-
PDF
- Split View
-
Views
-
Cite
Cite
Giuseppe Gatti, Petar Soso, Luca Dell'Angela, Luca Maschietto, Lorella Dreas, Bernardo Benussi, Roberto Luzzati, Gianfranco Sinagra, Aniello Pappalardo, Routine use of bilateral internal thoracic artery grafts for left-sided myocardial revascularization in insulin-dependent diabetic patients: early and long-term outcomes, European Journal of Cardio-Thoracic Surgery, Volume 48, Issue 1, July 2015, Pages 115–120, https://doi.org/10.1093/ejcts/ezu360
Close - Share Icon Share
Abstract
Despite encouraging late outcomes, the use of bilateral internal thoracic artery (BITA) grafting for myocardial revascularization in diabetic patients remains controversial because of an increased risk of sternal complications. In the present study, early and long-term outcomes of the routine use of left-sided BITA grafting in insulin-dependent diabetic patients were reviewed retrospectively.
Among the 2701 consecutive patients who underwent isolated BITA grafting at the authors' institution from 1999 throughout 2012, 188 (mean age: 67 ± 9 years) were insulin-dependent diabetic patients. The mean expected operative risk, calculated according to the European System for Cardiac Operative Risk Evaluation II, was 11 ± 10.8%.
There were 6 (3.2%) hospital deaths. Prolonged invasive ventilation (17.6%), multiple transfusion (16.5%), deep sternal wound infection (DSWI, 11.7%) and acute kidney injury (10.6%) were the most frequent major postoperative complications. Chronic lung disease (P = 0.08), low cardiac output (P = 0.039), multiple transfusion (P = 0.034) and mediastinal re-exploration (P = 0.071) were risk factors for DSWI. The mean follow-up was 5.7 ± 3.6 years. The 10-year non-parametric estimates of overall survival, freedom from cardiac and cerebrovascular death, and major adverse cardiac and cerebrovascular events were 57.7 [95% confidence interval (CI): 45.1–66.2], 83.6 (95% CI: 76.6–90.7) and 55.4% (95% CI: 44.7–66.1), respectively. Predictors of decreased late survival were old age (P = 0.013), chronic lung disease (P = 0.004), renal impairment (P = 0.009) and left ventricular dysfunction (P = 0.035).
Left-sided BITA grafting may be performed routinely even in insulin-dependent diabetic patients. The increased rates of postoperative complications do not prevent low early mortality and good long-term outcomes.
INTRODUCTION
In diabetic patients with multivessel coronary artery disease, coronary artery bypass graft (CABG) surgery offers better results than percutaneous coronary intervention [1, 2]. The use of bilateral internal thoracic artery (BITA) grafting for myocardial revascularization may additionally improve the long-term outcomes of surgery because of superior graft patency [3–6]. In diabetic patients, however, BITA grafting remains a controversial issue mainly due to the increased risk of sternal complications [7–9].
Some people who have type 2 diabetes mellitus need insulin treatment when glucose-lowering tablets are no longer effective in controlling blood glucose levels. These patients with type 2 diabetes on insulin, and the patients with type 1 diabetes, usually suffer from high rates of macro- and microvascular complications and all-cause mortality. Furthermore, early and late outcomes of cardiac and vascular operations for these insulin-dependent diabetic patients are generally worse than for their noninsulin-dependent counterparts [10].
To date there are a few reports that specifically analyse the outcomes of BITA grafting in insulin-dependent diabetic patients [11, 12]. The aim of the present study was to review the authors' experience in the routine use of BITA grafting in the difficult subset of patients suffering from diabetes and in need of insulin therapy.
PATIENTS AND METHODS
Study patients
From 1999 throughout 2012, a total of 3895 consecutive patients with multivessel coronary artery disease underwent isolated CABG surgery at the authors' institution (2701, 69.3% of patients received a BITA graft). Two hundred and eighty-eight (7.4%) of these 3895 patients suffered from diabetes on insulin, and were candidates for bilateral (n = 205) or single internal thoracic artery (ITA) grafting (n = 83) exclusively according to coronary artery anatomy and disease, without any other preoperative selection. However, among the candidates for BITA grafting, 17 (8.3%) received a single ITA graft (and saphenous vein or radial artery grafts in addition) or saphenous vein grafts alone: in 9 cases one or both ITAs were not suitable to be used as coronary bypass grafts because they were too thin (n = 3), inherently diseased (n = 1), damaged during harvesting (n = 3) or involved in a diffuse inflammatory process (n = 2); in 4 cases the appearance of myocardial ischaemia and severe hypotension following induction of anaesthesia (n = 2) or during left ITA harvesting (n = 2) needed immediate institution of cardiopulmonary bypass; in 4 cases, there were unexpected operative findings of severe cardiac dysfunction (n = 4). Thus, BITA grafts were finally used for left-sided myocardial revascularization in 188 insulin-dependent diabetic patients who were enrolled in this retrospective study (Table 1).
| Characteristic . | n = 188 . |
|---|---|
| Age (years) | 67 ± 9 (61–74) |
| <70 | 101 (53.7) |
| 70–75 | 47 (25) |
| >75 | 40 (21.3) |
| Female | 44 (23.4) |
| Hypertension | 137 (72.9) |
| Hyperlipidaemia | 171 (91) |
| Previous smoker | 50 (26.6) |
| BMI (kg/m2) | 27.3 ± 4 (24.3–29.4) |
| >30 | 39 (20.7) |
| Type 1 diabetes | 17 (9) |
| Type 2 diabetes on insulin | 171 (91) |
| Poor glycaemic controlb | 41 (21.8) |
| Poor mobilityc | 4 (2.1) |
| Chronic lung diseasec | 9 (4.8) |
| GFR (ml/min)d | 68.9 ± 32 (49.1–84.7) |
| 50–85d | 92 (48.9) |
| <50d | 50 (26.6) |
| Dialysis | 11 (5.8) |
| Extracardiac arteriopathyc | 75 (39.9) |
| Calcified ascending aorta | 29 (15.4) |
| Congestive heart failure | 8 (4.3) |
| Unstable angina | 64 (34) |
| Recent myocardial infarctc | 41 (21.8) |
| Left main CAD | 65 (34.6) |
| Three-system CAD | 140 (74.5) |
| Two-system CAD | 47 (25) |
| One-system CAD | 1 (0.5) |
| LVEF (%) | 51.7 ± 12.1 (42–60) |
| 40–50 | 37 (19.7) |
| <40 | 42 (22.3) |
| Previous percutaneous coronary intervention | 9 (4.8) |
| Previous cardiac surgery | 3 (1.6)e |
| Preoperative IABP | 16 (8.5) |
| Emergency operationc | 4 (2.1) |
| Expected operative risk (by EuroSCORE II) (%)f | 11 ± 10.8 (3.9–13.7)g |
| Characteristic . | n = 188 . |
|---|---|
| Age (years) | 67 ± 9 (61–74) |
| <70 | 101 (53.7) |
| 70–75 | 47 (25) |
| >75 | 40 (21.3) |
| Female | 44 (23.4) |
| Hypertension | 137 (72.9) |
| Hyperlipidaemia | 171 (91) |
| Previous smoker | 50 (26.6) |
| BMI (kg/m2) | 27.3 ± 4 (24.3–29.4) |
| >30 | 39 (20.7) |
| Type 1 diabetes | 17 (9) |
| Type 2 diabetes on insulin | 171 (91) |
| Poor glycaemic controlb | 41 (21.8) |
| Poor mobilityc | 4 (2.1) |
| Chronic lung diseasec | 9 (4.8) |
| GFR (ml/min)d | 68.9 ± 32 (49.1–84.7) |
| 50–85d | 92 (48.9) |
| <50d | 50 (26.6) |
| Dialysis | 11 (5.8) |
| Extracardiac arteriopathyc | 75 (39.9) |
| Calcified ascending aorta | 29 (15.4) |
| Congestive heart failure | 8 (4.3) |
| Unstable angina | 64 (34) |
| Recent myocardial infarctc | 41 (21.8) |
| Left main CAD | 65 (34.6) |
| Three-system CAD | 140 (74.5) |
| Two-system CAD | 47 (25) |
| One-system CAD | 1 (0.5) |
| LVEF (%) | 51.7 ± 12.1 (42–60) |
| 40–50 | 37 (19.7) |
| <40 | 42 (22.3) |
| Previous percutaneous coronary intervention | 9 (4.8) |
| Previous cardiac surgery | 3 (1.6)e |
| Preoperative IABP | 16 (8.5) |
| Emergency operationc | 4 (2.1) |
| Expected operative risk (by EuroSCORE II) (%)f | 11 ± 10.8 (3.9–13.7)g |
BMI: body mass index; CAD: coronary artery disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; GFR: glomerular filtration rate; IABP: intra-aortic balloon pumping; LVEF: left ventricular ejection fraction.
aThe values are the number of patients with the percentage in brackets, or the mean ± standard deviation with the interquartile range in brackets.
bBasal serum glucose >200 mg/dl at three consecutive measurements before surgery.
cThe definitions were those employed for EuroSCORE II [13].
dThe creatinine clearance rate, calculated according to the Cockcroft–Gault formula, was used for approximating the GFR.
eCoronary bypass surgery (n = 2) or aortic valve replacement (n = 1).
fRef. [13].
gMedian = 7.1%.
| Characteristic . | n = 188 . |
|---|---|
| Age (years) | 67 ± 9 (61–74) |
| <70 | 101 (53.7) |
| 70–75 | 47 (25) |
| >75 | 40 (21.3) |
| Female | 44 (23.4) |
| Hypertension | 137 (72.9) |
| Hyperlipidaemia | 171 (91) |
| Previous smoker | 50 (26.6) |
| BMI (kg/m2) | 27.3 ± 4 (24.3–29.4) |
| >30 | 39 (20.7) |
| Type 1 diabetes | 17 (9) |
| Type 2 diabetes on insulin | 171 (91) |
| Poor glycaemic controlb | 41 (21.8) |
| Poor mobilityc | 4 (2.1) |
| Chronic lung diseasec | 9 (4.8) |
| GFR (ml/min)d | 68.9 ± 32 (49.1–84.7) |
| 50–85d | 92 (48.9) |
| <50d | 50 (26.6) |
| Dialysis | 11 (5.8) |
| Extracardiac arteriopathyc | 75 (39.9) |
| Calcified ascending aorta | 29 (15.4) |
| Congestive heart failure | 8 (4.3) |
| Unstable angina | 64 (34) |
| Recent myocardial infarctc | 41 (21.8) |
| Left main CAD | 65 (34.6) |
| Three-system CAD | 140 (74.5) |
| Two-system CAD | 47 (25) |
| One-system CAD | 1 (0.5) |
| LVEF (%) | 51.7 ± 12.1 (42–60) |
| 40–50 | 37 (19.7) |
| <40 | 42 (22.3) |
| Previous percutaneous coronary intervention | 9 (4.8) |
| Previous cardiac surgery | 3 (1.6)e |
| Preoperative IABP | 16 (8.5) |
| Emergency operationc | 4 (2.1) |
| Expected operative risk (by EuroSCORE II) (%)f | 11 ± 10.8 (3.9–13.7)g |
| Characteristic . | n = 188 . |
|---|---|
| Age (years) | 67 ± 9 (61–74) |
| <70 | 101 (53.7) |
| 70–75 | 47 (25) |
| >75 | 40 (21.3) |
| Female | 44 (23.4) |
| Hypertension | 137 (72.9) |
| Hyperlipidaemia | 171 (91) |
| Previous smoker | 50 (26.6) |
| BMI (kg/m2) | 27.3 ± 4 (24.3–29.4) |
| >30 | 39 (20.7) |
| Type 1 diabetes | 17 (9) |
| Type 2 diabetes on insulin | 171 (91) |
| Poor glycaemic controlb | 41 (21.8) |
| Poor mobilityc | 4 (2.1) |
| Chronic lung diseasec | 9 (4.8) |
| GFR (ml/min)d | 68.9 ± 32 (49.1–84.7) |
| 50–85d | 92 (48.9) |
| <50d | 50 (26.6) |
| Dialysis | 11 (5.8) |
| Extracardiac arteriopathyc | 75 (39.9) |
| Calcified ascending aorta | 29 (15.4) |
| Congestive heart failure | 8 (4.3) |
| Unstable angina | 64 (34) |
| Recent myocardial infarctc | 41 (21.8) |
| Left main CAD | 65 (34.6) |
| Three-system CAD | 140 (74.5) |
| Two-system CAD | 47 (25) |
| One-system CAD | 1 (0.5) |
| LVEF (%) | 51.7 ± 12.1 (42–60) |
| 40–50 | 37 (19.7) |
| <40 | 42 (22.3) |
| Previous percutaneous coronary intervention | 9 (4.8) |
| Previous cardiac surgery | 3 (1.6)e |
| Preoperative IABP | 16 (8.5) |
| Emergency operationc | 4 (2.1) |
| Expected operative risk (by EuroSCORE II) (%)f | 11 ± 10.8 (3.9–13.7)g |
BMI: body mass index; CAD: coronary artery disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; GFR: glomerular filtration rate; IABP: intra-aortic balloon pumping; LVEF: left ventricular ejection fraction.
aThe values are the number of patients with the percentage in brackets, or the mean ± standard deviation with the interquartile range in brackets.
bBasal serum glucose >200 mg/dl at three consecutive measurements before surgery.
cThe definitions were those employed for EuroSCORE II [13].
dThe creatinine clearance rate, calculated according to the Cockcroft–Gault formula, was used for approximating the GFR.
eCoronary bypass surgery (n = 2) or aortic valve replacement (n = 1).
fRef. [13].
gMedian = 7.1%.
To evaluate the suitability of both ITAs to be used as coronary grafts, all patients had undergone bilateral selective angiography of the subclavian artery during preoperative coronary angiography. For those patients who were preoperatively in Canadian Cardiovascular Society class 1 or 2, dobutamine stress echocardiography demonstrated a viable myocardium in 20% or more of the left ventricular mass.
Surgery
Surgery was carried out via a median sternotomy either with cardiopulmonary bypass, with or without cross-clamping the aorta, or the off-pump technique. When a period of myocardial ischaemia was used, myocardial protection was usually achieved with multidose cold blood cardioplegia delivered both in an antegrade and retrograde mode. A single-dose crystalloid solution (Custodiol-HTK® solution; Essential Pharma, Newtown, PA, USA) was sometimes preferred, especially when longer ischaemic times were expected. Off-pump and beating-heart on-pump techniques were adopted only in the presence of a diffusely calcified ascending aorta (confirmed by epiaortic ultrasonography scanning) to avoid the risk of cracking atherosclerotic plaques by the aortic cross-clamp (Table 2).
| Parameters . | n = 188 . |
|---|---|
| No. of coronary anastomoses | 3.8 ± 1.2 (3–4) |
| No. of coronary anastomoses with bilateral ITA graft | 2.8 ± 0.8 (2–3) |
| Left ITA, in situ/free graft | 183 (97.3)/12 (6.4)b |
| Right ITA, in situ/free graft | 149 (79.3)/42 (22.3)b |
| Off-pump surgery | 22 (11.7) |
| On-pump surgery | 159 (84.6) |
| Cardiopulmonary bypass time (min) | 106.6 ± 33.3 (84–127.5) |
| Aortic cross-clamping time (min) | 84.0 ± 28.3 (64.5–99) |
| Cold blood cardioplegia | 115 (61.2) |
| Custodiol-HTK solution | 44 (23.4) |
| Beating-heart on-pump surgery | 7 (3.7) |
| Cardiopulmonary bypass time (min) | 102.6 ± 37.6 (70.5–114.5) |
| Parameters . | n = 188 . |
|---|---|
| No. of coronary anastomoses | 3.8 ± 1.2 (3–4) |
| No. of coronary anastomoses with bilateral ITA graft | 2.8 ± 0.8 (2–3) |
| Left ITA, in situ/free graft | 183 (97.3)/12 (6.4)b |
| Right ITA, in situ/free graft | 149 (79.3)/42 (22.3)b |
| Off-pump surgery | 22 (11.7) |
| On-pump surgery | 159 (84.6) |
| Cardiopulmonary bypass time (min) | 106.6 ± 33.3 (84–127.5) |
| Aortic cross-clamping time (min) | 84.0 ± 28.3 (64.5–99) |
| Cold blood cardioplegia | 115 (61.2) |
| Custodiol-HTK solution | 44 (23.4) |
| Beating-heart on-pump surgery | 7 (3.7) |
| Cardiopulmonary bypass time (min) | 102.6 ± 37.6 (70.5–114.5) |
ITA: internal thoracic artery.
aThe values are the number of patients with the percentages in brackets, or the mean ± standard deviation with the interquartile range in brackets.
bDistal segments of in situ grafts were sometimes used as free grafts.
| Parameters . | n = 188 . |
|---|---|
| No. of coronary anastomoses | 3.8 ± 1.2 (3–4) |
| No. of coronary anastomoses with bilateral ITA graft | 2.8 ± 0.8 (2–3) |
| Left ITA, in situ/free graft | 183 (97.3)/12 (6.4)b |
| Right ITA, in situ/free graft | 149 (79.3)/42 (22.3)b |
| Off-pump surgery | 22 (11.7) |
| On-pump surgery | 159 (84.6) |
| Cardiopulmonary bypass time (min) | 106.6 ± 33.3 (84–127.5) |
| Aortic cross-clamping time (min) | 84.0 ± 28.3 (64.5–99) |
| Cold blood cardioplegia | 115 (61.2) |
| Custodiol-HTK solution | 44 (23.4) |
| Beating-heart on-pump surgery | 7 (3.7) |
| Cardiopulmonary bypass time (min) | 102.6 ± 37.6 (70.5–114.5) |
| Parameters . | n = 188 . |
|---|---|
| No. of coronary anastomoses | 3.8 ± 1.2 (3–4) |
| No. of coronary anastomoses with bilateral ITA graft | 2.8 ± 0.8 (2–3) |
| Left ITA, in situ/free graft | 183 (97.3)/12 (6.4)b |
| Right ITA, in situ/free graft | 149 (79.3)/42 (22.3)b |
| Off-pump surgery | 22 (11.7) |
| On-pump surgery | 159 (84.6) |
| Cardiopulmonary bypass time (min) | 106.6 ± 33.3 (84–127.5) |
| Aortic cross-clamping time (min) | 84.0 ± 28.3 (64.5–99) |
| Cold blood cardioplegia | 115 (61.2) |
| Custodiol-HTK solution | 44 (23.4) |
| Beating-heart on-pump surgery | 7 (3.7) |
| Cardiopulmonary bypass time (min) | 102.6 ± 37.6 (70.5–114.5) |
ITA: internal thoracic artery.
aThe values are the number of patients with the percentages in brackets, or the mean ± standard deviation with the interquartile range in brackets.
bDistal segments of in situ grafts were sometimes used as free grafts.
Both ITAs were harvested as skeletonized conduits with low-intensity bipolar coagulation forceps, extending from the inferior border of the subclavian vein distally to the bifurcation into the superior epigastric and musculophrenic arteries [14]. Both ITAs were used as in situ grafts whenever possible. The right ITA was preferentially directed to the left anterior descending coronary artery and the left ITA to the posterolateral cardiac wall. The anteaortic crossover right ITA bypass graft was covered with a pedicled flap taken from the thymic remnants. Thus, in the event of an early or late mediastinal re-entry, the right ITA was protected by means of this pedicled thymus flap [15]. Additional coronary bypasses, usually for the right coronary artery, were performed with saphenous vein or (rarely) radial artery grafts. Sometimes, the ITA was taken down and used as a free graft either from the in situ contralateral ITA (Y-graft) or the proximal (aortic) end of a saphenous vein graft (Table 2).
The standard single-loop sternal wiring technique was preferentially used as a sternal closure system until 2009. Since 2010, the Erdinc double-loop sternal wiring technique was adopted systematically [16].
All patients were treated during operation and then in the intensive care unit with a continuous intravenous insulin infusion.
Follow-up
All perioperative data were prospectively recorded for every patient in a computerized data registry (FileMaker Pro 6.0; FileMaker, Inc., Santa Clara, CA, USA). The Centers for Disease Control and Prevention classification of surgical site infections was adopted to define sternal wound infections. Briefly, ‘superficial infections’ involve only skin or subcutaneous tissues, ‘deep infections’ involve deep soft tissues (fascial and muscle layers) and ‘organ/space infections’ involve tissues other than the incision [17]. For the purposes of this study, infections involving deep soft tissues, osteomyelitis and mediastinitis were ‘deep sternal wound infections (DSWIs)’. The surgeons' notes on wound revisions were reviewed to ensure that the definitions were in accordance with these classes.
An up-to-date clinical follow-up was obtained by a telephonic interview with the patient or her/his family. The occurrence of at least 1 postoperative major adverse cardiac and cerebrovascular event (MACCE)—defined as any of the following complications from hospital discharge to follow-up: sudden death, recurrent angina, myocardial infarction, congestive heart failure, percutaneous coronary intervention, repeat CABG and cerebrovascular accident—was recorded. For this study, follow-up was closed on 1 September 2013.
Approval to conduct the study was acquired from The Hospital Ethics Committee, based on retrospective data retrieval, having waived the need for patients to provide their individual consent.
Statistical methods
Data were expressed as number of patients or mean ± standard deviation, with the percentage or the range between the first and the third quartile (interquartile range) in brackets. Patients' clinical characteristics and operative data were compared using the χ2, Fisher exact or McNemar test for dichotomous variables, and the Student's t- or Mann–Whitney U-test for continuous variables, as appropriate. An odds ratio (OR) with a 95% confidence interval (CI) was given for each variable. Non-parametric estimates and curves of overall survival, freedom from cardiac and cerebrovascular death and MACCEs were generated with the Kaplan–Meier method. The Cox proportional hazards model was used to determine the influence of patients' characteristics and operative data on late survival. The hazard ratio with 95% CI was calculated for each variable. Comparisons between survival curves were made by the log-rank test. A P-value <0.1 was considered significant. All analyses were performed with the MINITAB release 16 statistical software (MINITAB, Inc., State College, PA, USA).
RESULTS
Early (hospital) outcomes
There were 6 (3.2%) hospital deaths: 4 patients died within postoperative day 30 (30-day mortality = 2.1%) and 2 patients later. Stroke, pneumonia, low cardiac output, acute kidney injury, sepsis and multiorgan failure were the causes of death. Age >75 years (OR = 8.11, 95% CI: 1.43–46; P = 0.019) and a glomerular filtration rate < 50 ml/min (OR = 15.2, 95% CI: 1.73–133.8; P = 0.005) were risk factors for hospital death according to the univariable analysis (Supplementary Table 1).
Prolonged (>48 h) invasive ventilation (17.6%), multiple transfusion (16.5%), DSWI (11.7%) and acute kidney injury (10.6%) were the most frequent major postoperative complications (Table 3). There were 3 cases of self-resolving phrenic nerve paralysis causing no slowdown in weaning from the ventilator. Chronic lung disease (OR = 4.05, 95% CI: 0.94–17.5; P = 0.08), low cardiac output (OR = 15.9, 95% CI: 1.38–183.4; P = 0.039), multiple transfusion (OR = 4.49, 95% CI: 1.2–16.8; P = 0.034) and mediastinal re-exploration for bleeding or tamponade (OR = 3.33, 95% CI: 0.95–11.7; P = 0.071) were risk factors for DSWI according to the univariable analysis (Supplementary Table 2). Every DSWI was treated with antibiotic therapy and a vacuum-assisted closure system (V.A.C.® Therapy; KCI Medical Srl, Assago, Milan, Italy), with the addition of surgical debridement and sternal reconstruction in the 15 (8%) patients with osteomyelitis or mediastinitis. Despite the fact that there were signs of overt infection, the presence of causative pathogens was confirmed in only 9 (40.9%) cases. Staphylococcus aureus (n = 5), Staphylococcus epidermidis (n = 3) and Enterococcus faecalis (n = 1) were the involved pathogens. The median length of hospital stay was 13.5 days (range: 6–116 days) in patients with DSWI and 11 days (range: 5–83 days) in patients without sternal complications (P < 0.0001).
| Complication . | n = 188 . |
|---|---|
| Neurological dysfunction | 4 (2.1)b |
| Prolonged (>48 h) invasive ventilation | 33 (17.6) |
| Atrial fibrillation, new-onset | 42/184c (22.8) |
| Perioperative myocardial infarct | 5 (2.7) |
| Low cardiac outputd | 3 (1.6) |
| Acute kidney injurye | 23 (10.6) |
| Multiple transfusion (3 or more units of RBCs) | 31 (16.5) |
| Mediastinal re-exploration for bleeding or tamponadef | 14 (7.4) |
| Sepsis | 2 (1.1) |
| Multiorgan failure | 1 (0.5) |
| Sternal wound infectiong | 28 (14.4) |
| Superficial incisional | 6 (3.2) |
| Deep incisional/osteomielitis/mediastinitish | 7 (3.7)/11 (5.8)/ 4 (2.1) |
| Complication . | n = 188 . |
|---|---|
| Neurological dysfunction | 4 (2.1)b |
| Prolonged (>48 h) invasive ventilation | 33 (17.6) |
| Atrial fibrillation, new-onset | 42/184c (22.8) |
| Perioperative myocardial infarct | 5 (2.7) |
| Low cardiac outputd | 3 (1.6) |
| Acute kidney injurye | 23 (10.6) |
| Multiple transfusion (3 or more units of RBCs) | 31 (16.5) |
| Mediastinal re-exploration for bleeding or tamponadef | 14 (7.4) |
| Sepsis | 2 (1.1) |
| Multiorgan failure | 1 (0.5) |
| Sternal wound infectiong | 28 (14.4) |
| Superficial incisional | 6 (3.2) |
| Deep incisional/osteomielitis/mediastinitish | 7 (3.7)/11 (5.8)/ 4 (2.1) |
DSWI: deep sternal wound infection; IABP: intra-aortic balloon pumping; RBCs: packed red blood cells.
aThe values are the number of patients with the percentage in brackets.
bDelayed awakening (n = 2), stroke (n = 1) or manifest psychiatric disorder (n = 1).
cPatients with preoperative stable sinus rhythm or paroxysmal atrial fibrillation.
dDefined as three consecutive cardiac index measurements <2.0 l/min/m2 despite adequate preload, afterload and inotropic support, or IABP.
eDefined as serum creatinine >2.0 mg/l in the patients without preoperative renal impairment, and increase in serum creatinine of at least 1.0 mg/l above baseline in the patients with preoperative renal impairment.
fThrough resternotomy.
gDefined according to the Centers for Disease Control and Prevention classification of surgical site infections [18].
hDSWI.
| Complication . | n = 188 . |
|---|---|
| Neurological dysfunction | 4 (2.1)b |
| Prolonged (>48 h) invasive ventilation | 33 (17.6) |
| Atrial fibrillation, new-onset | 42/184c (22.8) |
| Perioperative myocardial infarct | 5 (2.7) |
| Low cardiac outputd | 3 (1.6) |
| Acute kidney injurye | 23 (10.6) |
| Multiple transfusion (3 or more units of RBCs) | 31 (16.5) |
| Mediastinal re-exploration for bleeding or tamponadef | 14 (7.4) |
| Sepsis | 2 (1.1) |
| Multiorgan failure | 1 (0.5) |
| Sternal wound infectiong | 28 (14.4) |
| Superficial incisional | 6 (3.2) |
| Deep incisional/osteomielitis/mediastinitish | 7 (3.7)/11 (5.8)/ 4 (2.1) |
| Complication . | n = 188 . |
|---|---|
| Neurological dysfunction | 4 (2.1)b |
| Prolonged (>48 h) invasive ventilation | 33 (17.6) |
| Atrial fibrillation, new-onset | 42/184c (22.8) |
| Perioperative myocardial infarct | 5 (2.7) |
| Low cardiac outputd | 3 (1.6) |
| Acute kidney injurye | 23 (10.6) |
| Multiple transfusion (3 or more units of RBCs) | 31 (16.5) |
| Mediastinal re-exploration for bleeding or tamponadef | 14 (7.4) |
| Sepsis | 2 (1.1) |
| Multiorgan failure | 1 (0.5) |
| Sternal wound infectiong | 28 (14.4) |
| Superficial incisional | 6 (3.2) |
| Deep incisional/osteomielitis/mediastinitish | 7 (3.7)/11 (5.8)/ 4 (2.1) |
DSWI: deep sternal wound infection; IABP: intra-aortic balloon pumping; RBCs: packed red blood cells.
aThe values are the number of patients with the percentage in brackets.
bDelayed awakening (n = 2), stroke (n = 1) or manifest psychiatric disorder (n = 1).
cPatients with preoperative stable sinus rhythm or paroxysmal atrial fibrillation.
dDefined as three consecutive cardiac index measurements <2.0 l/min/m2 despite adequate preload, afterload and inotropic support, or IABP.
eDefined as serum creatinine >2.0 mg/l in the patients without preoperative renal impairment, and increase in serum creatinine of at least 1.0 mg/l above baseline in the patients with preoperative renal impairment.
fThrough resternotomy.
gDefined according to the Centers for Disease Control and Prevention classification of surgical site infections [18].
hDSWI.
Time-related survival
The follow-up was 100% complete. A total of 1037.4 cumulative patient-years were reviewed. The mean follow-up was 5.7 ± 3.6 years (range: 0.2–13.9 years). There were 23 cardiac deaths and 31 non-cardiac deaths. The causes of death were myocardial infarction (n = 10), congestive heart failure (n = 8), sudden death (n = 3), stroke (n = 2), malignancy (n = 12), pneumonia (n = 9), chronic renal failure (n = 2), acute diabetic complications (n = 2), trauma (n = 2), sepsis following urinary tract infection (n = 2) or hip surgery (n = 1) and gangrene of the leg (n = 1). The 1-, 5- and 10-year non-parametric estimates of overall survival (including hospital mortality) were 92.5 (95% CI: 88.8–96.3), 77.7 (95% CI: 71–84.4) and 57.7% (95% CI: 45.1–66.2), respectively (Fig. 1A). The predictors of decreased late survival were old age (P = 0.013), chronic lung disease (P = 0.004), renal impairment (P = 0.009) and left ventricular dysfunction (P = 0.035). DSWI was not a predictor of poor late survival (P = 0.4) (Table 4). The 1-, 5- and 10-year non-parametric estimates of freedom from cardiac and cerebrovascular death (including hospital mortality) were 94.6 (95% CI: 91.4–97.9), 89.9 (95% CI: 85–94.7) and 83.6% (95% CI: 76.6–90.7), respectively (Fig. 1B).
| Variables . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Age (years) | 1.05 | 1.01–1.1 | 0.013 |
| Female | 0.84 | 0.48–1.82 | 0.84 |
| Previous smoker | 1.16 | 0.61–2.18 | 0.66 |
| BMI >30 kg/m2 | 0.99 | 0.91–1.08 | 0.88 |
| Poor glycaemic controlb | 1.66 | 0.88–3.13 | 0.12 |
| Type 1 diabetes | 0.91 | 0.35–2.33 | 0.84 |
| Poor mobilityc | 1.63 | 0.33–8.1 | 0.55 |
| Chronic lung diseasec | 6.36 | 1.81–22.3 | 0.004 |
| GFR (ml/min)d | 0.98 | 0.96–0.99 | 0.009 |
| Dialysis | 1.34 | 0.32–5.63 | 0.69 |
| Extracardiac arteriopathyc | 1.05 | 0.59–1.87 | 0.88 |
| Congestive heart failure | 1.31 | 0.28–6.22 | 0.73 |
| Unstable angina | 1.12 | 0.61–2.05 | 0.72 |
| Recent myocardial infarctc | 0.87 | 0.42–1.8 | 0.71 |
| LVEF (%) | 0.97 | 0.95–1 | 0.035 |
| Previous cardiac surgery | 0 | – | 0.96 |
| Preoperative IABP | 0.81 | 0.21–3.1 | 0.76 |
| Emergency operationc | 0 | – | 0.98 |
| Off-pump surgery | 2.91 | 0.69–12.2 | 0.15 |
| DSWI | 1.44 | 0.62–3.31 | 0.4 |
| Variables . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Age (years) | 1.05 | 1.01–1.1 | 0.013 |
| Female | 0.84 | 0.48–1.82 | 0.84 |
| Previous smoker | 1.16 | 0.61–2.18 | 0.66 |
| BMI >30 kg/m2 | 0.99 | 0.91–1.08 | 0.88 |
| Poor glycaemic controlb | 1.66 | 0.88–3.13 | 0.12 |
| Type 1 diabetes | 0.91 | 0.35–2.33 | 0.84 |
| Poor mobilityc | 1.63 | 0.33–8.1 | 0.55 |
| Chronic lung diseasec | 6.36 | 1.81–22.3 | 0.004 |
| GFR (ml/min)d | 0.98 | 0.96–0.99 | 0.009 |
| Dialysis | 1.34 | 0.32–5.63 | 0.69 |
| Extracardiac arteriopathyc | 1.05 | 0.59–1.87 | 0.88 |
| Congestive heart failure | 1.31 | 0.28–6.22 | 0.73 |
| Unstable angina | 1.12 | 0.61–2.05 | 0.72 |
| Recent myocardial infarctc | 0.87 | 0.42–1.8 | 0.71 |
| LVEF (%) | 0.97 | 0.95–1 | 0.035 |
| Previous cardiac surgery | 0 | – | 0.96 |
| Preoperative IABP | 0.81 | 0.21–3.1 | 0.76 |
| Emergency operationc | 0 | – | 0.98 |
| Off-pump surgery | 2.91 | 0.69–12.2 | 0.15 |
| DSWI | 1.44 | 0.62–3.31 | 0.4 |
BMI: body mass index; CI: confidence interval; DSWI: sternal wound infection; GFR: glomerular filtration rate; HR: hazard ratio; IABP: intra-aortic balloon pumping; LVEF: left ventricular ejection fraction.
aThe hospital discharged patients.
bBasal serum glucose >200 mg/dl at three consecutive measurements before surgery.
cThe definitions were those employed for EuroSCORE II [13].
dThe creatinine clearance rate, calculated according to the Cockcroft–Gault formula, was used for approximating the GFR.
| Variables . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Age (years) | 1.05 | 1.01–1.1 | 0.013 |
| Female | 0.84 | 0.48–1.82 | 0.84 |
| Previous smoker | 1.16 | 0.61–2.18 | 0.66 |
| BMI >30 kg/m2 | 0.99 | 0.91–1.08 | 0.88 |
| Poor glycaemic controlb | 1.66 | 0.88–3.13 | 0.12 |
| Type 1 diabetes | 0.91 | 0.35–2.33 | 0.84 |
| Poor mobilityc | 1.63 | 0.33–8.1 | 0.55 |
| Chronic lung diseasec | 6.36 | 1.81–22.3 | 0.004 |
| GFR (ml/min)d | 0.98 | 0.96–0.99 | 0.009 |
| Dialysis | 1.34 | 0.32–5.63 | 0.69 |
| Extracardiac arteriopathyc | 1.05 | 0.59–1.87 | 0.88 |
| Congestive heart failure | 1.31 | 0.28–6.22 | 0.73 |
| Unstable angina | 1.12 | 0.61–2.05 | 0.72 |
| Recent myocardial infarctc | 0.87 | 0.42–1.8 | 0.71 |
| LVEF (%) | 0.97 | 0.95–1 | 0.035 |
| Previous cardiac surgery | 0 | – | 0.96 |
| Preoperative IABP | 0.81 | 0.21–3.1 | 0.76 |
| Emergency operationc | 0 | – | 0.98 |
| Off-pump surgery | 2.91 | 0.69–12.2 | 0.15 |
| DSWI | 1.44 | 0.62–3.31 | 0.4 |
| Variables . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Age (years) | 1.05 | 1.01–1.1 | 0.013 |
| Female | 0.84 | 0.48–1.82 | 0.84 |
| Previous smoker | 1.16 | 0.61–2.18 | 0.66 |
| BMI >30 kg/m2 | 0.99 | 0.91–1.08 | 0.88 |
| Poor glycaemic controlb | 1.66 | 0.88–3.13 | 0.12 |
| Type 1 diabetes | 0.91 | 0.35–2.33 | 0.84 |
| Poor mobilityc | 1.63 | 0.33–8.1 | 0.55 |
| Chronic lung diseasec | 6.36 | 1.81–22.3 | 0.004 |
| GFR (ml/min)d | 0.98 | 0.96–0.99 | 0.009 |
| Dialysis | 1.34 | 0.32–5.63 | 0.69 |
| Extracardiac arteriopathyc | 1.05 | 0.59–1.87 | 0.88 |
| Congestive heart failure | 1.31 | 0.28–6.22 | 0.73 |
| Unstable angina | 1.12 | 0.61–2.05 | 0.72 |
| Recent myocardial infarctc | 0.87 | 0.42–1.8 | 0.71 |
| LVEF (%) | 0.97 | 0.95–1 | 0.035 |
| Previous cardiac surgery | 0 | – | 0.96 |
| Preoperative IABP | 0.81 | 0.21–3.1 | 0.76 |
| Emergency operationc | 0 | – | 0.98 |
| Off-pump surgery | 2.91 | 0.69–12.2 | 0.15 |
| DSWI | 1.44 | 0.62–3.31 | 0.4 |
BMI: body mass index; CI: confidence interval; DSWI: sternal wound infection; GFR: glomerular filtration rate; HR: hazard ratio; IABP: intra-aortic balloon pumping; LVEF: left ventricular ejection fraction.
aThe hospital discharged patients.
bBasal serum glucose >200 mg/dl at three consecutive measurements before surgery.
cThe definitions were those employed for EuroSCORE II [13].
dThe creatinine clearance rate, calculated according to the Cockcroft–Gault formula, was used for approximating the GFR.
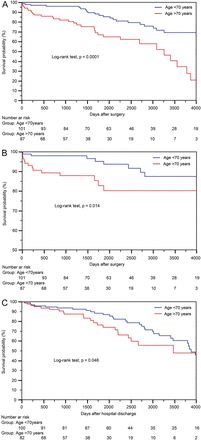
Non-parametric curves according to age (Kaplan–Meier model) of (A) overall survival (including hospital mortality), (B) freedom from cardiac and cerebrovascular death (including hospital mortality) and (C) freedom from MACCEs. The number of patients remaining at risk is reported. MACCE: major adverse cardiac and cerebrovascular event.
Functional status
During the follow-up, the New York Heart Association class was improved from 2.4 ± 0.6 preoperatively to 1.8 ± 0.9 (P = 0.001) postoperatively, and the Canadian Cardiovascular Society class from 3.1 ± 0.8 to 1.4 ± 0.7 (P < 0.001). There were 65 (35.7%) patients with at least 1 postoperative MACCE: sudden death (n = 3), recurrent angina (n = 22), myocardial infarct (n = 10), congestive heart failure (n = 44), percutaneous coronary intervention (n = 3) and stroke (n = 2). No patient underwent surgical reoperation. Of the 3 patients with unstable angina who underwent percutaneous coronary intervention during follow-up, coronary angiography had showed a critical lesion of the saphenous vein graft in 2 cases and a new critical lesion of the right coronary artery in 1; there was no disease of the BITA grafts. The 1-, 5- and 10-year non-parametric estimates of freedom from MACCEs were 96.1 (95% CI: 93.2–98.9), 83.6 (95% CI: 77.4–89.7) and 55.4% (95% CI: 44.7–66.1), respectively (Fig. 1C).
DISCUSSION
In CABG surgery, the use of bilateral versus single ITA grafts appears to offer superior long-term outcomes [3–6, 18, 19]. However, the rate of BITA use is still low, though it has been increasing over the years. According to recent reports, BITA grafting is performed in 4 and 12% of all CABG patients in North America and Europe, respectively, and less than 10% of the very young (<50 years of age) patients undergoing CABG surgery in the USA received the long-term survival benefits of BITA use [8, 20–22]. Concerns about the high risk of early complications, such as DSWI and perioperative bleeding, and the need for longer duration of surgery limit a more extensive use of BITA grafting. Indeed, most surgeons prudently prefer to avoid BITA use in elderly patients with high rates of the well-known predictors of sternal complications such as diabetes mellitus, morbid obesity, chronic lung disease and peripheral vascular disease [8]. Therefore, studies promoting benefits of BITA grafting arise necessarily from the preselected cohorts of patients where most of the patients have received single ITA grafts [18, 19].
Since 1986 the authors of the present study have been routinely performing BITA grafting at their Institution. Since 1999 they have been prospectively recording all perioperative data for every patient in a computerized data registry, the rate of BITA use being increased from about 60% in 1999 to over 80% in the last 2 years. All patients with multivessel coronary artery disease needing left-sided myocardial revascularization were potential candidates for BITA grafting. The sole exceptions have been the rare cases where one or both ITAs were unsuitable as coronary grafts, when there was an unexpected operative finding of severe cardiac dysfunction or when a rapid worsening of haemodynamics due to ischaemia needed immediate institution of cardiopulmonary bypass. Actually, there have been even some (exceptional) cases where the second ITA graft was harvested during cardiopulmonary bypass.
The present study retrospectively reviews the authors' experience in the routine use of skeletonized BITA grafts for left-sided myocardial revascularization in the difficult subset of patients suffering from diabetes mellitus and needing insulin treatment. The risk profiles of the present patients were generally superior to those from studies conducted on comparable populations of diabetics (with and without insulin dependence) with advanced ischaemic heart disease. This was due mainly to the higher rates of diabetes-related comorbidities such as renal failure and extracardiac arteriopathy [1–5, 7, 9, 11, 12]. There were a limited number of hospital deaths despite the high expected operative risk according to the EuroSCORE II. Old age (>75 years) and severe renal impairment were risk factors for hospital death according to the univariable analysis. No multivariable analysis was performed due to the limited number of deaths. There were generally frequent postoperative complications. Prolonged mechanical ventilation was favoured by a history of smoking, chronic lung disease, prolonged aortic cross-clamping and cardiopulmonary bypass times, diabetes-related airway infections, acute kidney injury and multiple transfusion. Fortunately, phrenic nerve paralysis was a rare and self-resolving complication. Acute kidney injury was facilitated by preoperative renal impairment, prolonged cardiopulmonary bypass time and multiple transfusion. Blood use was increased due to preoperative anaemia (chronic renal failure), perioperative bleeding and prolonged cardiopulmonary bypass time. Finally, DSWI was an expected and frequent postoperative complication. It was more frequent than reported in diabetic CABG patients by other authors, particularly with regard to the most serious forms such as osteomyelitis and mediastinitis. In the insulin-dependent diabetic patient, sternal wound infection might arise more frequently as DSWI or change quickly towards more serious forms of infection (immunodeficiency) [1–5, 7, 9, 11, 12]. The higher rate of sternal complications of the present series was in all probability due to the use of BITA grafts on a routine basis, without any preoperative selection of candidates for left-sided BITA grafting. Furthermore, only the results of insulin-dependent diabetic patients were reported. At the present authors' institution, the rates of DSWI following isolated BITA grafting in non-diabetic and noninsulin-dependent diabetic patients were 3.6 and 5.7%, respectively. Chronic lung disease, low cardiac output, multiple transfusion and mediastinal re-exploration for bleeding or tamponade were risk factors for DSWI according to the univariable analysis. No multivariable analysis was performed due to the limited number of events in relation to the number of variables that had to be considered in the analysis. Interestingly, old age, morbid obesity, extracardiac arteriopathy, renal impairment, poor preoperative glycaemic control and prolonged duration of surgery were not risk factors for DSWI. Although DSWIs were reduced by using the double-loop sternal wiring technique (see variable ‘early period (1999–2009)’ in Supplementary Table 2), the reduction was not significant (P = 0.15). Unlike what has previously been reported by some authors [9, 11], off-pump surgery was not a protective factor for DSWI. In the present study, however, off-pump and (rarely) beating-heart on-pump techniques have been used exclusively to avoid clamping a calcified ascending aorta. The two ITAs were simultaneously used as in situ grafts for up to 80% of all cases; when ITA was used as a free graft, it was preferably anastomosed to the in situ contralateral ITA (Y-graft). Probably due to both the institutional policy of reduced aortic manipulation and the routine use of epiaortic ultrasonography scanning, only one irreversible neurological complication occurred.
During the follow-up, there was significant symptomatic improvement. The coronary angiography did not show disease of the BITA grafts in any of the 3 patients who underwent percutaneous coronary intervention. No patient underwent repeat operation. Long-term overall survival, freedom from cardiac and cerebrovascular death and MACCEs were comparable with those cited in previous reports dealing with outcomes of diabetic patients who received BITA grafts [4–6], and superior to those of previous studies on outcomes following CABG surgery with single ITA grafts in diabetics [18, 19]. Old age, chronic lung disease, renal impairment and left ventricular dysfunction were predictors of decreased late survival. DSWI was not a predictor of poor late survival.
The primary limitations of the present study were the retrospective nature of the analysis and the fact that only a relatively small number of patients were evaluated at different times after surgery. All patients were insulin-dependent diabetics and underwent left-sided BITA grafting. No comparison was made between insulin-dependent and noninsulin-dependent diabetic patients, diabetic and non-diabetic patients, skeletonized and pedicled BITA grafts, off- and on-pump BITA grafting or, finally, between bilateral and single ITA use for myocardial revascularization. Since serum levels of glycated haemoglobin have not been available, preoperatively, for every patient of this retrospective study, basal serum glucose >200 mg/dl at three consecutive measurements before surgery was adopted as the marker of poor preoperative glycaemic control. No postoperative echocardiographic results were reported. Coronary angiography was performed only in (3) strongly symptomatic patients, and there was no direct information about patency of BITA grafts in all the remaining patients. Consequently, the results obtained can in no way be considered conclusive, and should be verified in a larger patient population by means of prospective controlled trials that include echocardiographic and angiographic evaluations.
In conclusion, left-sided BITA grafting may be performed routinely with low early (hospital) mortality even in insulin-dependent diabetic patients. Caution should be used in the elderly (>75 years) with severe renal impairment. The frequent postoperative complications are mainly due to high rates of diabetes-related comorbidities though the use of BITA grafts in these high-risk patients remains inevitably a strong predictor of sternal complications. The long-term outcomes are good.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Conflict of interest: none declared.
REFERENCES
- diabetes mellitus
- coronary revascularization
- ventricular dysfunction, left
- internal thoracic artery
- low cardiac output
- lung disease, chronic
- intrathoracic arterial bypass graft
- postoperative complications
- operative risk
- renal failure, acute
- follow-up
- objective (goal)
- sternum
- surgical procedures, operative
- heart
- insulin
- mediastinum
- mortality
- surgery specialty
- transplantation
- transfusion
- renal impairment
- older adult
- postoperative deep sternal wound infections




