-
PDF
- Split View
-
Views
-
Cite
Cite
Akiko Tanaka, Tomonori Shirasaka, Kenji Okada, Yutaka Okita, Aggressive multiple surgical interventions to pulmonary artery sarcoma, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 2, February 2015, Pages 384–385, https://doi.org/10.1093/ejcts/ezu184
Close - Share Icon Share
Abstract
We describe our experience with a patient who had metastasized pulmonary artery sarcoma, but survived 7 years after diagnosis. A 61-year-old man was diagnosed with pulmonary artery intimal sarcoma after resection of metastatic tumours to the bilateral lungs. The primary lesion in the pulmonary artery trunk extending into the bilateral branches was treated by tumour endoarterectomy followed by chemotherapy. He underwent resections of lung metastases two more times before detection of recurrent obstructive pulmonary artery sarcoma 4 years after the tumour endoarterectomy. En bloc resection of the tumour including the pulmonary artery trunk, valve and interventricular septum was performed, and the right ventricular out flow tract was reconstructed with a stentless pulmonary valve and equine pericardium. He died of the disease soon after an operation for metastatic brain tumour 3 years later. Pulmonary artery sarcoma has a dismal prognosis, but aggressively repeated surgical interventions may lengthen survival.
INTRODUCTION
Since the first description of primary pulmonary artery sarcoma by Mandelstamm [1], nearly 200 cases have been reported with dismal prognosis, and misdiagnosis as pulmonary thromboembolism is not uncommon [2]. Radical surgery provides the best chance of survival, however, surgical therapy is often abandoned for metastasized or recurrent tumours. We report here a case of pulmonary artery sarcoma, in which the patient survived 7 years through repeated resections of primary and recurrent sarcomas and metastatic lesions.
CASE
A 61-year-old male was operated on for multiple metastatic lung tumours in the bilateral lungs 5 years after a surgical resection of sigmoid cancer. Distal metastases of the cancer were suspected before the operation, but the pathological examination revealed that the metastatic tumours were sarcomas, and further evaluations for the primary lesion were performed. Enhanced computed tomography (CT) showed a defect in the main pulmonary trunk extending into the right pulmonary artery (Fig. 1A). A positron emission tomographic (PET)–CT scan also revealed increased fluorodeoxyglucose uptake in the pulmonary artery trunk and the right pulmonary artery. He was diagnosed with primary pulmonary artery sarcoma and transferred to our institute. Pulmonary tumour endoarterectomy was performed under deep hypothermic cardiac arrest (Fig. 1B). The surgical margins were confirmed negative for tumours by both intraoperative frozen section analysis and haematoxylin and eosin staining. The microscopic findings showed atypical spindle-shaped tumours with high mitosis activity, and fascicular growth and a pericytomatous pattern in a myxoid and collagenized background was present. Immunohistochemical staining of the tumour cells was positive for α-smooth muscle actin, HHF-35, CD34, CD117 and bcl-2, but negative for desmin, Myo-D1 and epithelial membrane antigen, and a diagnosis of intimal sarcoma was made accordingly. The patient underwent six cycles of adjuvant chemotherapy with interferon and doxorubicin. During the next 4 years, two additional operations for metastatic lung sarcomas were performed. Subsequently, the sarcoma relapsed as an obstructive mass in the right ventricular outflow tract, adhered to the pulmonary valve (Fig. 2). The tumour was resected en bloc including the main pulmonary artery trunk, the pulmonary valve and part of the interventricular septum, in the manner of harvesting a pulmonary autograft for the Ross procedure. The right ventricular outflow tract was reconstructed with a stentless valve and equine pericardium. The postoperative courses of both the first and second cardiac procedures were uneventful. No evidence of local recurrence was detected by echocardiography, CT and PET–CT after the second cardiac operation.
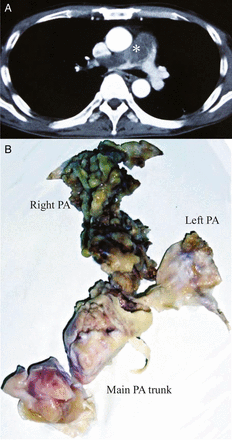
(A) A computed tomography image showing an obstructive pulmonary tumour extending from the main pulmonary trunk into the bilateral branches. Asterisk denotes the tumour. (B) The resected specimen of the tumour endoarterectomy. PA: pulmonary artery.
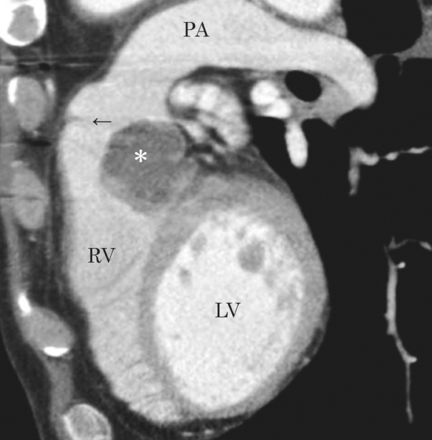
A computed tomography scan demonstrating a recurrent sarcoma in sagittal view. Asterisk denotes the tumour. Arrow: pulmonary valve; RV: right ventricle; LV: left ventricle; PA: pulmonary artery.
The patient was able to spend most of the time at home, and lived for 7 years after the first diagnosis of pulmonary sarcoma until he died of the disease soon after the palliative resection of metastatic brain tumour.
DISCUSSION
Although imaging tools have advanced considerably in recent years, pulmonary artery sarcoma grows so insidiously that the diagnosis is often delayed and pulmonary metastases are present in 50% of the patients at diagnosis [2]. In addition, pulmonary artery sarcomas are rare and are often misdiagnosed as pulmonary thromboembolism because of their similar clinical symptoms. Such delays in diagnosis and distal spread of the disease make the sarcoma a fatal disease, with survival of 36.5 ± 20.2 months for patients who underwent curative resection and 11 ± 3 months for those who underwent incomplete resection [3].
The tumour mostly originates in the pulmonary trunk, and both the right and left branches are likely involved, where pulmonary metastases are common. Although retrograde extension of the tumour to the right ventricle is less likely observed [2], the right ventricular outflow tract and the pulmonary artery trunk embryologically have the same origin, the bulbus cordis, where the tumour is suggested to originate from. Thus, it is necessary to be aware of the potential for local recurrence when the right ventricular outflow tract is left untouched as in our case and other's [4].
CT characteristics suggestive of pulmonary artery sarcomas include inhomogenous high or low attenuation, soft tissue density with the particular filling defect occupying the entire lumen of the pulmonary trunk with an increase in diameter of the involved vessel, and patchy and delayed contrast enhancement with CT angiography. Once suspicion is raised, PET–CT and magnetic resonance imaging are more helpful to differentiate between sarcoma and pulmonary embolism [5].
The typical cause of death in this entity is right-sided heart failure as a result of the right ventricular outflow obstruction. Curative surgical resection of the tumour is not always possible due to advanced disease, and simple debulking of the obstructive mass may be chosen to restore haemodynamics, but this incomplete resection may lead to an early relapse [3]. Reported curative surgical procedures for treating the tumour include tumour endoarterectomy, graft reconstruction of the pulmonary artery and pneumonectomy. The impact of chemotherapy and radiotherapy in treating pulmonary artery sarcoma remains unclear. There are several cases that report partial response or stable disease through chemotherapy or radiotherapy [4], but complete remission cannot be obtained without surgical resection.
In conclusion, aggressively repeated surgical interventions of the primary tumour, local recurrence and metastatic lesions were helpful to prolong the survival of a patient with pulmonary artery sarcoma. Patients with pulmonary artery sarcoma should be considered for a referral to an experienced centre to receive a complete resection.
Conflict of interest: none declared.
REFERENCES
- pericardial sac
- pulmonary artery
- lung
- metastatic malignant neoplasm to brain
- chemotherapy regimen
- heart ventricle
- equus caballus
- neoplasm metastasis
- pulmonary valve
- surgical procedures, operative
- diagnosis
- neoplasms
- metastasis to the lung
- interventricular septum
- trunk structure
- sarcoma, intimal
- malignant pulmonary artery neoplasm
- fluid flow




