-
PDF
- Split View
-
Views
-
Cite
Cite
Andrea Garatti, Serenella Castelvecchio, Michele Di Mauro, Francesco Bandera, Marco Guazzi, Lorenzo Menicanti, Impact of right ventricular dysfunction on the outcome of heart failure patients undergoing surgical ventricular reconstruction, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 2, February 2015, Pages 333–340, https://doi.org/10.1093/ejcts/ezu152
Close - Share Icon Share
Abstract
The aim was to assess the impact of right ventricular dysfunction (RVD) on the outcome of heart failure (HF) patients undergoing surgical ventricular reconstruction (SVR).
A total of 324 patients (65 ± 9 years) with previous myocardial infarction had an echocardiographic assessment of right ventricular (RV) function before and after SVR. RV function was assessed measuring the tricuspid annular plane systolic excursion (TAPSE) and RV dysfunction was defined by a TAPSE < 16 mm.
RV dysfunction was detected in 69 patients (Group A, mean age 64 ± 11 years), while 255 patients (Group B, mean age 65 ± 9 years) had a preserved RV function. Patients in Group A showed a higher New York Heart Association (NYHA) class (P = 0.01), larger left ventricular (LV) end-diastolic and end-systolic volumes (P = 0.01), a lower EF (P = 0.01), a higher percentage of moderate-to-severe mitral regurgitation (P = 0.01) and a higher systolic pulmonary artery pressure (PAPs; P = 0.01). Propensity score matching was applied in order to adjust for baseline differences. In the fully matched population, low-output syndrome (P = 0.01), inotropic support (P = 0.01) and intra-aortic balloon pump insertion (P = 0.03) were significantly more frequent in Group A compared with Group B. However, 30-day mortality was not significantly different between the two groups (P = 0.18). Kaplan-Meier 5- and 8-year survival rate (log-rank: P = 0.01) as well as freedom from cardiac events (log-rank: P = 0.02) were significantly lower in patients with RV dysfunction. At Cox regression analysis, preoperative RVD (P = 0.01) and NYHA class at admission >II (P = 0.02) resulted in independent predictor of late mortality.
RV dysfunction correlates with LV dysfunction and it is an important predictor of long-term outcome in HF patients undergoing SVR.
INTRODUCTION
Right ventricular (RV) dysfunction is a well-known predictor for mortality after acute myocardial infarction (MI) or coronary artery bypass grafting (CABG) and in chronic heart failure (HF) [1–3]. In patients with previous MI and left ventricular (LV) dysfunction, RV alterations can occur in a close relationship to the LV alterations that accompany the postischaemic remodelling process [4]. So far, RV systolic function is the result of a complex interaction with the remodelled and enlarged left ventricle, LV septal function, LV myocardial function and pulmonary artery systolic pressures (PAPs) with or without functional mitral regurgitation (MR). The complexity of such interaction suggests in turn a substantial variability in the response of the RV to LV dysfunction. Furthermore, biventricular impairment is a well-recognized pejorative prognostic factor in HF related to ischaemic or non-ischaemic disease [3–5].
Surgical ventricular reconstruction (SVR) is a specific procedure adopted to reverse LV remodelling in patients with ischaemic HF [6, 7]. The aim of SVR is to exclude scar tissue from the LV wall, thereby restoring the physiological volume and shape and improving LV systolic function, clinical status and survival. Furthermore, SVR has been proved to produce a mechanical intraventricular resynchronization that improves LV performance [8]. However, data on the relationship between RV function and SVR are lacking.
The purpose of this study was to assess the RV function before and after SVR and its relationship with the clinical outcome in patients with ischaemic LV dysfunction and HF.
MATERIALS AND METHODS
Study design
The present study is a retrospective case–control study. Between January 2002 and December 2011, 324 patients with previous MI and LV systolic dysfunction had an echocardiographic assessment of RV function before SVR performed by a single surgeon (Lorenzo Menicanti), eventually associated with CABG and mitral repair/replacement. All patients presented with symptoms of CHF and/or angina or intractable ventricular arrhythmias. Indications to SVR were a previous MI with LV dilatation and scarred tissue.
We wanted to test the hypothesis that preoperative RVD was an independent predictor of early and long-term mortality after SVR. RV function was assessed measuring the tricuspid annular plane systolic excursion (TAPSE), and RV dysfunction was defined by a TAPSE of ≤ 16 mm. According to this definition, the study population was divided into two groups (Group A—TAPSE ≤ 16 mm and Group B—TAPSE > 16 mm).
The primary end point of the present study was long-term mortality from any cause. Secondary end point was long-term freedom from cardiac events, defined as the sum of death of any cause and HF readmission (each patient included only once).
Demographic, clinical, echocardiographic and procedural data were retrospectively collected. The Istituto Policlinico San Donato Ethical Committee approved the study and waived the need for informed consent in consideration of the retrospective nature of the study. All patients admitted to the study gave their informed consent for the scientific analysis of their clinical data in an anonymous form.
Echocardiography
Echocardiography imaging was performed using a GE Vivid 7 (GE Healthcare, Waukesha, WI, USA) echocardiographic instrument. Both preoperative and postoperative echocardiographic exams were performed in our department by the same cardiologist (Serenella Castelvecchio). LV and RV chamber dimensions and function were measured according to the recommendations for chamber quantification from the American Society of Echocardiography/European Association of Echocardiography Guidelines [9].
Left ventricular analysis
LV end-diastolic and end-systolic internal diameters were obtained in parasternal long-axis view (mm) by using M-mode image. LV end-diastolic volume (EDV) and end-systolic volume (ESV) were calculated from the apical four-chamber view using the Simpson method, indexed to BSA (EDVI and ESVI, ml/m2). Ejection fraction (EF) was calculated as (EDV − ESV)/EDV × 100 (%). LA volume was measured at the end of LV systole from the apical four-chamber view (monoplane evaluation using the area-length method) and normalized for body surface area (BSA) (LAVI = LAV/BSA, ml/m2).
Right ventricular analysis
RV function was assessed measuring the TAPSE in the apical four-chamber view with an M-mode cursor placed through the lateral annulus in real time. TAPSE was measured as the total displacement of the tricuspid annulus (mm) from end-diastole to end-systole. PAPs were estimated by Doppler echocardiography recording the tricuspid regurgitant velocity from any view with continuous—wave Doppler (modified Bernoulli equation). MR was assessed using a four-degree scale, based on colour and continuous wave Doppler examination, independently by two different cardiologists.
All measurements were obtained from the mean of three beats for patients with sinus rhythm and from the mean of five beats for patients in atrial fibrillation. The intraclass correlation coefficient was determined for intraobserver variability. ICC for TAPSE measurement was 0.89, showing an excellent strength of agreement.
Surgical technique
Details of the surgical technique have been reported previously [6]. Briefly, the procedure was performed on the arrested heart with antegrade cold blood cardioplegia. Complete coronary revascularization was performed first. After completion of coronary grafting, the LV was opened with an incision parallel to the left anterior descending artery, starting at the middle scarred region and ending at the apex. Surgical ventricular reconstruction was performed using a mannequin (TRISVR, Chase Medical Richardson, TX, USA) filled at 50–60 ml/m2 to optimize the size and shape of the new ventricle. The mannequin shape helps in orienting the plane of the endoventricular circular suture at the transitional zone, obliquely towards the aortic flow tract and mainly in rebuilding the new apex. The device is also useful especially in leaving a residual chamber that is not too small. When needed, the mitral valve was repaired through the ventricular opening with a posterior annuloplasty. The indication to repair the valve was moderate-to-severe regurgitation or mild regurgitation associated with annulus dilatation (>40 mm).
Surgical risk estimation
The estimation of perioperative risk was based on the age, creatinine and ejection fraction (ACEF) score. The ACEF score was recently introduced by Ranucci et al. [10] in 2009. This score is based on three factors only: age, preoperative creatinine value and EF. The LV EF is included as a continuous variable to account for the extremely high risk attributable to patients with EF in the range of 10–30%. In this area, the risk increases exponentially with decreasing EF. The ACEF score does not pool together all patients with an EF <30%, as do the other risk scores. This fact probably explains the excellent accuracy of ACEF score in predicting 30-day mortality in patients with significantly depressed LV function.
Follow-up data collection
The follow-up was conducted either at the hospital during a routine clinical evaluation or by telephone contact with the patients, their relatives or family doctors, and was 100% complete. Mortality data were obtained from the National Registry of Death, or from direct relatives' interview.
The mean follow-up length in the overall population was 56.7 ± 39 months, without significant difference among the two study groups (P = 0.187). Long-term mortality included death of any cause. Readmission at follow-up included any episode of rehospitalization for HF. Cardiac event was defined as the sum of death of any cause and HF readmission (each patient included only once).
Statistical analysis
Normality distribution of the continuous variables was tested and validated with the Shapiro–Wilk test. Results were presented as mean values ± SD (median value). The parametric test (one-way analysis of variance) and non-parametric test (Mann–Whitney U-test) were both used and reported for comparison of continuous variables. The χ2 or Fisher exact test (two-tailed) was used for comparison of categorical variables. A P-value of <0.05 was considered statistically significant.
In order to adjust for significant unbalanced individual characteristics between patients with or without RVD, we used propensity scores (PSs) matching. We employed a multivariate logistic regression model to estimate PSs using all preoperative and perioperative variables. Patients with RVD were matched to a subset of patients without RVD on PS, using the nearest neighbour method. Covariate balance was measured using t-test or χ2 analysis, in case of numerical or categorical variable, respectively. Long-term survival analysis using the Kaplan–Meier estimator, log-rank test to compare survival/freedom curves and Cox regression were performed to compare groups and to identify possible significant risk factors. Statistical calculations were performed using a computerized statistical program (SPSS 17.0, Chicago, IL, USA).
RESULTS
Preoperative characteristics of the unmatched study population are depicted in Table 1. Among them, 69 patients presented with RV dysfunction (Group A, mean age 64 ± 11 years), while a normal RV function was detected in 255 patients (Group B, mean age 65 ± 9 years). The two groups were comparable in terms of preoperative cardiac risk factor. However, patients with RVD showed higher incidence of HF symptoms and atrial or ventricular arrhythmias at admission. Furthermore, patients in Group A presented with larger LV volumes, lower EF and higher incidence of severe MR.
| Variables . | RV dysfunction (n = 69) . | No RV dysfunction (n = 255) . | P-value . |
|---|---|---|---|
| Age (years) | 64 ± 11 | 65 ± 9 | 0.61 |
| Male gender | 55 (80%) | 213 (84%) | 0.55 |
| Cardiac risk factor | |||
| Family history of CAD | 26 (38%) | 110 (43%) | 0.27 |
| Hypertension | 39 (57%) | 139 (55%) | 0.40 |
| Dyslipidaemia | 36 (53%) | 151 (59%) | 0.20 |
| Diabetes | 22 (32%) | 69 (27%) | 0.23 |
| Chronic renal failure | 5 (7%) | 15 (6%) | 0.43 |
| Clinical presentation | |||
| Angina | 40 (60%) | 155 (64%) | 0.51 |
| NYHA class >2 | 48 (70%) | 119 (47%) | 0.01 |
| Atrial fibrillation | 10 (14%) | 34 (13%) | 0.47 |
| Ventricular arrhythmias | 4 (9%) | 28 (16%) | 0.19 |
| Preoperative echocardiography | |||
| EDV (ml) | 236 ± 103 | 209 ± 66 | 0.02 |
| ESV (ml) | 176 ± 89 | 144 ± 58 | 0.01 |
| Indexed EDV (ml/m2) | 128 ± 53 | 114 ± 36 | 0.02 |
| Indexed ESV (ml/m2) | 96 ± 47 | 79 ± 32 | 0.01 |
| EF (%) | 27 ± 7 | 32 ± 8 | 0.01 |
| TAPSE | 13 ± 1.3 | 21 ± 4 | 0.01 |
| E/A ratio | 1.8 ± 1.1 | 1.2 ± 0.8 | 0.01 |
| DTE | 163 ± 52 | 194 ± 60 | 0.01 |
| Systolic PAP (mmHg) | 44 ± 17 | 38 ± 13 | 0.01 |
| Mitral regurgitation (>2+) | 30 (43%) | 64 (25%) | 0.01 |
| Variables . | RV dysfunction (n = 69) . | No RV dysfunction (n = 255) . | P-value . |
|---|---|---|---|
| Age (years) | 64 ± 11 | 65 ± 9 | 0.61 |
| Male gender | 55 (80%) | 213 (84%) | 0.55 |
| Cardiac risk factor | |||
| Family history of CAD | 26 (38%) | 110 (43%) | 0.27 |
| Hypertension | 39 (57%) | 139 (55%) | 0.40 |
| Dyslipidaemia | 36 (53%) | 151 (59%) | 0.20 |
| Diabetes | 22 (32%) | 69 (27%) | 0.23 |
| Chronic renal failure | 5 (7%) | 15 (6%) | 0.43 |
| Clinical presentation | |||
| Angina | 40 (60%) | 155 (64%) | 0.51 |
| NYHA class >2 | 48 (70%) | 119 (47%) | 0.01 |
| Atrial fibrillation | 10 (14%) | 34 (13%) | 0.47 |
| Ventricular arrhythmias | 4 (9%) | 28 (16%) | 0.19 |
| Preoperative echocardiography | |||
| EDV (ml) | 236 ± 103 | 209 ± 66 | 0.02 |
| ESV (ml) | 176 ± 89 | 144 ± 58 | 0.01 |
| Indexed EDV (ml/m2) | 128 ± 53 | 114 ± 36 | 0.02 |
| Indexed ESV (ml/m2) | 96 ± 47 | 79 ± 32 | 0.01 |
| EF (%) | 27 ± 7 | 32 ± 8 | 0.01 |
| TAPSE | 13 ± 1.3 | 21 ± 4 | 0.01 |
| E/A ratio | 1.8 ± 1.1 | 1.2 ± 0.8 | 0.01 |
| DTE | 163 ± 52 | 194 ± 60 | 0.01 |
| Systolic PAP (mmHg) | 44 ± 17 | 38 ± 13 | 0.01 |
| Mitral regurgitation (>2+) | 30 (43%) | 64 (25%) | 0.01 |
Bold values were used to highlight the significant P-value (<0.05).
CAD: coronary artery disease; TAPSE: tricuspid annular plane systolic excursion; NYHA: New York Heart Association; EDV: end-diastolic volume; ESV: end-systolic volume; EF: ejection fraction; RV: right ventricular.
| Variables . | RV dysfunction (n = 69) . | No RV dysfunction (n = 255) . | P-value . |
|---|---|---|---|
| Age (years) | 64 ± 11 | 65 ± 9 | 0.61 |
| Male gender | 55 (80%) | 213 (84%) | 0.55 |
| Cardiac risk factor | |||
| Family history of CAD | 26 (38%) | 110 (43%) | 0.27 |
| Hypertension | 39 (57%) | 139 (55%) | 0.40 |
| Dyslipidaemia | 36 (53%) | 151 (59%) | 0.20 |
| Diabetes | 22 (32%) | 69 (27%) | 0.23 |
| Chronic renal failure | 5 (7%) | 15 (6%) | 0.43 |
| Clinical presentation | |||
| Angina | 40 (60%) | 155 (64%) | 0.51 |
| NYHA class >2 | 48 (70%) | 119 (47%) | 0.01 |
| Atrial fibrillation | 10 (14%) | 34 (13%) | 0.47 |
| Ventricular arrhythmias | 4 (9%) | 28 (16%) | 0.19 |
| Preoperative echocardiography | |||
| EDV (ml) | 236 ± 103 | 209 ± 66 | 0.02 |
| ESV (ml) | 176 ± 89 | 144 ± 58 | 0.01 |
| Indexed EDV (ml/m2) | 128 ± 53 | 114 ± 36 | 0.02 |
| Indexed ESV (ml/m2) | 96 ± 47 | 79 ± 32 | 0.01 |
| EF (%) | 27 ± 7 | 32 ± 8 | 0.01 |
| TAPSE | 13 ± 1.3 | 21 ± 4 | 0.01 |
| E/A ratio | 1.8 ± 1.1 | 1.2 ± 0.8 | 0.01 |
| DTE | 163 ± 52 | 194 ± 60 | 0.01 |
| Systolic PAP (mmHg) | 44 ± 17 | 38 ± 13 | 0.01 |
| Mitral regurgitation (>2+) | 30 (43%) | 64 (25%) | 0.01 |
| Variables . | RV dysfunction (n = 69) . | No RV dysfunction (n = 255) . | P-value . |
|---|---|---|---|
| Age (years) | 64 ± 11 | 65 ± 9 | 0.61 |
| Male gender | 55 (80%) | 213 (84%) | 0.55 |
| Cardiac risk factor | |||
| Family history of CAD | 26 (38%) | 110 (43%) | 0.27 |
| Hypertension | 39 (57%) | 139 (55%) | 0.40 |
| Dyslipidaemia | 36 (53%) | 151 (59%) | 0.20 |
| Diabetes | 22 (32%) | 69 (27%) | 0.23 |
| Chronic renal failure | 5 (7%) | 15 (6%) | 0.43 |
| Clinical presentation | |||
| Angina | 40 (60%) | 155 (64%) | 0.51 |
| NYHA class >2 | 48 (70%) | 119 (47%) | 0.01 |
| Atrial fibrillation | 10 (14%) | 34 (13%) | 0.47 |
| Ventricular arrhythmias | 4 (9%) | 28 (16%) | 0.19 |
| Preoperative echocardiography | |||
| EDV (ml) | 236 ± 103 | 209 ± 66 | 0.02 |
| ESV (ml) | 176 ± 89 | 144 ± 58 | 0.01 |
| Indexed EDV (ml/m2) | 128 ± 53 | 114 ± 36 | 0.02 |
| Indexed ESV (ml/m2) | 96 ± 47 | 79 ± 32 | 0.01 |
| EF (%) | 27 ± 7 | 32 ± 8 | 0.01 |
| TAPSE | 13 ± 1.3 | 21 ± 4 | 0.01 |
| E/A ratio | 1.8 ± 1.1 | 1.2 ± 0.8 | 0.01 |
| DTE | 163 ± 52 | 194 ± 60 | 0.01 |
| Systolic PAP (mmHg) | 44 ± 17 | 38 ± 13 | 0.01 |
| Mitral regurgitation (>2+) | 30 (43%) | 64 (25%) | 0.01 |
Bold values were used to highlight the significant P-value (<0.05).
CAD: coronary artery disease; TAPSE: tricuspid annular plane systolic excursion; NYHA: New York Heart Association; EDV: end-diastolic volume; ESV: end-systolic volume; EF: ejection fraction; RV: right ventricular.
After propensity score matching, preoperative characteristics, incidence of comorbidities (Table 2) and preoperative echocardiographic data (Table 3) were comparable between the two groups. Furthermore, preoperative medications were comparable between the two groups, with the exception of statins that were more frequently taken by patients without RV dysfunction (P = 0.02).
| Variables . | RV dysfunction (n = 69) . | No RV dysfunction (n = 69) . | P-value . |
|---|---|---|---|
| Age (years) | 65.8 ± 10.8 | 64.9 ± 9.2 | 0.60 |
| Gender | 0.41 | ||
| Male | 55 (80%) | 57 (83%) | |
| Female | 14 (20%) | 12 (17%) | |
| BSA | 1.8 ± 0.17 | 1.7 ± 0.21 | 0.32 |
| Cardiac risk factor | |||
| Family history of CAD | 26 (38%) | 28 (41%) | 0.46 |
| Hypertension | 39 (57%) | 37 (53%) | 0.39 |
| Dyslipidaemia | 36 (53%) | 39 (57%) | 0.40 |
| Diabetes | 17 (24%) | 13 (19%) | 0.18 |
| Active Smoking | 13 (19%) | 12 (17%) | 0.90 |
| CVA | 4 (6%) | 5 (7%) | 0.63 |
| Chronic renal failure | 5 (7%) | 3 (4%) | 0.36 |
| Clinical presentation | |||
| Angina | 45 (67%) | 53 (76%) | 0.16 |
| NYHA class >2 | 48 (69%) | 41 (60%) | 0.14 |
| Atrial fibrillation | 10 (14%) | 12 (17%) | 0.41 |
| QRS width (mV) | 120 ± 29 | 116 ± 28 | 0.37 |
| Preoperative medication | |||
| ACE-inhibitor | 62 (91%) | 58 (84%) | 0.16 |
| Beta-blockers | 48 (71%) | 51 (74%) | 0.40 |
| Amiodarone | 10 (16%) | 16 (23%) | 0.15 |
| Aspirin | 53 (78%) | 54 (78%) | 0.56 |
| Statins | 33 (48%) | 46 (67%) | 0.02 |
| Oral diuretics | 56 (82%) | 60 (87%) | 0.30 |
| Variables . | RV dysfunction (n = 69) . | No RV dysfunction (n = 69) . | P-value . |
|---|---|---|---|
| Age (years) | 65.8 ± 10.8 | 64.9 ± 9.2 | 0.60 |
| Gender | 0.41 | ||
| Male | 55 (80%) | 57 (83%) | |
| Female | 14 (20%) | 12 (17%) | |
| BSA | 1.8 ± 0.17 | 1.7 ± 0.21 | 0.32 |
| Cardiac risk factor | |||
| Family history of CAD | 26 (38%) | 28 (41%) | 0.46 |
| Hypertension | 39 (57%) | 37 (53%) | 0.39 |
| Dyslipidaemia | 36 (53%) | 39 (57%) | 0.40 |
| Diabetes | 17 (24%) | 13 (19%) | 0.18 |
| Active Smoking | 13 (19%) | 12 (17%) | 0.90 |
| CVA | 4 (6%) | 5 (7%) | 0.63 |
| Chronic renal failure | 5 (7%) | 3 (4%) | 0.36 |
| Clinical presentation | |||
| Angina | 45 (67%) | 53 (76%) | 0.16 |
| NYHA class >2 | 48 (69%) | 41 (60%) | 0.14 |
| Atrial fibrillation | 10 (14%) | 12 (17%) | 0.41 |
| QRS width (mV) | 120 ± 29 | 116 ± 28 | 0.37 |
| Preoperative medication | |||
| ACE-inhibitor | 62 (91%) | 58 (84%) | 0.16 |
| Beta-blockers | 48 (71%) | 51 (74%) | 0.40 |
| Amiodarone | 10 (16%) | 16 (23%) | 0.15 |
| Aspirin | 53 (78%) | 54 (78%) | 0.56 |
| Statins | 33 (48%) | 46 (67%) | 0.02 |
| Oral diuretics | 56 (82%) | 60 (87%) | 0.30 |
ACE: angiotensin-converting enzyme; BSA: body surface area; CAD: coronary artery disease; CVA: cerebrovascular accident; NYHA: New York Heart Association; RV: right ventricular.
| Variables . | RV dysfunction (n = 69) . | No RV dysfunction (n = 69) . | P-value . |
|---|---|---|---|
| Age (years) | 65.8 ± 10.8 | 64.9 ± 9.2 | 0.60 |
| Gender | 0.41 | ||
| Male | 55 (80%) | 57 (83%) | |
| Female | 14 (20%) | 12 (17%) | |
| BSA | 1.8 ± 0.17 | 1.7 ± 0.21 | 0.32 |
| Cardiac risk factor | |||
| Family history of CAD | 26 (38%) | 28 (41%) | 0.46 |
| Hypertension | 39 (57%) | 37 (53%) | 0.39 |
| Dyslipidaemia | 36 (53%) | 39 (57%) | 0.40 |
| Diabetes | 17 (24%) | 13 (19%) | 0.18 |
| Active Smoking | 13 (19%) | 12 (17%) | 0.90 |
| CVA | 4 (6%) | 5 (7%) | 0.63 |
| Chronic renal failure | 5 (7%) | 3 (4%) | 0.36 |
| Clinical presentation | |||
| Angina | 45 (67%) | 53 (76%) | 0.16 |
| NYHA class >2 | 48 (69%) | 41 (60%) | 0.14 |
| Atrial fibrillation | 10 (14%) | 12 (17%) | 0.41 |
| QRS width (mV) | 120 ± 29 | 116 ± 28 | 0.37 |
| Preoperative medication | |||
| ACE-inhibitor | 62 (91%) | 58 (84%) | 0.16 |
| Beta-blockers | 48 (71%) | 51 (74%) | 0.40 |
| Amiodarone | 10 (16%) | 16 (23%) | 0.15 |
| Aspirin | 53 (78%) | 54 (78%) | 0.56 |
| Statins | 33 (48%) | 46 (67%) | 0.02 |
| Oral diuretics | 56 (82%) | 60 (87%) | 0.30 |
| Variables . | RV dysfunction (n = 69) . | No RV dysfunction (n = 69) . | P-value . |
|---|---|---|---|
| Age (years) | 65.8 ± 10.8 | 64.9 ± 9.2 | 0.60 |
| Gender | 0.41 | ||
| Male | 55 (80%) | 57 (83%) | |
| Female | 14 (20%) | 12 (17%) | |
| BSA | 1.8 ± 0.17 | 1.7 ± 0.21 | 0.32 |
| Cardiac risk factor | |||
| Family history of CAD | 26 (38%) | 28 (41%) | 0.46 |
| Hypertension | 39 (57%) | 37 (53%) | 0.39 |
| Dyslipidaemia | 36 (53%) | 39 (57%) | 0.40 |
| Diabetes | 17 (24%) | 13 (19%) | 0.18 |
| Active Smoking | 13 (19%) | 12 (17%) | 0.90 |
| CVA | 4 (6%) | 5 (7%) | 0.63 |
| Chronic renal failure | 5 (7%) | 3 (4%) | 0.36 |
| Clinical presentation | |||
| Angina | 45 (67%) | 53 (76%) | 0.16 |
| NYHA class >2 | 48 (69%) | 41 (60%) | 0.14 |
| Atrial fibrillation | 10 (14%) | 12 (17%) | 0.41 |
| QRS width (mV) | 120 ± 29 | 116 ± 28 | 0.37 |
| Preoperative medication | |||
| ACE-inhibitor | 62 (91%) | 58 (84%) | 0.16 |
| Beta-blockers | 48 (71%) | 51 (74%) | 0.40 |
| Amiodarone | 10 (16%) | 16 (23%) | 0.15 |
| Aspirin | 53 (78%) | 54 (78%) | 0.56 |
| Statins | 33 (48%) | 46 (67%) | 0.02 |
| Oral diuretics | 56 (82%) | 60 (87%) | 0.30 |
ACE: angiotensin-converting enzyme; BSA: body surface area; CAD: coronary artery disease; CVA: cerebrovascular accident; NYHA: New York Heart Association; RV: right ventricular.
| Variables . | RV dysfunction (n = 69 patients) . | No RV dysfunction (n = 69 patients) . | Parametric P . | Non-parametric P . |
|---|---|---|---|---|
| Preoperative echocardiography | ||||
| Diastolic diameter (mm) | 65 ± 9 (64) | 65 ± 10 (65) | 0.92 | 0.91 |
| Systolic diameter (mm) | 53 ± 10 (53) | 53 ± 12 (52) | 0.86 | 0.75 |
| EDV (ml) | 228 ± 95 (223) | 219 ± 78 (206) | 0.56 | 0.61 |
| ESV (ml) | 171 ± 83 (167) | 162 ± 71 (149) | 0.53 | 0.49 |
| Indexed EDV (ml/m2) | 125 ± 51 (127) | 121 ± 44 (122) | 0.68 | 0.75 |
| Indexed ESV (ml/m2) | 93 ± 45 (95) | 90 ± 41 (91) | 0.64 | 0.59 |
| EF (%) | 26 ± 8 (24) | 27 ± 7 (25) | 0.57 | 0.36 |
| SV (ml) | 59 ± 22 (56) | 56 ± 15 (53) | 0.39 | 0.63 |
| Indexed SV (ml/m2) | 32 ± 11 (32) | 31 ± 8 (29) | 0.46 | 0.81 |
| Diastolic IV septum (mm) | 10 ± 2 (10) | 9 ± 3 (10) | 0.37 | 0.54 |
| Systolic IV septum (mm) | 12 ± 3 (12) | 11 ± 3 (12) | 0.62 | 0.75 |
| Dyast. LV posterior wall (mm) | 11 ± 2 (11) | 10 ± 1 (10) | 0.36 | 0.52 |
| Syst. LV posterior wall (mm) | 14 ± 3 (14) | 13 ± 2 (14) | 0.83 | 0.88 |
| Cardiac mass (g) | 310 ± 78 (314) | 307 ± 82 (307) | 0.84 | 0.71 |
| Indexed cardiac mass (g/m2) | 170 ± 44 (165) | 168 ± 44 (163) | 0.88 | 0.85 |
| RWT | 0.33 ± 0.09 (0.33) | 0.32 ± 0.07 (0.30) | 0.87 | 0.79 |
| Left atrium (diameter—mm) | 46 ± 9 (47) | 45 ± 7 (45) | 0.64 | 0.48 |
| DP | 2.2 ± 1.1 (2) | 1.9 ± 0.9 (2) | 0.20 | 0.24 |
| E/A ratio | 1.7 ± 1.1 (1.5) | 1.4 ± 0.9 (1.1) | 0.11 | 0.16 |
| DTE | 172 ± 59 (157) | 185 ± 54 (182) | 0.23 | 0.10 |
| TAPSE | 14 ± 1.5 (15) | 20 ± 2.7 (19) | 0.01 | 0.01 |
| Systolic PAP (mmHg) | 45 ± 18 (46) | 40 ± 12 (40) | 0.11 | 0.26 |
| Mitral annulus (mm) | 34 ± 6 (34) | 34 ± 5 (34) | 0.82 | 0.85 |
| Diastolic Sfericity index | 0.55 ± 0.11 (0.61) | 0.59 ± 0.12 (0.67) | 0.06 | 0.09 |
| Systolic Sfericity index | 0.48 ± 0.12 (0.55) | 0.51 ± 0.13 (0.59) | 0.19 | 0.27 |
| Mitral regurgitation (>2+) | 23 (33%) | 20 (29%) | 0.36 | – |
| Variables . | RV dysfunction (n = 69 patients) . | No RV dysfunction (n = 69 patients) . | Parametric P . | Non-parametric P . |
|---|---|---|---|---|
| Preoperative echocardiography | ||||
| Diastolic diameter (mm) | 65 ± 9 (64) | 65 ± 10 (65) | 0.92 | 0.91 |
| Systolic diameter (mm) | 53 ± 10 (53) | 53 ± 12 (52) | 0.86 | 0.75 |
| EDV (ml) | 228 ± 95 (223) | 219 ± 78 (206) | 0.56 | 0.61 |
| ESV (ml) | 171 ± 83 (167) | 162 ± 71 (149) | 0.53 | 0.49 |
| Indexed EDV (ml/m2) | 125 ± 51 (127) | 121 ± 44 (122) | 0.68 | 0.75 |
| Indexed ESV (ml/m2) | 93 ± 45 (95) | 90 ± 41 (91) | 0.64 | 0.59 |
| EF (%) | 26 ± 8 (24) | 27 ± 7 (25) | 0.57 | 0.36 |
| SV (ml) | 59 ± 22 (56) | 56 ± 15 (53) | 0.39 | 0.63 |
| Indexed SV (ml/m2) | 32 ± 11 (32) | 31 ± 8 (29) | 0.46 | 0.81 |
| Diastolic IV septum (mm) | 10 ± 2 (10) | 9 ± 3 (10) | 0.37 | 0.54 |
| Systolic IV septum (mm) | 12 ± 3 (12) | 11 ± 3 (12) | 0.62 | 0.75 |
| Dyast. LV posterior wall (mm) | 11 ± 2 (11) | 10 ± 1 (10) | 0.36 | 0.52 |
| Syst. LV posterior wall (mm) | 14 ± 3 (14) | 13 ± 2 (14) | 0.83 | 0.88 |
| Cardiac mass (g) | 310 ± 78 (314) | 307 ± 82 (307) | 0.84 | 0.71 |
| Indexed cardiac mass (g/m2) | 170 ± 44 (165) | 168 ± 44 (163) | 0.88 | 0.85 |
| RWT | 0.33 ± 0.09 (0.33) | 0.32 ± 0.07 (0.30) | 0.87 | 0.79 |
| Left atrium (diameter—mm) | 46 ± 9 (47) | 45 ± 7 (45) | 0.64 | 0.48 |
| DP | 2.2 ± 1.1 (2) | 1.9 ± 0.9 (2) | 0.20 | 0.24 |
| E/A ratio | 1.7 ± 1.1 (1.5) | 1.4 ± 0.9 (1.1) | 0.11 | 0.16 |
| DTE | 172 ± 59 (157) | 185 ± 54 (182) | 0.23 | 0.10 |
| TAPSE | 14 ± 1.5 (15) | 20 ± 2.7 (19) | 0.01 | 0.01 |
| Systolic PAP (mmHg) | 45 ± 18 (46) | 40 ± 12 (40) | 0.11 | 0.26 |
| Mitral annulus (mm) | 34 ± 6 (34) | 34 ± 5 (34) | 0.82 | 0.85 |
| Diastolic Sfericity index | 0.55 ± 0.11 (0.61) | 0.59 ± 0.12 (0.67) | 0.06 | 0.09 |
| Systolic Sfericity index | 0.48 ± 0.12 (0.55) | 0.51 ± 0.13 (0.59) | 0.19 | 0.27 |
| Mitral regurgitation (>2+) | 23 (33%) | 20 (29%) | 0.36 | – |
Bold values were used to highlight the significant P-value (<0.05).
TAPSE: tricuspid annular plane systolic excursion; EDV: end-diastolic volume; ESV: end-systolic volume; EF: ejection fraction; RV: right ventricular; LV: left ventricular; SV: stroke volume; IV: interventricular; RWT: relative wall thickness; DTE: deceleration time echocardiography; PAP: pulmonary artery pressure.
| Variables . | RV dysfunction (n = 69 patients) . | No RV dysfunction (n = 69 patients) . | Parametric P . | Non-parametric P . |
|---|---|---|---|---|
| Preoperative echocardiography | ||||
| Diastolic diameter (mm) | 65 ± 9 (64) | 65 ± 10 (65) | 0.92 | 0.91 |
| Systolic diameter (mm) | 53 ± 10 (53) | 53 ± 12 (52) | 0.86 | 0.75 |
| EDV (ml) | 228 ± 95 (223) | 219 ± 78 (206) | 0.56 | 0.61 |
| ESV (ml) | 171 ± 83 (167) | 162 ± 71 (149) | 0.53 | 0.49 |
| Indexed EDV (ml/m2) | 125 ± 51 (127) | 121 ± 44 (122) | 0.68 | 0.75 |
| Indexed ESV (ml/m2) | 93 ± 45 (95) | 90 ± 41 (91) | 0.64 | 0.59 |
| EF (%) | 26 ± 8 (24) | 27 ± 7 (25) | 0.57 | 0.36 |
| SV (ml) | 59 ± 22 (56) | 56 ± 15 (53) | 0.39 | 0.63 |
| Indexed SV (ml/m2) | 32 ± 11 (32) | 31 ± 8 (29) | 0.46 | 0.81 |
| Diastolic IV septum (mm) | 10 ± 2 (10) | 9 ± 3 (10) | 0.37 | 0.54 |
| Systolic IV septum (mm) | 12 ± 3 (12) | 11 ± 3 (12) | 0.62 | 0.75 |
| Dyast. LV posterior wall (mm) | 11 ± 2 (11) | 10 ± 1 (10) | 0.36 | 0.52 |
| Syst. LV posterior wall (mm) | 14 ± 3 (14) | 13 ± 2 (14) | 0.83 | 0.88 |
| Cardiac mass (g) | 310 ± 78 (314) | 307 ± 82 (307) | 0.84 | 0.71 |
| Indexed cardiac mass (g/m2) | 170 ± 44 (165) | 168 ± 44 (163) | 0.88 | 0.85 |
| RWT | 0.33 ± 0.09 (0.33) | 0.32 ± 0.07 (0.30) | 0.87 | 0.79 |
| Left atrium (diameter—mm) | 46 ± 9 (47) | 45 ± 7 (45) | 0.64 | 0.48 |
| DP | 2.2 ± 1.1 (2) | 1.9 ± 0.9 (2) | 0.20 | 0.24 |
| E/A ratio | 1.7 ± 1.1 (1.5) | 1.4 ± 0.9 (1.1) | 0.11 | 0.16 |
| DTE | 172 ± 59 (157) | 185 ± 54 (182) | 0.23 | 0.10 |
| TAPSE | 14 ± 1.5 (15) | 20 ± 2.7 (19) | 0.01 | 0.01 |
| Systolic PAP (mmHg) | 45 ± 18 (46) | 40 ± 12 (40) | 0.11 | 0.26 |
| Mitral annulus (mm) | 34 ± 6 (34) | 34 ± 5 (34) | 0.82 | 0.85 |
| Diastolic Sfericity index | 0.55 ± 0.11 (0.61) | 0.59 ± 0.12 (0.67) | 0.06 | 0.09 |
| Systolic Sfericity index | 0.48 ± 0.12 (0.55) | 0.51 ± 0.13 (0.59) | 0.19 | 0.27 |
| Mitral regurgitation (>2+) | 23 (33%) | 20 (29%) | 0.36 | – |
| Variables . | RV dysfunction (n = 69 patients) . | No RV dysfunction (n = 69 patients) . | Parametric P . | Non-parametric P . |
|---|---|---|---|---|
| Preoperative echocardiography | ||||
| Diastolic diameter (mm) | 65 ± 9 (64) | 65 ± 10 (65) | 0.92 | 0.91 |
| Systolic diameter (mm) | 53 ± 10 (53) | 53 ± 12 (52) | 0.86 | 0.75 |
| EDV (ml) | 228 ± 95 (223) | 219 ± 78 (206) | 0.56 | 0.61 |
| ESV (ml) | 171 ± 83 (167) | 162 ± 71 (149) | 0.53 | 0.49 |
| Indexed EDV (ml/m2) | 125 ± 51 (127) | 121 ± 44 (122) | 0.68 | 0.75 |
| Indexed ESV (ml/m2) | 93 ± 45 (95) | 90 ± 41 (91) | 0.64 | 0.59 |
| EF (%) | 26 ± 8 (24) | 27 ± 7 (25) | 0.57 | 0.36 |
| SV (ml) | 59 ± 22 (56) | 56 ± 15 (53) | 0.39 | 0.63 |
| Indexed SV (ml/m2) | 32 ± 11 (32) | 31 ± 8 (29) | 0.46 | 0.81 |
| Diastolic IV septum (mm) | 10 ± 2 (10) | 9 ± 3 (10) | 0.37 | 0.54 |
| Systolic IV septum (mm) | 12 ± 3 (12) | 11 ± 3 (12) | 0.62 | 0.75 |
| Dyast. LV posterior wall (mm) | 11 ± 2 (11) | 10 ± 1 (10) | 0.36 | 0.52 |
| Syst. LV posterior wall (mm) | 14 ± 3 (14) | 13 ± 2 (14) | 0.83 | 0.88 |
| Cardiac mass (g) | 310 ± 78 (314) | 307 ± 82 (307) | 0.84 | 0.71 |
| Indexed cardiac mass (g/m2) | 170 ± 44 (165) | 168 ± 44 (163) | 0.88 | 0.85 |
| RWT | 0.33 ± 0.09 (0.33) | 0.32 ± 0.07 (0.30) | 0.87 | 0.79 |
| Left atrium (diameter—mm) | 46 ± 9 (47) | 45 ± 7 (45) | 0.64 | 0.48 |
| DP | 2.2 ± 1.1 (2) | 1.9 ± 0.9 (2) | 0.20 | 0.24 |
| E/A ratio | 1.7 ± 1.1 (1.5) | 1.4 ± 0.9 (1.1) | 0.11 | 0.16 |
| DTE | 172 ± 59 (157) | 185 ± 54 (182) | 0.23 | 0.10 |
| TAPSE | 14 ± 1.5 (15) | 20 ± 2.7 (19) | 0.01 | 0.01 |
| Systolic PAP (mmHg) | 45 ± 18 (46) | 40 ± 12 (40) | 0.11 | 0.26 |
| Mitral annulus (mm) | 34 ± 6 (34) | 34 ± 5 (34) | 0.82 | 0.85 |
| Diastolic Sfericity index | 0.55 ± 0.11 (0.61) | 0.59 ± 0.12 (0.67) | 0.06 | 0.09 |
| Systolic Sfericity index | 0.48 ± 0.12 (0.55) | 0.51 ± 0.13 (0.59) | 0.19 | 0.27 |
| Mitral regurgitation (>2+) | 23 (33%) | 20 (29%) | 0.36 | – |
Bold values were used to highlight the significant P-value (<0.05).
TAPSE: tricuspid annular plane systolic excursion; EDV: end-diastolic volume; ESV: end-systolic volume; EF: ejection fraction; RV: right ventricular; LV: left ventricular; SV: stroke volume; IV: interventricular; RWT: relative wall thickness; DTE: deceleration time echocardiography; PAP: pulmonary artery pressure.
Early and late outcomes in the fully matched patient population
Perioperative characteristics are depicted in Table 4. Surgical coronary revascularization was accomplished in >90% of patients in both the groups (P = 0.50). The remaining patients had non-significant coronary lesions resulting from previous percutaneous transluminal coronary angioplasty. The mean number of CABG (P = 0.37) and the mitral valve repair rate (P = 0.50) were not significantly different between the two study groups.
| Variables . | RV dysfunction (n = 69 patients) . | No RV dysfunction (n = 69 patients) . | P-value . |
|---|---|---|---|
| CABG | 62 (90%) | 63 (91%) | 0.50 |
| Mean number of CABG | 2.5 ± 1.6 | 2.7 ± 1.6 | 0.37 |
| Use of left ITA | 61 (88%) | 63 (91%) | 0.39 |
| Patch closure | 40 (58%) | 36 (52%) | 0.30 |
| mitral valve repair | 25 (36%) | 24 (35%) | 0.50 |
| Postoperative inotropes | 54 (78%) | 40 (60% | 0.01 |
| Postoperative IABP | 22 (32%) | 11 (16%) | 0.03 |
| Postoperative complication | |||
| Low-output syndrome | 8 (12%) | 2 (4%) | 0.01 |
| CVA | 0 (0%) | 1 (2%) | 0.49 |
| Ventricular Arrhythmias | 8 (12%) | 5 (8%) | 0.30 |
| Atrial fibrillation | 14 (20%) | 15 (21%) | 0.55 |
| Acute renal failure | 7 (10%) | 3 (5%) | 0.17 |
| PM implant | 4 (6%) | 1 (2%) | 0.19 |
| Preoperative ACEF score | 2.7 ± 0.9 | 2.5 ± 0.9 | 0.23 |
| 30-day mortality | 10 (14%) | 7 (10%) | 0.18 |
| Long-term mortality | 22 (36%) | 6 (10%) | 0.01 |
| Preoperative TAPSE (1) | 14.0 | 20.0 | 0.01 |
| At discharge TAPSE (2) | 13.5 | 13.9 | 0.87 |
| At follow-up TAPSE (3) | 14.4 | 16.4 | 0.02 |
| P between (1) and (2) | 0.75 | 0.01 | |
| P between (2) and (3) | 0.68 | 0.03 | |
| Variables . | RV dysfunction (n = 69 patients) . | No RV dysfunction (n = 69 patients) . | P-value . |
|---|---|---|---|
| CABG | 62 (90%) | 63 (91%) | 0.50 |
| Mean number of CABG | 2.5 ± 1.6 | 2.7 ± 1.6 | 0.37 |
| Use of left ITA | 61 (88%) | 63 (91%) | 0.39 |
| Patch closure | 40 (58%) | 36 (52%) | 0.30 |
| mitral valve repair | 25 (36%) | 24 (35%) | 0.50 |
| Postoperative inotropes | 54 (78%) | 40 (60% | 0.01 |
| Postoperative IABP | 22 (32%) | 11 (16%) | 0.03 |
| Postoperative complication | |||
| Low-output syndrome | 8 (12%) | 2 (4%) | 0.01 |
| CVA | 0 (0%) | 1 (2%) | 0.49 |
| Ventricular Arrhythmias | 8 (12%) | 5 (8%) | 0.30 |
| Atrial fibrillation | 14 (20%) | 15 (21%) | 0.55 |
| Acute renal failure | 7 (10%) | 3 (5%) | 0.17 |
| PM implant | 4 (6%) | 1 (2%) | 0.19 |
| Preoperative ACEF score | 2.7 ± 0.9 | 2.5 ± 0.9 | 0.23 |
| 30-day mortality | 10 (14%) | 7 (10%) | 0.18 |
| Long-term mortality | 22 (36%) | 6 (10%) | 0.01 |
| Preoperative TAPSE (1) | 14.0 | 20.0 | 0.01 |
| At discharge TAPSE (2) | 13.5 | 13.9 | 0.87 |
| At follow-up TAPSE (3) | 14.4 | 16.4 | 0.02 |
| P between (1) and (2) | 0.75 | 0.01 | |
| P between (2) and (3) | 0.68 | 0.03 | |
Bold values were used to highlight the significant P-value (<0.05).
CVA: cerebrovascular accident; IABP: intra-aortic balloon pump; ITA: internal thoracic artery; TAPSE: tricuspid annular plane systolic excursion; RV: right ventricular; CABG: coronary artery bypass grafting; ACEF: age, creatinine and ejection fraction.
| Variables . | RV dysfunction (n = 69 patients) . | No RV dysfunction (n = 69 patients) . | P-value . |
|---|---|---|---|
| CABG | 62 (90%) | 63 (91%) | 0.50 |
| Mean number of CABG | 2.5 ± 1.6 | 2.7 ± 1.6 | 0.37 |
| Use of left ITA | 61 (88%) | 63 (91%) | 0.39 |
| Patch closure | 40 (58%) | 36 (52%) | 0.30 |
| mitral valve repair | 25 (36%) | 24 (35%) | 0.50 |
| Postoperative inotropes | 54 (78%) | 40 (60% | 0.01 |
| Postoperative IABP | 22 (32%) | 11 (16%) | 0.03 |
| Postoperative complication | |||
| Low-output syndrome | 8 (12%) | 2 (4%) | 0.01 |
| CVA | 0 (0%) | 1 (2%) | 0.49 |
| Ventricular Arrhythmias | 8 (12%) | 5 (8%) | 0.30 |
| Atrial fibrillation | 14 (20%) | 15 (21%) | 0.55 |
| Acute renal failure | 7 (10%) | 3 (5%) | 0.17 |
| PM implant | 4 (6%) | 1 (2%) | 0.19 |
| Preoperative ACEF score | 2.7 ± 0.9 | 2.5 ± 0.9 | 0.23 |
| 30-day mortality | 10 (14%) | 7 (10%) | 0.18 |
| Long-term mortality | 22 (36%) | 6 (10%) | 0.01 |
| Preoperative TAPSE (1) | 14.0 | 20.0 | 0.01 |
| At discharge TAPSE (2) | 13.5 | 13.9 | 0.87 |
| At follow-up TAPSE (3) | 14.4 | 16.4 | 0.02 |
| P between (1) and (2) | 0.75 | 0.01 | |
| P between (2) and (3) | 0.68 | 0.03 | |
| Variables . | RV dysfunction (n = 69 patients) . | No RV dysfunction (n = 69 patients) . | P-value . |
|---|---|---|---|
| CABG | 62 (90%) | 63 (91%) | 0.50 |
| Mean number of CABG | 2.5 ± 1.6 | 2.7 ± 1.6 | 0.37 |
| Use of left ITA | 61 (88%) | 63 (91%) | 0.39 |
| Patch closure | 40 (58%) | 36 (52%) | 0.30 |
| mitral valve repair | 25 (36%) | 24 (35%) | 0.50 |
| Postoperative inotropes | 54 (78%) | 40 (60% | 0.01 |
| Postoperative IABP | 22 (32%) | 11 (16%) | 0.03 |
| Postoperative complication | |||
| Low-output syndrome | 8 (12%) | 2 (4%) | 0.01 |
| CVA | 0 (0%) | 1 (2%) | 0.49 |
| Ventricular Arrhythmias | 8 (12%) | 5 (8%) | 0.30 |
| Atrial fibrillation | 14 (20%) | 15 (21%) | 0.55 |
| Acute renal failure | 7 (10%) | 3 (5%) | 0.17 |
| PM implant | 4 (6%) | 1 (2%) | 0.19 |
| Preoperative ACEF score | 2.7 ± 0.9 | 2.5 ± 0.9 | 0.23 |
| 30-day mortality | 10 (14%) | 7 (10%) | 0.18 |
| Long-term mortality | 22 (36%) | 6 (10%) | 0.01 |
| Preoperative TAPSE (1) | 14.0 | 20.0 | 0.01 |
| At discharge TAPSE (2) | 13.5 | 13.9 | 0.87 |
| At follow-up TAPSE (3) | 14.4 | 16.4 | 0.02 |
| P between (1) and (2) | 0.75 | 0.01 | |
| P between (2) and (3) | 0.68 | 0.03 | |
Bold values were used to highlight the significant P-value (<0.05).
CVA: cerebrovascular accident; IABP: intra-aortic balloon pump; ITA: internal thoracic artery; TAPSE: tricuspid annular plane systolic excursion; RV: right ventricular; CABG: coronary artery bypass grafting; ACEF: age, creatinine and ejection fraction.
Overall, perioperative low-output syndrome incidence was significantly higher in Group A (12 vs 5%; P = 0.01). As a consequence, postoperative inotropic support (P = 0.01) and intra-aortic balloon pump (IABP) insertion (P = 0.03) were significantly more frequent in patients with RV dysfunction compared with patients without RV dysfunction. The remaining perioperative complication incidence was comparable between the two groups. Preoperative ACEF score was not significantly different among the two study groups (P = 0.23). Thirty-day mortality was 14% (10/69) and 10% (7/69) in Groups A and B, respectively, and it did not reach statistical significance (P = 0.18).
Figure 1 shows the probability of death from any cause at follow-up, which occurred in 22 patients (36%) in Group A and in 6 patients (10%) in Group B (P = 0.01). Kaplan–Meier 5- and 8-year survival rate were significantly lower in patients with RV dysfunction (61 and 48% compared with 83 and 77% in Groups A and B, respectively; log-rank; P = 0.01). Furthermore, long-term freedom from cardiac events was significantly lower in patients with RVD (Kaplan–Meier 5- and 8-years freedom from cardiac events was 40 and 22% compared with 61 and 43% in Groups A and B, respectively; log-rank: P = 0.02; Fig. 2).
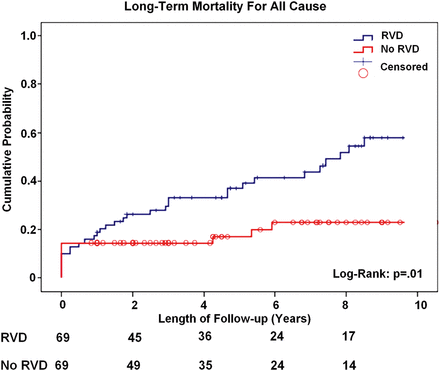
Kaplan–Meier long-term mortality plot for patients with RVD compared with patients without RVD. RVD: right ventricular dysfunction.
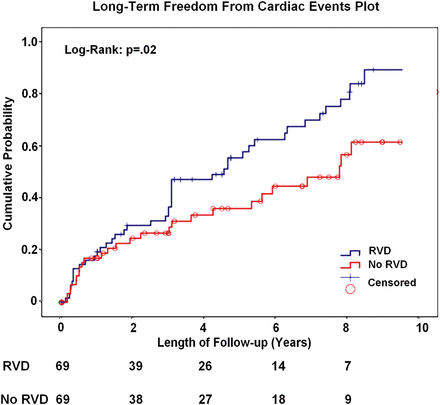
Kaplan–Meier long-term freedom from cardiac events (death or HF readmission) for patients with RVD compared with patients without RVD. RVD: right ventricular dysfunction.
Cox regression analysis (Fig. 3) demonstrated that the presence of RVD [hazard ratio (HR): 6.59 (95% confidence interval (CI): 1.97–9.05); P = 0.002] and New York Heart Association (NYHA) class class >II [HR: 3.09 (95% CI: 1.23–7.78); P = 0.02] were independent predictors of late mortality. In the same analysis, recurrence of moderate-to-severe MR at follow-up [HR: 1.98 (95% CI: 1.14–4.33); P = 0.04] was the only independent predictor of late freedom from cardiac events, irrespectively of biventricular function.
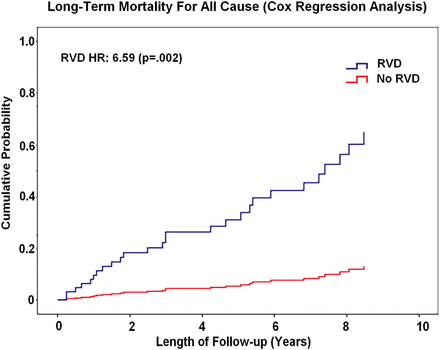
Long-term mortality plot for patients with RVD compared with patients without RVD. Cox regression multivariate analysis. RVD: right ventricular dysfunction.
The correlation between the probability of long-term mortality and the preoperative TAPSE value, and its interaction with the preoperative functional NYHA class are plotted in Fig. 4. The graph clearly shows the following: (i) long-term mortality significantly increases for TAPSE values <16, and this increase is exponential (R2 quadratic = 0.39); (ii) on equal TAPSE values, advanced preoperative NYHA class nearly doubles the probability of long-term mortality predicted by that given RV dysfunction rate and (iii) the concomitant presence of advanced NYHA class strengthens the exponential relationship between preoperative RVD and long-term mortality (R2 quadratic = 0.58).
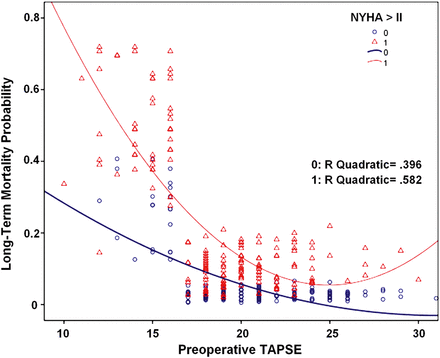
The correlation between the probability of long-term mortality and the preoperative TAPSE value, and its interaction with the preoperative functional New York Heart Association class. TAPSE: tricuspid annular plane systolic excursion.
Changes in RV function over time, as expressed by the TAPSE values, are depicted in Table 4. In patients with RVD, RV function remained steadily depressed over time, without significant improvement late after surgery. In contrast, patients in Group B experienced a significant impairment of RV function postoperatively, but TAPSE showed a progressive improvement over time.
DISCUSSION
In the present study, RV dysfunction, as reflected by an impaired TAPSE, emerged as a major predictor of both all-cause mortality and HF hospitalization in patients with ischaemic cardiomyopathy submitted to SVR.
The right ventricle has been considered for many years the ‘dark side of the moon’, as its contribution to the underlying mechanisms of various cardiac diseases has been obscured by the extensive research on LV pathology. Cardiac surgeons shared the same attitude, as preoperative RV dysfunction has been generally neglected as compared with traditional LV-related predictors of perioperative and postoperative outcomes.
However, several studies have recently shown that RV dysfunction is a prognostic marker both in patients submitted to cardiac surgery and in congestive HF patients, whatever technique is used to study the right ventricle [11]. Furthermore, many evidences suggest that RV impairment in cardiomyopathy is not only the simple consequence of pulmonary venous and arterial hypertension due to LV chronic dysfunction. Indeed, in patients with chronic HF, RV function can be preserved despite the presence of pulmonary hypertension, while on the contrary, RV dysfunction can occur with normal pulmonary artery pressure [12].
Right ventricular dysfunction in post- myocardial infarction remodelled heart
RV load, myocardial function and ventricular interaction are known as the three main determinants of RV function [13]. Compared with the LV, the RV is a thin-walled structure. Since pulmonary resistance level is low in normal subjects, the RV is accustomed to low afterload and functions with low cavity pressure and high chamber compliance. Hence, the right ventricle is a very sensitive chamber to changes in load conditions, but is much more sensitive to pressure overload than to volume overload. RV systolic function is reduced in pulmonary hypertension related to primary or secondary pulmonary disease as well as to left HF [14]. Increase in LV filling pressure and/or left atrial pressure elicits a backward rise in pulmonary venous pressure, pulmonary capillary wedge and artery pressure. This is consistent with the present study, where we observed that patients with RV dysfunction exhibited significantly larger left atrium dimensions (P = 0.02) and higher value of systolic pulmonary pressure (P = 0.01). Pulmonary hypertension in this group of patients was determined by two different mechanisms that are strictly interrelated: firstly, the higher incidence of severe MR and secondly, the increase LV filling pressures determined by a significant impairment of diastolic function. Indeed, few data have previously suggested that RV function in left heart HF is not a simple function of PAPs, which accounted for less than one quarter of RV EF alteration [15]. LV function and size influence RV function. First, the LV acts on RV function through interventricular septum [16]. The septum transmits systolic and diastolic pressure between right and left cavities. Although pressure interaction through the interventricular septum is considered less important, RV pressure is well known to influence LV function. Septal contraction contributes to both RV and LV functions, and is regarded a major determinant of RV performance [17]. Interventricular septum contraction is able to maintain RV function and cardiac output despite RV free wall impairment. The contribution of septal contraction to RV systolic function ranges from 24% in a normal RV to 35% in pathology.
Recently, Le Tourneau et al. [18], in a study of RV function by means of radionuclide angiography on 208 patients with MR, were able to demonstrate that alteration of RV systolic performance was clearly associated with the systolic impairment of LV septal region. In the present study, data on regional wall motion abnormality were not available, so it was not possible to identify patients in both groups with evident septal contraction impairment. However, LV remodelling after anterior acute MI often involves the interventricular septum, which appears thinner and fibrotic. Therefore, it is reasonable to conclude that in our population, severe impairment of septal contraction was an important mechanism of RV dysfunction, even if we were not able to quantitatively assess its contribution to RV systolic impairment.
Right ventricular dysfunction and outcome after cardiac surgery
The prognostic role of preoperative RV dysfunction in predicting early and long-term outcomes after cardiac surgery has been recently highlighted by several authors. Maslow et al. [19], in a retrospective series of 41 patients with severe LV systolic dysfunction operated of isolated CABG, demonstrated that RV dysfunction defined by a RV fractional area change (FAC) < 35% was a major determinant of postoperative outcome. Furthermore, Haddad et al. [20], in a series of patients operated of mitral and mitroaortic surgery, showed that preoperative RV dysfunction was the strongest predictor of postoperative low-output syndrome (LOS) and 30-day mortality. Several reasons can be advocated to explain the impact of RV dysfunction on early outcomes. Indeed, RV function is often impaired after cardiac surgery owing to ineffective myocardial protection (i.e. retrograde cardioplegia), air embolism, pulmonary vasoconstriction related to protamine and the ECC inflammatory response.
More difficult is to figure out the reasons underlying the prognostic impact of RV dysfunction in mid-term period. Di Mauro et al. [21], in a series of 111 patients with dilated cardiomyopathy submitted to MV repair, showed that severe preoperative RV dysfunction, defined by a TAPSE <12 mm, significantly affected early and 5-year survival. Chrustowicz et al. [22], in a consecutive series of patients with depressed LV function operated of MV repair, demonstrated that preoperative RV dilatation (RV diameter >35 mm) was associated with decreased long-term mortality.
In the present study, RV dysfunction was not associated with increased 30-day mortality, despite these patients experiencing higher postoperative LOS, with higher rate of inotropic support and IABP insertion. However, this result should be cautiously interpreted for several reasons. First, patients with RV dysfunction presented with all the risk factors (greater NYHA class, larger LV volumes, lower EF and higher incidence of severe MR) that have been reported to be independent predictors of in-hospital mortality after SVR [23]. Finally, the P-level of comparison of in-hospital mortality (P = 0.18) was not so far from the significance level. This suggests, probably, that the lack of difference we observed could be related to the small population, and a larger observation should be carried on before concluding on the impact of RV dysfunction on early mortality.
Conversely, we showed that concomitant baseline RV dysfunction had an unfavourable effect on long-term prognosis in HF patients undergoing SVR. A TAPSE <16 mm proved to be associated with a worse outcome at 5 and 10 years in the present population. Furthermore, this is the first time, to the best of our knowledge, that RVD has been analysed in relation to the long-term outcome in a surgical population of patients undergoing SVR. The reasons of RVD impact on late outcomes are not completely elucidated. It is evident that RVD associated with LV systolic HF defines a population of high-risk patients that carries the worst prognosis. Indeed, in the fully unmatched population, patients with RV dysfunction showed larger LV volumes, lower EF, higher pulmonary pressure and higher rate of severe MR. However, after matching, RVD emerged as an independent predictor of adverse outcome. It is notable that RVD continues to negatively impact long-term outcomes despite the fact that SVR corrects many of the negative prognostic factors related to LV dysfunction. Furthermore, despite SVR could theoretically determine an improvement in RV function (reducing left ventricular wall stress and mechanical dyssynchrony, decreasing pulmonary hypertension and improving LV filling pressures [24]), in our experience, patients with RVD failed to improve RV performance early and late after surgery. In contrast, a transient RV dysfunction early after surgery was observed in patients with normal preoperative TAPSE values. However, in this group, RV performance significantly improved in the late follow-up, even if it did not returned to preoperative levels. This is consistent with the observations of Raina et al. [25] who showed that after cardiac surgery, the RV contractile pattern changes, with a relative loss of longitudinal shortening—as measured by TAPSE—and gain in transverse shortening despite normal RV function.
Despite that we did not analyse the correlations between TAPSE changes over time and clinical outcomes, it could be reasonable to conclude that the lack of late improvement in RV performance after SVR could be the patho-physiological basis of the prognostic negative impact of preoperative RVD on late clinical outcomes.
For these reasons, a further analysis of changes in TAPSE value over time and its correlation with clinical events is the object of an ongoing study.
STUDY LIMITATIONS
TAPSE is only a rough indicator of RV function. However, the complex geometry of the right ventricle makes it difficult to assess the systolic function of this cardiac chamber, and no technique or parameter is exempt from potential criticism. Cardiac magnetic resonance imaging is the gold standard for quantifying RV size and function; however, access to the technology may be limited, and it may be contraindicated in many of the present patients due to pacemaker or defibrillator. As the present study is a 10-year retrospective study, our intention was to use a method that can be easily applied but is reliable to provide a quick assessment of RV function. Indeed, although it measures longitudinal function, it has shown good correlation with more complex techniques estimating RV global systolic function, such as radionuclide-derived RV EF, 2D RV FAC and 2D RV EF.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr P. Ferrazzi(Monza, Italy): In your paper, you have selected 50 patients with very bad right ventricular function. You report a hospital mortality of 12%, and 50% during 5 years of follow-up.
So this data, in my opinion, suggests that SVR is an operation for the early stage of the disease because the patients selected are probably in very poor condition. They have associated very poor left ventricular function during this time. We have the same results from the STICH trial database. But in the group of patients without SVR, undergoing coronary bypass only, the mortality is significantly lower.
So for the take home message, if tomorrow we have the kind of patient that you presented with tricuspid excursion less than 16, what do you suggest: to avoid SVR, to do only coronary bypass, to switch to ventricular assist device, or to transplantation?
Dr Garatti: Coming to the last observation, the patient who is eligible for heart transplant or VAD must be evaluated. But you know that there are scarce resources, especially regarding heart transplantation, which are probably limited to and available for young people. The mean age of this population, anyway, was older than 65 years which is the cut-off for heart transplant. So this kind of surgery, generally speaking, bypass, mitral valve, and aneurysm, is the only way we can treat these older people.
I don't really know. I mean, the problem is that these are very large ventricles, so the idea of performing only the bypass and leaving a very big ventricle I think has a negative impact on the prognosis.
The other important fact is that avoiding SVR probably has less impact on the early mortality, but at least half of these patients had mitral regurgitation. And I think it is quite mandatory to treat the mitral regurgitation because otherwise we have no improvement in the right ventricular function. So if we do the mitral with the CABG, I think that the surgical risk is not very different compared to CABG plus SVR plus the mitral.
So I think that probably we must evaluate patient by patient, even though I know that it is not such a scientific answer. But the real problem in these patients is the mitral valve that impacts the mortality.
Dr R. Voces(Bilbao, Spain): Do you recommend closure of the pericardium after remodelling surgery with no grafts in case of right ventricular dysfunction?
Dr Garatti: We close the pericardium every time, even with the grafts, because we pass the mammary artery just above the phrenic nerve, so we are not worried about closing the pericardium. I know what your question means. I don't have data to say that closing or not closing the pericardium impacts right ventricular dysfunction. But for protocol in everyday practice, we close the pericardium.
Dr M. Zembala(Zabrze, Poland): You said that RV function is certainly an important predictor of a good outcome, but do you agree that mitral regurgitation after SVR surgery, as you said already, is affecting and elevating pulmonary hypertension, but is it also possibly due to restriction of the LV after surgery? There's good work done by Dr Roman Przybylski from our group on factors affecting the outcome. Did you utilize the SVR Mannequin? Can you comment on the effect when you are sometimes excessively restrictive with the SVR?
Dr Garatti: Yes, we always use the Mannequin inside, so we try to be not so restrictive. The real problem is to evaluate the diastolic function before and after, and this could be a problem because, of course, impairment of diastolic function adds to pulmonary hypertension in affecting both left ventricular and right ventricular function.
To my knowledge, this problem of diastolic dysfunction after SVR is not completely clear. I mean, it is not so clear how to identify good predictors of diastolic dysfunction after surgical ventricular restoration, except if you leave a small chamber. And that's likely a problem in screening patients.
Author notes
Presented at the 27th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 5–9 October 2013.
- myocardial infarction
- mitral valve insufficiency
- intra-aortic balloon pumping
- echocardiography
- transesophageal atrial pacing stress echocardiography
- ventricular dysfunction, left
- ventricular dysfunction, right
- heart failure
- left ventricle
- cardiac event
- diastole
- objective (goal)
- heart ventricle
- phosphoadenosine phosphosulfate
- preoperative care
- survival rate
- systole
- heart
- mortality
- cox proportional hazards models
- new york heart association classification
- surgical ventricular reconstruction
- pulmonary artery systolic pressure
- pediatric acute pancreatitis severity scoring system
- inotropic support




