-
PDF
- Split View
-
Views
-
Cite
Cite
Brendon M. Stiles, Gregory G. Salzler, Abu Nasar, Subroto Paul, Paul C. Lee, Jeffrey L. Port, Nasser K. Altorki, Clinical predictors of early cancer-related mortality following neoadjuvant therapy and oesophagectomy, European Journal of Cardio-Thoracic Surgery, Volume 48, Issue 3, September 2015, Pages 455–460, https://doi.org/10.1093/ejcts/ezu479
Close - Share Icon Share
Abstract
Although oesophagectomy can be curative for patients with oesophageal cancer (OC), it may be associated with high morbidity and decreased quality of life. Identifying risk factors for early systemic progression or death after oesophagectomy may help to guide treatment choices in at-risk patients.
Patients undergoing oesophagectomy following neoadjuvant therapy for OC (November 1987 to January 2013) were reviewed, excluding deaths ≤3 months. Univariate predictors of death ≤1 year of operation were explored by logistic regression. Significant predictors (P ≤ 0.10) were included in a multivariate model. A risk factor index was created based on the number of significant risk factors in individual patients.
Of 581 patients, 238 had neoadjuvant chemotherapy or chemotherapy and radiation followed by oesophagectomy. Of these, 15% (n = 36) died ≤1 year following oesophagectomy and 69% of those from documented cancer recurrence. Clinical predictors of death ≤1 year by multivariate analysis included performance status >0 (HR 2.19; CI 1.02–4.69), poor (G3) tumour differentiation (HR 2.67; CI 1.14–6.21) and lack of clinical response (no response or progression versus complete and partial response) to neoadjuvant therapy (HR 2.77; CI 1.07–7.15). For patients with all factors evaluable (n = 167), variables were summed to derive a cumulative risk factor index, 0–3. An increased risk factor index (≥2) was highly associated with increased risk of death ≤1 year postoperatively (HR 4.84; CI 1.93–12.16), as well as with poor overall survival.
Clinically defined risk factors that predict early mortality following oesophagectomy include performance status, poor tumour differentiation and clinical response. In patients with at least two of these risk factors, 29% will die within 1 year of surgery. These patients should be identified and individual consideration given to less morbid surgical strategies or to alternative treatments.
INTRODUCTION
Although oesophagectomy following neoadjuvant treatment can be curative for oesophageal cancer (OC) patients, multimodality treatment may come at the expense of significant treatment-related morbidity and a decreased quality of life. Additionally, it is not unusual for patients presenting with locally advanced OC to have early cancer recurrence following treatment [1–4]. Disease progression in this setting typically leads to death. Death within 1 year should be considered a treatment failure as this would approximate the natural history of the disease. Consideration should therefore be given to less morbid treatment strategies in OC patients who are unlikely to survive more than 1 year after surgery.
Unfortunately, it is difficult to predict which OC patients will suffer early recurrence or death. A number of pathological factors have been identified, which are associated with poor long-term survival [5]. Many of these factors form the basis of the TNM staging system [6]. Similarly, several series have reported factors associated with early (less than 12 months) recurrence and death after oesophagectomy [1–4]. These studies included patients with early stage disease and those with and without neoadjuvant therapy. Not surprisingly, prognostic factors in these analyses were heavily weighted towards pathological variables such as involved margins, lymph node classification and final pathological stage. However, the question of whether or not to operate is less relevant in early stage disease for which surgical resection is the standard of care. For locally advanced disease, multiple treatment modalities are frequently integrated into the treatment plan, making the question more relevant. We therefore sought to examine clinically useful predictors of early death in a more homogenous population of patients undergoing neoadjuvant therapy followed by surgery for OC. Identifying clinical risk factors for early disease progression or death after oesophagectomy may help to guide subsequent treatment choices in a similar patient population. We focused on the time period from 3 to 12 months following surgery in order to exclude early deaths, which are typically treatment-related [7].
MATERIALS AND METHODS
Study design, preoperative evaluation and patient follow-up
We conducted an institutional review board-approved review of a prospectively assembled thoracic surgery database (November 1987 to January 13). Patient consent was waived. Patients were considered eligible for this review if they had carcinoma of the oesophagus or gastroesophageal junction, treated with neoadjuvant chemotherapy and/or radiation therapy followed by oesophagectomy. Patients who died in the hospital or less than 3 months following resection were excluded. Demographics, staging, surgical and survival data were reviewed. All data were collected and entered into a prospective database, and updated at regular intervals.
Following hospital discharge, patients were seen at intervals of 3 months for the first 2 years and every 6 months thereafter. Patients from distant geographic regions were followed by contacting their local physician as well as direct patient contact. Computed tomography of the chest and upper abdomen was obtained every 6 months for 2 years and every year thereafter. Other studies such as endoscopy and PET scanning were done when recurrence was suspected.
Statistical analysis
Descriptive statistics (including frequency, percent, mean, median, standard deviation and range) are presented for demographic and clinical factors. Variables of interest were examined by the χ2 test or Fisher's exact test for categorical variables and the two-sample t-test or Wilcoxon rank-sum test for continuous variables. For analysis of tumour differentiation, patients with well or moderately differentiated tumours (G1 and G2) were compared against those with poorly differentiated tumours (G3).
Univariate predictors of death ≤1 year were explored by logistic regression. Factors identified at P < 0.20 by univariate analysis were selected for inclusion in a Cox regression model to assess the independent effect of these variables on early death. Potential collinearity between univariate predictors was assessed by the Pearson (or Spearman-rank) correlation coefficient (for continuous predictors) or the kappa statistic (for categorical predictors) prior to the final specification of the multivariate model. If two variables were highly correlated, only one of the variables was evaluated in the multivariate model.
Overall survival (OS) for the cohort (from the date of surgery) was evaluated by Kaplan–Meier survival analysis. The log-rank test was employed to compare OS between different cohorts of patients. All P-values are two-sided with statistical significance evaluated at the 0.05 alpha level. Ninety-five percent confidence intervals (95% CI) for adjusted odds ratios (from the multivariate model) and for Kaplan–Meier survival probability estimates were calculated to assess the precision of the obtained estimates. All analyses were performed in SAS Version 9.2 (SAS Institute, Inc., Cary, NC, USA), SPSS Version 18.0 (SPSS, Inc., Chicago, IL, USA) and STATA Version 11.0 (StataCorp, College Station, TX, USA).
RESULTS
Demographics
Among 581 patients operated on at our institution over the time period (November 1987 to January 13), 253 patients (44%) received neoadjuvant chemotherapy alone or chemoradiation (CRT) prior to oesophagectomy. Among these patients, 5 hospital deaths (2.0%) and 10 additional deaths within 3 months (4.0%) were excluded from the analysis. The remaining 238 patients served as the cohort for this study. The majority of these patients (80%, n = 191) were treated after 2001. Characteristics of the patients are reported in Table 1. Most were male (n = 193, 81%), had adenocarcinoma (n = 165, 69%), had T3 or T4 tumours (82%), received neoadjuvant chemotherapy (n = 188, 79%) as opposed to CRT and underwent transthoracic en bloc resection with two- or three-field lymphadenectomy (n = 202, 86%). Characterization of clinical response to induction therapy was documented in 167 patients (70%). In the remaining patients, we could not accurately infer this information from the historical charts. The group with the clinical response assessed was representative of the group as a whole with no significant differences in clinical or pathological factors between the groups. The majority of entire cohort of patients (n = 223, 94%) had R0 resections, whereas 9 patients (3.8%) had R1 resection and 6 (2.5%) had R2 resections. Final pathological stages of all 238 patients are listed in Table 1.
| Oesophageal cancer patients undergoing neoadjuvant therapy followed by surgical resection . | |
|---|---|
| Median age | 62 (56–74) |
| Gender | |
| Female | 45 (19%) |
| Male | 193 (81%) |
| Performance status | |
| 0 | 165 (69%) |
| 1, 2 | 73 (31%) |
| Histology | |
| Adenocarcinoma | 165 (69%) |
| Squamous cell | 61 (26%) |
| Other | 4 (2%) |
| Differentiation | |
| Well-moderate | 120 (50%) |
| Poor | 118 (50% |
| Clinical T classification | |
| T1/T2 | 39 (16%) |
| T3/T4 | 194 (82%) |
| Missing | 5 (2%) |
| Neoadjuvant therapy | |
| Chemotherapy | 188 (79%) |
| Chemoradiation | 50 (31%) |
| Clinical response (evaluated in 167) | |
| Progression/no or minimal | 50 (30%) |
| Partial or complete | 117 (70%) |
| Surgical approach | |
| Transhiatal | 23 (10%) |
| Transthoracic non-en bloc | 11 (5%) |
| En bloc, two-field dissection | 72 (30%) |
| En bloc, three-field dissection | 132 (55%) |
| Pathological stage | |
| 0 (CPR) | 28 (12%) |
| I | 28 (12%) |
| II | 61 (25%) |
| III | 117 (49%) |
| IV | 4 (2%) |
| ‘R’ status | |
| R0 | 223 (94%) |
| R1 | 15 (65) |
| Oesophageal cancer patients undergoing neoadjuvant therapy followed by surgical resection . | |
|---|---|
| Median age | 62 (56–74) |
| Gender | |
| Female | 45 (19%) |
| Male | 193 (81%) |
| Performance status | |
| 0 | 165 (69%) |
| 1, 2 | 73 (31%) |
| Histology | |
| Adenocarcinoma | 165 (69%) |
| Squamous cell | 61 (26%) |
| Other | 4 (2%) |
| Differentiation | |
| Well-moderate | 120 (50%) |
| Poor | 118 (50% |
| Clinical T classification | |
| T1/T2 | 39 (16%) |
| T3/T4 | 194 (82%) |
| Missing | 5 (2%) |
| Neoadjuvant therapy | |
| Chemotherapy | 188 (79%) |
| Chemoradiation | 50 (31%) |
| Clinical response (evaluated in 167) | |
| Progression/no or minimal | 50 (30%) |
| Partial or complete | 117 (70%) |
| Surgical approach | |
| Transhiatal | 23 (10%) |
| Transthoracic non-en bloc | 11 (5%) |
| En bloc, two-field dissection | 72 (30%) |
| En bloc, three-field dissection | 132 (55%) |
| Pathological stage | |
| 0 (CPR) | 28 (12%) |
| I | 28 (12%) |
| II | 61 (25%) |
| III | 117 (49%) |
| IV | 4 (2%) |
| ‘R’ status | |
| R0 | 223 (94%) |
| R1 | 15 (65) |
Patients undergoing neoadjuvant therapy followed by oesophagectomy, excluding deaths within 3 months of surgery.
| Oesophageal cancer patients undergoing neoadjuvant therapy followed by surgical resection . | |
|---|---|
| Median age | 62 (56–74) |
| Gender | |
| Female | 45 (19%) |
| Male | 193 (81%) |
| Performance status | |
| 0 | 165 (69%) |
| 1, 2 | 73 (31%) |
| Histology | |
| Adenocarcinoma | 165 (69%) |
| Squamous cell | 61 (26%) |
| Other | 4 (2%) |
| Differentiation | |
| Well-moderate | 120 (50%) |
| Poor | 118 (50% |
| Clinical T classification | |
| T1/T2 | 39 (16%) |
| T3/T4 | 194 (82%) |
| Missing | 5 (2%) |
| Neoadjuvant therapy | |
| Chemotherapy | 188 (79%) |
| Chemoradiation | 50 (31%) |
| Clinical response (evaluated in 167) | |
| Progression/no or minimal | 50 (30%) |
| Partial or complete | 117 (70%) |
| Surgical approach | |
| Transhiatal | 23 (10%) |
| Transthoracic non-en bloc | 11 (5%) |
| En bloc, two-field dissection | 72 (30%) |
| En bloc, three-field dissection | 132 (55%) |
| Pathological stage | |
| 0 (CPR) | 28 (12%) |
| I | 28 (12%) |
| II | 61 (25%) |
| III | 117 (49%) |
| IV | 4 (2%) |
| ‘R’ status | |
| R0 | 223 (94%) |
| R1 | 15 (65) |
| Oesophageal cancer patients undergoing neoadjuvant therapy followed by surgical resection . | |
|---|---|
| Median age | 62 (56–74) |
| Gender | |
| Female | 45 (19%) |
| Male | 193 (81%) |
| Performance status | |
| 0 | 165 (69%) |
| 1, 2 | 73 (31%) |
| Histology | |
| Adenocarcinoma | 165 (69%) |
| Squamous cell | 61 (26%) |
| Other | 4 (2%) |
| Differentiation | |
| Well-moderate | 120 (50%) |
| Poor | 118 (50% |
| Clinical T classification | |
| T1/T2 | 39 (16%) |
| T3/T4 | 194 (82%) |
| Missing | 5 (2%) |
| Neoadjuvant therapy | |
| Chemotherapy | 188 (79%) |
| Chemoradiation | 50 (31%) |
| Clinical response (evaluated in 167) | |
| Progression/no or minimal | 50 (30%) |
| Partial or complete | 117 (70%) |
| Surgical approach | |
| Transhiatal | 23 (10%) |
| Transthoracic non-en bloc | 11 (5%) |
| En bloc, two-field dissection | 72 (30%) |
| En bloc, three-field dissection | 132 (55%) |
| Pathological stage | |
| 0 (CPR) | 28 (12%) |
| I | 28 (12%) |
| II | 61 (25%) |
| III | 117 (49%) |
| IV | 4 (2%) |
| ‘R’ status | |
| R0 | 223 (94%) |
| R1 | 15 (65) |
Patients undergoing neoadjuvant therapy followed by oesophagectomy, excluding deaths within 3 months of surgery.
Early death following oesophagectomy
Excluding the early treatment-related deaths, another 36 patients (15%) died within 1 year of surgical resection. Characteristics of these patients versus those who did not expire in this time period are reported in Table 2. Patients who died early were more likely to have performance status of 1 or 2 (P = 0.006), more often had high grade, poorly differentiated tumours (P = 0.001), were more likely to have a poor clinical response to therapy (P = 0.012), were less likely to have R0 resection (P = 0.014) and had higher pathological stage (P = 0.001). Univariate clinical predictors of death between 3 and 12 months were explored (Table 3). Age, gender and histological subtype were not predictive of outcome, whereas performance status greater than zero (HR 2.67, CI 1.30–5.51, P = 0.008), poor tumour differentiation (HR 3.66, CI 1.63–8.17, P = 0.002), induction chemotherapy (HR 5.30, CI 1.23–22.9) as opposed to induction CRT alone and lack of response (including disease progression, stable disease and minimal response) to induction therapy (HR 3.04, CI 1.24–7.47, P = 0.015) all predicted early death with a P-value of <0.20. Two distinct multivariate analyses were performed. The first included all 238 patients, but did not include the variable ‘clinical response to induction therapy’ as it was not evaluated in all patients. In this model, multivariate predictors of death within 1 year included performance status greater than zero (HR 2.19, CI 1.02–4.69, P = 0.044) and poor tumour differentiation (HR 2.67, CI 1.14–6.21, P = 0.023). In the second model, only patients in which clinical response to induction therapy was recorded (n = 167) were evaluated. In this model, minimal or no clinical response to induction therapy was predictive of death within 1 year (HR 2.77, CI 1.07–7.15, P = 0.035), as was poor tumour differentiation (HR 3.01, CI 1.08–8.38, P = 0.035), whereas poor performance status (HR 1.85, CI 0.70–4.88, P = 0.217) failed to reach statistical significance.
Comparison of patients who died within 1 year versus those surviving greater than 1 year
| . | Dead 3–12 months (N = 36) . | Survived >1 year (N = 202) . | P-value . |
|---|---|---|---|
| Median age [in years, (IQR)] | 62 (57–69) | 62 (55–68) | 0.659 |
| Performance status | |||
| 0 | 18 (11%) | 147 (89%) | 0.006 |
| 1, 2 | 18 (25%) | 55 (75%) | |
| Tumour differentiation | |||
| Low to moderate | 9 (8%) | 111 (92%) | 0.001 |
| Poor/high grade | 27 (23%) | 91 (77%) | |
| Clinical T classification | |||
| Tx, T1 or T2 | 3 (8%) | 36 (92%) | 0.142 |
| T3 or T4 | 33 (17%) | 161 (83%) | |
| Induction | |||
| Chemo | 34 (18%) | 154 (82%) | 0.013 |
| Chemo-RT | 2 (4%) | 48 (96%) | |
| Clinical response | |||
| Minimal/stable/progression | 12 (24%) | 38 (76%) | 0.012 |
| Partial/complete | 11 (9%) | 106 (91%) | |
| Missing or no data | N = 13 | N = 58 | |
| ‘R’ status | |||
| R0 | 30 (13%) | 193 (87%) | 0.014 |
| R1 | 6 (40%) | 9 (60%) | |
| Path stage | |||
| 0 (CPR) | 0 (0%) | 28 (100%) | 0.001 |
| I | 2 (7%) | 26 (93%) | |
| II | 5 (8%) | 56 (92%) | |
| III | 27 (23%) | 90 (77%) | |
| IV | 2 (50%) | 2 (50%) | |
| . | Dead 3–12 months (N = 36) . | Survived >1 year (N = 202) . | P-value . |
|---|---|---|---|
| Median age [in years, (IQR)] | 62 (57–69) | 62 (55–68) | 0.659 |
| Performance status | |||
| 0 | 18 (11%) | 147 (89%) | 0.006 |
| 1, 2 | 18 (25%) | 55 (75%) | |
| Tumour differentiation | |||
| Low to moderate | 9 (8%) | 111 (92%) | 0.001 |
| Poor/high grade | 27 (23%) | 91 (77%) | |
| Clinical T classification | |||
| Tx, T1 or T2 | 3 (8%) | 36 (92%) | 0.142 |
| T3 or T4 | 33 (17%) | 161 (83%) | |
| Induction | |||
| Chemo | 34 (18%) | 154 (82%) | 0.013 |
| Chemo-RT | 2 (4%) | 48 (96%) | |
| Clinical response | |||
| Minimal/stable/progression | 12 (24%) | 38 (76%) | 0.012 |
| Partial/complete | 11 (9%) | 106 (91%) | |
| Missing or no data | N = 13 | N = 58 | |
| ‘R’ status | |||
| R0 | 30 (13%) | 193 (87%) | 0.014 |
| R1 | 6 (40%) | 9 (60%) | |
| Path stage | |||
| 0 (CPR) | 0 (0%) | 28 (100%) | 0.001 |
| I | 2 (7%) | 26 (93%) | |
| II | 5 (8%) | 56 (92%) | |
| III | 27 (23%) | 90 (77%) | |
| IV | 2 (50%) | 2 (50%) | |
Comparison of patients who died within 1 year versus those surviving greater than 1 year
| . | Dead 3–12 months (N = 36) . | Survived >1 year (N = 202) . | P-value . |
|---|---|---|---|
| Median age [in years, (IQR)] | 62 (57–69) | 62 (55–68) | 0.659 |
| Performance status | |||
| 0 | 18 (11%) | 147 (89%) | 0.006 |
| 1, 2 | 18 (25%) | 55 (75%) | |
| Tumour differentiation | |||
| Low to moderate | 9 (8%) | 111 (92%) | 0.001 |
| Poor/high grade | 27 (23%) | 91 (77%) | |
| Clinical T classification | |||
| Tx, T1 or T2 | 3 (8%) | 36 (92%) | 0.142 |
| T3 or T4 | 33 (17%) | 161 (83%) | |
| Induction | |||
| Chemo | 34 (18%) | 154 (82%) | 0.013 |
| Chemo-RT | 2 (4%) | 48 (96%) | |
| Clinical response | |||
| Minimal/stable/progression | 12 (24%) | 38 (76%) | 0.012 |
| Partial/complete | 11 (9%) | 106 (91%) | |
| Missing or no data | N = 13 | N = 58 | |
| ‘R’ status | |||
| R0 | 30 (13%) | 193 (87%) | 0.014 |
| R1 | 6 (40%) | 9 (60%) | |
| Path stage | |||
| 0 (CPR) | 0 (0%) | 28 (100%) | 0.001 |
| I | 2 (7%) | 26 (93%) | |
| II | 5 (8%) | 56 (92%) | |
| III | 27 (23%) | 90 (77%) | |
| IV | 2 (50%) | 2 (50%) | |
| . | Dead 3–12 months (N = 36) . | Survived >1 year (N = 202) . | P-value . |
|---|---|---|---|
| Median age [in years, (IQR)] | 62 (57–69) | 62 (55–68) | 0.659 |
| Performance status | |||
| 0 | 18 (11%) | 147 (89%) | 0.006 |
| 1, 2 | 18 (25%) | 55 (75%) | |
| Tumour differentiation | |||
| Low to moderate | 9 (8%) | 111 (92%) | 0.001 |
| Poor/high grade | 27 (23%) | 91 (77%) | |
| Clinical T classification | |||
| Tx, T1 or T2 | 3 (8%) | 36 (92%) | 0.142 |
| T3 or T4 | 33 (17%) | 161 (83%) | |
| Induction | |||
| Chemo | 34 (18%) | 154 (82%) | 0.013 |
| Chemo-RT | 2 (4%) | 48 (96%) | |
| Clinical response | |||
| Minimal/stable/progression | 12 (24%) | 38 (76%) | 0.012 |
| Partial/complete | 11 (9%) | 106 (91%) | |
| Missing or no data | N = 13 | N = 58 | |
| ‘R’ status | |||
| R0 | 30 (13%) | 193 (87%) | 0.014 |
| R1 | 6 (40%) | 9 (60%) | |
| Path stage | |||
| 0 (CPR) | 0 (0%) | 28 (100%) | 0.001 |
| I | 2 (7%) | 26 (93%) | |
| II | 5 (8%) | 56 (92%) | |
| III | 27 (23%) | 90 (77%) | |
| IV | 2 (50%) | 2 (50%) | |
| Independents variables . | Univariate predictors . | Multivariate predictors* . | ||
|---|---|---|---|---|
| HR (95% CI), N = 513 . | P-value . | HR (95% CI), N = 471 . | P-value . | |
| Age group | ||||
| 65 years and below [n = 156] | Reference: 1.00 | |||
| Above 65 years [n = 82] | 0.94 (0.45–2.00) | 0.878 | ||
| Performance status (PS) | ||||
| PS = 0 [n = 165] | Reference: 1.00 | |||
| PS greater than zero [n = 73] | 2.67 (1.30–5.51) | 0.008 | 2.19 (1.02–4.69) | 0.044 |
| Histology grade | ||||
| Low-grade tumours [n = 120] | Reference: 1.00 | |||
| Poorly differentiated [n = 118] | 3.66 (1.63–8.17) | 0.002 | 2.67 (1.14–6.21) | 0.023 |
| Clinic ‘T’ stage | ||||
| Clinical Tx, Tis, T1, T2 [n = 39] | Reference: 1.00 | |||
| Clinical T3 and T4 [n = 194] | 2.46 (0.72–8.46) | 0.153 | 2.38 (0.66–8.65) | 0.186 |
| Histology group | ||||
| Adeno [n = 165] | Reference: 1.00 | |||
| Squamous [n = 61] | 1.23 (0.57–2.68) | 0.600 | ||
| Neoadjuvant therapy | ||||
| Chemotherapy [n = 188] | Reference: 1.00 | |||
| Chemoradiation [n = 50] | 5.30 (1.23–22.87) | 0.025 | 0.27 (0.58–1.21) | 0.086 |
| ‘R’ status | ||||
| R0 [n = 223] | Reference: 1.00 | |||
| R1/R2 [n = 15] | 4.29 (1.42–12.91) | 0.010 | 3.09 (0.95–10.06) | 0.061 |
| Clinical response | Only evaluated in 167 patients and evaluated in a distinct multivariate analysis | |||
| Partial/complete [n = 117] | Reference: 1.00 | |||
| Minimal/stable/progression [n = 50] | 3.04 (1.24–7.47) | 0.015 | 2.77 (1.07–7.15) | 0.035 |
| Independents variables . | Univariate predictors . | Multivariate predictors* . | ||
|---|---|---|---|---|
| HR (95% CI), N = 513 . | P-value . | HR (95% CI), N = 471 . | P-value . | |
| Age group | ||||
| 65 years and below [n = 156] | Reference: 1.00 | |||
| Above 65 years [n = 82] | 0.94 (0.45–2.00) | 0.878 | ||
| Performance status (PS) | ||||
| PS = 0 [n = 165] | Reference: 1.00 | |||
| PS greater than zero [n = 73] | 2.67 (1.30–5.51) | 0.008 | 2.19 (1.02–4.69) | 0.044 |
| Histology grade | ||||
| Low-grade tumours [n = 120] | Reference: 1.00 | |||
| Poorly differentiated [n = 118] | 3.66 (1.63–8.17) | 0.002 | 2.67 (1.14–6.21) | 0.023 |
| Clinic ‘T’ stage | ||||
| Clinical Tx, Tis, T1, T2 [n = 39] | Reference: 1.00 | |||
| Clinical T3 and T4 [n = 194] | 2.46 (0.72–8.46) | 0.153 | 2.38 (0.66–8.65) | 0.186 |
| Histology group | ||||
| Adeno [n = 165] | Reference: 1.00 | |||
| Squamous [n = 61] | 1.23 (0.57–2.68) | 0.600 | ||
| Neoadjuvant therapy | ||||
| Chemotherapy [n = 188] | Reference: 1.00 | |||
| Chemoradiation [n = 50] | 5.30 (1.23–22.87) | 0.025 | 0.27 (0.58–1.21) | 0.086 |
| ‘R’ status | ||||
| R0 [n = 223] | Reference: 1.00 | |||
| R1/R2 [n = 15] | 4.29 (1.42–12.91) | 0.010 | 3.09 (0.95–10.06) | 0.061 |
| Clinical response | Only evaluated in 167 patients and evaluated in a distinct multivariate analysis | |||
| Partial/complete [n = 117] | Reference: 1.00 | |||
| Minimal/stable/progression [n = 50] | 3.04 (1.24–7.47) | 0.015 | 2.77 (1.07–7.15) | 0.035 |
*Only variables with a P-value of <0.20 were included in the multivariable analysis.
| Independents variables . | Univariate predictors . | Multivariate predictors* . | ||
|---|---|---|---|---|
| HR (95% CI), N = 513 . | P-value . | HR (95% CI), N = 471 . | P-value . | |
| Age group | ||||
| 65 years and below [n = 156] | Reference: 1.00 | |||
| Above 65 years [n = 82] | 0.94 (0.45–2.00) | 0.878 | ||
| Performance status (PS) | ||||
| PS = 0 [n = 165] | Reference: 1.00 | |||
| PS greater than zero [n = 73] | 2.67 (1.30–5.51) | 0.008 | 2.19 (1.02–4.69) | 0.044 |
| Histology grade | ||||
| Low-grade tumours [n = 120] | Reference: 1.00 | |||
| Poorly differentiated [n = 118] | 3.66 (1.63–8.17) | 0.002 | 2.67 (1.14–6.21) | 0.023 |
| Clinic ‘T’ stage | ||||
| Clinical Tx, Tis, T1, T2 [n = 39] | Reference: 1.00 | |||
| Clinical T3 and T4 [n = 194] | 2.46 (0.72–8.46) | 0.153 | 2.38 (0.66–8.65) | 0.186 |
| Histology group | ||||
| Adeno [n = 165] | Reference: 1.00 | |||
| Squamous [n = 61] | 1.23 (0.57–2.68) | 0.600 | ||
| Neoadjuvant therapy | ||||
| Chemotherapy [n = 188] | Reference: 1.00 | |||
| Chemoradiation [n = 50] | 5.30 (1.23–22.87) | 0.025 | 0.27 (0.58–1.21) | 0.086 |
| ‘R’ status | ||||
| R0 [n = 223] | Reference: 1.00 | |||
| R1/R2 [n = 15] | 4.29 (1.42–12.91) | 0.010 | 3.09 (0.95–10.06) | 0.061 |
| Clinical response | Only evaluated in 167 patients and evaluated in a distinct multivariate analysis | |||
| Partial/complete [n = 117] | Reference: 1.00 | |||
| Minimal/stable/progression [n = 50] | 3.04 (1.24–7.47) | 0.015 | 2.77 (1.07–7.15) | 0.035 |
| Independents variables . | Univariate predictors . | Multivariate predictors* . | ||
|---|---|---|---|---|
| HR (95% CI), N = 513 . | P-value . | HR (95% CI), N = 471 . | P-value . | |
| Age group | ||||
| 65 years and below [n = 156] | Reference: 1.00 | |||
| Above 65 years [n = 82] | 0.94 (0.45–2.00) | 0.878 | ||
| Performance status (PS) | ||||
| PS = 0 [n = 165] | Reference: 1.00 | |||
| PS greater than zero [n = 73] | 2.67 (1.30–5.51) | 0.008 | 2.19 (1.02–4.69) | 0.044 |
| Histology grade | ||||
| Low-grade tumours [n = 120] | Reference: 1.00 | |||
| Poorly differentiated [n = 118] | 3.66 (1.63–8.17) | 0.002 | 2.67 (1.14–6.21) | 0.023 |
| Clinic ‘T’ stage | ||||
| Clinical Tx, Tis, T1, T2 [n = 39] | Reference: 1.00 | |||
| Clinical T3 and T4 [n = 194] | 2.46 (0.72–8.46) | 0.153 | 2.38 (0.66–8.65) | 0.186 |
| Histology group | ||||
| Adeno [n = 165] | Reference: 1.00 | |||
| Squamous [n = 61] | 1.23 (0.57–2.68) | 0.600 | ||
| Neoadjuvant therapy | ||||
| Chemotherapy [n = 188] | Reference: 1.00 | |||
| Chemoradiation [n = 50] | 5.30 (1.23–22.87) | 0.025 | 0.27 (0.58–1.21) | 0.086 |
| ‘R’ status | ||||
| R0 [n = 223] | Reference: 1.00 | |||
| R1/R2 [n = 15] | 4.29 (1.42–12.91) | 0.010 | 3.09 (0.95–10.06) | 0.061 |
| Clinical response | Only evaluated in 167 patients and evaluated in a distinct multivariate analysis | |||
| Partial/complete [n = 117] | Reference: 1.00 | |||
| Minimal/stable/progression [n = 50] | 3.04 (1.24–7.47) | 0.015 | 2.77 (1.07–7.15) | 0.035 |
*Only variables with a P-value of <0.20 were included in the multivariable analysis.
Cumulative risk factors
Based on both multivariate analyses, we chose performance status >0, poor tumour differentiation and lack of response to neoadjuvant therapy as clinically useful factors for inclusion into a cumulative risk factor index. Each factor accounted for one point and each patient with all factors evaluable was given a score of 0–3. Results are presented in Table 4. Most patients (70%, n = 111) had at least one risk factor, whereas 30% (n = 49) had two or three. With an index of 0 or 1, only 2% (n = 1) and 12% (n = 8) of patients, respectively, died within 1 year of surgery. However, with an index of 2 or 3, 29% (n = 11) and 27% (n = 3) of each group died within 1 year. Combining patients with an index of 0 and 1 for comparison with patients with an index of 2 and 3 for analysis, patients in the second group (n = 47) had a 4.84-fold (CI 1.93–12.16, P = 0.001) risk of death.
| Risk factor index . | Number of patients (% of total) . | Alive >1 year . | Dead within 1 year . |
|---|---|---|---|
| 0 | 50 (30%) | 49 (98%) | 1 (2%) |
| 1 | 68 (41%) | 60 (88%) | 8 (12%) |
| 2 | 38 (23%) | 27 (71%) | 11 (29%) |
| 3 | 11 (7%) | 8 (73%) | 3 (27%) |
| Risk factor index . | Number of patients (% of total) . | Alive >1 year . | Dead within 1 year . |
|---|---|---|---|
| 0 | 50 (30%) | 49 (98%) | 1 (2%) |
| 1 | 68 (41%) | 60 (88%) | 8 (12%) |
| 2 | 38 (23%) | 27 (71%) | 11 (29%) |
| 3 | 11 (7%) | 8 (73%) | 3 (27%) |
| Risk factor index . | Number of patients (% of total) . | Alive >1 year . | Dead within 1 year . |
|---|---|---|---|
| 0 | 50 (30%) | 49 (98%) | 1 (2%) |
| 1 | 68 (41%) | 60 (88%) | 8 (12%) |
| 2 | 38 (23%) | 27 (71%) | 11 (29%) |
| 3 | 11 (7%) | 8 (73%) | 3 (27%) |
| Risk factor index . | Number of patients (% of total) . | Alive >1 year . | Dead within 1 year . |
|---|---|---|---|
| 0 | 50 (30%) | 49 (98%) | 1 (2%) |
| 1 | 68 (41%) | 60 (88%) | 8 (12%) |
| 2 | 38 (23%) | 27 (71%) | 11 (29%) |
| 3 | 11 (7%) | 8 (73%) | 3 (27%) |
Causes of death and survival
Median and mean follow-up were 18.5 and 34.5 months, respectively. Tumour recurrence was documented by CT scan and confirmed by PET scan or tissue biopsy. Of the entire cohort of 238 patients, 51 patients (21%) had tumour recurrence documented within the first year (median recurrence 6.7 months). Among the 36 patients who died between 3 and 12 months were 25 (49%) of the patients who recurred, in which case death was thought to be directly related to the recurrence. Most patients had distant recurrence (n = 16, 64%) or distant and local recurrences (n = 6, 24%), whereas 3 patients (12%) had local recurrence only. An additional 9 patients were thought to have died from their cancer, although there were no imaging studies that documented tumour recurrence. The cause of death in the remaining 2 patients was respiratory failure in one and unknown in the other.
Mortality was also high among the remaining 26 patients with early disease recurrence who did not die within the first year. Their median survival was only 15.7 months, with 81% (21 of 26) dead at the time of analysis. For this entire cohort of patients undergoing oesophagectomy after neoadjuvant therapy, disease-free survival at 3 and 5 years was 40 and 34%, respectively. OS at 3 and 5 years was 50 and 35%, respectively. In evaluable patients, those with a risk factor index of 0 or 1 had improved 3-year (62 vs 35%) and 5-year (45 vs 28%) OS compared with patients with a risk factor index of 2 or 3 (Fig. 1; P = 0.006).
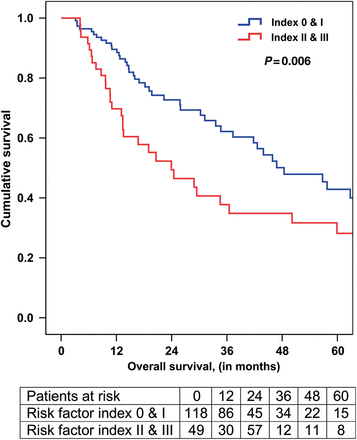
Overall survival among patients with a cumulative risk factor index of 0 and 1 versus those patients with 2 and 3 risk factors.
DISCUSSION
OC is a common neoplasm worldwide. As most patients present with advanced, metastatic disease, the overall 5-year survival rate is only 11% [8]. However, even for those patients presenting with only local or regional disease, the 5-year OS still approximates just 21–39%. For patients with early stage, node-negative disease, the treatment of choice remains surgical resection [9, 10]. However, for patients with deeper tumour invasion and particularly for those with clinically positive nodal disease, multimodality treatment options have been extensively investigated. For operable patients, surgery is usually performed after induction chemotherapy or CRT [11–14]. A meta-analysis has shown that multimodality therapy results in a decreased risk of death (HR 0.81–0.90), compared with surgery alone [15]. Some have suggested that definitive CRT (dCRT) should be considered equivalent to multimodality therapy or even suggest that it should be the standard of care for locally advanced OC [16]. They base this argument on lower rates of treatment-related morbidity in the dCRT patient populations. However, most studies, although admittedly retrospective and prone to selection bias, have demonstrated that patients who undergo surgical resection as part of their therapy have improved OS [9, 17, 18].
Our approach to patients with locally advanced OC is therefore induction chemotherapy or chemoradiation followed by surgical resection in operable patients. Even with this strategy, a significant number of patients will recur. Recurrence of tumour after treatment is the leading cause of death in these patients. Previous studies have suggested that as many as 12–22% of patients may recur even within the first year after oesophagectomy [2–4]. Early recurrence is particularly troubling as it represents a staging and/or treatment failure and likely does not extend the lives of patients beyond the natural course of the disease. The pattern of recurrence is typically systemic, estimated to comprise between 60 and 83% of recurrences in previous studies [1–4]. These previous reviews were performed on mixed populations of patients, not all undergoing neadjuvant therapy prior to resection. In this study, we sought to determine the rate of early recurrence following neoadjuvant chemotherapy or chemoradiation followed by surgical resection. We also sought to identify clinical predictors of early death. Previous studies have identified tumour differentiation, chemotherapy response, tumour size and depth, number of lymph node metastases, margin status and intramural metastases as predictors of early recurrence and death [2, 3]. Unfortunately, apart from tumour differentiation and chemotherapy response that can be determined preoperatively, the other factors are definitively evaluable only after the tumour has been resected and do not particularly help with surgical decision-making. We therefore attempted to focus on clinical predictors of early death in order to better risk-stratify patients following neoadjuvant therapy.
We found that 15% (n = 36) of our patient cohort died between 3 and 12 months. We excluded another 15 patients who had undergone neoadjuvant therapy and oesophagectomy who had died in the hospital (2%) or within 3 months of operation (4%) as we have previously found in our own database that the majority of these deaths are treatment-related (unpublished). Of the 36 patients who died between 3 and 12 months, 25 had documented disease recurrence within this short-time period, at a median of just 6.7 months following surgery. As with previous studies, the majority of the recurrences were systemic (88%). Another 9 patients were thought to have died from their cancer, although they did not have documented disease recurrence by radiological studies at our institution. As it is rare for patients to die from loco-regional recurrences, we would assume that many of these patients also had systemic disease. This suggests that many of these patients may have had occult metastases unrecognized at the time of clinical staging. Clinical risk factors for early death included performance status, which although not thought to be reflective of cancer burden, may have implications for the patients' ability to tolerate systemic therapy or for the host immune response. Other clinical risk factors included poor tumour differentiation and poor response to neoadjuvant therapy, both risk factors for systemic progression. If a patient had any two or had three of these predictors present, he or she has a 29% chance of dying within 1 year from surgery, an almost 5-fold risk over patients with only 1 or with no risk factors.
A high risk of death within 1 year following surgery poses the obvious question of whether surgery should be undertaken in these patients with multiple clinical risk factors. Previous studies have suggested that patients who expire within 1 year are unlikely to have regained their preoperative quality of life and physical activity level [19–21]. Based on current data, patients treated with dCRT could be expected to have less treatment-related morbidity, which would lead to the assumption that they have a better quality of life [22]. If OS between dCRT and multimodality strategies including surgery is similar in these high-risk patients, an argument could therefore be made to forego surgery. However, it is difficult to withhold surgery from a patient with an otherwise resectable tumour based on prediction models alone. This is particularly true when the balance of the literature suggests improved outcomes in patients undergoing surgical resection. At best, we should educate these high-risk patients regarding the potential for recurrence and let them evaluate the potential risks and benefits of different treatment strategies. It should be noted that our 5-year survival even for the high-risk group of patients with 2–3 risk factors was 28%, a number equivalent to the risk of dying in the first year. Clearly, even some of these high-risk patients are cured. The challenge therefore is to determine which patients would not be cured. We believe that patients with poor performance status should be carefully evaluated. In some instances, it may be advantageous to delay surgery if the poor performance status is secondary to the neoadjuvant therapy, in order to allow for physiological recovery. Certainly, any new finding on restaging PET or CT scans should be evaluated thoroughly and not ignored, particularly if thought to possibly represent systemic disease. A need also exists to develop blood- or tissue-based biomarkers and to refine tumour imaging to improve prognostication.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr T. Lerut(Leuven, Belgium): This very interesting presentation focuses on something that is attracting more attention, which is performance status. Yesterday we made a presentation on the influence of age, gender and BMI. So I just wanted to know from your study whether you could say how many patients had shifted in their performance score from higher to lower, or maybe vice versa, during the induction course, and how that influenced the final risk score. What you can do, for instance, is systematic placement of a feeding jejunostomy upfront at the start of your induction therapy, to change the performance status. So I just wanted to ask your opinion and comments on how you would implement some measures to change performance status.
Dr Stiles: Those are great points and we appreciate your thinking about it. Unfortunately, one of the problems with our database is that we actually capture performance status after induction therapy. So we don't have a great sense of what induction therapy has done to that performance status. I suspect, as you suggest, though, a lot of patients may have decreased performance status, although a few may improve. It is sort of curious why performance status would affect oncologic recurrence in the early time period. We have talked about that as well. Presumably those patients tolerate their induction therapy less well, need more dose reduction, and sort of struggle through the therapy. Maybe they are immune-suppressed. But I agree with you on all those points.
Dr M. Okumura(Osaka, Japan): Do you not use PET-CT scanning before surgery after pre-induction chemoradiotherapy?
Dr Stiles: Yes, we do. Some of the patients early on in this series didn't all get a pre- and a post-induction PET. Now we do it routinely. Approximately 70% of the patients in the whole study had both. We didn't look at the effect of downstaging by PET, but for this paper we included it into our general impression of clinical downstaging.
Dr C. Kang(Seoul, South Korea): I have several questions. First, in your data, many patients received neoadjuvant chemotherapy and a small group received neoadjuvant chemoradiation. Can you tell me the indication for the different neoadjuvant protocol?
Secondly, did you find any difference in terms of complications or quality of life difference between neoadjuvant chemotherapy and neoadjuvant chemoradiation?
And lastly, in your data, in univariate analysis, neoadjuvant chemoradiation was better but in multivariate analysis the difference disappeared. Can you explain why that happened, for example, like a complete resection rate and a downstaging rate?
Dr Stiles: We have historically used chemotherapy, although we have gone to more chemoradiation. Now typically we use chemoradiation based on the CROSS protocol for squamous cell patients. For adenocarcinoma we take it individually but tend to do more chemotherapy alone. In that analysis, there was no difference in early recurrence and death between the adenocarcinomas and the squamous, although in our group, approximately 50% of the squamous got chemoradiation whereas only about 15 to 18% of the adenocarcinomas got it.
Again, I still don't understand it. I think there are other variables in play in terms of why that protective effect that we saw with the chemoradiation in the first year comes into play. We have recently partnered with MD Anderson and Montreal to try to put this together and look at chemotherapy versus chemoradiation and the pattern of recurrence in those patients as well as the long-term effect. Again, for our data with an en bloc resection, we don't see any long-term difference in survival between chemotherapy versus chemoradiation, and that holds true for both adenocarcinoma and squamous cell cancer patients.
Author notes
Presented at the 22nd European Conference on General Thoracic Surgery, Copenhagen, Denmark, 15–18 June 2014.




