-
PDF
- Split View
-
Views
-
Cite
Cite
Miriam Patella, Marco Anile, Paola Del Porto, Daniele Diso, Ylenia Pecoraro, Ilaria Onorati, Sara Mantovani, Tiziano De Giacomo, Fiorentina Ascenzioni, Erino A. Rendina, Federico Venuta, Role of cytokine profile in the differential diagnosis between acute lung rejection and pulmonary infections after lung transplantation†, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 6, June 2015, Pages 1031–1036, https://doi.org/10.1093/ejcts/ezu395
Close - Share Icon Share
Abstract
Acute lung rejection (ALR) is a relatively frequent complication during the first year after lung transplantation (LT). It is characterized by perivascular/bronchial mononuclear inflammation mediated by several cytokines. The aim of our study was to monitor a panel of cytokines extracted from the bronchoalveolar lavage (BAL) during the first year after LT and correlate them with clinical ALR.
Twenty double lung transplant recipients were prospectively assessed. Fifteen (75%) were affected by cystic fibrosis (CF). BAL was collected at seven different steps (pretransplant, immediately post-transplant, after 1 week, 1, 3, 6 months and 1 year). A panel of six cytokines was analysed: tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, macrophage inflammatory protein (MIP)-1α and IL-10. We correlated the cytokine levels with clinical ALR episodes, bacterial and cytomegalovirus (CMV) infections.
One hundred and thirty-eight BAL samples were collected and analysed. In CF patients, the levels of proinflammatory cytokines significantly dropped immediately after the transplant while they increased in all the other patients. Four patients (20%) died between 6 months and 1 year. Nine patients (45%) showed one clinical ALR episode within 6 months; in 6 (30%) patients, a bacterial pneumonia was diagnosed and 5 (25%) developed CMV infection. No differences with the complication rate between CF and non-CF patients were observed. During the infection episodes, all proinflammatory cytokines increased with low levels of IL-10; in case of ALR, levels of IL-1β and MIP-1α increased significantly (P = 0.01 and P < 0.0001), IL-10 levels were higher compared with the infection episodes (P = 0.03). No significant changes were observed for TNF-α, IL-6 and IL-8.
The BAL cytokine profile (IL-1β, MIP-1α and IL-10) seems useful to differentiate ALR and infections.
INTRODUCTION
According to the Registry of the International Society for Heart and Lung Transplantation [1], 33% of adult recipients experience at least one episode of acute lung rejection (ALR) during the first year after lung transplantation (LT).
From the clinical point of view, ALR can mimic pulmonary infection, with dyspnoea, low-grade fever, hypoxia, high white blood cell count and infiltrates on chest X-ray; however, it can also be asymptomatic. Resolution or strong improvement of symptoms after administration of steroids is considered a reliable ‘ex adiuvantibus’ diagnosis [2]. Tissue samples should be obtained for confirmation by trans-bronchial biopsy (TBB) as a routine surveillance or at the onset of symptoms [3]. The Lung Rejection Study Group recommended five parenchymal biopsies to adequately identify rejection [4]. However, the use of surveillance bronchoscopy to detect clinically silent episodes of ALR is controversial [5, 6]. Fibre-optic bronchoscopy with bronchoalveolar lavage (BAL) carries very low risks; TBB adds the risk of bleeding, pneumothorax and oversedation. Additionally, the interpretation of biopsies may be difficult since rejection and infection often coexist.
Considerable efforts have been made to discover surrogate or ancillary markers of ALR, in order to avoid the risks related to TBB and improve prompt and accurate diagnosis.
Several authors studied BAL to correlate cytological findings with histology at TBB. Total cellularity and percentage of lymphocytes, neutrophils, eosinophils, CD25+, CD4+ and CD8+ cells have been fully evaluated and correlated with rejection and infection [3, 7–10]. However, cytological and immunophenotypical profiles have proven to be neither reproducible nor to be sensitive and specific enough to be relied upon for accurate diagnosis [8, 11].
It is currently believed that ALR is orchestrated by the helper subset of T lymphocytes involving mainly the small pulmonary arterioles and veins. The cytokines produced by these activated cells stimulate proliferation, chemotaxis and activation of cytotoxic T lymphocytes, neutrophils and alveolar macrophages. Thus, several studies evaluated the cytokines profile in BAL of patients affected by ALR or lung infections. The potential role of interleukin (IL)-17 [10, 12], tumour necrosis factor (TNF)-α [13] and interferon (IFN)-γ [14] has been assessed without univocal results.
We evaluated a panel of proinflammatory [IL-1β, TNF-α, IL-6, IL-8 and macrophage inflammatory protein (MIP)-1α] and anti-inflammatory cytokines (IL-10) in the BAL of lung recipients collected before transplantation and during the first year of follow-up. We correlated the cytokine levels with the episodes of ALR, bacterial pneumonia and CMV infection in the attempt to provide a useful tool to differentiate ALR from infection.
PATIENTS AND METHODS
Study protocol
We prospectively enrolled in this study 20 patients undergoing LT at our institution; their characteristics are reported in Table 1. These patients went through a surveillance protocol consisting in bronchoscopy with BAL performed just before and immediately after transplantation, 7 days, 30 days, 3, 6 and 12 months after surgery. Additional bronchoscopies were performed when ALR or infection was clinically suspected. At the same time steps, cytomegalovirus (CMV) DNA polymerase chain reaction (PCR) on blood samples was assessed.
| Age, years (standard deviation) | 40.4 (±13.3) |
| Sex, n (%) | |
| Female | 11 (55) |
| Male | 9 (45) |
| Transplant indication, n (%) | |
| Cystic fibrosis | 15 (75) |
| Emphysema | 2 (10) |
| Idiopathic pulmonary fibrosis | 3 (15) |
| Type of transplant, n (%) | |
| Single | 3 (15) |
| Double | 17 (85) |
| Cytomegalovirus serology, n (%) | |
| D+/R− | 3 (15) |
| Complications, n (%) | |
| Symptomatic acute lung rejection | 9 (45) |
| Asymptomatic acute lung rejection | 2 (10) |
| Pneumonia | 6 (30) |
| Cytomegalovirus infection | 5 (25) |
| Age, years (standard deviation) | 40.4 (±13.3) |
| Sex, n (%) | |
| Female | 11 (55) |
| Male | 9 (45) |
| Transplant indication, n (%) | |
| Cystic fibrosis | 15 (75) |
| Emphysema | 2 (10) |
| Idiopathic pulmonary fibrosis | 3 (15) |
| Type of transplant, n (%) | |
| Single | 3 (15) |
| Double | 17 (85) |
| Cytomegalovirus serology, n (%) | |
| D+/R− | 3 (15) |
| Complications, n (%) | |
| Symptomatic acute lung rejection | 9 (45) |
| Asymptomatic acute lung rejection | 2 (10) |
| Pneumonia | 6 (30) |
| Cytomegalovirus infection | 5 (25) |
| Age, years (standard deviation) | 40.4 (±13.3) |
| Sex, n (%) | |
| Female | 11 (55) |
| Male | 9 (45) |
| Transplant indication, n (%) | |
| Cystic fibrosis | 15 (75) |
| Emphysema | 2 (10) |
| Idiopathic pulmonary fibrosis | 3 (15) |
| Type of transplant, n (%) | |
| Single | 3 (15) |
| Double | 17 (85) |
| Cytomegalovirus serology, n (%) | |
| D+/R− | 3 (15) |
| Complications, n (%) | |
| Symptomatic acute lung rejection | 9 (45) |
| Asymptomatic acute lung rejection | 2 (10) |
| Pneumonia | 6 (30) |
| Cytomegalovirus infection | 5 (25) |
| Age, years (standard deviation) | 40.4 (±13.3) |
| Sex, n (%) | |
| Female | 11 (55) |
| Male | 9 (45) |
| Transplant indication, n (%) | |
| Cystic fibrosis | 15 (75) |
| Emphysema | 2 (10) |
| Idiopathic pulmonary fibrosis | 3 (15) |
| Type of transplant, n (%) | |
| Single | 3 (15) |
| Double | 17 (85) |
| Cytomegalovirus serology, n (%) | |
| D+/R− | 3 (15) |
| Complications, n (%) | |
| Symptomatic acute lung rejection | 9 (45) |
| Asymptomatic acute lung rejection | 2 (10) |
| Pneumonia | 6 (30) |
| Cytomegalovirus infection | 5 (25) |
Dyspnoea, hypoxia, perihilar infiltrates at chest X-ray and forced expiratory volume in 1 second (FEV1) decline were considered clinical signs of ALR in patients without any evidence of infection. Infection was defined by ≥105 colony-forming units (CFU) of bacterial respiratory pathogens per ml of BAL fluid. More than 250 DNA copies per millilitre of plasma identified CMV viraemia.
BAL collection and processing
After wedging the bronchoscope in the lobe with radiological anomalies, or if absent, in the middle lobe or in the lingula, BAL was performed by instilling two 75-ml aliquots of sterile isotonic saline at room temperature. The fluid was aspirated and divided into two separate containers. The first sample was sent for microbiological analysis and the second one was stored at 0–4°C and processed for cytokines assay within 30 min.
Cytokine expression was analysed in undiluted BAL supernatants using the custom bead-based Multiplex kits for the Luminex Platform (Laboscope, Milan, Italy). Human IL-1β, TNF-α, IL-8, IL-6, MIP-1α and IL-10 were analysed using custom base kit (Human Fluorokine MAP Base Kit, PanelA, R&D System, Minneapolis, MN, USA) with the following analyte-specific bead sets: human TNF-α LUH210, human IL-1β/IL-1F2 LUH201, human CCL3/MIP-1α LUH270, human CXCL8/IL-8 LUH208, human IL-10 LUH217, human IL-6 LUH206 (R&D System) and analysed on a Bio-Plex (Bio-Rad, Hercules, CA, USA). The assays were performed in duplicate. The sensitivity of the assay was 1.50 pg/ml for TNF-α, 0.57 pg/ml for IL-1β, 0.30 pg/ml for IL-10, 1.45 pg/ml for MIP-1α, 1.97 pg/ml for IL-8 and 1.11 pg/ml for IL-6.
Immunosuppression and cytomegalovirus prophylaxis
Patients received a combination of either cyclosporine A (CsA) or tacrolimus (Tac) with methylprednisolone and azathioprine. Through plasma drug, levels of CsA and Tac were kept within the recommended therapeutic range (CsA: 200–400 µg/l or CsA C2: 800–1200 µg/l; Tac: 8–12 µg/l). Intravenous methylprednisolone was administrated in bolus doses of 125 mg t.i.d. for postoperative days 1–3, tapering it down to a final dose of 0.5 mg/kg/day orally. Azathioprine was administered at 2 mg/kg/day.
Patients with primary CMV mismatch (donor CMV+/recipient CMV−) received CMV hyperimmune gammaglobuline 0.5 ml/kg/day for the first 3 postoperative days, intravenous ganciclovir 5 mg/kg b.i.d. for 2 weeks and oral valganciclovir for the following 6 weeks.
All the patients started the prophylaxis against the bronchiolitis obliterans 3 weeks after the transplant, introducing the azithromycin at the dose of 500 mg for the first 5 days and tapering it down at 250 mg 3 days per week sine die.
Acute lung rejection, cytomegalovirus and bacterial infections treatment
At the onset of signs or symptoms as fever, leucopenia or leucocytosis, change in character/quantity of sputum, worsening gas exchange, new/worsening radiographic changes on chest X-ray or computed tomography scan, we performed the bronchoscopy with BAL (10 patients presented this clinical status at the time of the planned bronchoscopies). We immediately administrated to the patients a broad spectrum antibiotic coverage. After 72 h, we obtained the final microbiological culture with possibly antibiogram, which may confirm the presence of ≥105 CFU of bacterial respiratory pathogens per ml of BAL fluid. In this case, we continued the antibiotic therapy on the base of the new antibiogram. Patients did not receive any additional steroids out of the standard immunosuppressive therapy (methylprednisolone 0.5 mg/kg/day). If the microbiological culture was negative and the patients did not show any improvement with antibiotic administration, we considered the diagnosis of ALR and we switched to steroids boli. Episodes of clinical ALR were treated with intravenous methylprednisolone (1000 mg on Day 1, 500 mg on Days 2 and 3). Patients with CMV reactivations or primary infections received intravenous ganciclovir 5 mg/kg b.i.d. for 4–6 weeks or oral valganciclovir 900 mg b.i.d. for 8 weeks.
Statistical analysis
Data were reported as mean ± standard deviation and the differences were analysed with the one-way ANOVA analysis and post hoc tests. Correlation analysis was performed between cytokines during ALR and infection episodes with Pearson's correlation coefficient. Statistical significance was considered for P < 0.05. Statistical analysis was performed with SPSS Statistics version 17.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Of the 140 BAL samples planned (seven steps in 20 patients), we were able to collect and analyse 133 (95%) of them. Four samples at 1 year were not obtained because patients died between 6 months and 1 year after the transplant, and three were not processed for technical problems during storage. Of the planned bronchoscopies, 10 were performed for the presence of dyspnoea, hypoxia, decline of FEV1 and/or the presence of pulmonary infiltrates and 123 in asymptomatic patients. Five additional bronchoscopies with BAL were performed for the presence of clinical signs of ALR or infection at a time different from the planned bronchoscopies. Five patients had positive CMV PCR at the time of BAL; none of them showed any respiratory symptom. Thus, the overall number of samples processed was 138.
All the 15 patients affected by cystic fibrosis (CF) showed a lung bacterial colonization before the transplant. We observed the presence of Pseudomonas aeruginosa mucosa in 10 patients, Staphylococcus aureus in 4 patients and a combinations of both bacteria in 1 patient. One patient with emphysema showed the presence of P. aeruginosa rugosa in the BAL before the transplant. Six of the 15 BAL obtained in the presence of symptoms showed the presence of lung pathogens (≥105 CFU/ml of BAL fluid) such as P. aeruginosa, Haemophilus influenzae, S. aureus and Stenotrophomonas maltophilia, and these patients received a course of antibiotics. We observed five episodes of pneumonia occurring in CF patients; three of them represented a reactivation of pretransplant colonizing pathogens (P. aeruginosa in all cases). Two different episodes were observed in the same patient. One patient affected by idiopathic pulmonary fibrosis (IPF) experienced a pulmonary infection caused by a multidrug resistant S. aureus, which was not present in the pretransplant BAL culture. Symptomatic patients without any evidence of infection were treated with steroids with subsequent clinical and radiological improvement. Overall, no difference in the incidence of complications between CF and non-CF patients was observed (P = 0.08). During follow-up, we identified nine episodes of clinical ALR (two of them occurred in the same patient), six pulmonary bacterial infections and five CMV reactivations. All the ALR episodes occurred within the first 6 months after transplantation. Three patients developed separately ARL and infection at different time points during follow-up.
Pretransplant levels of all proinflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α and MIP1-α) were significantly higher in CF patients and dramatically dropped after surgery (P < 0.0001); on the other hand, non-CF patients presented an opposite trend of this group of cytokines (P = 0.03) (Fig. 1). Postoperative levels of proinflammatory cytokines reached similar values in both groups (P = 0.09). No differences between the two groups were observed in the preoperative and postoperative anti-inflammatory IL-10 levels. The BAL obtained during clinical episodes of ALR constantly showed higher levels of IL-1β and MIP-1α compared with those harvested in patients without ALR (Figs. 2 and 3). Particularly, patients with ALR presented levels of IL-1β and MIP-1α significantly increased when compared with those measured before the episode of ALR (254.2 vs 1731.5 pg/ml P < 0.0001 and 388.1 vs 1097.8 pg/ml, respectively; P = 0.01).

Pre- and post-transplant bronchoalveolar lavage levels of proinflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α and MIP1-α) in CF (P < 0.0001) and non-CF patients (P = 0.03). Postoperative levels of proinflammatory cytokines reached similar values in both groups (P = 0.09). Cytokines levels expressed in pg/ml on the Y-axis. CF: cystic fibrosis; IL: interleukin; TNF: tumour necrosis factor; MIP: macrophage inflammatory protein.

Bronchoalveolar lavage levels of IL-1β at the time of planned bronchoscopies. ALR versus non-ALR: P < 0.01. Cytokines levels expressed in pg/ml on the Y-axis. ALR: acute lung rejection; IL: interleukin.

Bronchoalveolar lavage levels of MIP-1α at the time of planned bronchoscopies. ALR versus non-ALR: P < 0.001. Cytokines levels expressed in pg/ml on the Y-axis. ALR: acute lung rejection; MIP: macrophage inflammatory protein.
During clinical ALR episodes, IL-6, IL-8 and TNF-α levels were higher when compared with stable patients, but this difference did not reach statistical significance.
In patients with infections, proinflammatory cytokine levels univocally increased; particularly, bacterial infections showed higher levels of TNF-α, IL-1β, IL-6 and IL-8 when compared with CMV infections, whereas MIP-1α was similar in both situations (Fig. 4).

Bronchoalveolar lavage levels of proinflammatory cytokines during bacterial and CMV infection episodes. IL-1β: P < 0.001, IL-6: P = 0.002, IL-8: P = 0.003, TNF-α = 0.005, MIP-1α: P = 0.07. Cytokines levels expressed in pg/ml on the Y-axis. CMV: cytomegalovirus; IL: interleukin; TNF: tumour necrosis factor; MIP: macrophage inflammatory protein.
The trend of IL-10 was completely different in patients with infections and ALR (Fig. 5). In case of CMV or bacterial infection, IL-10 remains low with an increase of proinflammatory cytokines. On the other hand, during ALR episodes, we observed an increase in IL-10 levels compared with baseline and to patients with infections (3.54 vs 14.22 pg/ml P = 0.002) (Fig. 6). During the surveillance bronchoscopies, performed in asymptomatic patients, we observed the presence of P. aeruginosa or S. aureus at low count in 80% of CF patients. At the same time, we did not observe any substantial increasing of the proinflammatory cytokines so we did not treat them as infections episodes. During the surveillance bronchoscopies, we also identified in two patients a cytokine BAL pattern similar to those with clinical ALR. These patients underwent TBB that showed histological grade A1 rejection and received steroid therapy.
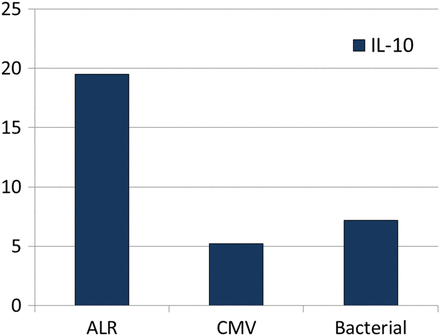
Bronchoalveolar lavage levels of IL-10 during ALR, CMV and bacterial infections. During ALR episodes IL-10 levels were higher compared with CMV and bacterial infections (P = 0.005). Cytokines levels expressed in pg/ml on the Y-axis. IL: interleukin; TNF: tumour necrosis factor; MIP: macrophage inflammatory protein; ALR: acute lung rejection; CMV: cytomegalovirus.
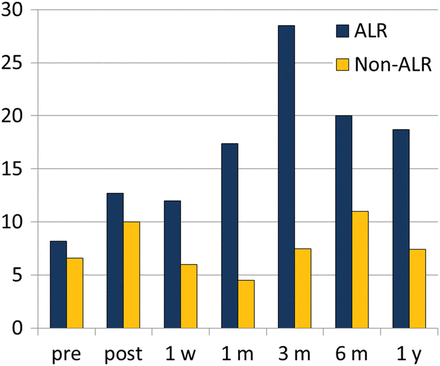
Bronchoalveolar lavage levels of IL-10 at the time of planned bronchoscopies. ALR versus non-ALR: P = 0.002. Cytokines levels expressed in pg/ml on the Y-axis. ALR: acute lung rejection; IL: interleukin.
DISCUSSION
ALR and pulmonary infections represent the most frequent early complications after LT [1]; repeated episodes of ALR predispose to the subsequent onset of bronchiolitis obliterans syndrome (BOS). Thus, it is mandatory to promptly diagnose and differentiate these two clinical entities and start early treatment.
BAL is recognized as the more reliable, accurate and safe method for routine microbiological detection of infections; at many centres, the gold standard to diagnose ALR episodes is still histology at TBB. However, despite the standardization of pathological grading, few studies have addressed the reproducibility of histological interpretation of ALR at TBB [15, 16]. Furthermore, TBB can potentially present a number of complications including bleeding and pneumothorax and there is a considerable exposure to radiation both for the patient and the staff. Consequently, BAL appears to be an attractive and less invasive method to study the graft immunological profile.
Cytokine production following transplantation occurs at two steps: the early antigen-independent cascade regulated by the recipient immunological status, surgical trauma, donor brain death and ischaemia–reperfusion injury and the late antigen-dependent cascade that directs the activated recipient lymphocytes (predominantly effector T cells) into the graft. Different networks existing between proinflammatory and anti-inflammatory mediators lead to adequate responses to infection, graft tolerance or rejection. The pattern of cytokines released into the microenvironment dictates the direction of the immune reaction towards the type of response. We have chosen to study a panel of cytokine including IL-1β, IL-6, IL-8, TNF-α, MIP-1α and IL-10 on the basis of their characteristics. IL-1β, IL-6, IL-8, TNF-α and MIP-1α are considered proinflammatory cytokines, particularly involved in the acute phase of immune reaction by promoting the differentiation of Th1 lymphocytes. IL-10 is considered an anti-inflammatory cytokine that inhibits the development of alloreactive Th1 cells and deviates the immune reaction towards a Th2-type response.
In CF patients, we observed a significant drop in all the proinflammatory cytokines immediately after surgery while in the other patients the same molecules increased. This finding can be explained by the recipient immunological status at the time of transplantation. Airway inflammation and high levels of IL-6, IL-8 and MIP-1α have been identified in patients with CF even in the absence of active infection [17, 18]. These changes in the basal cytokines profile may explain our post-surgical findings. In these patients, even if surgical trauma and ischaemia–reperfusion injury cause an important inflammatory response, the benefit brought by the removal of the infected lungs seems to be more effective on the immunological status. In contrast, for patients with IPF and emphysema, the effects of surgery are clearly visible through the profile of proinflammatory mediators when compared with the preoperatory status. Other authors reported similar results: Teixeira et al. [19] analysed the expression of inflammatory cytokines in the pleural fluid and they observed increased levels of IL-1β, IL-6 and IL-8 6 h after transplantation with a progressive decrease over time.
In case of infection, high proinflammatory cytokine levels were recorded. These mediators are strongly related with the acute inflammatory response and they drive the immune system towards the acute phase and the Th1 type of reaction. Thus, it is not surprising to observe this pattern.
During ALR, the BAL levels of IL-1β and MIP-1α increased significantly; also IL-10 levels were higher compared with infection episodes in all patients. The production of IL-1β has been associated with ALR, ischaemia–reperfusion injury and BOS in several studies: in fact, it can act as proinflammatory cytokine associated with epithelial damage [20]. Interestingly, we observed that the level of TNF-α does not increase significantly during ALR episodes. IL-1β and TNFα mediate the acute inflammatory response through neutrophils and monocyte recruitment. They induce the expression of adhesion molecules on vascular epithelium, which could lead to cell adhesion and extravasation resulting in lung allograft injury. They have an apparently redundant function, but they differ because IL-1β can be produced by macrophages as well as by neutrophils, epithelial and endothelial cells. This could reflect the impact of time on the cellular population and cytokine production. In fact, all the ALR episodes occurred within 6 months and we can speculate that the IL-1β is produced from activated macrophages and epithelial cells of the graft, while the later-occurring rejection could be associated with production of inflammatory cytokines from other cells.
The role of MIP-1α is also interesting. It has been demonstrated that infection might induce the expression of MIP-1α and that IL-10 is capable to block the production of MIP-1α released by LPS-stimulated macrophages [21]. Moreover, MIP-1α is secreted at low concentrations in the presence of CMV infection and it should play a role in containing viral infection [22]. In our patients, high levels of IL-10 detected in BAL of patient with ALR did not influence the concomitant secretion of MIP-1α, suggesting a different response to noninfectious stimuli.
Concerning IL-10, we observed a lower expression in the BAL of infected patients compared with ALR episodes. IL-10, a type 2 cytokine with immunomodulatory activity has an unclear role in allograft rejection [23, 24]. It is possible that the immune response caused by symptomatic pulmonary infections is strongly polarized through the Th1 type and, compared with ALR episodes, the level of IL-10 remains lower.
According to our policy, we identified and treated symptomatic ALR episodes based on clinical and radiological findings without pathological confirmation. Based on the preliminary results of the study that suggested a specific cytokine pattern related with ALR, we also identified two clinical silent episodes of ALR. These patients had a cytokine BAL profile with increased levels of IL-1β, MIP-1α and IL-10, negative culture for lung pathogens and negative CMV PCR; they underwent a second fibre-optic bronchoscopy with TBB, showing the presence of perivascular mononuclear infiltrates compatible with grade A1 rejection. These patients received intravenous steroids and the following BAL showed normal levels of cytokines. Looking back to the first enrolled patient, we retrospectively identified another sample with a cytokine milieu compatible with an episode of ALR that corresponds to a patient with signs of BOS 30 months after the transplantation.
Colombat et al. [15] published a study correlating histology at TBB with the clinical status. Despite the lack of histological lesions compatible with ALR at TBB of symptomatic patients, and taking into account the worsening or the absence of improvement after antibiotics administration, treatment against rejection was administered with a positive response in 90% of the patients. McWilliams et al. [5] reported a positive correspondence between clinical suspicion of ALR and ≥A2 grade rejection at TBB of 15.7%, and this finding was able to modify clinical management in 43.8% of the patients. In our series, all the symptomatic patients treated for suspected ALR improved with steroids from both the clinical and radiological point of view.
On the other hand, clinical judgement does not identify asymptomatic ALR episodes; in these patients, the value of surveillance bronchoscopy to identify and treat ALR with the goal of reducing development of BOS is still unclear [6]. Results are often controversial particularly concerning the benefits on survival [25]. A screening programme based on less invasive diagnostic methods like cytokines assay on BAL should avoid unnecessary TBB and focus the attention on the group of patients at risk.
The major limitation of our study is represented by the lack of pathological confirmation of rejection that it is still considered undisputed. However, based on the positive response to steroid treatment in all the patients, we can assume that clinical suspicion of ARL was reliable. Another limitation is the small number of patients enrolled in the study; in addition, the vast majority of this group includes CF patients and the immunological status related to the disease could potentially influence the results.
In conclusion, notwithstanding these limitations, our study supports the value of cytokine BAL profile in the early diagnosis of symptomatic episodes of ALR, in the screening of silent episodes of ALR and to differentiate rejection and infection. Even if both ALR and infections elicit the Th1 type of response, the underlying mechanisms should be different, and it is mandatory to understand them to better evaluate and treat these complications.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 22nd European Conference on General Thoracic Surgery, Copenhagen, Denmark, 15–18 June 2014.
Fondazione Eleonora Lorillard Spencer Cenci.
- cytokine
- tumor necrosis factors
- lung transplantation
- lung
- bronchial lavage
- cystic fibrosis
- differential diagnosis
- interleukin-10
- interleukin-8
- interleukins
- macrophage inflammatory proteins
- rejection (psychology)
- infections
- cytomegalovirus infections
- cytomegalovirus
- interleukin-6
- transplantation
- lung infections




