-
PDF
- Split View
-
Views
-
Cite
Cite
Hans-Hinrich Sievers, Ulrich Stierle, Efstratios I. Charitos, Johanna J.M. Takkenberg, Jürgen Hörer, Rüdiger Lange, Ulrich Franke, Marc Albert, Armin Gorski, Rainer G. Leyh, Arlindo Riso, Jörg Sachweh, Anton Moritz, Roland Hetzer, Wolfgang Hemmer, A multicentre evaluation of the autograft procedure for young patients undergoing aortic valve replacement: update on the German Ross Registry, European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 1, January 2016, Pages 212–218, https://doi.org/10.1093/ejcts/ezv001
Close - Share Icon Share
Abstract
Conventional aortic valve replacement (AVR) in young, active patients represents a suboptimal solution in terms of long-term survival, durability and quality of life. The aim of the present work is to present an update on the multicentre experience with the pulmonary autograft procedure in young, adult patients.
Between 1990–2013, 1779 adult patients (1339 males; 44.7 ± 11.6 years) underwent the pulmonary autograft procedure in 8 centres. All patients underwent prospective clinical and echocardiographic examinations annually. The mean follow-up was 8.3 ± 5.1 years (range 0–24.3 years) with a total cumulative follow-up of 14 288 years and 662 patients having a follow-up of at least 10 years.
The early (30-day) mortality rate was 1.1% (n = 19). Late (>30 day) survival of the adult population was comparable with the age- and gender-matched general population (observed deaths: 101, expected deaths: 91; P = 0.29). Freedom from autograft reoperation at 5, 10 and 15 years was 96.8, 94.7 and 86.7%, respectively, whereas freedom from homograft reoperation was 97.6, 95.5 and 92.3%, respectively. The overall freedom from reoperation was 94.9, 91.1 and 82.7%, respectively. Longitudinal modelling of functional valve performance revealed a low (<5%) probability of a patient being in higher autograft regurgitation grades throughout the first decade. Similarly, excellent homograft function was observed throughout the first 15 years.
The autograft principle results in postoperative long-term survival comparable with that of the age- and gender-matched general population and reoperation rates within the 1%/patient-year boundaries and should be considered in young, active patients who want to avoid the shortcomings of conventional prostheses.
INTRODUCTION
Although significant advances in prosthetic heart valves have been introduced in the last couple of decades, in young patients with aortic valve disease conventional aortic valve replacement represents a suboptimal solution. Mechanical aortic valve replacement still requires lifelong anticoagulation and brings lifestyle restrictions [1] and a potential for bleeding or thrombotic complications [2], whereas biological prostheses in young patients have disappointing durability [3]. Despite the fact that young adults (>16 and <60 years of age) represent only a small minority of all the patients requiring aortic valve replacement, these patients have different needs, expectations and wishes so that data and outcomes from the 60- and 70-year old patient populations cannot be extrapolated to the former.
The aim of the present study is to present an update to our previous report [4] on patients operated with the autograft principle (Ross procedure) in centres participating in the German Ross Registry. For the purposes of the present report, only adult patients (≥16 years old) were included.
METHODS
Patient population
Details on the German–Dutch Ross Registry have been previously reported [4]. In the present report, we do not include the Dutch population of the German–Dutch Ross Registry because due to revised institutional regulations the transfer of the updated follow-up data could not be provided in time for the drafting of the present manuscript. The present work focuses on the 1779 adult (>16 years old) patients operated in 8 German centres. Between the present and the last update [4], four centres (270 patients) were permanently removed from the Registry due to their inability to comply with the Registry's follow-up requirements. For the present study, the completeness of follow-up was 95%. At the time of database closure, 87 patients had at least one documented episode of atrial fibrillation, and 236 patients were on Coumadin or Aspirin.
Indications for the primary procedure, technical details, definition of events and methods of outcome reporting have been reported previously [4–7]. In brief, a Ross reintervention was defined as any surgical or interventional procedure performed after the initial Ross procedure on the autograft or homograft. A Ross reoperation was defined as a surgical session that included at least one Ross reintervention on the autograft or homograft, or both (1, 1 and 2 reinterventions, respectively) and could include concomitant interventions to other cardiac structures. Demographics of the patient population are presented in Table 1.
| . | Total . | SC . | RR . | RR + R . |
|---|---|---|---|---|
| Patients (n) | 1779 | 823 | 249 | 707 |
| Follow-up (years) | ||||
| Mean | 8.3 ± 5.1 | 8.7 ± 5.0 | 9.5 ± 6.1 | 7.4 ± 4.7 |
| Range | 0–24.3 | 0–19.4 | 0–17.8 | 0.2–24.3 |
| Age (years) | 44.7 ± 11.6 | 45.1 ± 11.2 | 40.3 ± 12.8 | 45.7 ± 11.3 |
| Male gender | 1339 (75.3) | 626 (76.1) | 187 (75.1) | 526 (74.4) |
| Age group (years) | ||||
| 16–40 | 568 (31.9) | 253 (30.7) | 117 (47.0) | 198 (28.0) |
| 41–60 | 1100 (61.8) | 509 (61.8) | 124 (49.8) | 467 (66.1) |
| >60 | 111 (6.2) | 61 (7.4) | 8 (3.2) | 42 (5.9) |
| Predominant aortic haemodynamics | ||||
| Regurgitation | 398 (22.4) | 205 (24.9) | 54 (21.7) | 139 (19.7) |
| Stenosis | 423 (23.8) | 158 (19.2) | 52 (20.9) | 213 (30.1) |
| Combined | 920 (51.7) | 447 (54.3) | 132 (53.0) | 341 (48.2) |
| Aortic valve type | ||||
| Bicuspid | 1152 (64.8) | 585 (71.1) | 136 (54.6) | 431 (61.0) |
| Tricuspid | 367 (20.6) | 161 (19.6) | 77 (30.9) | 129 (18.2) |
| Other | 163 (9.2) | 37 (4.5) | 11 (4.4) | 115 (16.3) |
| Unknown | 97 (5.5) | 40 (4.9) | 25 (10.0) | 32 (4.5) |
| Atrial fibrillation | 16 (0.9) | 11 (1.3) | 2 (0.8) | 3 (0.4) |
| Concomitant procedures (n) | ||||
| Total | 854 (48.0) | 362 (44.0) | 69 (27.7) | 423 (59.8) |
| CABG | 93 (5.2) | 27 (3.3) | 14 (5.6) | 52 (7.4) |
| Previous cardiac interventions (n) | 139 (7.8) | 45 (5.5) | 33 (13.3) | 61 (8.6) |
| Circulatory arrest | ||||
| Patients (n) | 130 (7.3) | 81 (9.8) | 10 (4.0) | 39 (5.5) |
| Mean ± SD | 16.5 ± 7.0 | 17.7 ± 3.9 | 17.9 ± 20.8 | 13.8 ± 5.6 |
| Range | 2–64 | 12–33 | 2–64 | 3–33 |
| CPB time (min) | ||||
| Mean ± SD | 193.4 ± 45.5 | 215.6 ± 36.2 | 177.9 ± 46.1 | 174.4 ± 43.4 |
| Range | 71–544 | 71–460 | 95–482 | 71–544 |
| Cross-clamp time (min) | ||||
| Mean ± SD | 153.7 ± 36.6 | 176.8 ± 34.3 | 126.8 ± 25.8 | 137.8 ± 26.0 |
| Range | 38–309 | 43–309 | 38–269 | 79–258 |
| In-hospital (<30 day) mortality | 19 (1.1) | 8 (1.0) | 0 | 11 (1.6) |
| . | Total . | SC . | RR . | RR + R . |
|---|---|---|---|---|
| Patients (n) | 1779 | 823 | 249 | 707 |
| Follow-up (years) | ||||
| Mean | 8.3 ± 5.1 | 8.7 ± 5.0 | 9.5 ± 6.1 | 7.4 ± 4.7 |
| Range | 0–24.3 | 0–19.4 | 0–17.8 | 0.2–24.3 |
| Age (years) | 44.7 ± 11.6 | 45.1 ± 11.2 | 40.3 ± 12.8 | 45.7 ± 11.3 |
| Male gender | 1339 (75.3) | 626 (76.1) | 187 (75.1) | 526 (74.4) |
| Age group (years) | ||||
| 16–40 | 568 (31.9) | 253 (30.7) | 117 (47.0) | 198 (28.0) |
| 41–60 | 1100 (61.8) | 509 (61.8) | 124 (49.8) | 467 (66.1) |
| >60 | 111 (6.2) | 61 (7.4) | 8 (3.2) | 42 (5.9) |
| Predominant aortic haemodynamics | ||||
| Regurgitation | 398 (22.4) | 205 (24.9) | 54 (21.7) | 139 (19.7) |
| Stenosis | 423 (23.8) | 158 (19.2) | 52 (20.9) | 213 (30.1) |
| Combined | 920 (51.7) | 447 (54.3) | 132 (53.0) | 341 (48.2) |
| Aortic valve type | ||||
| Bicuspid | 1152 (64.8) | 585 (71.1) | 136 (54.6) | 431 (61.0) |
| Tricuspid | 367 (20.6) | 161 (19.6) | 77 (30.9) | 129 (18.2) |
| Other | 163 (9.2) | 37 (4.5) | 11 (4.4) | 115 (16.3) |
| Unknown | 97 (5.5) | 40 (4.9) | 25 (10.0) | 32 (4.5) |
| Atrial fibrillation | 16 (0.9) | 11 (1.3) | 2 (0.8) | 3 (0.4) |
| Concomitant procedures (n) | ||||
| Total | 854 (48.0) | 362 (44.0) | 69 (27.7) | 423 (59.8) |
| CABG | 93 (5.2) | 27 (3.3) | 14 (5.6) | 52 (7.4) |
| Previous cardiac interventions (n) | 139 (7.8) | 45 (5.5) | 33 (13.3) | 61 (8.6) |
| Circulatory arrest | ||||
| Patients (n) | 130 (7.3) | 81 (9.8) | 10 (4.0) | 39 (5.5) |
| Mean ± SD | 16.5 ± 7.0 | 17.7 ± 3.9 | 17.9 ± 20.8 | 13.8 ± 5.6 |
| Range | 2–64 | 12–33 | 2–64 | 3–33 |
| CPB time (min) | ||||
| Mean ± SD | 193.4 ± 45.5 | 215.6 ± 36.2 | 177.9 ± 46.1 | 174.4 ± 43.4 |
| Range | 71–544 | 71–460 | 95–482 | 71–544 |
| Cross-clamp time (min) | ||||
| Mean ± SD | 153.7 ± 36.6 | 176.8 ± 34.3 | 126.8 ± 25.8 | 137.8 ± 26.0 |
| Range | 38–309 | 43–309 | 38–269 | 79–258 |
| In-hospital (<30 day) mortality | 19 (1.1) | 8 (1.0) | 0 | 11 (1.6) |
SC: sub-coronary; RR + R: root replacement with additional root reinforcement; RR: root replacement without additional root reinforcement; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass.
| . | Total . | SC . | RR . | RR + R . |
|---|---|---|---|---|
| Patients (n) | 1779 | 823 | 249 | 707 |
| Follow-up (years) | ||||
| Mean | 8.3 ± 5.1 | 8.7 ± 5.0 | 9.5 ± 6.1 | 7.4 ± 4.7 |
| Range | 0–24.3 | 0–19.4 | 0–17.8 | 0.2–24.3 |
| Age (years) | 44.7 ± 11.6 | 45.1 ± 11.2 | 40.3 ± 12.8 | 45.7 ± 11.3 |
| Male gender | 1339 (75.3) | 626 (76.1) | 187 (75.1) | 526 (74.4) |
| Age group (years) | ||||
| 16–40 | 568 (31.9) | 253 (30.7) | 117 (47.0) | 198 (28.0) |
| 41–60 | 1100 (61.8) | 509 (61.8) | 124 (49.8) | 467 (66.1) |
| >60 | 111 (6.2) | 61 (7.4) | 8 (3.2) | 42 (5.9) |
| Predominant aortic haemodynamics | ||||
| Regurgitation | 398 (22.4) | 205 (24.9) | 54 (21.7) | 139 (19.7) |
| Stenosis | 423 (23.8) | 158 (19.2) | 52 (20.9) | 213 (30.1) |
| Combined | 920 (51.7) | 447 (54.3) | 132 (53.0) | 341 (48.2) |
| Aortic valve type | ||||
| Bicuspid | 1152 (64.8) | 585 (71.1) | 136 (54.6) | 431 (61.0) |
| Tricuspid | 367 (20.6) | 161 (19.6) | 77 (30.9) | 129 (18.2) |
| Other | 163 (9.2) | 37 (4.5) | 11 (4.4) | 115 (16.3) |
| Unknown | 97 (5.5) | 40 (4.9) | 25 (10.0) | 32 (4.5) |
| Atrial fibrillation | 16 (0.9) | 11 (1.3) | 2 (0.8) | 3 (0.4) |
| Concomitant procedures (n) | ||||
| Total | 854 (48.0) | 362 (44.0) | 69 (27.7) | 423 (59.8) |
| CABG | 93 (5.2) | 27 (3.3) | 14 (5.6) | 52 (7.4) |
| Previous cardiac interventions (n) | 139 (7.8) | 45 (5.5) | 33 (13.3) | 61 (8.6) |
| Circulatory arrest | ||||
| Patients (n) | 130 (7.3) | 81 (9.8) | 10 (4.0) | 39 (5.5) |
| Mean ± SD | 16.5 ± 7.0 | 17.7 ± 3.9 | 17.9 ± 20.8 | 13.8 ± 5.6 |
| Range | 2–64 | 12–33 | 2–64 | 3–33 |
| CPB time (min) | ||||
| Mean ± SD | 193.4 ± 45.5 | 215.6 ± 36.2 | 177.9 ± 46.1 | 174.4 ± 43.4 |
| Range | 71–544 | 71–460 | 95–482 | 71–544 |
| Cross-clamp time (min) | ||||
| Mean ± SD | 153.7 ± 36.6 | 176.8 ± 34.3 | 126.8 ± 25.8 | 137.8 ± 26.0 |
| Range | 38–309 | 43–309 | 38–269 | 79–258 |
| In-hospital (<30 day) mortality | 19 (1.1) | 8 (1.0) | 0 | 11 (1.6) |
| . | Total . | SC . | RR . | RR + R . |
|---|---|---|---|---|
| Patients (n) | 1779 | 823 | 249 | 707 |
| Follow-up (years) | ||||
| Mean | 8.3 ± 5.1 | 8.7 ± 5.0 | 9.5 ± 6.1 | 7.4 ± 4.7 |
| Range | 0–24.3 | 0–19.4 | 0–17.8 | 0.2–24.3 |
| Age (years) | 44.7 ± 11.6 | 45.1 ± 11.2 | 40.3 ± 12.8 | 45.7 ± 11.3 |
| Male gender | 1339 (75.3) | 626 (76.1) | 187 (75.1) | 526 (74.4) |
| Age group (years) | ||||
| 16–40 | 568 (31.9) | 253 (30.7) | 117 (47.0) | 198 (28.0) |
| 41–60 | 1100 (61.8) | 509 (61.8) | 124 (49.8) | 467 (66.1) |
| >60 | 111 (6.2) | 61 (7.4) | 8 (3.2) | 42 (5.9) |
| Predominant aortic haemodynamics | ||||
| Regurgitation | 398 (22.4) | 205 (24.9) | 54 (21.7) | 139 (19.7) |
| Stenosis | 423 (23.8) | 158 (19.2) | 52 (20.9) | 213 (30.1) |
| Combined | 920 (51.7) | 447 (54.3) | 132 (53.0) | 341 (48.2) |
| Aortic valve type | ||||
| Bicuspid | 1152 (64.8) | 585 (71.1) | 136 (54.6) | 431 (61.0) |
| Tricuspid | 367 (20.6) | 161 (19.6) | 77 (30.9) | 129 (18.2) |
| Other | 163 (9.2) | 37 (4.5) | 11 (4.4) | 115 (16.3) |
| Unknown | 97 (5.5) | 40 (4.9) | 25 (10.0) | 32 (4.5) |
| Atrial fibrillation | 16 (0.9) | 11 (1.3) | 2 (0.8) | 3 (0.4) |
| Concomitant procedures (n) | ||||
| Total | 854 (48.0) | 362 (44.0) | 69 (27.7) | 423 (59.8) |
| CABG | 93 (5.2) | 27 (3.3) | 14 (5.6) | 52 (7.4) |
| Previous cardiac interventions (n) | 139 (7.8) | 45 (5.5) | 33 (13.3) | 61 (8.6) |
| Circulatory arrest | ||||
| Patients (n) | 130 (7.3) | 81 (9.8) | 10 (4.0) | 39 (5.5) |
| Mean ± SD | 16.5 ± 7.0 | 17.7 ± 3.9 | 17.9 ± 20.8 | 13.8 ± 5.6 |
| Range | 2–64 | 12–33 | 2–64 | 3–33 |
| CPB time (min) | ||||
| Mean ± SD | 193.4 ± 45.5 | 215.6 ± 36.2 | 177.9 ± 46.1 | 174.4 ± 43.4 |
| Range | 71–544 | 71–460 | 95–482 | 71–544 |
| Cross-clamp time (min) | ||||
| Mean ± SD | 153.7 ± 36.6 | 176.8 ± 34.3 | 126.8 ± 25.8 | 137.8 ± 26.0 |
| Range | 38–309 | 43–309 | 38–269 | 79–258 |
| In-hospital (<30 day) mortality | 19 (1.1) | 8 (1.0) | 0 | 11 (1.6) |
SC: sub-coronary; RR + R: root replacement with additional root reinforcement; RR: root replacement without additional root reinforcement; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass.
Statistical analysis
Frequencies are given as absolute numbers and percentages. Continuous data are expressed as the mean ± standard deviation. The actuarial estimates of freedom from reoperation events were calculated using the Kaplan–Meier method. The instantaneous risk of reoperation is presented as the smoothed instantaneous rate that a patient will require reoperation within the interval (t, t ± dt) provided the patient was not censored until the beginning of t [8–10].
Given our understanding of the risk of autograft and homograft reinterventions and the postoperative survival function of the Ross patients that has been observed until now, we attempted to extrapolate the estimated risk of reoperation for a Ross patient up to 75 years of age according to the patient's age at the initial Ross procedure, under a competing risk and event redistribution framework [11–13]. This calculation attempts to answer the question what is the probability that a patient will experience at least one reoperation when simultaneously considering the competing risk of death, which may serve some purposes for decision-making prior to aortic valve replacement. Several studies have failed to show excess mortality for the Ross population compared with that of the general population [4, 14, 15]; however, this is expected to deviate in the near future [13] at least to a small degree. For this calculation, we penalized the mortality hazard of the Ross patients by a factor of 1.25. For the calculation of the risk for autograft or homograft reoperation, a Weibull survival model was utilized (Intercept: 4.20, Scale = 0.82, P < 0.001) [16]. To calculate the probability of experiencing at least one reoperation till the age of 75 allowing for the competing risk of death, a Markov model was utilized [13] using the above-mentioned assumptions on the incidence of death and reoperations.
Modelling of continuous echocardiographic data (homograft transvalvular gradient) was performed using multivariate mixed-model regression allowing for a random patient intercept and slope. Extensions of mixed models for ordinal data were employed for the analysis of the time course of autograft and homograft regurgitation with time.
All statistical analyses were performed using R version 3.1.1 (R Development Core Team 2014; R: A Language and Environment for Statistical Computing, Vienna, Austria; 2014, http://www.R-project.org/).
RESULTS
The reasons for reoperations as well as their incidence are presented in Table 2. The late (>30 days) survival of the Ross population followed closely the expected survival of the general German population [observed late (>30 days) deaths: 101, expected: 91.1; P = 0.29); Fig. 1.
| . | Total . | SC . | RR . | RR + R . |
|---|---|---|---|---|
| Patients with Ross-related reoperation (% of total) | 147 (8.3) | 61 (7.4) | 31 (12.4) | 55 (7.8) |
| Follow-up (years) | ||||
| Mean ± SD | 8.3 ± 5.1 | 8.7 ± 5.0 | 9.5 ± 6.1 | 7.4 ± 4.7 |
| Range | 0–24.3 | 0–19.4 | 0–17.8 | 0.2–24.3 |
| Cumulative follow-up (patient-year) | 14,228.6 | 7069.3 | 2178.1 | 4981.1 |
| Ross-related reoperations | 175 | 77 | 37 | 61 |
| Reoperation mortality (n) | 5 | 2 | 0 | 3 |
| Ross-related reinterventions | 197 | 88 | 41 | 68 |
| Reoperation type | ||||
| Autograft | 84 | 29 | 18 | 37 |
| Homograft | 69 | 37 | 15 | 17 |
| Combined | 22 | 11 | 4 | 7 |
| Autograft reinterventions | 106 | 40 | 22 | 44 |
| Endocarditis | 30 | 14 | 2 | 14 |
| SVD | 29 | 19 | 4 | 6 |
| NSVD | 44 | 6 | 16 | 22 |
| Technical | 3 | 1 | 2 | |
| Homograft reinterventions | 91 | |||
| SVD | ||||
| Stenosis | 62 | |||
| Regurgitation | 7 | |||
| NSVD | 5 | |||
| Endocarditis | 17 | |||
| . | Total . | SC . | RR . | RR + R . |
|---|---|---|---|---|
| Patients with Ross-related reoperation (% of total) | 147 (8.3) | 61 (7.4) | 31 (12.4) | 55 (7.8) |
| Follow-up (years) | ||||
| Mean ± SD | 8.3 ± 5.1 | 8.7 ± 5.0 | 9.5 ± 6.1 | 7.4 ± 4.7 |
| Range | 0–24.3 | 0–19.4 | 0–17.8 | 0.2–24.3 |
| Cumulative follow-up (patient-year) | 14,228.6 | 7069.3 | 2178.1 | 4981.1 |
| Ross-related reoperations | 175 | 77 | 37 | 61 |
| Reoperation mortality (n) | 5 | 2 | 0 | 3 |
| Ross-related reinterventions | 197 | 88 | 41 | 68 |
| Reoperation type | ||||
| Autograft | 84 | 29 | 18 | 37 |
| Homograft | 69 | 37 | 15 | 17 |
| Combined | 22 | 11 | 4 | 7 |
| Autograft reinterventions | 106 | 40 | 22 | 44 |
| Endocarditis | 30 | 14 | 2 | 14 |
| SVD | 29 | 19 | 4 | 6 |
| NSVD | 44 | 6 | 16 | 22 |
| Technical | 3 | 1 | 2 | |
| Homograft reinterventions | 91 | |||
| SVD | ||||
| Stenosis | 62 | |||
| Regurgitation | 7 | |||
| NSVD | 5 | |||
| Endocarditis | 17 | |||
SC: sub-coronary; RR + R: root replacement with additional root reinforcement; RR: root replacement without additional root reinforcement; SD: standard deviation; SVD: structural valve deterioration; NSVD: non-structural valve deterioration.
| . | Total . | SC . | RR . | RR + R . |
|---|---|---|---|---|
| Patients with Ross-related reoperation (% of total) | 147 (8.3) | 61 (7.4) | 31 (12.4) | 55 (7.8) |
| Follow-up (years) | ||||
| Mean ± SD | 8.3 ± 5.1 | 8.7 ± 5.0 | 9.5 ± 6.1 | 7.4 ± 4.7 |
| Range | 0–24.3 | 0–19.4 | 0–17.8 | 0.2–24.3 |
| Cumulative follow-up (patient-year) | 14,228.6 | 7069.3 | 2178.1 | 4981.1 |
| Ross-related reoperations | 175 | 77 | 37 | 61 |
| Reoperation mortality (n) | 5 | 2 | 0 | 3 |
| Ross-related reinterventions | 197 | 88 | 41 | 68 |
| Reoperation type | ||||
| Autograft | 84 | 29 | 18 | 37 |
| Homograft | 69 | 37 | 15 | 17 |
| Combined | 22 | 11 | 4 | 7 |
| Autograft reinterventions | 106 | 40 | 22 | 44 |
| Endocarditis | 30 | 14 | 2 | 14 |
| SVD | 29 | 19 | 4 | 6 |
| NSVD | 44 | 6 | 16 | 22 |
| Technical | 3 | 1 | 2 | |
| Homograft reinterventions | 91 | |||
| SVD | ||||
| Stenosis | 62 | |||
| Regurgitation | 7 | |||
| NSVD | 5 | |||
| Endocarditis | 17 | |||
| . | Total . | SC . | RR . | RR + R . |
|---|---|---|---|---|
| Patients with Ross-related reoperation (% of total) | 147 (8.3) | 61 (7.4) | 31 (12.4) | 55 (7.8) |
| Follow-up (years) | ||||
| Mean ± SD | 8.3 ± 5.1 | 8.7 ± 5.0 | 9.5 ± 6.1 | 7.4 ± 4.7 |
| Range | 0–24.3 | 0–19.4 | 0–17.8 | 0.2–24.3 |
| Cumulative follow-up (patient-year) | 14,228.6 | 7069.3 | 2178.1 | 4981.1 |
| Ross-related reoperations | 175 | 77 | 37 | 61 |
| Reoperation mortality (n) | 5 | 2 | 0 | 3 |
| Ross-related reinterventions | 197 | 88 | 41 | 68 |
| Reoperation type | ||||
| Autograft | 84 | 29 | 18 | 37 |
| Homograft | 69 | 37 | 15 | 17 |
| Combined | 22 | 11 | 4 | 7 |
| Autograft reinterventions | 106 | 40 | 22 | 44 |
| Endocarditis | 30 | 14 | 2 | 14 |
| SVD | 29 | 19 | 4 | 6 |
| NSVD | 44 | 6 | 16 | 22 |
| Technical | 3 | 1 | 2 | |
| Homograft reinterventions | 91 | |||
| SVD | ||||
| Stenosis | 62 | |||
| Regurgitation | 7 | |||
| NSVD | 5 | |||
| Endocarditis | 17 | |||
SC: sub-coronary; RR + R: root replacement with additional root reinforcement; RR: root replacement without additional root reinforcement; SD: standard deviation; SVD: structural valve deterioration; NSVD: non-structural valve deterioration.

Probability of survival of the Ross patients compared with the general German population; 17 patients with <30 day mortality were excluded.
Actuarial estimates of freedom of autograft, homograft and combined are presented in Figs 2 and 3 and in Supplementary Figs E1 and E2. In the present patient population, although the sub-coronary technique resulted in higher freedom from reoperation (Figs 2 and 4), this did not reach the level of statistical significance (P = 0.132). However, since the instantaneous hazard for reoperation of the root replacement technique (Fig. 2) exhibits the same accelerated failure pattern during the second decade similar to that seen in previous publications [4] (which included the Dutch population of the Registry), it can be assumed that the effect of the operative technique will reach in due time the level of statistical significance also in this population. Primarily due to homograft shortage, 149 patients received a bioprosthesis instead of a homograft for RVOT reconstruction. However, the durability of bioprosthesis implantation in the RVOT seems to be unacceptably low, with the hazard function for reoperation being almost an order of magnitude higher than with a homograft (Fig. 3).
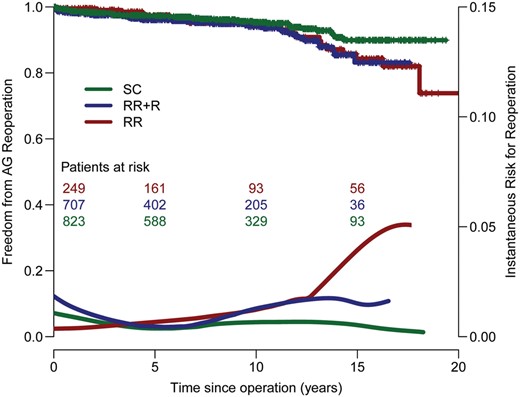
Freedom from autograft reoperation stratified by technique. SC: sub-coronary; RR + R: root replacement with additional root reinforcement; RR: root replacement without additional root reinforcement.
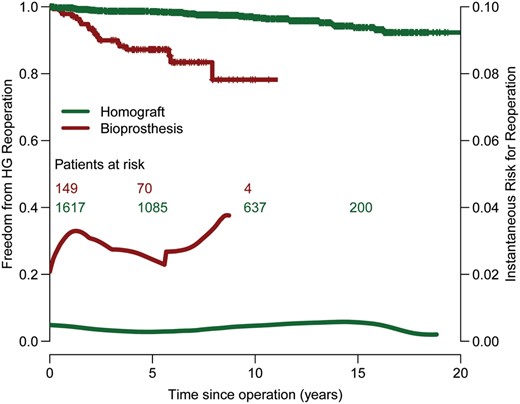
Freedom from homograft reoperation stratified by type of prosthesis.
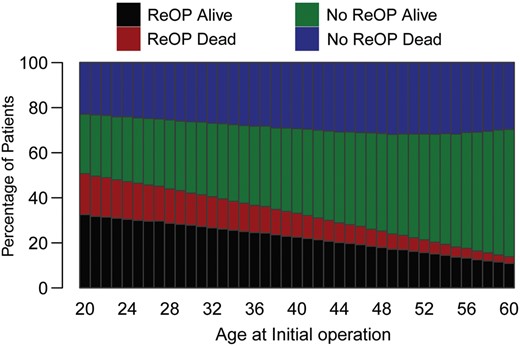
Projections for the need for a reoperation according to the age at the initial operation under a competing risk framework are presented in Fig. 4.
Homograft function concerning the development of pulmonary insufficiency and stenosis remained relatively constant; the probability of pulmonary insufficiency Grade I increased slightly over the years and also the pressure gradient over the right ventricular outflow tract increased slightly with time without however haemodynamic significance (Fig. 5). Aortic valve function was stable in the second decade, with a slight increase in aortic insufficiency Grade I (Fig. 6). Of all patients with known NYHA status at the latest follow-up, 88.9% had NYHA class I (NYHA class II: 9.1%; NYHA class: III 1.8%; NYHA class IV: 0.2%).
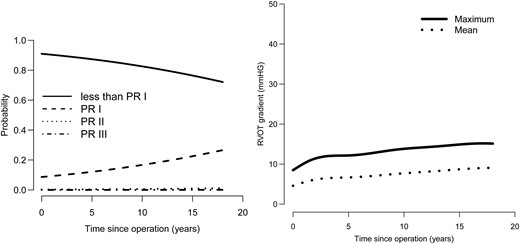
(Left) The time course of transvalvular mean and maximum homograft gradients. (Right) Longitudinal probability of being in each pulmonary regurgitation grade with time. PR: pulmonary regurgitation.
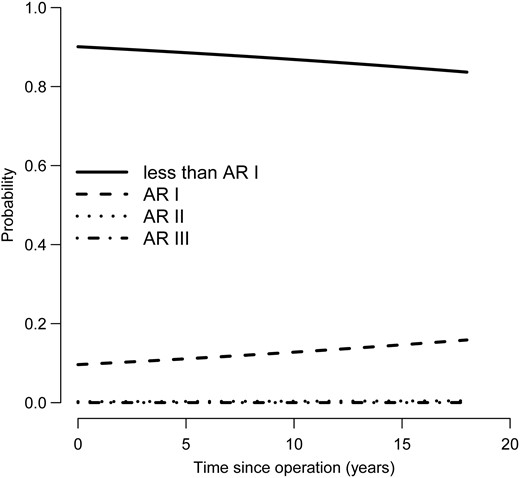
Longitudinal probability of being in each autograft regurgitation grade with time. AR: aortic regurgitation.
DISCUSSION
These updated registry results show that the favourable outcome after the Ross procedure continues with increase in the follow-up time now up to 24 years, with a considerable number of patients in the second decade after the operation.
After all improvements in valve surgery including mechanical valves with lower anticoagulation needs, bioprostheses with assumed increased durability and the advances in transcatheter aortic valve replacement as an option in the elderly, the choice for an aortic valve substitute in young patients remains difficult for both patient and surgeon. Mechanical valves still require lifelong anticoagulation therapy and may produce disturbing noise, while bioprostheses fail prematurely especially in young patients [3]. In the Ross procedure, the autologous living pulmonary valve is used for aortic valve replacement, thus potentially offering an extended durability and near-normal valve function [15]. Continuous follow-up with high patient numbers are necessary to evaluate this technique. We implemented the Ross Registry in 2001 covering now 14.133 patient × years with up to 24 years of follow-up. Although registry results have their own shortcomings, they provide a more general and real-world view on a specific operation.
With respect to the total number of aortic valve replacement procedures, the Ross operation was less frequently performed in only a small number of patients showing the high selection background. In two centres, the frequency of the Ross procedure was roughly 5% of the total aortic valve replacement volume and in the other six centres less than 2%. It remains unclear if other techniques like bioprostheses would have provided even better outcomes. To our knowledge, however, there is no report on such low freedom from reoperation (82.7%) with bioprostheses in these young patients after 15 years, knowing that the comparability lacks non-randomization [3]. Nevertheless, it remains speculative that the selection criteria (wish of the patient, pulmonary autograft integrity, aortic valve phenotype, ejection fraction etc.) would have an influence on the known premature degeneration process of bioprostheses in young patients. On the other hand, there is some evidence that the quality of life in young active patients with mechanical valves is inferior to that after a Ross operation [1]. Nevertheless, a randomized trial would be favourable. Another point why the Ross procedure is performed in few centres only is probably the considerable experience needed to obtain acceptable results. Furthermore, young patients receive bioprostheses with the perspective of getting a transcatheter redo after failure of the bioprosthesis; the results of this procedure however are not validated. Because there is a tremendous amount of open questions with the Ross procedure in particular, we carefully looked after each patient now over a time period of more than 20 years so that these data may serve as some kind of a benchmark in this highly selected group of patients.
The early hospital mortality rate was 1.1%, which is comparable with that of conventional aortic valve replacement. In these selected patients, operative mortality should be 0% for all types of aortic valve replacement techniques. Indeed, in the first author's series, there was no operative mortality in the latest 420 Ross patients. The sometimes reported objection against the Ross operation concerning high early mortality cannot be substantiated by this registry's results [17].
The late survival after the Ross procedure was similar for the age- and gender-matched general population. Nevertheless, there seems to arise some deviation between the survival curves of the normal population and the Ross patients later than 15 years of follow-up, which however until now does not reach the level of statistical significance.
However, it should be stated that morbid events in young patients such as the present cohort are rare events and the primary cause of mortality in the general population of that age are accidents and malignancies. Cardiac events in the matched general population are seldom. On the other hand, cardiac-related mortality accounts for about 50% of all deaths observed in the registry. Assuming that the Ross procedure cannot influence the incidence of accidents or malignancies, it is speculated that with time the observed deaths in the Registry will exceed those expected in the general population, and reach the level of statistical significance.
Nevertheless, as recently published, this long-term survival was statistically significantly better than that in patients with a mechanical valve [18]. Only anticoagulation self-management and optimal anticoagulation monitoring in patients with mechanical valves seems to result in similar good survival compared with Ross patients at least during 7 years postoperatively [19].
In the sub-coronary autograft technique, the risk for reoperation seems to be linear with time resulting in a 86.7% freedom from autograft reoperation at 15 years and 92.3% freedom from homograft reoperation, whereas the overall freedom from reoperation was 82.7% after 15 years. Thus, there is a constant rate of roughly 1% per patient-year for the reoperation. This risk is not trivial and is thoroughly communicated to the patients prior to the operation.
The RR + R technique is a very heterogeneous group which essentially includes all patients who receive some kind of prophylactic or therapeutic reinforcement during the Ross procedure. This technique is a relatively young technique. Although we have observed some increase in the risk of reoperation, we do not observe the accelerated pattern of failure in the second decade as with the RR technique. Longer follow-up time is required to answer whether the RR + R technique can reduce the incidence of reoperation or it just postpones an increase in the reoperation hazard by some time frame. However, it must be considered that four centres with 270 patients were eliminated from the registry and we cannot exclude the possibility that the eliminated centres may have some influence on this issue or that RR in general will fail at a faster rate.
Bioprostheses in the RVOT as an alternative to homografts showed less favourable results, although in almost all cases a stentless valve was used. The influence of the size of the heterograft and the different implications of stentless heterograft conduits and stented valve conduits could not be evaluated in this study.
During the time frame of the registry of over 24 years, alternative techniques have developed such as aortic valve repair and use of bioprostheses in younger patients. Several studies even in very experienced centres have shown that, after aortic valve repair, the risk for reoperation can be higher than 1% per patient-year, even reaching up to 5% per patient × year [20]. Also aortic valve repair in patients with a significant stenotic component or calcification cannot be performed. On the other side, well-designed studies and retrospective reports still document a high deterioration rate for even modern bioprostheses in young patients [3]. Nevertheless, it should be taken into consideration that alternatives like repair and bioprostheses in patients of younger age are less complex operations. The outcome over 20 years, however, remains to be elucidated. The longitudinal modelling of functional valve characteristics revealed a low (<5%) probability of a patient with a higher autograft regurgitation throughout the first and first half of the second decade (Fig. 6). Also the homograft function regarding development of insufficiency or stenosis was relatively constant up to 17 years after the operation. Together, these excellent haemodynamic results may be one reason for the excellent long-term survival of these patients and the excellent functional capacity with 88.9% of patients in NYHA class I in long-term follow-up.
Given our understanding thus far of the risk for autograft and homograft reoperation, we attempted to estimate the probability of experiencing at least one reoperation till the age of 75 according to the age at initial operation as shown in Fig. 4. The risk rate for a patient being 45 years at the initial operation to be alive at 75 years and having had a reoperation was roughly 19% and without reoperation 41%. The risk for reoperation up to the age of 75 years declines with increase in age at the initial operation.
From four centres, 270 patients were eliminated, leaving 1797 patients of the registry for the analysis. These centres did not provide follow-up data. The reason for that is unclear and may range from absence of manpower to unfavourable outcome, vanishing interest or risk avoidance [21]. Thus, the comparison of actual data with previous Ross reports is limited, although we did not find a significant influence after eliminating the 270 patients from the Ross Registry.
Despite all shortcomings of the registry, we must thank the participants for their unique efforts in collecting the Ross data prospectively over 13 years and retrospectively over 11 years on a voluntary, non-industry-financed basis with 95% follow-up completeness, which is a unique worldwide achievement, adding to the excellent results in single experienced centres [22–24] of promoted experts on this field, commenting on the underuse of Ross operation as a lost opportunity [25].
In conclusion, for the young active patient, the autograft principle results in postoperative long-term survival comparable with that of the age- and gender-matched general population with reoperation rates in the 1% per patient × year range. The functional results were encouraging. Altogether, the autograft principle for the treatment of aortic valve disease in young, active patients, who wish to avoid the shortcoming of conventional prostheses, should be strongly considered.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
ACKNOWLEDGEMENTS
The authors are thankful to Michael Diwoky and Tobias Frin for their invaluable support in the management and analysis of the data and their assistance in preparing our manuscript and figures.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr G. Luciani(Verona, Italy): This is yet another very important contribution of the German Ross registry, which set out about seven years ago to provide evidence-based information regarding recommendations for aortic valve replacement with a pulmonary autograft in the young. Obviously we understand and appreciate the tremendous effort that is required to start and maintain such a registry. Some of us have tried the same method in our own countries and we understand it very clearly. This leads to my very first question, which is related to the methodology of the study and of this presentation.
Now, I have noticed in the last few years that the registry, which set out as a Dutch-German or German-Dutch Ross registry, has dropped the Dutch arm and, as very honestly stated by Professor Sievers, Dr Charitos and the co-workers in the methods of this manuscript, four, I believe, of the original 11 units in Germany have also been permanently deleted, comprising I believe around 270 patients from the original database. My first question is, has this to do exclusively with a lack of reliable follow-up information, which I believe may be a problem with these large registries, or is there an issue in these units, starting with the Dutch units and continuing on with some of the German units, of divergent clinical outcomes observed that has led, and this may reasonably happen, these units to change their policy as compared with regards to the Ross procedure?
The second question is related to the results, and this is also very interesting. There is a lot of information in the presentation, as you also appreciate, but the one that struck me the most is that the so-called reinforced root technique, which had shown to be protective from autograft reoperation in all the series of publications from the same registry, in this very last presentation has not shown to be so. So the two DK or Kaplan-Meier survival curves of the supported and unsupported are overlapping now.
Now, my question to Dr Charitos is, do you believe this is only inherent with the change of the denominator because of the dropout of the four German cardiac units or do you extrapolate, in spite of the beautiful statistical simulation you did, that all Ross roots regardless of support and technical modifications later than the second decade are doomed to fail after the second decade of follow-up?
The very last question is, the overall freedom from reoperation, and we have to sum up autograft and homograft in this case, is starting to resemble, unfortunately, more and more so, the freedom from reoperation after elective aortic valve repair in the young having obviously only aortic insufficiency or in the older patients also in your registry the freedom from replacement in a patient carrying a bioprosthetic valve in the older patients. So the question obviously you imagine is, where do we draw the line of contraindications to the Ross procedure in patients younger than 40 as when compared to valve repair and in patients older than 50 patients as when compared to bioprosthetic aortic valve replacement?
Once again, I thank the scientific committee for this opportunity and congratulations on an outstanding presentation.
Dr Charitos: Regarding your first question, there is a population change between this presentation and our last presentation published in 2012. The main reason why the Dutch population was not included in this update is purely bureaucratic. It has to do with revising institutional regulations regarding the transfer of data internationally. We were unable to get this data from our colleagues in Erasmus in time in order to include them in this work. They will be included in future works, but unfortunately, due to these revised regulations, some bureaucratic procedures are necessary before patient data can be transferred to another country.
As you specify and as we write also in our manuscript, we have dropped some German centres. This has to do with a lack of follow-up. These centres haven't provided follow-up to the registry for some years, and they are less willing to perform follow-up in these patients. There are not many patients. We wish we could include them in the follow-up, but we cannot have these patients reduce the overall follow-up completeness, especially if the centres do not have an interest anymore in performing follow-up in these patients.
It was not an easy decision to remove patients from a registry. Fortunately There are not many patients, but this is something that we have to live with and this is something that we specifically state in the manuscript. Whether this is a limitation or not, I am not sure. At least for the first decade and because of the large number of patients in the Registry, the removal of these patients shouldn't have a big effect in changing any conclusions.
Your second question was about the root reinforcement technique. As we have specified, this is a very homogeneous group. It is essentially all patients that receive some kind of prophylactic or therapeutic reinforcement during the Ross procedure. This is a relatively young technique. We don't observe this accelerated pattern of failure in the second decade. We have observed some increase in the risk of reoperation. We have to see how this will develop in the next years. This is too early to talk about the second decade in this, again, rather inhomogeneous collection of patients.
And your third question was?
Dr Luciani: The overall freedom from any reoperation is about 82.7% I think in the second decade. So this resembles, unfortunately, some of the bad outcomes after elective valve repair in the presence of pure AI or AVR with a bioprosthesis.
Dr Charitos: This is also an important comment, and this is also a population change in the last couple of years. We don't see the Ross technique as a competitor of the aortic valve repair anymore; at least this has changed in the last seven years. Usually in most centres a repair is preferred to a Ross; Ross is an aortic valve replacement procedure and as such, it is a second choice.
Author notes
Presented at the 28th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Milan, Italy, 11–15 October 2014.




