the period of lung collapse was less than 3 days [ 1–3 ]. However, RPO can be infrequently observed after open heart surgery with a relatively short period of ventilation of one lung. Although mechanical stress during re-expansion and histological abnormalities of the pulmonary microvessels in a collapsed lung were reported as the mechanisms of RPO, the risk factors for RPO after cardiac surgery with single-lung ventilation are still unknown [ 1–9 ].
Recently, excellent outcomes of minimally invasive cardiac surgery (MICS) conducted by single-lung ventilation were reported, and indication for MICS has been expanded [ 10 , 11 ]. There are several advantages of MICS, such as early social rehabilitation, cosmetic merit and reduced risk of transfusion and serious infection [ 12–14 ]. On the other hand, distinctive complications sometimes occur after MICS [ 15 , 16 ]. Postoperative neurological impairment is one of the distinctive complications after MICS with mini-thoracotomy. RPO is rarely observed after MICS with mini-thoracotomy, but undoubtedly has serious adverse effects on the postoperative course (prolonged ventilation time, postoperative hospital stay and rehabilitation) [ 17–19 ]. Although there are a lot of articles on RPO after drainage of pneumothorax or pleural effusion [ 2 , 4 , 5 ], RPO is not generally recognized as one of the most critical complications after MICS, and the risk factors for RPO are still unclear. Therefore, we present a single institution's experience of MICS with right mini-thoracotomy to evaluate the occurrence rate and risk factors of RPO after MICS.
PATIENTS AND METHODS
Between March 2005 and October 2013, a total of 388 consecutive patients were scheduled for MICS conducted by single-lung ventilation with right mini-thoracotomy at a single cardiovascular institute. Of these, 7 patients who were converted to median sternotomy were excluded. The remaining 381 patients who underwent MICS with right mini-thoracotomy were included in the cohort. RPO was diagnosed by expert radiologists as a new and apparent unilateral pulmonary oedema on a chest radiography and computed tomography after operation (Fig. 1 A and B) [ 20 , 21 ]. We did not include asymptomatic and uneventful cases with an ambiguous unilateral change, in which radiologists could not conclusively identify postoperative RPO. Additionally, when an abnormal unilateral shadow was observed on chest X-ray due to pulmonary atelectasis, it was improved by bronchoscopic aspiration. Therefore, we deduced that pulmonary atelectasis was excluded by using bronchoscopy. We observed RPO in 8 patients (2.1% [8/381]), and compared the perioperative data between patients with and without RPO, and evaluated the risk factors. This study was approved by the institutional review board.
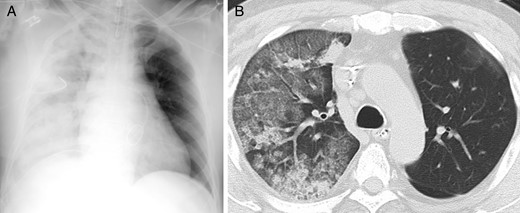
( A ) Chest radiography of Case 1 shows a diffuse ground-glass attenuation of the right lung. ( B ) Chest computed tomography of Case 5 shows obvious unilateral change with alveolar consolidation and septal thickening.
Surgical technique
Our procedures of MICS with right mini-thoracotomy were previously reported; therefore, we briefly describe our techniques, including mitral and aortic valve surgeries, below [ 13 ]. After intubation with a double-lumen endotracheal tube, transoesophageal echocardiography and a Swan–Ganz catheter were routinely used for cardiac monitoring. The surgical approach was through the third or fourth anterolateral intercostal space with a 5- to 6-cm skin incision. The right femoral artery and vein were used to establish cardiopulmonary bypass (CPB). Superior vena cava drainage was additionally used in mitral valve surgery. We used a vacuum-assisted venous drainage system for venous drainage, as necessary. After direct aortic cross-clamping (ACC), cardiac arrest was obtained by antegrade cardioplegic solution through the aortic root. Selective deliveries of cardioplegic solution into both coronary orifices were used in patients with aortic insufficiency. A left atrial vent tube was inserted from a 5-mm port at the fifth intercostal space, and the thoracic cavity was filled with carbon dioxide from the side tube of the 5-mm port. After standard valve surgeries were performed, de-airing was established by the aortic root and left atrial vents.
Regarding respiratory management, single-lung ventilation was started at the time of opening the visceral pleura. Lung ventilation was stopped after the establishment of CPB. Positive end-expiratory pressure (PEEP) of the left lung was kept to 8–10 cmH 2 O during cardiac surgery to obtain an excellent surgical exposure. Double-lung ventilation was restarted after weaning off CPB.
Statistical analysis
Continuous data are presented as mean ± standard deviation, and were analysed using two-tailed t -tests or compared with the Mann–Whitney test for independent data, as appropriate. Categorical variables are given as a count and percentage of patients, and were compared using χ2 or Fisher's exact test. Univariate analysis was performed on all variables to detect potential risk factors for postoperative RPO. All continuous parameters were dichotomized at the value with the lowest P -value obtained by receiver operating characteristic curve and the value was chosen as the threshold for logistic regression analysis. The univariate predictors with a P- value of <0.1 were selected by the stepwise method and entered into the multivariate logistic regression. A P- value of <0.05 was considered significant. All data were analysed using the Statistical Analysis Systems software JMP 9.0 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Patient demographics
A total of 381 patients underwent MICS through right mini-thoracotomy, including 6 patients with cardiac tumour or mobile thrombus, 28 with congenital heart diseases and 347 with heart valve diseases (91 aortic valve replacement [AVR], 52 mitral valve replacement [MVR], 196 mitral valve repair [MVP], 1 tricuspid valve replacement, 5 AVR plus MVP and 2 AVR plus MVR). Concomitant tricuspid annuloplasty was performed in 43 patients. Concomitant full maze procedure, left atrial maze procedure and pulmonary vein isolation were performed in 32, 9 and 20 patients, respectively. The patients' mean age was 57 ± 11.8 years and the proportion of female patients was 40%. There were no significant differences in major preoperative data between patients with and without RPO. Regarding respiratory function, there were no significant differences in the percentage of chronic obstructive pulmonary disease (COPD) and smoking history between the two groups (0% [0/8] vs 3% [11/373]; P = 1.000 and 25% [2/8] vs 35% [129/373]; P = 0.720). Preoperative spirometry was performed in all patients and there were no significant abnormal findings in patients with RPO (%vital capacity: 84.5 ± 6.4% and forced expiratory volume 1.0%: 76.6 ± 6.2%). However, the greater percentage of a preoperative use of steroid or immunosuppressant was found in patients with RPO (25% [2/8] vs 1% [4/373]; P = 0.006). Table 1 gives a comparison of preoperative data between patients with and without RPO.
| . | RPO ( n = 8) . | Non-RPO ( n = 373) . | P -value . |
|---|---|---|---|
| Age (years) | 52.4 ± 12.0 | 57.1 ± 14.85 | 0.247 |
| Female gender | 5 (63%) | 149 (40%) | 0.277 |
| BSA (m 2 ) | 1.65 ± 0.17 | 1.64 ± 0.19 | 0.707 |
| DM | 2 (25%) | 32 (8.6%) | 0.154 |
| COPD | 0 (0%) | 11 (3%) | 1.000 |
| Smoking | 2 (25%) | 129 (35%) | 0.720 |
| LVEF (%) | 59 ± 20 | 66 ± 10 | 0.220 |
| Creatinine (mg/dl) | 0.99 ± 0.6 | 0.92 ± 0.8 | 0.968 |
| Steroid or immunosuppressant | 2 (25%) | 4 (1%) | 0.006 |
| ASD | 0 | 27 | |
| VSD | 0 | 1 | |
| Cardiac tumour | 0 | 6 | |
| AI | 0 | 50 | |
| AS | 0 | 41 | |
| MR | 6 | 228 | |
| MS | 1 | 20 | |
| Isolated TR | 0 | 1 | |
| AI + MR | 1 | 4 | |
| AS + MR | 0 | 1 | |
| AS + MS | 0 | 2 |
| . | RPO ( n = 8) . | Non-RPO ( n = 373) . | P -value . |
|---|---|---|---|
| Age (years) | 52.4 ± 12.0 | 57.1 ± 14.85 | 0.247 |
| Female gender | 5 (63%) | 149 (40%) | 0.277 |
| BSA (m 2 ) | 1.65 ± 0.17 | 1.64 ± 0.19 | 0.707 |
| DM | 2 (25%) | 32 (8.6%) | 0.154 |
| COPD | 0 (0%) | 11 (3%) | 1.000 |
| Smoking | 2 (25%) | 129 (35%) | 0.720 |
| LVEF (%) | 59 ± 20 | 66 ± 10 | 0.220 |
| Creatinine (mg/dl) | 0.99 ± 0.6 | 0.92 ± 0.8 | 0.968 |
| Steroid or immunosuppressant | 2 (25%) | 4 (1%) | 0.006 |
| ASD | 0 | 27 | |
| VSD | 0 | 1 | |
| Cardiac tumour | 0 | 6 | |
| AI | 0 | 50 | |
| AS | 0 | 41 | |
| MR | 6 | 228 | |
| MS | 1 | 20 | |
| Isolated TR | 0 | 1 | |
| AI + MR | 1 | 4 | |
| AS + MR | 0 | 1 | |
| AS + MS | 0 | 2 |
RPO: re-expansion pulmonary oedema; BSA: body surface area; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; ASD: atrial septal defect; VSD: ventricular septal defect; AI: aortic insufficiency; AS: aortic stenosis; MR: mitral regurgitation; MS: mitral stenosis; TR: tricuspid regurgitation.
| . | RPO ( n = 8) . | Non-RPO ( n = 373) . | P -value . |
|---|---|---|---|
| Age (years) | 52.4 ± 12.0 | 57.1 ± 14.85 | 0.247 |
| Female gender | 5 (63%) | 149 (40%) | 0.277 |
| BSA (m 2 ) | 1.65 ± 0.17 | 1.64 ± 0.19 | 0.707 |
| DM | 2 (25%) | 32 (8.6%) | 0.154 |
| COPD | 0 (0%) | 11 (3%) | 1.000 |
| Smoking | 2 (25%) | 129 (35%) | 0.720 |
| LVEF (%) | 59 ± 20 | 66 ± 10 | 0.220 |
| Creatinine (mg/dl) | 0.99 ± 0.6 | 0.92 ± 0.8 | 0.968 |
| Steroid or immunosuppressant | 2 (25%) | 4 (1%) | 0.006 |
| ASD | 0 | 27 | |
| VSD | 0 | 1 | |
| Cardiac tumour | 0 | 6 | |
| AI | 0 | 50 | |
| AS | 0 | 41 | |
| MR | 6 | 228 | |
| MS | 1 | 20 | |
| Isolated TR | 0 | 1 | |
| AI + MR | 1 | 4 | |
| AS + MR | 0 | 1 | |
| AS + MS | 0 | 2 |
| . | RPO ( n = 8) . | Non-RPO ( n = 373) . | P -value . |
|---|---|---|---|
| Age (years) | 52.4 ± 12.0 | 57.1 ± 14.85 | 0.247 |
| Female gender | 5 (63%) | 149 (40%) | 0.277 |
| BSA (m 2 ) | 1.65 ± 0.17 | 1.64 ± 0.19 | 0.707 |
| DM | 2 (25%) | 32 (8.6%) | 0.154 |
| COPD | 0 (0%) | 11 (3%) | 1.000 |
| Smoking | 2 (25%) | 129 (35%) | 0.720 |
| LVEF (%) | 59 ± 20 | 66 ± 10 | 0.220 |
| Creatinine (mg/dl) | 0.99 ± 0.6 | 0.92 ± 0.8 | 0.968 |
| Steroid or immunosuppressant | 2 (25%) | 4 (1%) | 0.006 |
| ASD | 0 | 27 | |
| VSD | 0 | 1 | |
| Cardiac tumour | 0 | 6 | |
| AI | 0 | 50 | |
| AS | 0 | 41 | |
| MR | 6 | 228 | |
| MS | 1 | 20 | |
| Isolated TR | 0 | 1 | |
| AI + MR | 1 | 4 | |
| AS + MR | 0 | 1 | |
| AS + MS | 0 | 2 |
RPO: re-expansion pulmonary oedema; BSA: body surface area; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; ASD: atrial septal defect; VSD: ventricular septal defect; AI: aortic insufficiency; AS: aortic stenosis; MR: mitral regurgitation; MS: mitral stenosis; TR: tricuspid regurgitation.
Operative data
In the operative data, significantly longer operation, CPB and ACC time were found in patients with RPO (388 ± 80 vs 272 ± 61 min; P = 0.0002, 253 ± 79 vs 158 ± 50 min; P = 0.0009 and 162 ± 65 vs 108 ± 38 min; P = 0.0204, respectively). Additionally, a significantly greater percentage of second CPB run was observed in patients with RPO (38% [3/8] vs 1.3% [5/373]; P = 0.0003; Table 2 ).
| . | RPO ( n = 8) . | Non-RPO ( n = 373) . | P -value . |
|---|---|---|---|
| Operation time (min) | 388 ± 80 | 272 ± 61 | <0.001 |
| CPB time (min) | 253 ± 79 | 158 ± 50 | <0.001 |
| ACC time (min) | 162 ± 65 | 108 ± 38 | 0.020 |
| Second pump run | 3 (38%) | 5 (1.3%) | <0.001 |
| Initial ventilation time (h) | 147.3 ± 241.6 | 8.3 ± 53.74 | 0.002 |
| ICU stay (days) | 9.5 ± 8.5 | 1.5 ± 0.9 | <0.001 |
| Hospital stay (days) | 41.3 ± 21.0 | 20.3 ± 9.8 | <0.001 |
| 30-day death | 1 (12.5%) | 2 (0.5%) | 0.062 |
| . | RPO ( n = 8) . | Non-RPO ( n = 373) . | P -value . |
|---|---|---|---|
| Operation time (min) | 388 ± 80 | 272 ± 61 | <0.001 |
| CPB time (min) | 253 ± 79 | 158 ± 50 | <0.001 |
| ACC time (min) | 162 ± 65 | 108 ± 38 | 0.020 |
| Second pump run | 3 (38%) | 5 (1.3%) | <0.001 |
| Initial ventilation time (h) | 147.3 ± 241.6 | 8.3 ± 53.74 | 0.002 |
| ICU stay (days) | 9.5 ± 8.5 | 1.5 ± 0.9 | <0.001 |
| Hospital stay (days) | 41.3 ± 21.0 | 20.3 ± 9.8 | <0.001 |
| 30-day death | 1 (12.5%) | 2 (0.5%) | 0.062 |
CPB: cardiopulmonary bypass; ACC: aortic cross-clamping; ICU: intensive care unit.
| . | RPO ( n = 8) . | Non-RPO ( n = 373) . | P -value . |
|---|---|---|---|
| Operation time (min) | 388 ± 80 | 272 ± 61 | <0.001 |
| CPB time (min) | 253 ± 79 | 158 ± 50 | <0.001 |
| ACC time (min) | 162 ± 65 | 108 ± 38 | 0.020 |
| Second pump run | 3 (38%) | 5 (1.3%) | <0.001 |
| Initial ventilation time (h) | 147.3 ± 241.6 | 8.3 ± 53.74 | 0.002 |
| ICU stay (days) | 9.5 ± 8.5 | 1.5 ± 0.9 | <0.001 |
| Hospital stay (days) | 41.3 ± 21.0 | 20.3 ± 9.8 | <0.001 |
| 30-day death | 1 (12.5%) | 2 (0.5%) | 0.062 |
| . | RPO ( n = 8) . | Non-RPO ( n = 373) . | P -value . |
|---|---|---|---|
| Operation time (min) | 388 ± 80 | 272 ± 61 | <0.001 |
| CPB time (min) | 253 ± 79 | 158 ± 50 | <0.001 |
| ACC time (min) | 162 ± 65 | 108 ± 38 | 0.020 |
| Second pump run | 3 (38%) | 5 (1.3%) | <0.001 |
| Initial ventilation time (h) | 147.3 ± 241.6 | 8.3 ± 53.74 | 0.002 |
| ICU stay (days) | 9.5 ± 8.5 | 1.5 ± 0.9 | <0.001 |
| Hospital stay (days) | 41.3 ± 21.0 | 20.3 ± 9.8 | <0.001 |
| 30-day death | 1 (12.5%) | 2 (0.5%) | 0.062 |
CPB: cardiopulmonary bypass; ACC: aortic cross-clamping; ICU: intensive care unit.
Postoperative data
The overall 30-day mortality rate was 0.8% (3/381) and the 30-day mortality rate of patients with RPO was 12.5% (1/8). Initial ventilation time was significantly longer in the RPO group (147.3 ± 241.6 vs 8.3 ± 53.74 h; P = 0.0022). In addition, prolonged intensive care unit (ICU) and postoperative hospital stay were found in patients with RPO (9.5 ± 8.5 vs 1.5 ± 0.9 days; P < 0.0001 and 41.3 ± 21.0 vs 20.3 ± 9.8 days; P = 0.0003; Table 2 and Fig. 2 ). Table 3 summarizes the characteristics of 8 patients with RPO after MICS. All of them had mitral valve disease, and 1 patient underwent AVR plus MVP. There was no history of COPD in the RPO group. In these patients with RPO, ventilator settings in the operation theatre and the ICU were as follows: FiO 2 : 0.93 ± 0.15, respiration rate (RR): 10.6 ± 1.5, tidal volume (TV): 419 ± 27 ml, PEEP: 4.6 ± 1.3 cmH 2 O and FiO 2 : 0.61 ± 0.10, RR: 11.3 ± 1.0, TV: 501 ± 105 ml, PEEP: 6.0 ± 1.4 cmH 2 O, respectively. Moreover, arterial blood gas test findings in the operation theatre and the ICU were as follows: potential hydrogen (PH): 7.35 ± 0.03, PaO 2 : 164.1 ± 128.4 mmHg, PaCO 2 : 42.2 ± 4.8 mmHg, PaO 2 /FiO 2 (P/F) ratio: 205 ± 159 mmHg and PH: 7.39 ± 0.07, PaO 2 : 130.6 ± 72.7 mmHg, PaCO 2 : 40.4 ± 3.7 mmHg, P/F ratio: 224 ± 141 mmHg, respectively. There were 5 patients with a P/F ratio of <200 mmHg. Obvious unilateral findings of pulmonary oedema were found within 2 days after operation, and RPO occurred 2 days after operation in 3 patients. Death within 30 days was observed in 1 patient with low left ventricular ejection fraction (13%; Case 3) who required MVP for severe mitral regurgitation after CABG using the left internal mammary artery. In this case, RPO occurred immediately after MICS-MVP. The patient's respiratory function did not improve, and he died due to low cardiac output syndrome by septic shock at 29 days after the surgery. The RPO grade in this case was severe, and moderate-grade RPO was observed in 2 patients (Cases 1 and 8). In the other 5 patients, postoperative ventilation time was less than 48 h and their RPO grades were found to be mild. Although operation and CPB times in Case 6 were relatively short, RPO occurred 2 days after surgery. This patient had taken an immunosuppressant for years due to rheumatoid arthritis. Mild-grade RPO improved by ventilatory support and conservative management. In patients with moderate-grade RPO, the initiation of differential lung ventilation or steroid pulse therapy was performed.
| Patient . | Gender . | Age (years) . | BSA (m 2 ) . | Smoking . | LVEF (%) . | Immunosuppressant . | Procedure . | OP time . | CPB time . | Second run . | Day of onset from surgery . | Death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 40 | 1.87 | − | 70 | − | MVP + TAP | 420 | 255 | − | 0 | − |
| 2 | F | 55 | 1.61 | − | 78 | − | MVP | 414 | 288 | + | 0 | − |
| 3 | M | 73 | 1.57 | + | 13 | − | MVP | 329 | 225 | − | 0 | + |
| 4 | F | 56 | 1.36 | − | 62 | − | MVR + TAP | 289 | 194 | − | 2 | − |
| 5 | M | 58 | 1.90 | + | 63 | − | MVP + TAP | 413 | 273 | − | 0 | − |
| 6 | F | 37 | 1.63 | − | 58 | + | MVP | 279 | 107 | − | 2 | − |
| 7 | F | 42 | 1.58 | − | 64 | − | MVR | 471 | 327 | + | 2 | − |
| 8 | M | 58 | 1.71 | − | 65 | + | AVR + MVP | 489 | 356 | + | 1 | − |
| Mean ± SD | 52 ± 12 | 1.65 ± 0.17 | 59 ± 20 | 388 ± 80 | 253 ± 79 |
| Patient . | Gender . | Age (years) . | BSA (m 2 ) . | Smoking . | LVEF (%) . | Immunosuppressant . | Procedure . | OP time . | CPB time . | Second run . | Day of onset from surgery . | Death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 40 | 1.87 | − | 70 | − | MVP + TAP | 420 | 255 | − | 0 | − |
| 2 | F | 55 | 1.61 | − | 78 | − | MVP | 414 | 288 | + | 0 | − |
| 3 | M | 73 | 1.57 | + | 13 | − | MVP | 329 | 225 | − | 0 | + |
| 4 | F | 56 | 1.36 | − | 62 | − | MVR + TAP | 289 | 194 | − | 2 | − |
| 5 | M | 58 | 1.90 | + | 63 | − | MVP + TAP | 413 | 273 | − | 0 | − |
| 6 | F | 37 | 1.63 | − | 58 | + | MVP | 279 | 107 | − | 2 | − |
| 7 | F | 42 | 1.58 | − | 64 | − | MVR | 471 | 327 | + | 2 | − |
| 8 | M | 58 | 1.71 | − | 65 | + | AVR + MVP | 489 | 356 | + | 1 | − |
| Mean ± SD | 52 ± 12 | 1.65 ± 0.17 | 59 ± 20 | 388 ± 80 | 253 ± 79 |
SD: standard deviation; BSA: body surface area; LVEF: left ventricular ejection fraction; MVP: mitral valve plasty; TAP : tricuspid annuloplasty; MVR : mitral valve replacement; AVR : aortic valve replacement; OP : operative; CPB: cardiopulmonary bypass.
| Patient . | Gender . | Age (years) . | BSA (m 2 ) . | Smoking . | LVEF (%) . | Immunosuppressant . | Procedure . | OP time . | CPB time . | Second run . | Day of onset from surgery . | Death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 40 | 1.87 | − | 70 | − | MVP + TAP | 420 | 255 | − | 0 | − |
| 2 | F | 55 | 1.61 | − | 78 | − | MVP | 414 | 288 | + | 0 | − |
| 3 | M | 73 | 1.57 | + | 13 | − | MVP | 329 | 225 | − | 0 | + |
| 4 | F | 56 | 1.36 | − | 62 | − | MVR + TAP | 289 | 194 | − | 2 | − |
| 5 | M | 58 | 1.90 | + | 63 | − | MVP + TAP | 413 | 273 | − | 0 | − |
| 6 | F | 37 | 1.63 | − | 58 | + | MVP | 279 | 107 | − | 2 | − |
| 7 | F | 42 | 1.58 | − | 64 | − | MVR | 471 | 327 | + | 2 | − |
| 8 | M | 58 | 1.71 | − | 65 | + | AVR + MVP | 489 | 356 | + | 1 | − |
| Mean ± SD | 52 ± 12 | 1.65 ± 0.17 | 59 ± 20 | 388 ± 80 | 253 ± 79 |
| Patient . | Gender . | Age (years) . | BSA (m 2 ) . | Smoking . | LVEF (%) . | Immunosuppressant . | Procedure . | OP time . | CPB time . | Second run . | Day of onset from surgery . | Death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 40 | 1.87 | − | 70 | − | MVP + TAP | 420 | 255 | − | 0 | − |
| 2 | F | 55 | 1.61 | − | 78 | − | MVP | 414 | 288 | + | 0 | − |
| 3 | M | 73 | 1.57 | + | 13 | − | MVP | 329 | 225 | − | 0 | + |
| 4 | F | 56 | 1.36 | − | 62 | − | MVR + TAP | 289 | 194 | − | 2 | − |
| 5 | M | 58 | 1.90 | + | 63 | − | MVP + TAP | 413 | 273 | − | 0 | − |
| 6 | F | 37 | 1.63 | − | 58 | + | MVP | 279 | 107 | − | 2 | − |
| 7 | F | 42 | 1.58 | − | 64 | − | MVR | 471 | 327 | + | 2 | − |
| 8 | M | 58 | 1.71 | − | 65 | + | AVR + MVP | 489 | 356 | + | 1 | − |
| Mean ± SD | 52 ± 12 | 1.65 ± 0.17 | 59 ± 20 | 388 ± 80 | 253 ± 79 |
SD: standard deviation; BSA: body surface area; LVEF: left ventricular ejection fraction; MVP: mitral valve plasty; TAP : tricuspid annuloplasty; MVR : mitral valve replacement; AVR : aortic valve replacement; OP : operative; CPB: cardiopulmonary bypass.
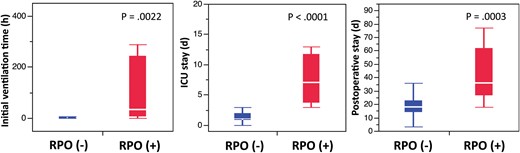
Comparison of postoperative data between patients with and without re-expansion pulmonary oedema (RPO). Significantly prolonged initial ventilation time, intensive care unit (ICU) and postoperative hospital stay were found in patients with RPO.
Risk factors for RPO after MICS
On univariate logistic analysis, preoperative use of steroid or immunosuppressant, longer operation time (≥329 min), longer CPB time (≥194 min), longer ACC time (≥156 min) and second CPB run were identified as perioperative risk factors for RPO after MICS with P- values of <0.1 (odds ratio [OR]: 30.8 [95% confidence interval, CI: 3.8–194.5]; P = 0.004, OR: 20.8 [95% CI: 4.6–144.8]; P < 0.001, OR: 35.7 [95% CI: 6.2–673.3]; P < 0.001, OR: 31.0 [95% CI: 6.8–217.8]; P < 0.001 and OR: 44.2 [95% CI: 7.5–241.8]; P = 0.0002, respectively). Multivariate logistic analysis detected preoperative use of steroid or immunosuppressant and longer ACC time (≥156 min) as independent risk factors for RPO after MICS (OR: 87.6 [95% CI: 4.1–2463.8]; P = 0.006 and OR: 36.0 [95% CI: 4.8–731.4]; P < 0.001; Table 4 ).
| Variables . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| OR (CI) . | P -value . | OR (CI) . | P -value . | |
| Preoperative use of steroid or immunosuppressant | 30.8 (3.8–194.5) | 0.004 | 87.6 (4.1–2463.8) | 0.006 |
| Operation time (≥329 min) | 20.8 (4.6–144.8) | <0.001 | – | – |
| CPB time (≥194 min) | 35.7 (6.2–673.3) | <0.001 | – | – |
| ACC time (≥156 min) | 31.0 (6.8–217.8) | <0.001 | 36.0 (4.8–731.4) | <0.001 |
| Second CPB run | 44.2 (7.5–241.8) | <0.001 | – | – |
| Variables . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| OR (CI) . | P -value . | OR (CI) . | P -value . | |
| Preoperative use of steroid or immunosuppressant | 30.8 (3.8–194.5) | 0.004 | 87.6 (4.1–2463.8) | 0.006 |
| Operation time (≥329 min) | 20.8 (4.6–144.8) | <0.001 | – | – |
| CPB time (≥194 min) | 35.7 (6.2–673.3) | <0.001 | – | – |
| ACC time (≥156 min) | 31.0 (6.8–217.8) | <0.001 | 36.0 (4.8–731.4) | <0.001 |
| Second CPB run | 44.2 (7.5–241.8) | <0.001 | – | – |
OR: odds ratio; CI: confidence interval. For other abbreviations, see Table 2 .
| Variables . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| OR (CI) . | P -value . | OR (CI) . | P -value . | |
| Preoperative use of steroid or immunosuppressant | 30.8 (3.8–194.5) | 0.004 | 87.6 (4.1–2463.8) | 0.006 |
| Operation time (≥329 min) | 20.8 (4.6–144.8) | <0.001 | – | – |
| CPB time (≥194 min) | 35.7 (6.2–673.3) | <0.001 | – | – |
| ACC time (≥156 min) | 31.0 (6.8–217.8) | <0.001 | 36.0 (4.8–731.4) | <0.001 |
| Second CPB run | 44.2 (7.5–241.8) | <0.001 | – | – |
| Variables . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| OR (CI) . | P -value . | OR (CI) . | P -value . | |
| Preoperative use of steroid or immunosuppressant | 30.8 (3.8–194.5) | 0.004 | 87.6 (4.1–2463.8) | 0.006 |
| Operation time (≥329 min) | 20.8 (4.6–144.8) | <0.001 | – | – |
| CPB time (≥194 min) | 35.7 (6.2–673.3) | <0.001 | – | – |
| ACC time (≥156 min) | 31.0 (6.8–217.8) | <0.001 | 36.0 (4.8–731.4) | <0.001 |
| Second CPB run | 44.2 (7.5–241.8) | <0.001 | – | – |
OR: odds ratio; CI: confidence interval. For other abbreviations, see Table 2 .
DISCUSSION
By Riesman et al . in 1853, Pinault et al . reported that pulmonary oedema occurs in a re-expanded lung after drainage of pleural effusion. This is believed to be the first report of RPO [ 22 ]. Mechanical stress exerted during expansion and histological abnormalities of the pulmonary microvessels in a chronically collapsed lung are known as an important factor of RPO; however, the cause of these abnormalities has not been fully determined. Therefore, it is still difficult to choose the optimal treatment for RPO. Sohara [ 3 ] reported the following theory on the mechanisms. First of all, microvascular endothelium is thickened in a collapsed lung, the re-expansion of the chronically collapsed lung is stretched and the pulmonary microvessels are damaged. Subsequently, decreasing alveolar surfactant activity in the re-expanded lung reduces the perivascular pressure and increases injury of the microvessels. When reperfusion occurs in the injured pulmonary microvessels, oxygen-derived free radicals are produced. Consequently, leucocyte sequestration is induced by free radicals and microvascular injury. These biological damages due to free radicals and leucocytes lead to additional adverse effects on the pulmonary microvessels and RPO.
As mentioned above, there are reports of RPO after drainage of pneumothorax or pleural effusion, with a mortality rate of up to 21% [ 2 , 4 , 5 ]. However, there are still only a few reports on RPO after MICS. The incidence is relatively low, but it should be recognized as one of the serious complications after MICS. Generally, Keyl et al . reported RPO within 24 h after MICS, which was observed in 7.9% of patients, and postoperative mechanical ventilation length of 16.0 h. Additionally, 1.5% simultaneously developed postoperative ventilation time [ 19 ]. In our experience, the mortality rate was 12.5% (1 of 8), postoperative prolonged ventilatory support, ICU and hospital stay were required, and the advantages of MICS were completely forfeited. Mahfood et al . reported 47 cases of RPO associated with pneumothorax, and the period of lung collapse was more than 3 days in 83% of patients. On the other hand, RPO after MICS was observed in a relatively short period of lung collapse time. The shortest operative time was 279 min in our study, and there is a case report of RPO after thoracoscopic mediastinal tumour resection in 90 min of lung collapse time [ 23 ]. Regarding risk factors for RPO, prolonged operative, CPB and ACC time were detected as risk factors. However, preoperative smoking history and respiratory function were not significantly associated with the incidence of RPO. These results show that the mechanisms of RPO seem to be different in MICS compared with other situations. Although it is still unclear, specific factors in MICS, such as the use of CPB, systemic full heparinization, continuous PEEP in the left lung and decreased pulmonary circulation, may influence pulmonary conditions. Since prolonged ACC time was isolated as one of the risk factors by multivariate analysis, not only longer operation and CPB times, but also a combination of malperfusion and collapse of the lung was thought to be a main difference and cause of RPO. Therefore, avoiding prolonged lung ischaemia and intermittent ventilation may be important strategies to prevent postoperative RPO. On the other hand, preoperative steroid or immunosuppressant intake was also detected as a risk factor for RPO. A single dose of dexamethasone administration after anaesthesia induction was reported to be effective for prevention of RPO after MICS by Keyl et al . [ 19 ]. Considering this result, preoperative discontinuation of steroid intake might influence the postoperative activity of endogenous steroid. Therefore, we also think that steroid use just before starting operation could potentially reduce the risk of RPO.
RPO is classified according to severity, and conservative management is the first choice of treatment for mild cases. On the other hand, prolonged mechanical ventilation may be required for moderate grade RPO, depending on the situation. In patients with severe RPO, extracorporeal membrane oxygenation was reported to be a useful option [ 17 , 18 ]. Also, high-frequency jet ventilation was reported to be effective for severe RPO [ 24 ]. Furthermore, steroids could be effective in stabilizing the pulmonary microvessel membrane. However, there are still only a few reports on this topic, and an optimal strategy for the treatment of RPO has not been determined. Recently, an asynchronous differential lung ventilation has been reported as a new therapeutic modality for treating severe RPO [ 25 ]. Regarding respiratory management, 5 cmH 2 O of PEEP was applied to only the collapsed unilateral lung, and other respiratory settings, such as TV, respiratory rate and fraction of inspired oxygen, were approximately the same as for a normal lung (350 ml, 25 per min, and 1.0, respectively). We applied this differential lung ventilation for Case 1 with severe RPO, which occurred immediately after surgery. After the initiation of differential lung ventilation and steroid pulse therapy from postoperative day 2, the patient was weaned from the mechanical ventilator successfully on postoperative day 12.
Study limitations
There were several limitations in this study. First, RPO was not clearly defined, and it was difficult to diagnose this accurately. Additionally, it is very difficult to delineate the differences in pathological changes between diffuse pulmonary damage and surgical traumatic damage. Therefore, we may not be able to exclude the influences of unexpected factors. Secondly, since the occurrence rate of RPO was relatively low, there was a significant difference in the sample number between patients with and without RPO. Since the number of patients with RPO was small, multivariate analysis to detect the risk factors of postoperative RPO may not be adequate. Finally, this study did not include long-term outcomes.
CONCLUSIONS
RPO is one of the most serious complications after MICS with right mini-thoracotomy. Although the occurrence rate is relatively low, RPO can be a life-threatening complication and can prolong hospitalization. Therefore, RPO should be recognized as a possible complication, and preventive strategies of RPO after MICS were very important issues. According to the present study, preoperative steroid or immunosuppressant intake and prolonged ACC time could potentially be risk factors for RPO. However, the sample number was not sufficient, and further evaluations will be required.
Funding
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the article.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the Annual Scientific Meeting of the International Society for Minimally Invasive Cardiothoracic Surgery, Boston, MA, USA, 28–31 May 2014.




