-
PDF
- Split View
-
Views
-
Cite
Cite
David R. Koolbergen, Johan S. J. Manshanden, Berto J. Bouma, Nico A. Blom, Barbara J. M. Mulder, Bas A. J. M. de Mol, Mark G. Hazekamp, Valve-sparing aortic root replacement, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 2, February 2015, Pages 348–354, https://doi.org/10.1093/ejcts/ezu167
Close - Share Icon Share
Abstract
To evaluate our results of valve-sparing aortic root replacement and associated (multiple) valve repair.
From September 2003 to September 2013, 97 patients had valve-sparing aortic root replacement procedures. Patient records and preoperative, postoperative and recent echocardiograms were reviewed. Median age was 40.3 (range: 13.4–68.6) years and 67 (69.1%) were male. Seven (7.2%) patients were younger than 18 years, the youngest being 13.4 years. Fifty-four (55.7%) had Marfan syndrome, 2 (2.1%) other fibrous tissue diseases, 15 (15.5%) bicuspid aortic valve and 3 (3.1%) had earlier Fallot repair. The reimplantation technique was used in all, with a straight vascular prosthesis in 11 (26–34 mm) and the Valsalva prosthesis in 86 (26–32 mm). Concomitant aortic valve repair was performed in 43 (44.3%), mitral valve repair in 10 (10.3%), tricuspid valve repair in 5 (5.2%) and aortic arch replacement in 3 (3.1%).
Mean follow-up was 4.2 ± 2.4 years. Follow-up was complete in all. One 14-year old patient died 1.3 years post-surgery presumably of ventricular arrhythmia. One patient underwent reoperation for aneurysm of the proximal right coronary artery after 4.9 years and 4 patients required aortic valve replacement, 3 of which because of endocarditis after 0.1, 0.8 and 1.3 years and 1 because of cusp prolapse after 3.8 years. No thrombo-embolic complications occurred. Mortality, root reoperation and aortic regurgitation were absent in 88.0 ± 0.5% at 5-year follow-up.
Results of valve-sparing root replacement are good, even in association with a high incidence of concomitant valve repair. Valve-sparing aortic root replacement can be performed at a very young age as long as an adult size prosthesis can be implanted.
INTRODUCTION
Long-term results of valve-sparing root replacement (VSRR) are very encouraging, both in degenerative aneurysm disease [1, 2] as well as in connective tissue disorder [3, 4]. Consequently, indications and applications are expanding [5–7] and the valve-sparing operation is incorporated more and more into the routine practice of the general and congenital cardiac surgeon. Nonetheless, different surgeons are still searching for further improvements to optimize the valve-sparing aortic root replacement technique [8, 9]. In our centre for congenital heart disease Amsterdam–Leiden (CAHAL), we are gaining experience in performing elective valve-sparing operations for treatment of aortic root aneurysm in adults and adolescents with connective tissue disorders, valve pathology and congenital heart defects. In this series, a high number of concomitant valve repairs and other surgical procedures were performed. With the use of the Valsalva graft (Gelweave Valsalva™ by Sultzer Vascutek, Renfrewshire, Scotland) a fast and reproducible technique was developed in order to keep cross-clamp and bypass times within safe limits for multiple procedure cardiac surgery. An efficient modification of sub-commissural annuloplasty is presented that we used in patients in whom aortic annulus reduction was required. With this study our short to mid-term results are evaluated.
PATIENTS AND METHODS
Study design
Aortic valve-sparing operations were performed in a total number of 97 adolescent and adult patients (mean age 38.4 ± 12.8 years; range: 13.4–68.6 years; minimal body weight ≥50 kg) with congenital heart disease at the Academic Medical Centre in Amsterdam and Leiden University Medical Center from September 2003 to September 2013. All patients had aortic valve-sparing root replacement by means of reimplantation of the aortic valve into a vascular prosthesis. Two surgeons at two locations performed the surgery. A retrospective study was performed analysing patient records and preoperative, postoperative and recent echocardiograms.
Patient characteristics
Patient characteristics and preoperative clinical profile are listed in Table 1. Clinical diagnoses were Marfan syndrome in 54 (55.7%), Loeys–Dietz in 2 (2.2%) and bicuspid aortic valve in 15 (15.5%). Three patients received VSRR late after tetralogy of Fallot correction; one after a previous Yacoub procedure; another one late after a Ross procedure and five after repair of aortic coarctation in childhood. Preoperative aortic root diameter was 49.5 ± 4.9 mm and mean preoperative annular diameter was 27.1 ± 3.0 mm. Preoperative left ventricular ejection fraction (LVEF) was >50% in 90 (92.8%), 30–50% in 7 (7.2%) and <30% in none. Aortic regurgitation (AR) was moderate or severe in 20 (20.6%), mild in 18 (18.6%) and absent in 59 (60.8%) patients on preoperative echocardiograms.
Clinical profile of all adolescent and adult patients with aortic root aneurysm
| . | All patients, n = 97 (%) . |
|---|---|
| Mean age (years ± SD) | 38.4 ± 12.8 |
| Male gender | 67 (69.1) |
| Diagnoses | |
| Marfan syndrome | 54 (55.7) |
| Loeys–Dietz syndrome | 2 (2.1) |
| Suspect tissue disorder | 11 (11.3) |
| Associated diseases | |
| Body mass index >30 | 10 (10.3) |
| Smoking | 28 (28.9) |
| Diabetes | 1 (1.0) |
| Hypertension | 21 (21.6) |
| High cholesterol | 2 (2.1) |
| Chronic obstructive lung disease | 2 (2.1) |
| EuroSCORE (r) ± SD | 6.6 ± 4.7 |
| Previous surgery: | |
| Total Fallot correction | 3 (3.1) |
| Ross procedure | 1 (1.0) |
| Aortic coarctationa | 5 (5.2) |
| VSRR (Yacoub) | 1 (1.0) |
| Bicuspid aortic valve | 15 (15.5) |
| Left ventricular ejection fraction | |
| >50% | 90 (92.8) |
| 30–50% | 7 (7.2) |
| <30% | 0 (0.0) |
| Aortic root ø, mm (mean ± SD) | 49.5 ± 4.9 |
| Aortic annulus ø, mm (mean ± SD) | 27.1 ± 3.0 |
| Aortic regurgitation | |
| None/trivial (0) | 59 (60.8) |
| Mild (1+) | 18 (18.6) |
| Moderate (2+) | 10 (10.3) |
| Severe (3+) | 10 (10.3) |
| Urgent/emergency surgery | 0 (0.0) |
| . | All patients, n = 97 (%) . |
|---|---|
| Mean age (years ± SD) | 38.4 ± 12.8 |
| Male gender | 67 (69.1) |
| Diagnoses | |
| Marfan syndrome | 54 (55.7) |
| Loeys–Dietz syndrome | 2 (2.1) |
| Suspect tissue disorder | 11 (11.3) |
| Associated diseases | |
| Body mass index >30 | 10 (10.3) |
| Smoking | 28 (28.9) |
| Diabetes | 1 (1.0) |
| Hypertension | 21 (21.6) |
| High cholesterol | 2 (2.1) |
| Chronic obstructive lung disease | 2 (2.1) |
| EuroSCORE (r) ± SD | 6.6 ± 4.7 |
| Previous surgery: | |
| Total Fallot correction | 3 (3.1) |
| Ross procedure | 1 (1.0) |
| Aortic coarctationa | 5 (5.2) |
| VSRR (Yacoub) | 1 (1.0) |
| Bicuspid aortic valve | 15 (15.5) |
| Left ventricular ejection fraction | |
| >50% | 90 (92.8) |
| 30–50% | 7 (7.2) |
| <30% | 0 (0.0) |
| Aortic root ø, mm (mean ± SD) | 49.5 ± 4.9 |
| Aortic annulus ø, mm (mean ± SD) | 27.1 ± 3.0 |
| Aortic regurgitation | |
| None/trivial (0) | 59 (60.8) |
| Mild (1+) | 18 (18.6) |
| Moderate (2+) | 10 (10.3) |
| Severe (3+) | 10 (10.3) |
| Urgent/emergency surgery | 0 (0.0) |
VSSR: Valve-sparing root replacement.
aAortic coarctation: end-to-end anastomosis.
Clinical profile of all adolescent and adult patients with aortic root aneurysm
| . | All patients, n = 97 (%) . |
|---|---|
| Mean age (years ± SD) | 38.4 ± 12.8 |
| Male gender | 67 (69.1) |
| Diagnoses | |
| Marfan syndrome | 54 (55.7) |
| Loeys–Dietz syndrome | 2 (2.1) |
| Suspect tissue disorder | 11 (11.3) |
| Associated diseases | |
| Body mass index >30 | 10 (10.3) |
| Smoking | 28 (28.9) |
| Diabetes | 1 (1.0) |
| Hypertension | 21 (21.6) |
| High cholesterol | 2 (2.1) |
| Chronic obstructive lung disease | 2 (2.1) |
| EuroSCORE (r) ± SD | 6.6 ± 4.7 |
| Previous surgery: | |
| Total Fallot correction | 3 (3.1) |
| Ross procedure | 1 (1.0) |
| Aortic coarctationa | 5 (5.2) |
| VSRR (Yacoub) | 1 (1.0) |
| Bicuspid aortic valve | 15 (15.5) |
| Left ventricular ejection fraction | |
| >50% | 90 (92.8) |
| 30–50% | 7 (7.2) |
| <30% | 0 (0.0) |
| Aortic root ø, mm (mean ± SD) | 49.5 ± 4.9 |
| Aortic annulus ø, mm (mean ± SD) | 27.1 ± 3.0 |
| Aortic regurgitation | |
| None/trivial (0) | 59 (60.8) |
| Mild (1+) | 18 (18.6) |
| Moderate (2+) | 10 (10.3) |
| Severe (3+) | 10 (10.3) |
| Urgent/emergency surgery | 0 (0.0) |
| . | All patients, n = 97 (%) . |
|---|---|
| Mean age (years ± SD) | 38.4 ± 12.8 |
| Male gender | 67 (69.1) |
| Diagnoses | |
| Marfan syndrome | 54 (55.7) |
| Loeys–Dietz syndrome | 2 (2.1) |
| Suspect tissue disorder | 11 (11.3) |
| Associated diseases | |
| Body mass index >30 | 10 (10.3) |
| Smoking | 28 (28.9) |
| Diabetes | 1 (1.0) |
| Hypertension | 21 (21.6) |
| High cholesterol | 2 (2.1) |
| Chronic obstructive lung disease | 2 (2.1) |
| EuroSCORE (r) ± SD | 6.6 ± 4.7 |
| Previous surgery: | |
| Total Fallot correction | 3 (3.1) |
| Ross procedure | 1 (1.0) |
| Aortic coarctationa | 5 (5.2) |
| VSRR (Yacoub) | 1 (1.0) |
| Bicuspid aortic valve | 15 (15.5) |
| Left ventricular ejection fraction | |
| >50% | 90 (92.8) |
| 30–50% | 7 (7.2) |
| <30% | 0 (0.0) |
| Aortic root ø, mm (mean ± SD) | 49.5 ± 4.9 |
| Aortic annulus ø, mm (mean ± SD) | 27.1 ± 3.0 |
| Aortic regurgitation | |
| None/trivial (0) | 59 (60.8) |
| Mild (1+) | 18 (18.6) |
| Moderate (2+) | 10 (10.3) |
| Severe (3+) | 10 (10.3) |
| Urgent/emergency surgery | 0 (0.0) |
VSSR: Valve-sparing root replacement.
aAortic coarctation: end-to-end anastomosis.
Indication for surgery
Primary indication for VSRR in all patients was aneurysm of the aortic root. The criteria we used to recommend surgical intervention were based on the international guidelines of the European Society of Cardiology; thresholds for surgery were 50 mm root diameter at the sinus level in patients with connective tissue disease and 45 mm if risk factors like family history of aortic dissection, progressive dilatation (>2 mm/year), severe AR or mitral valve regurgitation and desire for pregnancy were present [10]. For patients with bicuspid valves, the threshold recently changed from 50 to 55 mm in the absence of other risk factors according to the latest ESC guidelines on valvular heart disease (version 2012) [11]. The aortic annulus was considered dilated >25 mm for adult patients.
Surgical technique
The first 11 patients had the aortic valve reimplanted into a straight vascular prosthesis (Hemashield™ by Maquet, Rastat, Germany) (size range: 26–34 mm) of which one-third (4) were implanted in adolescents. In these patients, the Stanford modification of the ‘David-V’ valve-sparing procedure was performed as described by Demers and Miller [12]. Since 2003, the next 86 patients had the aortic valve implanted into the Valsalva prosthesis (Gelweave Valsalva™ by Sultzer Vascutek, Renfrewshire, Scotland) (size range: 26–32 mm) with a modified reimplantation technique as described by Pacini et al. [13] and the group of de Kerchove et al. [14].
Graft sizing and preserving root geometry
After installing cardiopulmonary bypass, aortic clamping and complete cardioplegic arrest a transverse aortotomy was performed ∼1 cm above the sinotubular junction (STJ). The ascending aorta was resected, leaving a 1 cm collar for the distal anastomosis. Stay sutures were placed at the top of the commissures (4–0 polypropylene) and notice was taken whether the tops of the commissures were situated in one horizontal plane or not. Cusps were inspected for fenestrations that might interfere with a valve-sparing procedure, after which cuspal geometric height was measured. Large central fenestrations (>50% of coapatation area) were regarded as contraindication for a valve-sparing procedure while smaller fenestrations (<50% of coaptation area) in the commissural area were accepted. Cuspal geometric height was measured from the nadir of the cusp up to the central free edge with the cusp gently stretched. Short cuspal geometric height (<17 mm) of one or more cusps was regarded as contraindication for valve-sparing procedure. The coronary buttons were cut out and the base of the aorta was dissected externally down to sub-annular level, followed by excision of sinus tissue; leaving ∼4–5 mm of remnant tissue attached to the annulus. While holding the commissural stay sutures in upward position and creating low pressure in the left ventricle using the left vent, coaptation of the cusps was installed and facilitated. Now, while using valve sizers and moving the stay sutures in- and outwards, we determined the diameter of the sinotubular junction that provides optimal leaflet apposition. The selected Valsalva graft was consistently 4 mm larger, as the prosthesis was fitted outside of the aortic valve complex, usually a 28 or 30 mm graft for adult patients. The graft was roughly trimmed at both ends. Next, 2–0 braided polyester pledged horizontal mattress sutures were passed under the annulus to the outside of the root (normally between 12 and 15 sutures). While gently stretching the commissures, the distance between the suture beneath the commissure and the suture at the top of the commissures was measured; this distance was marked on the prosthesis. The level of the ‘neo-sinotubular junction’ on the Valsalva graft was used as a reference point to create the new sinotubular junction; hereby respecting and restoring root geometry as much as possible. A line was drawn on the graft indicating the expected placement of the mattresses according to the observed root geometry. The commissural stay sutures were passed proximal to distal through the graft lumen and the mattress sutures were passed to the graft at the marked line. Now, a Hegar dilator with the desired annulus size was placed through the aortic valve after which the graft was tied down. A Hegar 23 mm was used for patients <70 kg and a Hegar 24 mm for patients >70 kg. The three stay sutures were now passed through the graft at the level of the neo-sinotubular junction and again kept in upward position while creating low pressure in the left ventricle; the coaptation length and height was judged and corrected if necessary. With 4–0 polypropylene sutures, the rim of sinus tissue was sutured to the graft, making sure that the needle passes in and out in line with each other to avoid consumption of graft wall and allow neo-sinuses to unfold. If necessary, the STJ could be brought down to the desired size by putting three plication sutures at the commissure level. The coronary buttons were sewn in with a strip of autologous pericardium in the suture line for reinforcement and haemostasis. The distal suture line was done with a small strip of xenopericardium on the inside of the suture line to prevent intimal tears. As a final step, we evacuated air from the heart, removed the aortic cross clamp and resuscitated the heart.
Surgical techniques for aortic cusp repair
Techniques used for correcting leaflet prolapse include triangular resection (with or without pericardial patch repair), leaflet plication and free margin resuspension as described by Boodhwani et al. [15] for both bicuspid and tricuspid valves [14]. Restrictive leaflet disease was corrected by shaving and decalcification of affected leaflets, with or without patching. In some cases, adjunctive repair techniques needed to be used after intraoperative transoesophageal echocardiographic assessment of AR, orientation of the regurgitant jet (if present), coaptation length and coaptation level of the aortic valve cusps. Coaptation length of at least 7 mm at the mid-portion of the free margin and a coaptation level above the aortic valve annulus were considered as prerequisites for a successful repair. The presence of an eccentric residual AR jet was an indication for re-exploration of the aortic valve.
Sub-commissural annuloplasty
In cases of aortic annulus dilatation with or without cusp repair, a new technique was applied to reduce annulus size, improve cusp coaptation and stabilize the repair. A schematic representation of this technique is shown in Figs 1 and 2. After placement of all sub-annular pledged sutures below the cusps (with exclusion of the sub-commissural area), three non-pledged sutures (2–0 prolene) were placed through the holes of the six pledges that were placed next to the commissures on annular level, forming three pairs in this manner. Next, the Hegar dilator with the desired annulus size was placed through the aortic valve and the sub-commissural sutures were tightened first, in order to have most annular reduction in this area and to improve cusp coaptation at the same time.
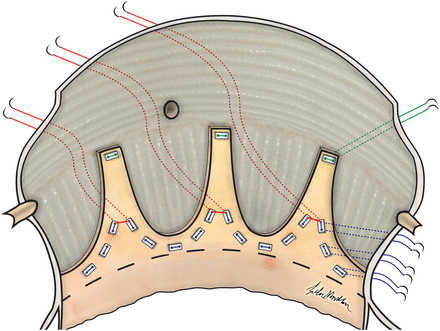
Sub-commissural annuloplasty in tricuspid aortic valve is performed by placement of three non-pledged sutures (2–0 prolene) through the holes of all six pledges that are placed next to the commissures on annular level; forming three pairs.
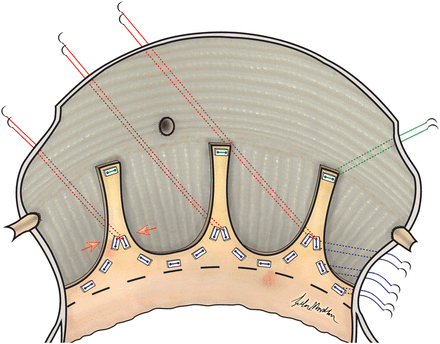
Annular reduction as a result of sub-commissural annuloplasty in tricuspid aortic valve.
Follow-up
Transthoracic echocardiograms (TTEs) were performed for assessing preoperative, immediate postoperative and most recent postoperative aortic valve function. All patients had a TTE before discharge from hospital. Patients were followed by the referring cardiologists in our adult congenital heart unit and were contacted for echocardiographic analysis 6 months postoperatively and annually thereafter. Echocardiographic data included dimensions of maximum aortic root diameter, annular diameter and the degree of AR. AR was entered into the database as: none/trace, mild, moderate or severe on the basis of information from colour flow mapping and continuous-wave Doppler echocardiography. If the echo report read ‘trace to mild’, it was entered as mild, ‘mild to moderate’ was entered as moderate and ‘moderate to severe’ was entered as severe. A recent echocardiogram was made of all patients 6 months before closing this study. Progression of AR to moderate or severe was an indication for reoperation.
Statistical analysis
The Kaplan–Meier method was used to calculate estimates for long-term survival and freedom from morbid events and their survival curves were plotted using the GraphPad Prism® 6.0 software for Macintosh. All data are presented as mean ± standard deviation, unless otherwise noted. All data analyses were performed using SPSS 20.0 for Macintosh (IBM® SPSS® Software).
RESULTS
Operative data
In addition to aortic valve-sparing operation, the most common concomitant procedures were: aortic valve repair in 19 (28.4%), simple (annuloplasty ring only) and complex mitral valve repair in 7 (7.2%) and 3 (3.1%), respectively, and simple tricuspid valve repair in 5 (5.2%) patients. When the valve-sparing operation was performed with concomitant procedures, cardiopulmonary bypass and cross-clamp times increase with 39 and 20 min, respectively. All other concomitant surgical procedures are listed together with cardiopulmonary bypass (CPB) and cross-clamp (AoX) times in Table 2. There were no operative deaths.
Operative data of adolescent and adult patients undergoing valve-sparing aortic root replacement
| . | All patients, n = 97 (%) . |
|---|---|
| Mean CPB time (min) ± SD | |
| Overall | 182 ± 41 |
| VSRR only | 168 ± 28 |
| VSRR with concomitant procedures | 207 ± 49 |
| Mean cross-clamp time (min) ± SD | |
| Overall | 148 ± 28 |
| VSRR only | 140 ± 22 |
| VSRR with concomitant procedures | 161 ± 33 |
| Proceduresa | |
| VSRR only(1 Fallot; 1 Ross-Coarct; 1 Yacoub; 1 Coarct-BAV; 4 BAV) | 63 (64.9) |
| VSRR + AVP(1 Fallot; 1 Coarct-BAV; 5 BAV) | 13 (13.4) |
| VSRR + AVP + MVP(1 BAV) | 4 (4.1) |
| VSRR + AVP + TVP + VSD closure(1 BAV) | 2 (2.1) |
| VSRR + CABG(1 Coarct-BAV) | 2 (2.1) |
| VSRR + MVP | 4 (4.1) |
| VSRR + MVP + TVP | 2 (2.1) |
| VSRR + TVP + PVR(1 Fallot) | 1 (1.0) |
| VSRR + PFO closure | 3 (3.1) |
| VSRR + Arch replacement(1 Coarct-BAV) | 3 (3.1) |
| Aortic valve plasty | |
| AVP | 13 (13.4) |
| AVP + SCA | 6 (6.2) |
| SCA | 24 (24.7) |
| . | All patients, n = 97 (%) . |
|---|---|
| Mean CPB time (min) ± SD | |
| Overall | 182 ± 41 |
| VSRR only | 168 ± 28 |
| VSRR with concomitant procedures | 207 ± 49 |
| Mean cross-clamp time (min) ± SD | |
| Overall | 148 ± 28 |
| VSRR only | 140 ± 22 |
| VSRR with concomitant procedures | 161 ± 33 |
| Proceduresa | |
| VSRR only(1 Fallot; 1 Ross-Coarct; 1 Yacoub; 1 Coarct-BAV; 4 BAV) | 63 (64.9) |
| VSRR + AVP(1 Fallot; 1 Coarct-BAV; 5 BAV) | 13 (13.4) |
| VSRR + AVP + MVP(1 BAV) | 4 (4.1) |
| VSRR + AVP + TVP + VSD closure(1 BAV) | 2 (2.1) |
| VSRR + CABG(1 Coarct-BAV) | 2 (2.1) |
| VSRR + MVP | 4 (4.1) |
| VSRR + MVP + TVP | 2 (2.1) |
| VSRR + TVP + PVR(1 Fallot) | 1 (1.0) |
| VSRR + PFO closure | 3 (3.1) |
| VSRR + Arch replacement(1 Coarct-BAV) | 3 (3.1) |
| Aortic valve plasty | |
| AVP | 13 (13.4) |
| AVP + SCA | 6 (6.2) |
| SCA | 24 (24.7) |
aCongenital heart disease diagnoses.
AVP: aortic valve plasty; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; MVP: mitral valve plasty; PFO: persistent foramen ovale; PVR: pulmonary valve replacement; SCA: sub-commissural annuloplasty (Fig. 1 and 2); TVP: tricuspid valve plasty; VSD: ventricular septal defect; VSRR: valve-sparing root replacement.
Operative data of adolescent and adult patients undergoing valve-sparing aortic root replacement
| . | All patients, n = 97 (%) . |
|---|---|
| Mean CPB time (min) ± SD | |
| Overall | 182 ± 41 |
| VSRR only | 168 ± 28 |
| VSRR with concomitant procedures | 207 ± 49 |
| Mean cross-clamp time (min) ± SD | |
| Overall | 148 ± 28 |
| VSRR only | 140 ± 22 |
| VSRR with concomitant procedures | 161 ± 33 |
| Proceduresa | |
| VSRR only(1 Fallot; 1 Ross-Coarct; 1 Yacoub; 1 Coarct-BAV; 4 BAV) | 63 (64.9) |
| VSRR + AVP(1 Fallot; 1 Coarct-BAV; 5 BAV) | 13 (13.4) |
| VSRR + AVP + MVP(1 BAV) | 4 (4.1) |
| VSRR + AVP + TVP + VSD closure(1 BAV) | 2 (2.1) |
| VSRR + CABG(1 Coarct-BAV) | 2 (2.1) |
| VSRR + MVP | 4 (4.1) |
| VSRR + MVP + TVP | 2 (2.1) |
| VSRR + TVP + PVR(1 Fallot) | 1 (1.0) |
| VSRR + PFO closure | 3 (3.1) |
| VSRR + Arch replacement(1 Coarct-BAV) | 3 (3.1) |
| Aortic valve plasty | |
| AVP | 13 (13.4) |
| AVP + SCA | 6 (6.2) |
| SCA | 24 (24.7) |
| . | All patients, n = 97 (%) . |
|---|---|
| Mean CPB time (min) ± SD | |
| Overall | 182 ± 41 |
| VSRR only | 168 ± 28 |
| VSRR with concomitant procedures | 207 ± 49 |
| Mean cross-clamp time (min) ± SD | |
| Overall | 148 ± 28 |
| VSRR only | 140 ± 22 |
| VSRR with concomitant procedures | 161 ± 33 |
| Proceduresa | |
| VSRR only(1 Fallot; 1 Ross-Coarct; 1 Yacoub; 1 Coarct-BAV; 4 BAV) | 63 (64.9) |
| VSRR + AVP(1 Fallot; 1 Coarct-BAV; 5 BAV) | 13 (13.4) |
| VSRR + AVP + MVP(1 BAV) | 4 (4.1) |
| VSRR + AVP + TVP + VSD closure(1 BAV) | 2 (2.1) |
| VSRR + CABG(1 Coarct-BAV) | 2 (2.1) |
| VSRR + MVP | 4 (4.1) |
| VSRR + MVP + TVP | 2 (2.1) |
| VSRR + TVP + PVR(1 Fallot) | 1 (1.0) |
| VSRR + PFO closure | 3 (3.1) |
| VSRR + Arch replacement(1 Coarct-BAV) | 3 (3.1) |
| Aortic valve plasty | |
| AVP | 13 (13.4) |
| AVP + SCA | 6 (6.2) |
| SCA | 24 (24.7) |
aCongenital heart disease diagnoses.
AVP: aortic valve plasty; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; MVP: mitral valve plasty; PFO: persistent foramen ovale; PVR: pulmonary valve replacement; SCA: sub-commissural annuloplasty (Fig. 1 and 2); TVP: tricuspid valve plasty; VSD: ventricular septal defect; VSRR: valve-sparing root replacement.
Early results
No early mortality occurred. Re-exploration for bleeding (2) or pericardial tamponade (2) was needed in 4 (4.1%) patients. One patient suffered a perioperative myocardial infarction and needed an implantable cardioverter defibrillator because of poor left ventricular function. Two pericardial effusions needed drainage (2.1%). During hospitalization, there were no thrombo-embolic complications. Median intensive care unit stay was 1.0 (range: 1–16) day and median hospital stay was 7.0 (range: 4–31) days. At discharge, postoperative echocardiograms showed that AR was absent or trace in 64 (66.0%), mild in 30 (30.9%) and moderate in 3 (3.3%) patients.
Late mortality and morbidity
Late complications include the death of a 14-year old Marfan patient 1.3 years post-surgery, presumably due to ventricular arrhythmia. He had dilated left ventricle even before surgery. Late morbidity consisted of 5 patients who needed to be reoperated on. One patient underwent reoperation for an aneurysm of the right coronary artery 4.9 years postoperatively. Four patients required late aortic valve replacement and/or Bentall procedure. The first patient received an aortic valve homograft 1 month postoperatively due to active endocarditis, affecting both the left and right coronary cusp without causing AR. After 6.1 years, the aortic homograft was replaced by a Bentall prosthesis because of degeneration. The second patient developed endocarditis with vegetation on the mitral valve and an abscess near the Valsalva graft. A Bentall procedure and mitral valve plasty were performed 0.8 year postoperatively, followed by a mechanical mitral valve at 1.0 year due to another episode of active endocarditis. The third patient underwent VSRR with sub-commissural annuloplasty (SCA), mitral valve plasty and tricuspid valve plasty and after being lost to follow-up for almost 1 year, the patient needed a Bentall procedure 1.3 years postoperatively due to severe AR. At reoperation, the complete non-coronary cusp seemed to have disappeared most likely due to endocarditis. The fourth patient with Marfan syndrome underwent VSRR with aortic valve plasty (plication of the non coronary cusp [NCC]) and mitral valve plasty and needed Bentall procedure 3.8 years postoperative after release of the plication suture that was placed on the NCC, causing recurrent prolapse with severe aorticregurgitation. No late thrombo-embolic complications occurred.
Mid-term results
Estimated survival at 5-year follow-up was 98.9 ± 0.1%. Estimated freedom from reoperation is shown in Fig. 3 and was 93.7 ± 0.3% at 5-year follow-up. Echocardiograms at the last follow-up and before death or reoperation in all patients showed that AR was none or trace in 58 (59.8%), mild in 34 (35.1%), moderate in 2 (2.1%) and severe in 3 (3.1%). Thus, a total number of 5 (5.2%) patients had greater than mild AR at the last follow-up and 2 of these 5 patients underwent reoperation (Patients 3 and 4 from the previous paragraph). Estimated freedom from mortality, root reoperation and moderate and/or severe AR was 88.0 ± 0.5% at 5 years postoperatively (Fig. 3).
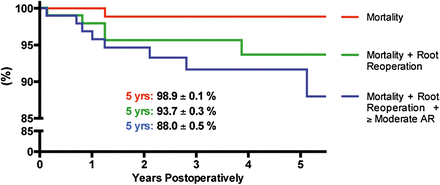
Three Kaplan–Meier estimates on mortality (red line); mortality and root reoperation (green line) and mortality, root reoperation and freedom from moderate and/or severe AR (blue line) after valve-sparing operations.
Sub-commissural annuloplasty results
Sub-commissural annuloplasty (Figs 1 and 2) was performed concomitantly in 30 (30.9%) exclusively adult patients. Of all 19 patients who needed aortic valve plasty, additional SCA was performed in 6 (31.6%). Cosgrove's sub-commissural annuloplasty [16] was performed in 1 (5.3%) patient. Patients who underwent VSRR with SCA needed 164 ± 31 min CPB and 137 ± 24 min cross-clamp time to complete the procedure, compared with 170 ± 26 and 142 ± 21 min, respectively, in those who underwent VSRR only. Immediate postoperative echocardiograms showed adequate annular diameter reduction and coaptation improvement. Echocardiograms at the last follow-up showed that among the 6 patients that needed both aortic valve repair and SCA concomitantly to VSRR, AR was absent in 2 (33.3%) and mild in 4 (66.6%). Among the 24 patients in whom SCA was performed concomitantly to VSRR, AR was absent in 17 (70.8%), mild in 5 (20.8%), moderate in 1 (4.2%) and 1 (4.2%) patient in this group developed severe AR due to endocarditis.
COMMENT
After the introduction of the VSRR technique by Yacoub (remodelling) and David (reimplantation), this surgical approach has gained wide acceptance as an alternative to composite graft replacement. Often the aortic valve itself is not or only mildly diseased and the anticoagulation and prosthesis-related complications can be avoided with this approach. It is a challenge, however, to improve the results of composite graft replacement in particular with respect to overall survival and freedom from reoperation. In large series with long-term follow-up, survival rates almost comparable with that of the general population were reported [17]. The use of Yacoub's remodelling technique comes with a certain risk of progressive dilatation of the aortic annulus over time that could cause AR as the technique lacks effective fixation of the annulus, especially in the group of patients with connective tissue disease [18, 19]. The one patient in our series who received VSRR after the Yacoub procedure in the past elsewhere came back with exactly this problem. On the other hand, the remodelling technique has the theoretical advantage of recreating sinuses of Valsalva in the graft which might improve long-term valve durability by decreasing leaflet stresses [20]. With respect to early postoperative bleeding and freedom from reoperation for recurrent AR, the reimplantation technique as described by David appears to be superior over the remodelling technique [21]. But, also in larger series of experienced centres, a small percentage of patients remain at risk of valve failure and replacement in the long term [1]. Therefore, the technique of reimplantation has evolved over the last two decades in the hands of the inventor himself and multiple modifications were made by other surgeons [8, 9]. The main concern is that when the aortic cusps are placed inside a cylindrical structure without aortic sinuses and the annulus becomes rigid, this may lead to increased stress on the aortic cusps and higher risk of late valve failure [20].
Thus, like others, our goal has been to preserve or restore natural root geometry as much as possible with a technique based on original description by Pacini et al. [13] and later further optimized by de Kerchove et al. [14, 15]. At the same time, we aimed for optimal cusp coaptation well above the annular plane and at least 7 mm of coaptation length, as a minimum. The use of the Valsalva prothesis (Gelweave Valsalva™ by Sultzer Vascutek, Renfrewshire, Scotland) has facilitated the creation of the neo-sinusses and exact measurements in longitudinal direction are more precise because the material has no longitudinal stretch at the root level. A fast and reproducible technique was developed as we have described above. The mean aortic cross-clamp time for VSRR alone was very acceptable and allowed us to perform (multiple) additional procedures when required; so cross-clamp times were easily kept within safe limits. This is particularly important for patients with connective tissue disease, because they often have multiple affected valves that are in need of correction, as was also shown in our series. This also applies to patients with congenital heart defects who may suffer from (multiple) lesions that need complex surgery simultaneously with VSRR, for example, arch repair related to post-coarctation pathology. Although our follow-up time is still relatively short, the results are satisfying and comparable with the literature. The freedom from moderate or severe AR and freedom from reoperation were negatively influenced by an unfortunately high incidence of endocarditis in our series. Leaving these out of account, there was only one late failure.
It has been demonstrated that aortic annular dilatation (aortic annulus >25 mm) is a risk factor for early and late failure [8] and therefore, there is a frequent need to downsize the aortic annulus. Sub-commissural annuloplasty as described by Fraser and Cosgrove [16] is frequently used in aortic valve repair and in combination with aortic valve-sparing techniques. It is clear that it increases the area of cusp coaptation and that it is helpful in preventing or treating small residual leaks, especially if the exact mechanism is not completely understood. A drawback of this technique is that abnormal cusp motion and restriction, especially in the commissural area, may induce outflow gradient across the valve and it takes 10–15 min to perform the technique properly. The sub-commissural annuloplasty technique that we have presented (Figs 1 and 2) has proved to be efficient because it costs no extra time to perform. Besides this, the effect on the aortic valve tissue itself is less aggressive and cusp geometry and motion are not or only minimally altered. At the same time, an improvement of cusp coaptation area is achieved as the desired reduction in annular size is accomplished mainly in the interleaflet triangle area, in combination with a strong fixation of the root in the sub-annular plane.
However, most reported technical modifications by our self or others rely for a significant part on personal judgement or eye balling and less on exact measurement and standardization. This hampers the evaluation of our own results and the comparison with the results of other groups. In recent years, new insights into and concepts in valve-sparing surgery have been published where the need for a standardized approach is emphasised [8, 22]. The introduction of the concept of effective coaptation height and its importance with respect to late outcome was clearly demonstrated by Schäfers group [22]. Besides, the introduction by Schäfers of the caliper to measure effective coaptation height during surgery seems to be an important tool in achieving and predicting the desired operative result. By the group of Lansac, extensive work on the topic of standardization of the procedure has been done [8]. Their systematic approach and remodelling technique in combination with external aortic ring is showing promising results. Regardless of the techniques we choose, technical standardization is a requirement for evidence-based improvements in valve and root surgery.
Unfortunately, in 2 patients the spared valve was proved to be destructed by endocarditis and in 1 there was a strong suspicion. The cause of this high incidence of endocarditis is unclear. Some surgeons state that the use of Teflon pledges in valve-sparing procedures increases the postoperative risk of endocarditis and that their use should be avoided for this reason. Although this may be theoretically reasonable, there is no evidence for this and we never observed a similar high incidence in composite graft replacement where we use the same suturing material. Especially for patients who need reduction of the aortic annulus and those with connective tissue disease, we think that Teflon pledges are essential for strong and reliable fixation of the annulus. After complete epitheliazation, the risk of endocarditis can be expected to be minimal and not more than in conventional aortic valve or root replacement.
Limitations
Like most retrospective studies, ours has several limitations. All echocardiographic data are based on written reports from multiple cardiologists within our institution, and to level out any difference in interpretation, we personally reviewed all studies that showed moderate or severe AR, together with one cardiologist. This is a retrospective review of a cohort of patients who have been operated on by two surgeons at a single institution (two centres) and therefore, the results may not be generalizable. Finally, we have not looked for risk factors (e.g. for reoperation, endocarditis or mortality) in this study.
CONCLUSION
Aortic valve-sparing root replacement is a valuable technique for large groups of patients with excellent results in experienced hands. It is a technically demanding operation and still in development in order to optimize long-term results. The results of our series with high percentage of associated valve repair are good, although mean follow-up time is still limited. The contribution of minor or major technical improvements made by others or us is difficult to establish due to lack of standardization and should be improved in the future.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr F. Santini(Genova, Italy): The aim of this retrospective study was to evaluate the surgical results achieved in the last ten years of valve-sparing aortic root replacement and associated multiple valve repairs performed in a quite heterogeneous group of paediatric and adult patients with dissimilar connective tissue disorders, different valve pathologies and congenital heart defects, a few after previous surgical procedures. The primary indication for valve-sparing root replacement was aneurysm of the aortic root, and all patients had an aortic valve-sparing procedure by means of reimplantation of the aortic valve into a vascular prosthesis. During the experience, surgery was accomplished by two surgeons by means of evolving root techniques, different shape vascular grafts, and associated evolving surgical strategies for both aortic and other types of valve repair.
While the authors are to be congratulated for their excellent results with no early mortality and a remarkable mid-term outcome, the very heterogeneous patient population studied, the different techniques utilized in view of the wide spectrum of associated aortic and other valve pathologies included, make an overall interpretation of data difficult and uncertain. Indeed, a few and quite different subcategories of patients were compared in this analysis over a period of time during which surgical techniques evolved quite significantly.
I have four questions for the authors. You mentioned in your manuscript that for adult patients with bicuspid aortic valves, the threshold for surgery recently changed from 50 to 55 mm in the absence of other risk factors. How much is ‘recently’ in your 10 years' experience, and based on what evidence did you change your policy?
The second question is built into the first as well. You also mentioned that the aortic annulus was considered dilated in adult patients when above 25 mm. Did you always use the absolute dimension or did you normalize the dimension for either patient body surface area or height, or did you use any other indexing criteria?
In the description of your technique, you stated that cusps were inspected for fenestrations that might interfere with a valve-sparing procedure. What was, and what currently is, your decision-making process as far as fenestrations are concerned?
You mentioned also that at discharge postoperative echocardiograms showed that aortic regurgitation was mild in one-third of the patients. Did you evaluate the impact of this so-called "mild aortic regurgitation" on mid-term results, and what are your current criteria for redo operation in these patients?
Mr. Manshanden: Your last remark, the aortic regurgitation at discharge, if it read mild to moderate, we had to enter it as moderate. So at discharge they were not reviewed by one and the same cardiologist, so they probably had somewhere in between so that we had to enter it as if being worse than they had.
You had one surgical technical question about the fenestrations; I would like to pass that up to Dr Koolbergen. And let's see, the guidelines from the European Society of Cardiology, they recently changed, indicating operative indication from 55 mm, so that's what we're sticking to. And did I answer all your questions now or have I missed one?
Dr Santini: Did you use index measurement as far as annulus dimension is concerned?
Mr Manshanden: We only use the absolute measurement of 25 mm. But we're not working with Z-scores in our hospital, so we're dependent on what the cardiologists judge upon. It would be more elegant to use Z-scores, yes, and to look at the body surface area. I totally agree on that if that's what you mean.
Dr D. Koolbergen(Amsterdam, Netherlands): And we didn't index the annulus diameter, and this is, as I said in the manuscript, a major drawback until now. The technique we used relied in large measure on eyeballing, and there is not much standardization until now. Concerning your remark about the fenestrations, of course, small fenestrations in the area of the commissures we accepted, and large fenestrations in the central part of the cusps were seen as a contraindication for valve-sparing surgery.
Dr Santini: Did you use the same approach as far as fenestration is considered despite the age of the patient? I mean, the same rules for paediatric and adult?
Dr Koolbergen: Yes, despite the age of the patient.
Dr J. Hörer(Munich, Germany): Do you make any difference in the indication in patients presenting with different types of connective tissue diseases?
Mr Manshanden: Not within this paper, no.
Dr Koolbergen: Well, for the one child with the Loeys-Dietz syndrome, we went rather early for surgery because they are known to have dissection earlier than the others.
Author notes
Presented at the 27th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 5–9 October 2013.
The first two authors contributed equally to this work.
- aortic arch
- aortic valve
- aortic valve insufficiency
- endocarditis
- marfan syndrome
- tetralogy of fallot
- echocardiography
- mitral valve repair
- medical records
- repair of tricuspid valve
- right coronary artery
- bicuspid aortic valve
- aneurysm
- repair of aortic valve
- aortic valve replacement
- adult
- follow-up
- objective (goal)
- preoperative care
- repeat surgery
- surgical replantation
- surgical procedures, operative
- blood vessel prosthesis
- mortality
- surgery specialty
- ventricular arrhythmia
- embolism
- aortic root replacement
- prostheses




