-
PDF
- Split View
-
Views
-
Cite
Cite
Masahiro Dohi, Hiroaki Miyata, Kiyoshi Doi, Kazunari Okawa, Noboru Motomura, Shinichi Takamoto, Hitoshi Yaku, on behalf of the Japan Cardiovascular Surgery Database, The off-pump technique in redo coronary artery bypass grafting reduces mortality and major morbidities: propensity score analysis of data from the Japan Cardiovascular Surgery Database, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 2, February 2015, Pages 299–308, https://doi.org/10.1093/ejcts/ezu081
Close - Share Icon Share
Abstract
The benefits of off-pump coronary artery grafting (OPCAB) have been demonstrated. Especially in patients with a high number of comorbidities, redo coronary artery bypass grafting (CABG) remains a difficult entity of CABG, because patients are likely to have multiple risk factors and often have diseased patent grafts with adhesions. The aim of the present study was to evaluate the effects of the OPCAB technique in redo CABG on mortality and morbidity using data from the Japan Cardiovascular Surgery Database (JCVSD).
We analysed 34 980 patients who underwent isolated CABG between 2008 and 2011, as reported in the JCVSD. Of these, 1.8% of patients (n = 617/34980) had undergone redo CABG, including those who underwent OPCAB (n = 364; 69%) and on-pump CABG (n = 253; 41%). We used propensity score (PS) matching with 13 preoperative risk factors to adjust for differences in baseline characteristics between the redo OPCAB and on-pump redo CABG groups. By one-to-one PS matching, we selected 200 pairs from each group.
There were no significant differences in patient background between the redo OPCAB and on-pump redo CABG groups after PS matching. There was no significant difference in the mean number of distal anastomoses after matching (2.41 ± 1.00 vs 2.21 ± 1.04, P = 0.074); nevertheless, the mean operation time was significantly shorter in the redo OPCAB than the on-pump redo CABG group (353.7 vs 441.3 min, P < 0.00010). Patients in the redo OPCAB group had a lower 30-day mortality rate (3.5 vs 7.0%, P = 0.18), a significantly lower rate of composite mortality or major morbidities (11.0 vs 21.5%, P = 0.0060), a significantly lower rate of prolonged ventilation (>24 h) (7.0 vs 15.0%, P = 0.016), a significantly shorter duration of intensive care unit (ICU) stay (ICU stay ≥8 days) (7.0 vs 14.5%, P = 0.023) and a significantly decreased need for blood transfusions (71.5 vs 94.0%, P < 0.00010) than patients in the on-pump redo CABG group.
The off-pump technique reduced early operative mortality and the incidences of major complications in redo CABG.
INTRODUCTION
Although recent reoperative coronary artery bypass grafting (redo CABG) has been associated with improved outcomes, it remains a difficult procedure of CABG because patients are likely to have multiple risk factors. On the other hand, off-pump coronary artery grafting (OPCAB) has been reported to be beneficial [1–3], especially in patients with several comorbidities, such as those undergoing redo CABG [4, 5].
In fact, retrospective clinical studies comparing OPCAB and on-pump CABG in redo surgery suggest that OPCAB can reduce morbidity among redo CABG patients, while providing equivalent or superior operative results [6–14]. However, no comparative clinical studies utilizing data from a large nationwide database have validated the superiority of various variables of the off-pump technique in redo CABG.
In this study, we present the contemporary results of redo CABG registered in the Japan Cardiovascular Surgery Database (JCVSD), which currently contains clinical data from almost all Japanese hospitals where cardiovascular surgery is performed. Our aim was to evaluate the superiority of the off-pump technique in redo CABG, and to retrospectively compare isolated redo CABG with or without cardiopulmonary bypass (CPB) using data from the JCVSD through propensity score (PS) matching.
MATERIALS AND METHODS
Study population
The JCVSD was established in 2000 with the goal of evaluating surgical outcomes after cardiovascular procedures in many centres throughout Japan [15, 16]. As of January 2013, JCVSD has recorded clinical information from over 500 hospitals, which comprises almost all hospitals with cardiovascular surgery units in Japan. The data collection form has 255 variables that are almost identical to those in the Society of Thoracic Surgeons (STS) National Database [available at: http://www.sts.org (25 February 2014, date last accessed)]. The definitions of JCVSD variables [available at: http://www.jacvsd.umin.jp (25 February 2014, date last accessed)] are also based on those of The STS National Database (Tables 1 and 2). Through the JCVSD web-based system, each participating hospital enters data and uses a feedback report in real time that includes risk-adjusted outcomes compared with all participating hospitals. Although participation in the JCVSD is voluntary, submissions tend to be thorough, with overall preoperative risk factors used in risk models missing in fewer than 3% of the entries. The accuracy of the submitted data is verified through monthly visits to each hospital by The Site Visit Working Group. The Site Visit Working Group members verify that the number of procedures from the original operative record list in the hospital matches that reported in the JCVSD. These members also examine each clinical chart of procedures and compare it with the JCVSD data.
| Variables . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Patients | 617 | 253 | 364 | |
| Sex (male) | 78.8 | 79.8 | 78.0 | 0.62 |
| Age (years), mean (SD) | 69.35 ± 9.2 | 68.87 ± 9.74 | 69.68 ± 8.81 | 0.28 |
| Body surface area, mean (SD) | 1.64 ± 0.18 | 1.64 ± 0.18 | 1.64 ± 0.18 | 0.54 |
| Smoking | 52.4 | 49.8 | 54.1 | 0.33 |
| Body mass index >30 kg/m2 | 5.2 | 5.1 | 5.2 | 1.00 |
| Diabetes | 46.2 | 45.5 | 46.7 | 0.81 |
| Preoperative creatinine value | 1.73 ± 2.29 | 1.68 ± 2.29 | 1.76 ± 2.29 | 0.70 |
| Renal failure | 18.5 | 18.2 | 18.7 | 0.92 |
| Dialysis | 9.1 | 9.6 | 9.4 | 0.89 |
| Hyperlipidaemia | 60.8 | 59.3 | 61.8 | 0.56 |
| Hypertension | 77.6 | 80.2 | 75.8 | 0.20 |
| Cerebrovascular disease | 11.0 | 12.6 | 9.9 | 0.30 |
| Carotid stenosis | 7.9 | 7.9 | 8.0 | 1.00 |
| COPD (moderate and severe) | 11.3 | 10.3 | 12.1 | 0.52 |
| Extracardiac disease | 20.9 | 23.7 | 19.0 | 0.16 |
| Peripheral | 18.0 | 19.8 | 16.8 | 0.34 |
| Myocardial infarction | 44.9 | 46.6 | 43.7 | 0.51 |
| Previous coronary artery intervention | 45.4 | 44.7 | 45.9 | 0.81 |
| Unstable angina | 32.9 | 38.3 | 29.1 | 0.019 |
| Shock | 4.9 | 7.9 | 2.7 | 0.0040 |
| Congestive heart failure | 14.7 | 17.0 | 13.2 | 0.21 |
| Arrhythmia | 11.7 | 13.8 | 10.2 | 0.16 |
| NYHA class III or IV | 28.4 | 31.2 | 26.4 | 0.20 |
| Diseased vessels, % | ||||
| Left main disease | 37.0 | 42.7 | 33.0 | 0.017 |
| 1 | 13.9 | 9.9 | 16.8 | 0.018 |
| 2 | 25.0 | 20.2 | 28.3 | 0.023 |
| Triple-vessel disease | 55.3 | 62.1 | 50.5 | 0.0050 |
| LV impairment, % | ||||
| Good >EF:60% | 34.5 | 35.2 | 34.1 | 0.80 |
| ≤EF:60% | 65.2 | 64.0 | 65.9 | 0.67 |
| Bad <EF:30% | 8.1 | 9.9 | 6.9 | 0.18 |
| Mitral valve insufficiency II–IV | 21.1 | 20.9 | 21.2 | 1.00 |
| Tricuspid valve insufficiency II–IV | 14.6 | 17.4 | 12.6 | 0.11 |
| Status, % | ||||
| Urgent | 8.3 | 9.9 | 7.1 | 0.24 |
| Emergency/salvage | 8.1 | 10.7 | 6.3 | 0.071 |
| Japan SCORE | ||||
| Expected 30 days operative mortality | 7.66 ± 0.14 | 5.51 ± 0.1 | 0.023 | |
| Expected composite 30-day mortality or major morbidity | 25.1 ± 0.18 | 21.9 ± 0.15 | 0.019 | |
| Variables . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Patients | 617 | 253 | 364 | |
| Sex (male) | 78.8 | 79.8 | 78.0 | 0.62 |
| Age (years), mean (SD) | 69.35 ± 9.2 | 68.87 ± 9.74 | 69.68 ± 8.81 | 0.28 |
| Body surface area, mean (SD) | 1.64 ± 0.18 | 1.64 ± 0.18 | 1.64 ± 0.18 | 0.54 |
| Smoking | 52.4 | 49.8 | 54.1 | 0.33 |
| Body mass index >30 kg/m2 | 5.2 | 5.1 | 5.2 | 1.00 |
| Diabetes | 46.2 | 45.5 | 46.7 | 0.81 |
| Preoperative creatinine value | 1.73 ± 2.29 | 1.68 ± 2.29 | 1.76 ± 2.29 | 0.70 |
| Renal failure | 18.5 | 18.2 | 18.7 | 0.92 |
| Dialysis | 9.1 | 9.6 | 9.4 | 0.89 |
| Hyperlipidaemia | 60.8 | 59.3 | 61.8 | 0.56 |
| Hypertension | 77.6 | 80.2 | 75.8 | 0.20 |
| Cerebrovascular disease | 11.0 | 12.6 | 9.9 | 0.30 |
| Carotid stenosis | 7.9 | 7.9 | 8.0 | 1.00 |
| COPD (moderate and severe) | 11.3 | 10.3 | 12.1 | 0.52 |
| Extracardiac disease | 20.9 | 23.7 | 19.0 | 0.16 |
| Peripheral | 18.0 | 19.8 | 16.8 | 0.34 |
| Myocardial infarction | 44.9 | 46.6 | 43.7 | 0.51 |
| Previous coronary artery intervention | 45.4 | 44.7 | 45.9 | 0.81 |
| Unstable angina | 32.9 | 38.3 | 29.1 | 0.019 |
| Shock | 4.9 | 7.9 | 2.7 | 0.0040 |
| Congestive heart failure | 14.7 | 17.0 | 13.2 | 0.21 |
| Arrhythmia | 11.7 | 13.8 | 10.2 | 0.16 |
| NYHA class III or IV | 28.4 | 31.2 | 26.4 | 0.20 |
| Diseased vessels, % | ||||
| Left main disease | 37.0 | 42.7 | 33.0 | 0.017 |
| 1 | 13.9 | 9.9 | 16.8 | 0.018 |
| 2 | 25.0 | 20.2 | 28.3 | 0.023 |
| Triple-vessel disease | 55.3 | 62.1 | 50.5 | 0.0050 |
| LV impairment, % | ||||
| Good >EF:60% | 34.5 | 35.2 | 34.1 | 0.80 |
| ≤EF:60% | 65.2 | 64.0 | 65.9 | 0.67 |
| Bad <EF:30% | 8.1 | 9.9 | 6.9 | 0.18 |
| Mitral valve insufficiency II–IV | 21.1 | 20.9 | 21.2 | 1.00 |
| Tricuspid valve insufficiency II–IV | 14.6 | 17.4 | 12.6 | 0.11 |
| Status, % | ||||
| Urgent | 8.3 | 9.9 | 7.1 | 0.24 |
| Emergency/salvage | 8.1 | 10.7 | 6.3 | 0.071 |
| Japan SCORE | ||||
| Expected 30 days operative mortality | 7.66 ± 0.14 | 5.51 ± 0.1 | 0.023 | |
| Expected composite 30-day mortality or major morbidity | 25.1 ± 0.18 | 21.9 ± 0.15 | 0.019 | |
Values are percentage or mean ± SD values.
COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association; LV: left ventricular; EF: ejection fraction.
| Variables . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Patients | 617 | 253 | 364 | |
| Sex (male) | 78.8 | 79.8 | 78.0 | 0.62 |
| Age (years), mean (SD) | 69.35 ± 9.2 | 68.87 ± 9.74 | 69.68 ± 8.81 | 0.28 |
| Body surface area, mean (SD) | 1.64 ± 0.18 | 1.64 ± 0.18 | 1.64 ± 0.18 | 0.54 |
| Smoking | 52.4 | 49.8 | 54.1 | 0.33 |
| Body mass index >30 kg/m2 | 5.2 | 5.1 | 5.2 | 1.00 |
| Diabetes | 46.2 | 45.5 | 46.7 | 0.81 |
| Preoperative creatinine value | 1.73 ± 2.29 | 1.68 ± 2.29 | 1.76 ± 2.29 | 0.70 |
| Renal failure | 18.5 | 18.2 | 18.7 | 0.92 |
| Dialysis | 9.1 | 9.6 | 9.4 | 0.89 |
| Hyperlipidaemia | 60.8 | 59.3 | 61.8 | 0.56 |
| Hypertension | 77.6 | 80.2 | 75.8 | 0.20 |
| Cerebrovascular disease | 11.0 | 12.6 | 9.9 | 0.30 |
| Carotid stenosis | 7.9 | 7.9 | 8.0 | 1.00 |
| COPD (moderate and severe) | 11.3 | 10.3 | 12.1 | 0.52 |
| Extracardiac disease | 20.9 | 23.7 | 19.0 | 0.16 |
| Peripheral | 18.0 | 19.8 | 16.8 | 0.34 |
| Myocardial infarction | 44.9 | 46.6 | 43.7 | 0.51 |
| Previous coronary artery intervention | 45.4 | 44.7 | 45.9 | 0.81 |
| Unstable angina | 32.9 | 38.3 | 29.1 | 0.019 |
| Shock | 4.9 | 7.9 | 2.7 | 0.0040 |
| Congestive heart failure | 14.7 | 17.0 | 13.2 | 0.21 |
| Arrhythmia | 11.7 | 13.8 | 10.2 | 0.16 |
| NYHA class III or IV | 28.4 | 31.2 | 26.4 | 0.20 |
| Diseased vessels, % | ||||
| Left main disease | 37.0 | 42.7 | 33.0 | 0.017 |
| 1 | 13.9 | 9.9 | 16.8 | 0.018 |
| 2 | 25.0 | 20.2 | 28.3 | 0.023 |
| Triple-vessel disease | 55.3 | 62.1 | 50.5 | 0.0050 |
| LV impairment, % | ||||
| Good >EF:60% | 34.5 | 35.2 | 34.1 | 0.80 |
| ≤EF:60% | 65.2 | 64.0 | 65.9 | 0.67 |
| Bad <EF:30% | 8.1 | 9.9 | 6.9 | 0.18 |
| Mitral valve insufficiency II–IV | 21.1 | 20.9 | 21.2 | 1.00 |
| Tricuspid valve insufficiency II–IV | 14.6 | 17.4 | 12.6 | 0.11 |
| Status, % | ||||
| Urgent | 8.3 | 9.9 | 7.1 | 0.24 |
| Emergency/salvage | 8.1 | 10.7 | 6.3 | 0.071 |
| Japan SCORE | ||||
| Expected 30 days operative mortality | 7.66 ± 0.14 | 5.51 ± 0.1 | 0.023 | |
| Expected composite 30-day mortality or major morbidity | 25.1 ± 0.18 | 21.9 ± 0.15 | 0.019 | |
| Variables . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Patients | 617 | 253 | 364 | |
| Sex (male) | 78.8 | 79.8 | 78.0 | 0.62 |
| Age (years), mean (SD) | 69.35 ± 9.2 | 68.87 ± 9.74 | 69.68 ± 8.81 | 0.28 |
| Body surface area, mean (SD) | 1.64 ± 0.18 | 1.64 ± 0.18 | 1.64 ± 0.18 | 0.54 |
| Smoking | 52.4 | 49.8 | 54.1 | 0.33 |
| Body mass index >30 kg/m2 | 5.2 | 5.1 | 5.2 | 1.00 |
| Diabetes | 46.2 | 45.5 | 46.7 | 0.81 |
| Preoperative creatinine value | 1.73 ± 2.29 | 1.68 ± 2.29 | 1.76 ± 2.29 | 0.70 |
| Renal failure | 18.5 | 18.2 | 18.7 | 0.92 |
| Dialysis | 9.1 | 9.6 | 9.4 | 0.89 |
| Hyperlipidaemia | 60.8 | 59.3 | 61.8 | 0.56 |
| Hypertension | 77.6 | 80.2 | 75.8 | 0.20 |
| Cerebrovascular disease | 11.0 | 12.6 | 9.9 | 0.30 |
| Carotid stenosis | 7.9 | 7.9 | 8.0 | 1.00 |
| COPD (moderate and severe) | 11.3 | 10.3 | 12.1 | 0.52 |
| Extracardiac disease | 20.9 | 23.7 | 19.0 | 0.16 |
| Peripheral | 18.0 | 19.8 | 16.8 | 0.34 |
| Myocardial infarction | 44.9 | 46.6 | 43.7 | 0.51 |
| Previous coronary artery intervention | 45.4 | 44.7 | 45.9 | 0.81 |
| Unstable angina | 32.9 | 38.3 | 29.1 | 0.019 |
| Shock | 4.9 | 7.9 | 2.7 | 0.0040 |
| Congestive heart failure | 14.7 | 17.0 | 13.2 | 0.21 |
| Arrhythmia | 11.7 | 13.8 | 10.2 | 0.16 |
| NYHA class III or IV | 28.4 | 31.2 | 26.4 | 0.20 |
| Diseased vessels, % | ||||
| Left main disease | 37.0 | 42.7 | 33.0 | 0.017 |
| 1 | 13.9 | 9.9 | 16.8 | 0.018 |
| 2 | 25.0 | 20.2 | 28.3 | 0.023 |
| Triple-vessel disease | 55.3 | 62.1 | 50.5 | 0.0050 |
| LV impairment, % | ||||
| Good >EF:60% | 34.5 | 35.2 | 34.1 | 0.80 |
| ≤EF:60% | 65.2 | 64.0 | 65.9 | 0.67 |
| Bad <EF:30% | 8.1 | 9.9 | 6.9 | 0.18 |
| Mitral valve insufficiency II–IV | 21.1 | 20.9 | 21.2 | 1.00 |
| Tricuspid valve insufficiency II–IV | 14.6 | 17.4 | 12.6 | 0.11 |
| Status, % | ||||
| Urgent | 8.3 | 9.9 | 7.1 | 0.24 |
| Emergency/salvage | 8.1 | 10.7 | 6.3 | 0.071 |
| Japan SCORE | ||||
| Expected 30 days operative mortality | 7.66 ± 0.14 | 5.51 ± 0.1 | 0.023 | |
| Expected composite 30-day mortality or major morbidity | 25.1 ± 0.18 | 21.9 ± 0.15 | 0.019 | |
Values are percentage or mean ± SD values.
COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association; LV: left ventricular; EF: ejection fraction.
| . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Intraoperative variables | ||||
| Operation time (min) | 375.21 ± 147.0 | 427.61 ± 151.23 | 339.06 ± 132.61 | <0.00010 |
| Distal anastomoses mean (SD) | 2.12 ± 1.10 | 2.30 ± 1.04 | 2.00 ± 1.12 | 0.0010 |
| Number of distal anastomoses | ||||
| 1, 2 | 66.9 | 60.1 | 71.7 | 0.0030 |
| 3 | 21.9 | 26.9 | 18.4 | 0.013 |
| 4, 5 | 10.7 | 13.0 | 9.1 | 0.15 |
| 6 | 0.5 | 0.0 | 0.8 | 0.27 |
| Early outcomes | ||||
| 30-day mortality | 5.2 | 8.3 | 3.1 | 0.0050 |
| Operative mortality | 7.0 | 10.7 | 4.4 | 0.0040 |
| Blood transfusion | 74.2 | 88.9 | 64.0 | <0.00010 |
| Initial ventilator time (h) | 45.21 ± 147.79 | 65.49 ± 160.32 | 31.17 ± 136.92 | 0.0050 |
| Intensive care unit stay ≥8 days | 10.2 | 15.4 | 6.6 | 0.0010 |
| Postoperative morbidity | ||||
| Stroke | 2.1 | 2.8 | 1.6 | 0.40 |
| Transient | 1.8 | 2.8 | 1.1 | 0.14 |
| Continuous coma ≥24 h | 1.8 | 3.2 | 0.8 | 0.058 |
| Renal failure requiring dialysis | 3.9 | 5.9 | 2.5 | 0.034 |
| Renal failure | 9.9 | 14.6 | 6.6 | 0.0010 |
| Deep sternal wound infection | 1.3 | 1.6 | 1.1 | 0.72 |
| Prolonged ventilation | 10.0 | 16.6 | 5.5 | <0.00010 |
| Reoperation for bleeding | 2.4 | 3.6 | 1.6 | 0.18 |
| Perioperative myocardial infarction | 2.8 | 5.1 | 1.1 | 0.0040 |
| Atrial fibrillation | 12.3 | 13.8 | 11.3 | 0.38 |
| Heart block | 0.3 | 0.8 | 0.0 | 0.17 |
| Gastrointestinal complication | 3.1 | 2.8 | 3.3 | 0.82 |
| Pneumonia | 3.9 | 4.0 | 3.8 | 1.00 |
| Readmission within 30 days | 2.9 | 4.0 | 2.2 | 0.23 |
| Composite 30-day mortality or major morbidity | 14.9 | 22.9 | 9.3 | <0.00010 |
| . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Intraoperative variables | ||||
| Operation time (min) | 375.21 ± 147.0 | 427.61 ± 151.23 | 339.06 ± 132.61 | <0.00010 |
| Distal anastomoses mean (SD) | 2.12 ± 1.10 | 2.30 ± 1.04 | 2.00 ± 1.12 | 0.0010 |
| Number of distal anastomoses | ||||
| 1, 2 | 66.9 | 60.1 | 71.7 | 0.0030 |
| 3 | 21.9 | 26.9 | 18.4 | 0.013 |
| 4, 5 | 10.7 | 13.0 | 9.1 | 0.15 |
| 6 | 0.5 | 0.0 | 0.8 | 0.27 |
| Early outcomes | ||||
| 30-day mortality | 5.2 | 8.3 | 3.1 | 0.0050 |
| Operative mortality | 7.0 | 10.7 | 4.4 | 0.0040 |
| Blood transfusion | 74.2 | 88.9 | 64.0 | <0.00010 |
| Initial ventilator time (h) | 45.21 ± 147.79 | 65.49 ± 160.32 | 31.17 ± 136.92 | 0.0050 |
| Intensive care unit stay ≥8 days | 10.2 | 15.4 | 6.6 | 0.0010 |
| Postoperative morbidity | ||||
| Stroke | 2.1 | 2.8 | 1.6 | 0.40 |
| Transient | 1.8 | 2.8 | 1.1 | 0.14 |
| Continuous coma ≥24 h | 1.8 | 3.2 | 0.8 | 0.058 |
| Renal failure requiring dialysis | 3.9 | 5.9 | 2.5 | 0.034 |
| Renal failure | 9.9 | 14.6 | 6.6 | 0.0010 |
| Deep sternal wound infection | 1.3 | 1.6 | 1.1 | 0.72 |
| Prolonged ventilation | 10.0 | 16.6 | 5.5 | <0.00010 |
| Reoperation for bleeding | 2.4 | 3.6 | 1.6 | 0.18 |
| Perioperative myocardial infarction | 2.8 | 5.1 | 1.1 | 0.0040 |
| Atrial fibrillation | 12.3 | 13.8 | 11.3 | 0.38 |
| Heart block | 0.3 | 0.8 | 0.0 | 0.17 |
| Gastrointestinal complication | 3.1 | 2.8 | 3.3 | 0.82 |
| Pneumonia | 3.9 | 4.0 | 3.8 | 1.00 |
| Readmission within 30 days | 2.9 | 4.0 | 2.2 | 0.23 |
| Composite 30-day mortality or major morbidity | 14.9 | 22.9 | 9.3 | <0.00010 |
Values are percentage or mean ± SD values.
| . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Intraoperative variables | ||||
| Operation time (min) | 375.21 ± 147.0 | 427.61 ± 151.23 | 339.06 ± 132.61 | <0.00010 |
| Distal anastomoses mean (SD) | 2.12 ± 1.10 | 2.30 ± 1.04 | 2.00 ± 1.12 | 0.0010 |
| Number of distal anastomoses | ||||
| 1, 2 | 66.9 | 60.1 | 71.7 | 0.0030 |
| 3 | 21.9 | 26.9 | 18.4 | 0.013 |
| 4, 5 | 10.7 | 13.0 | 9.1 | 0.15 |
| 6 | 0.5 | 0.0 | 0.8 | 0.27 |
| Early outcomes | ||||
| 30-day mortality | 5.2 | 8.3 | 3.1 | 0.0050 |
| Operative mortality | 7.0 | 10.7 | 4.4 | 0.0040 |
| Blood transfusion | 74.2 | 88.9 | 64.0 | <0.00010 |
| Initial ventilator time (h) | 45.21 ± 147.79 | 65.49 ± 160.32 | 31.17 ± 136.92 | 0.0050 |
| Intensive care unit stay ≥8 days | 10.2 | 15.4 | 6.6 | 0.0010 |
| Postoperative morbidity | ||||
| Stroke | 2.1 | 2.8 | 1.6 | 0.40 |
| Transient | 1.8 | 2.8 | 1.1 | 0.14 |
| Continuous coma ≥24 h | 1.8 | 3.2 | 0.8 | 0.058 |
| Renal failure requiring dialysis | 3.9 | 5.9 | 2.5 | 0.034 |
| Renal failure | 9.9 | 14.6 | 6.6 | 0.0010 |
| Deep sternal wound infection | 1.3 | 1.6 | 1.1 | 0.72 |
| Prolonged ventilation | 10.0 | 16.6 | 5.5 | <0.00010 |
| Reoperation for bleeding | 2.4 | 3.6 | 1.6 | 0.18 |
| Perioperative myocardial infarction | 2.8 | 5.1 | 1.1 | 0.0040 |
| Atrial fibrillation | 12.3 | 13.8 | 11.3 | 0.38 |
| Heart block | 0.3 | 0.8 | 0.0 | 0.17 |
| Gastrointestinal complication | 3.1 | 2.8 | 3.3 | 0.82 |
| Pneumonia | 3.9 | 4.0 | 3.8 | 1.00 |
| Readmission within 30 days | 2.9 | 4.0 | 2.2 | 0.23 |
| Composite 30-day mortality or major morbidity | 14.9 | 22.9 | 9.3 | <0.00010 |
| . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Intraoperative variables | ||||
| Operation time (min) | 375.21 ± 147.0 | 427.61 ± 151.23 | 339.06 ± 132.61 | <0.00010 |
| Distal anastomoses mean (SD) | 2.12 ± 1.10 | 2.30 ± 1.04 | 2.00 ± 1.12 | 0.0010 |
| Number of distal anastomoses | ||||
| 1, 2 | 66.9 | 60.1 | 71.7 | 0.0030 |
| 3 | 21.9 | 26.9 | 18.4 | 0.013 |
| 4, 5 | 10.7 | 13.0 | 9.1 | 0.15 |
| 6 | 0.5 | 0.0 | 0.8 | 0.27 |
| Early outcomes | ||||
| 30-day mortality | 5.2 | 8.3 | 3.1 | 0.0050 |
| Operative mortality | 7.0 | 10.7 | 4.4 | 0.0040 |
| Blood transfusion | 74.2 | 88.9 | 64.0 | <0.00010 |
| Initial ventilator time (h) | 45.21 ± 147.79 | 65.49 ± 160.32 | 31.17 ± 136.92 | 0.0050 |
| Intensive care unit stay ≥8 days | 10.2 | 15.4 | 6.6 | 0.0010 |
| Postoperative morbidity | ||||
| Stroke | 2.1 | 2.8 | 1.6 | 0.40 |
| Transient | 1.8 | 2.8 | 1.1 | 0.14 |
| Continuous coma ≥24 h | 1.8 | 3.2 | 0.8 | 0.058 |
| Renal failure requiring dialysis | 3.9 | 5.9 | 2.5 | 0.034 |
| Renal failure | 9.9 | 14.6 | 6.6 | 0.0010 |
| Deep sternal wound infection | 1.3 | 1.6 | 1.1 | 0.72 |
| Prolonged ventilation | 10.0 | 16.6 | 5.5 | <0.00010 |
| Reoperation for bleeding | 2.4 | 3.6 | 1.6 | 0.18 |
| Perioperative myocardial infarction | 2.8 | 5.1 | 1.1 | 0.0040 |
| Atrial fibrillation | 12.3 | 13.8 | 11.3 | 0.38 |
| Heart block | 0.3 | 0.8 | 0.0 | 0.17 |
| Gastrointestinal complication | 3.1 | 2.8 | 3.3 | 0.82 |
| Pneumonia | 3.9 | 4.0 | 3.8 | 1.00 |
| Readmission within 30 days | 2.9 | 4.0 | 2.2 | 0.23 |
| Composite 30-day mortality or major morbidity | 14.9 | 22.9 | 9.3 | <0.00010 |
Values are percentage or mean ± SD values.
Study end points
The study outcomes measured from the JCVSD were as follows: operation time and the number of distal anastomoses as the intraoperative variable, 30-day mortality, operative mortality, initial ventilation time, the number of patients who stayed in the intensive care unit ([ICU] stay) ≥8 days and blood transfusion as early outcomes. The 30-day mortality was defined as death within 30 days of the operation, regardless of the patient's geographic location, even if the patient had been discharged from the hospital. Operative mortality included any patient who died within the index hospitalization, regardless of the length of hospital stay, and including any patient who died after being discharged from hospital up to 30 days from the date of the operation. Hospital-to-hospital transfer was not considered discharge [17].
Major morbidity was defined as any of the five following postoperative in-hospital complications: stroke, reoperation for any reason, need for mechanical ventilation for more than 24 h postoperatively, renal failure with newly required dialysis or deep sternal wound infection [18]. In addition to the above complications, the following complications were defined as postoperative morbidities (Table 2): (i) renal failure (defined as an increase in serum creatinine value to twice preoperative levels or to >2.0 mg/dl, or new requirement for dialysis or haemofiltration); (ii) continuous coma for >24 h; (iii) perioperative myocardial infarction (defined as at least two of the following: continuous angina for >20 min regardless of nitrite treatment or rest, elevation of cardiac enzyme levels [creatine kinase (CK)-MB level >1/20 of the total CK level or twice preoperative levels and/or lactate dehydrogenase isozyme subtype 1 > subtype 2 and/or positive troponin]; new cardiac wall motion abnormalities; Q waves or ST-T elevation on more than 2 serial leads in the electrocardiogram); (iv) atrial fibrillation (new onset); (v) heart block requiring permanent pacemaker; (v) gastrointestinal complications; (vi) pneumonia and (vii) readmission within 30 days of discharge. In this analysis, composite 30-day mortality or major morbidity was considered a postoperative morbidity (Table 2).
Statistical analyses
We compared the baseline demographics of patients who underwent off-pump surgery with those of patients who underwent on-pump surgery. The PS matching [19] method was used for adjusting differences in baseline characteristics because the patients were not randomly assigned to receive redo OPCAB.
PS were calculated for each patient to adjust for confounders of group assignment using the following 13 preoperative variables, which include the circumstances under which surgeons generally tend to avoid or prefer to use CPB: age; presence of unstable angina; extracardiac disease; cerebrovascular disease (the presence of stroke or a history of transient ischaemic attack); a history of non-invasive carotid stenosis >75%; diabetes; renal failure; chronic lung disease (mild, moderate or severe); cardiogenic shock; a history of myocardial infarction; ejection fraction <30%; triple-vessel disease and left main disease (variables at the basis of the model are given in Table 3).
| Variables . | B Value . | P-value . |
|---|---|---|
| Age | 1.013 | 0.16 |
| Carotid stenosis | 0.978 | 0.94 |
| Renal failure | 1.067 | 0.77 |
| Diabetes (insulin) | 1.212 | 0.45 |
| Cerebrovascular disease | 0.853 | 0.56 |
| COPD (mild, moderate or severe) | 1.340 | 0.29 |
| Extracardiac disease | 0.740 | 0.44 |
| Myocardial infarction | 1.005 | 0.98 |
| Unstable angina | 0.807 | 0.25 |
| Shock | 0.401 | 0.035 |
| Left main disease | 0.749 | 0.36 |
| Triple-vessel disease | 0.627 | 0.0090 |
| <EF:30% | 0.720 | 0.067 |
| Variables . | B Value . | P-value . |
|---|---|---|
| Age | 1.013 | 0.16 |
| Carotid stenosis | 0.978 | 0.94 |
| Renal failure | 1.067 | 0.77 |
| Diabetes (insulin) | 1.212 | 0.45 |
| Cerebrovascular disease | 0.853 | 0.56 |
| COPD (mild, moderate or severe) | 1.340 | 0.29 |
| Extracardiac disease | 0.740 | 0.44 |
| Myocardial infarction | 1.005 | 0.98 |
| Unstable angina | 0.807 | 0.25 |
| Shock | 0.401 | 0.035 |
| Left main disease | 0.749 | 0.36 |
| Triple-vessel disease | 0.627 | 0.0090 |
| <EF:30% | 0.720 | 0.067 |
COPD: chronic obstructive pulmonary disease; EF: ejection fraction.
| Variables . | B Value . | P-value . |
|---|---|---|
| Age | 1.013 | 0.16 |
| Carotid stenosis | 0.978 | 0.94 |
| Renal failure | 1.067 | 0.77 |
| Diabetes (insulin) | 1.212 | 0.45 |
| Cerebrovascular disease | 0.853 | 0.56 |
| COPD (mild, moderate or severe) | 1.340 | 0.29 |
| Extracardiac disease | 0.740 | 0.44 |
| Myocardial infarction | 1.005 | 0.98 |
| Unstable angina | 0.807 | 0.25 |
| Shock | 0.401 | 0.035 |
| Left main disease | 0.749 | 0.36 |
| Triple-vessel disease | 0.627 | 0.0090 |
| <EF:30% | 0.720 | 0.067 |
| Variables . | B Value . | P-value . |
|---|---|---|
| Age | 1.013 | 0.16 |
| Carotid stenosis | 0.978 | 0.94 |
| Renal failure | 1.067 | 0.77 |
| Diabetes (insulin) | 1.212 | 0.45 |
| Cerebrovascular disease | 0.853 | 0.56 |
| COPD (mild, moderate or severe) | 1.340 | 0.29 |
| Extracardiac disease | 0.740 | 0.44 |
| Myocardial infarction | 1.005 | 0.98 |
| Unstable angina | 0.807 | 0.25 |
| Shock | 0.401 | 0.035 |
| Left main disease | 0.749 | 0.36 |
| Triple-vessel disease | 0.627 | 0.0090 |
| <EF:30% | 0.720 | 0.067 |
COPD: chronic obstructive pulmonary disease; EF: ejection fraction.
We performed a one-to-one matched analysis on the basis of estimated PS of each patient. The log odds of the probability that a patient underwent redo OPCAB was modelled as a function of the confounders, which we identified and included in our data set. C-statistics were calculated for evaluating the goodness of fit. The area under the curve of this PS model was 0.62. The estimated PS were compared between the redo OPCAB and on-pump redo CABG groups, and a ‘match’ occurred when 1 patient in the redo OPCAB had an estimated score within 0.6 standard deviation (SD) of another patient in the on-pump redo CABG. If two or more patients in the redo OPCAB group met this criterion, we randomly selected 1 patient for matching.
We performed univariate comparisons of patient characteristics and outcome variables between the PS-matched groups of redo OPCAB and on-pump redo CABG, using Fisher's exact tests or Pearson's χ2 test and t-tests, as appropriate. A P-value of <0.05 was considered statistically significant. All statistical analyses were conducted using PASW version 18.0 (SPSS, Inc.; Chicago, IL, USA).
RESULTS
We analysed the data of 34 980 patients who underwent isolated CABG and were included in the JCVSD between 2008 and 2011. Of these, 1.8% of patients [n = 617/34980] had undergone redo CABG.
Of these, 253 (41%) underwent an on-pump procedure, whereas the other 364 (69%) underwent an off-pump procedure (Fig. 1). Of note, 7.7% of patients (n = 28/364) who required intraoperative conversion from redo OPCAB to on-pump redo CABG were included in the redo OPCAB group for final analysis.
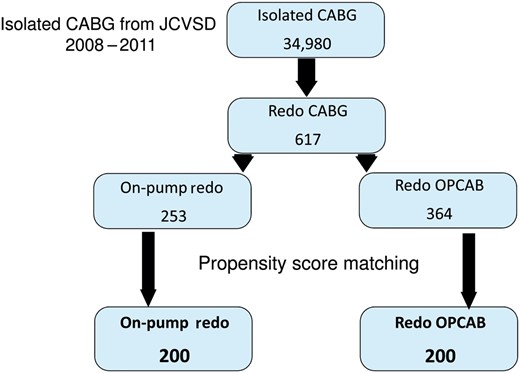
Patient selection. JCVSD: Japan Cardiovascular Surgery Database.
Unadjusted outcomes
The demographic and preoperative characteristics of patients undergoing redo OPCAB versus on-pump redo CABG before propensity matching are listed in Table 1. The on-pump redo CABG group had a significantly higher preoperative incidence of unstable angina and shock than the redo OPCAB group (38.3 vs 29.1%, P = 0.019; 7.9 vs 2.7%, P = 0.0040). The other preoperative data were similar between the two groups.
As for diseased vessels, the rates of triple-vessel and left main disease were significantly higher in the on-pump redo CABG group than in the redo OPCAB group (62.1 vs 50.5%, P = 0.0050; 42.7 vs 33.0%, P = 0.017). On the other hand, the redo OPCAB group had a lower number of diseased vessels compared with the on-pump redo CABG group (single-vessel disease: 9.9% in the on-pump redo CABG group versus 16.8% in the redo OPCAB group; double-vessel disease: 20.2% in the on-pump redo CABG group versus 28.3% in the redo OPCAB group). The Japan SCORE, (The Japan SCORE is a risk model based on the JCVSD. Expected mortality and expected composite 30-day mortality or major morbidity are calculated from this risk model.) [15, 16] were significantly higher in the on-pump redo CABG group than in the redo OPCAB group (7.66 ± 0.14% vs 5.51 ± 0.1, P = 0.023; 25.1 ± 0.18% vs 21.9 ± 0.15%, P = 0.019, respectively).
There were significant differences in intraoperative variables between the two groups (Table 2). The redo OPCAB group underwent a fewer mean number of distal anastomoses compared with the on-pump redo CABG group (2.00 ± 1.12 vs 2.30 ± 1.04, P = 0.0010). Operation times were significantly longer in the on-pump redo CABG group than in the redo OPCAB group, at 427.61 ± 151.23 vs 339.06 ± 132.61 min (P < 0.00010).
Significant differences between the groups were observed in early outcomes (Table 2). All of the outcomes were significantly worse in the on-pump redo CABG group than in the redo OPCAB group. The 30-day mortality was 8.3% in the on-pump redo CABG group and 3.1% in the redo OPCAB group (P = 0.0050).
Postoperative morbidities are given in Table 2. The incidences of renal failure, renal failure requiring dialysis, prolonged ventilation, perioperative myocardial infarction and composite 30-day mortality or major morbidity were higher in the on-pump redo CABG group than in the redo OPCAB group.
Propensity-matched pairs
We selected 13 preoperative risk factors to eliminate the differences between the two groups (Table 3) and used PS matching with these preoperative risk factors to adjust for differences in baseline characteristics between the two groups. Using one-to-one matching, we selected 200 pairs from each group (Fig. 1).
There were no significant differences among all preoperative factors, including shock, unstable angina, number of diseased vessels (94.3%: multivessel disease), left ventricular impairment and preoperative status between the two groups. Moreover, there were no significant differences in the Japan SCORE (expected mortality: 6.62 ± 0.12% vs 6.37 ± 0.11%, P = 0.85; expected 30-day mortality or composite major morbidity: 24.95 ± 0.17% vs 24.01 ± 0.17%, P = 0.58) (Table 4).
| Variables . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Patients | 400 | 200 | 200 | |
| Sex (male) | 80.5 | 83.0 | 78.0 | 0.26 |
| Age (years), mean (SD) | 68.4 ± 9.4 | 68.7 ± 9.4 | 68.1 ± 9.3 | 0.52 |
| Body surface area, mean (SD) | 1.65 ± 0.17 | 1.66 ± 0.2 | 1.63 ± 0.2 | 0.19 |
| Smoking | 53.3 | 52.5 | 54.0 | 0.84 |
| Body mass index >30 kg/m2 | 6.5 | 6.0 | 7.0 | 0.84 |
| Diabetes | 46.8 | 46.5 | 47.0 | 1.00 |
| Preoperative creatinine value | 1.7 ± 2.24 | 1.7 ± 2.3 | 1.7 ± 2.2 | 0.91 |
| Renal failure | 17.8 | 17.5 | 18.0 | 1.00 |
| Dialysis | 8.3 | 8.0 | 8.5 | 1.00 |
| Hyperlipidaemia | 60.8 | 61.0 | 60.5 | 1.00 |
| Hypertension | 76.0 | 80.0 | 72.0 | 0.079 |
| Cerebrovascular disease | 12.0 | 10.5 | 13.5 | 0.44 |
| Carotid stenosis | 8.5 | 8.0 | 9.0 | 0.86 |
| COPD (moderate and severe) | 2.3 | 2.5 | 2.0 | 1.00 |
| Extracardiac disease | 21.8 | 21.0 | 22.5 | 0.81 |
| Peripheral | 18.8 | 18.5 | 19.0 | 1.00 |
| Myocardial infarction | 47.5 | 47.0 | 48.0 | 0.92 |
| Previous coronary artery intervention | 46.3 | 45.0 | 47.5 | 0.69 |
| Unstable angina | 41.8 | 40.5 | 43.0 | 0.69 |
| Shock | 5.5 | 6.0 | 5.0 | 0.83 |
| Congestive heart failure | 16.5 | 16.5 | 16.5 | 1.00 |
| Arrhythmia | 12.8 | 13.5 | 12.0 | 0.77 |
| NYHA class III or IV | 34.5 | 32.0 | 37.0 | 0.34 |
| Diseased vessels, % | ||||
| Left main disease | 47.5 | 45.0 | 50.0 | 0.37 |
| 1 | 5.8 | 6.0 | 5.5 | 1.00 |
| 2 | 16.3 | 18.0 | 14.5 | 0.42 |
| Triple-vessel disease | 74.3 | 70.0 | 78.5 | 0.067 |
| LV impairment, % | ||||
| Good >EF:60% | 30.8 | 35.5 | 26.0 | 0.051 |
| ≤EF:60% | 69.0 | 64.0 | 74.0 | 0.040 |
| Bad <EF:30% | 9.0 | 8.0 | 10.0 | 0.60 |
| Mitral valve insufficiency II–IV | 22.5 | 20.0 | 25.0 | 0.28 |
| Tricuspid valve insufficiency II–IV | 15.5 | 18.0 | 13.0 | 0.21 |
| Status, % | ||||
| Urgent | 10.0 | 10.0 | 10.0 | 1.00 |
| Emergency/salvage | 8.5 | 8.5 | 8.5 | 1.00 |
| Japan SCORE | ||||
| Expected 30 days operative mortality | 6.62 ± 0.12 | 6.37 ± 0.11 | 0.85 | |
| Expected composite 30-day mortality or major morbidity | 24.95 ± 0.17 | 24.01 ± 0.17 | 0.58 | |
| Variables . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Patients | 400 | 200 | 200 | |
| Sex (male) | 80.5 | 83.0 | 78.0 | 0.26 |
| Age (years), mean (SD) | 68.4 ± 9.4 | 68.7 ± 9.4 | 68.1 ± 9.3 | 0.52 |
| Body surface area, mean (SD) | 1.65 ± 0.17 | 1.66 ± 0.2 | 1.63 ± 0.2 | 0.19 |
| Smoking | 53.3 | 52.5 | 54.0 | 0.84 |
| Body mass index >30 kg/m2 | 6.5 | 6.0 | 7.0 | 0.84 |
| Diabetes | 46.8 | 46.5 | 47.0 | 1.00 |
| Preoperative creatinine value | 1.7 ± 2.24 | 1.7 ± 2.3 | 1.7 ± 2.2 | 0.91 |
| Renal failure | 17.8 | 17.5 | 18.0 | 1.00 |
| Dialysis | 8.3 | 8.0 | 8.5 | 1.00 |
| Hyperlipidaemia | 60.8 | 61.0 | 60.5 | 1.00 |
| Hypertension | 76.0 | 80.0 | 72.0 | 0.079 |
| Cerebrovascular disease | 12.0 | 10.5 | 13.5 | 0.44 |
| Carotid stenosis | 8.5 | 8.0 | 9.0 | 0.86 |
| COPD (moderate and severe) | 2.3 | 2.5 | 2.0 | 1.00 |
| Extracardiac disease | 21.8 | 21.0 | 22.5 | 0.81 |
| Peripheral | 18.8 | 18.5 | 19.0 | 1.00 |
| Myocardial infarction | 47.5 | 47.0 | 48.0 | 0.92 |
| Previous coronary artery intervention | 46.3 | 45.0 | 47.5 | 0.69 |
| Unstable angina | 41.8 | 40.5 | 43.0 | 0.69 |
| Shock | 5.5 | 6.0 | 5.0 | 0.83 |
| Congestive heart failure | 16.5 | 16.5 | 16.5 | 1.00 |
| Arrhythmia | 12.8 | 13.5 | 12.0 | 0.77 |
| NYHA class III or IV | 34.5 | 32.0 | 37.0 | 0.34 |
| Diseased vessels, % | ||||
| Left main disease | 47.5 | 45.0 | 50.0 | 0.37 |
| 1 | 5.8 | 6.0 | 5.5 | 1.00 |
| 2 | 16.3 | 18.0 | 14.5 | 0.42 |
| Triple-vessel disease | 74.3 | 70.0 | 78.5 | 0.067 |
| LV impairment, % | ||||
| Good >EF:60% | 30.8 | 35.5 | 26.0 | 0.051 |
| ≤EF:60% | 69.0 | 64.0 | 74.0 | 0.040 |
| Bad <EF:30% | 9.0 | 8.0 | 10.0 | 0.60 |
| Mitral valve insufficiency II–IV | 22.5 | 20.0 | 25.0 | 0.28 |
| Tricuspid valve insufficiency II–IV | 15.5 | 18.0 | 13.0 | 0.21 |
| Status, % | ||||
| Urgent | 10.0 | 10.0 | 10.0 | 1.00 |
| Emergency/salvage | 8.5 | 8.5 | 8.5 | 1.00 |
| Japan SCORE | ||||
| Expected 30 days operative mortality | 6.62 ± 0.12 | 6.37 ± 0.11 | 0.85 | |
| Expected composite 30-day mortality or major morbidity | 24.95 ± 0.17 | 24.01 ± 0.17 | 0.58 | |
Values are percentage or mean ± SD values.
COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association; LV: left ventricular; EF: ejection fraction.
| Variables . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Patients | 400 | 200 | 200 | |
| Sex (male) | 80.5 | 83.0 | 78.0 | 0.26 |
| Age (years), mean (SD) | 68.4 ± 9.4 | 68.7 ± 9.4 | 68.1 ± 9.3 | 0.52 |
| Body surface area, mean (SD) | 1.65 ± 0.17 | 1.66 ± 0.2 | 1.63 ± 0.2 | 0.19 |
| Smoking | 53.3 | 52.5 | 54.0 | 0.84 |
| Body mass index >30 kg/m2 | 6.5 | 6.0 | 7.0 | 0.84 |
| Diabetes | 46.8 | 46.5 | 47.0 | 1.00 |
| Preoperative creatinine value | 1.7 ± 2.24 | 1.7 ± 2.3 | 1.7 ± 2.2 | 0.91 |
| Renal failure | 17.8 | 17.5 | 18.0 | 1.00 |
| Dialysis | 8.3 | 8.0 | 8.5 | 1.00 |
| Hyperlipidaemia | 60.8 | 61.0 | 60.5 | 1.00 |
| Hypertension | 76.0 | 80.0 | 72.0 | 0.079 |
| Cerebrovascular disease | 12.0 | 10.5 | 13.5 | 0.44 |
| Carotid stenosis | 8.5 | 8.0 | 9.0 | 0.86 |
| COPD (moderate and severe) | 2.3 | 2.5 | 2.0 | 1.00 |
| Extracardiac disease | 21.8 | 21.0 | 22.5 | 0.81 |
| Peripheral | 18.8 | 18.5 | 19.0 | 1.00 |
| Myocardial infarction | 47.5 | 47.0 | 48.0 | 0.92 |
| Previous coronary artery intervention | 46.3 | 45.0 | 47.5 | 0.69 |
| Unstable angina | 41.8 | 40.5 | 43.0 | 0.69 |
| Shock | 5.5 | 6.0 | 5.0 | 0.83 |
| Congestive heart failure | 16.5 | 16.5 | 16.5 | 1.00 |
| Arrhythmia | 12.8 | 13.5 | 12.0 | 0.77 |
| NYHA class III or IV | 34.5 | 32.0 | 37.0 | 0.34 |
| Diseased vessels, % | ||||
| Left main disease | 47.5 | 45.0 | 50.0 | 0.37 |
| 1 | 5.8 | 6.0 | 5.5 | 1.00 |
| 2 | 16.3 | 18.0 | 14.5 | 0.42 |
| Triple-vessel disease | 74.3 | 70.0 | 78.5 | 0.067 |
| LV impairment, % | ||||
| Good >EF:60% | 30.8 | 35.5 | 26.0 | 0.051 |
| ≤EF:60% | 69.0 | 64.0 | 74.0 | 0.040 |
| Bad <EF:30% | 9.0 | 8.0 | 10.0 | 0.60 |
| Mitral valve insufficiency II–IV | 22.5 | 20.0 | 25.0 | 0.28 |
| Tricuspid valve insufficiency II–IV | 15.5 | 18.0 | 13.0 | 0.21 |
| Status, % | ||||
| Urgent | 10.0 | 10.0 | 10.0 | 1.00 |
| Emergency/salvage | 8.5 | 8.5 | 8.5 | 1.00 |
| Japan SCORE | ||||
| Expected 30 days operative mortality | 6.62 ± 0.12 | 6.37 ± 0.11 | 0.85 | |
| Expected composite 30-day mortality or major morbidity | 24.95 ± 0.17 | 24.01 ± 0.17 | 0.58 | |
| Variables . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Patients | 400 | 200 | 200 | |
| Sex (male) | 80.5 | 83.0 | 78.0 | 0.26 |
| Age (years), mean (SD) | 68.4 ± 9.4 | 68.7 ± 9.4 | 68.1 ± 9.3 | 0.52 |
| Body surface area, mean (SD) | 1.65 ± 0.17 | 1.66 ± 0.2 | 1.63 ± 0.2 | 0.19 |
| Smoking | 53.3 | 52.5 | 54.0 | 0.84 |
| Body mass index >30 kg/m2 | 6.5 | 6.0 | 7.0 | 0.84 |
| Diabetes | 46.8 | 46.5 | 47.0 | 1.00 |
| Preoperative creatinine value | 1.7 ± 2.24 | 1.7 ± 2.3 | 1.7 ± 2.2 | 0.91 |
| Renal failure | 17.8 | 17.5 | 18.0 | 1.00 |
| Dialysis | 8.3 | 8.0 | 8.5 | 1.00 |
| Hyperlipidaemia | 60.8 | 61.0 | 60.5 | 1.00 |
| Hypertension | 76.0 | 80.0 | 72.0 | 0.079 |
| Cerebrovascular disease | 12.0 | 10.5 | 13.5 | 0.44 |
| Carotid stenosis | 8.5 | 8.0 | 9.0 | 0.86 |
| COPD (moderate and severe) | 2.3 | 2.5 | 2.0 | 1.00 |
| Extracardiac disease | 21.8 | 21.0 | 22.5 | 0.81 |
| Peripheral | 18.8 | 18.5 | 19.0 | 1.00 |
| Myocardial infarction | 47.5 | 47.0 | 48.0 | 0.92 |
| Previous coronary artery intervention | 46.3 | 45.0 | 47.5 | 0.69 |
| Unstable angina | 41.8 | 40.5 | 43.0 | 0.69 |
| Shock | 5.5 | 6.0 | 5.0 | 0.83 |
| Congestive heart failure | 16.5 | 16.5 | 16.5 | 1.00 |
| Arrhythmia | 12.8 | 13.5 | 12.0 | 0.77 |
| NYHA class III or IV | 34.5 | 32.0 | 37.0 | 0.34 |
| Diseased vessels, % | ||||
| Left main disease | 47.5 | 45.0 | 50.0 | 0.37 |
| 1 | 5.8 | 6.0 | 5.5 | 1.00 |
| 2 | 16.3 | 18.0 | 14.5 | 0.42 |
| Triple-vessel disease | 74.3 | 70.0 | 78.5 | 0.067 |
| LV impairment, % | ||||
| Good >EF:60% | 30.8 | 35.5 | 26.0 | 0.051 |
| ≤EF:60% | 69.0 | 64.0 | 74.0 | 0.040 |
| Bad <EF:30% | 9.0 | 8.0 | 10.0 | 0.60 |
| Mitral valve insufficiency II–IV | 22.5 | 20.0 | 25.0 | 0.28 |
| Tricuspid valve insufficiency II–IV | 15.5 | 18.0 | 13.0 | 0.21 |
| Status, % | ||||
| Urgent | 10.0 | 10.0 | 10.0 | 1.00 |
| Emergency/salvage | 8.5 | 8.5 | 8.5 | 1.00 |
| Japan SCORE | ||||
| Expected 30 days operative mortality | 6.62 ± 0.12 | 6.37 ± 0.11 | 0.85 | |
| Expected composite 30-day mortality or major morbidity | 24.95 ± 0.17 | 24.01 ± 0.17 | 0.58 | |
Values are percentage or mean ± SD values.
COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association; LV: left ventricular; EF: ejection fraction.
As for intraoperative variables (Table 5), there was no significant difference in the mean number of distal anastomoses after matching (2.41 ± 1.00 vs 2.21 ± 1.04, P = 0.074). The data of our study show that, in the on-pump redo CABG group, 35.0% of patients were given blood cardioplegia and 10.0% were given the crystalloid cardioplegia solution for myocardial protection. No cardioplegia was provided in 45.5% patients in the on-pump redo CABG group. In almost all patients in whom no cardioplegia was provided, the on-pump beating procedure was performed. Half of the on-pump redo CABG procedures are performed using the beating heart technique in Japan [20]. The JCVSD does not capture whether adequate myocardial protection was provided during CABG, particularly in the setting of patent mammary graft, for example, whether retrograde cardioplegia or systemic hyperkalaemia is used for achieving diastolic arrest and myocardial protection. The distribution of arterial versus vein grafts in the two groups is given in Table 5. There were no significant differences in the distribution of internal thoracic artery graft and the absence of ITA graft use in the two groups. The causes of all deaths in the two groups registered in the JCVSD are given in Table 6.
| . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Intraoperative variables | ||||
| Operation time (min) | 397.5 ± 148.16 | 441.3 ± 146.3 | 353.7 ± 136.9 | <0.00010 |
| Distal anastomoses mean (SD) | 2.31 ± 1.12 | 2.41 ± 1.0 | 2.21 ± 1.2 | 0.074 |
| Number of distal anastomoses | ||||
| 1,2 | 60.0 | 56.5 | 63.5 | 0.18 |
| 3 | 26.3 | 28.5 | 24.0 | 0.36 |
| 4,5 | 13.0 | 15.0 | 11.0 | 0.30 |
| 6 | 0.8 | 0.0 | 1.5 | 0.25 |
| The distribution of grafts | ||||
| Left ITA | 36.3 | 36.5 | 36.0 | 1.00 |
| Right ITA | 32.3 | 27.5 | 37.0 | 0.054 |
| Bilateral ITA | 11.5 | 9.0 | 14.0 | 0.16 |
| No ITA use | 43.0 | 45.0 | 41.0 | 0.48 |
| . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Intraoperative variables | ||||
| Operation time (min) | 397.5 ± 148.16 | 441.3 ± 146.3 | 353.7 ± 136.9 | <0.00010 |
| Distal anastomoses mean (SD) | 2.31 ± 1.12 | 2.41 ± 1.0 | 2.21 ± 1.2 | 0.074 |
| Number of distal anastomoses | ||||
| 1,2 | 60.0 | 56.5 | 63.5 | 0.18 |
| 3 | 26.3 | 28.5 | 24.0 | 0.36 |
| 4,5 | 13.0 | 15.0 | 11.0 | 0.30 |
| 6 | 0.8 | 0.0 | 1.5 | 0.25 |
| The distribution of grafts | ||||
| Left ITA | 36.3 | 36.5 | 36.0 | 1.00 |
| Right ITA | 32.3 | 27.5 | 37.0 | 0.054 |
| Bilateral ITA | 11.5 | 9.0 | 14.0 | 0.16 |
| No ITA use | 43.0 | 45.0 | 41.0 | 0.48 |
Values are percentage or mean ± SD values.
ITA: internal thoracic artery.
| . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Intraoperative variables | ||||
| Operation time (min) | 397.5 ± 148.16 | 441.3 ± 146.3 | 353.7 ± 136.9 | <0.00010 |
| Distal anastomoses mean (SD) | 2.31 ± 1.12 | 2.41 ± 1.0 | 2.21 ± 1.2 | 0.074 |
| Number of distal anastomoses | ||||
| 1,2 | 60.0 | 56.5 | 63.5 | 0.18 |
| 3 | 26.3 | 28.5 | 24.0 | 0.36 |
| 4,5 | 13.0 | 15.0 | 11.0 | 0.30 |
| 6 | 0.8 | 0.0 | 1.5 | 0.25 |
| The distribution of grafts | ||||
| Left ITA | 36.3 | 36.5 | 36.0 | 1.00 |
| Right ITA | 32.3 | 27.5 | 37.0 | 0.054 |
| Bilateral ITA | 11.5 | 9.0 | 14.0 | 0.16 |
| No ITA use | 43.0 | 45.0 | 41.0 | 0.48 |
| . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Intraoperative variables | ||||
| Operation time (min) | 397.5 ± 148.16 | 441.3 ± 146.3 | 353.7 ± 136.9 | <0.00010 |
| Distal anastomoses mean (SD) | 2.31 ± 1.12 | 2.41 ± 1.0 | 2.21 ± 1.2 | 0.074 |
| Number of distal anastomoses | ||||
| 1,2 | 60.0 | 56.5 | 63.5 | 0.18 |
| 3 | 26.3 | 28.5 | 24.0 | 0.36 |
| 4,5 | 13.0 | 15.0 | 11.0 | 0.30 |
| 6 | 0.8 | 0.0 | 1.5 | 0.25 |
| The distribution of grafts | ||||
| Left ITA | 36.3 | 36.5 | 36.0 | 1.00 |
| Right ITA | 32.3 | 27.5 | 37.0 | 0.054 |
| Bilateral ITA | 11.5 | 9.0 | 14.0 | 0.16 |
| No ITA use | 43.0 | 45.0 | 41.0 | 0.48 |
Values are percentage or mean ± SD values.
ITA: internal thoracic artery.
| . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Early outcomes | ||||
| 30-day mortality | 5.3 | 7.0 | 3.5 | 0.18 |
| Operative mortality | 7.5 | 9.5 | 5.5 | 0.18 |
| Blood transfusion | 82.8 | 94.0 | 71.5 | <0.00010 |
| Initial ventilator time (h) | 47.8 ± 146.08 | 58.9 ± 137.5 | 36.8 ± 153.7 | 0.13 |
| Intensive care unit stay ≥8 days | 10.8 | 14.5 | 7.0 | 0.023 |
| Cause of all death | 0.59 | |||
| Arrhythmia | 1.0 | 1.0 | 1.0 | |
| Bleeding | 0.3 | 0.5 | 0.0 | |
| Infection | 0.8 | 0.5 | 1.0 | |
| Low output syndrome | 4.0 | 5.0 | 3.0 | |
| Pulmonary | 0.5 | 0.5 | 0.5 | |
| Renal | 0.3 | 0.5 | 0.0 | |
| Others | 2.0 | 3.0 | 1.0 | |
| Postoperative morbidity | ||||
| Stroke | 1.5 | 2.5 | 0.5 | 0.22 |
| Transient | 1.5 | 2.0 | 1.0 | 0.69 |
| Continuous coma ≥24 h | 1.5 | 2.0 | 1.0 | 0.69 |
| Renal failure requiring dialysis | 3.8 | 5.0 | 2.5 | 0.29 |
| Renal failure | 9.5 | 12.0 | 7.0 | 0.12 |
| Deep sternal wound infection | 1.0 | 1.0 | 1.0 | 1.00 |
| Prolonged ventilation | 11.0 | 15.0 | 7.0 | 0.016 |
| Reoperation for bleeding | 3.5 | 4.0 | 3.0 | 0.79 |
| Perioperative myocardial infarction | 3.0 | 4.5 | 1.5 | 0.14 |
| Atrial fibrillation | 12.5 | 14.0 | 11.0 | 0.45 |
| Heart block | 0.3 | 0.5 | 0.0 | 1.00 |
| Gastrointestinal complication | 2.0 | 2.0 | 2.0 | 1.00 |
| Pneumonia | 3.3 | 4.0 | 2.5 | 0.58 |
| Readmission within 30 days | 3.5 | 4.5 | 2.5 | 0.42 |
| Composite 30-day mortality or major morbidity | 16.3 | 21.5 | 11.0 | 0.0060 |
| . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Early outcomes | ||||
| 30-day mortality | 5.3 | 7.0 | 3.5 | 0.18 |
| Operative mortality | 7.5 | 9.5 | 5.5 | 0.18 |
| Blood transfusion | 82.8 | 94.0 | 71.5 | <0.00010 |
| Initial ventilator time (h) | 47.8 ± 146.08 | 58.9 ± 137.5 | 36.8 ± 153.7 | 0.13 |
| Intensive care unit stay ≥8 days | 10.8 | 14.5 | 7.0 | 0.023 |
| Cause of all death | 0.59 | |||
| Arrhythmia | 1.0 | 1.0 | 1.0 | |
| Bleeding | 0.3 | 0.5 | 0.0 | |
| Infection | 0.8 | 0.5 | 1.0 | |
| Low output syndrome | 4.0 | 5.0 | 3.0 | |
| Pulmonary | 0.5 | 0.5 | 0.5 | |
| Renal | 0.3 | 0.5 | 0.0 | |
| Others | 2.0 | 3.0 | 1.0 | |
| Postoperative morbidity | ||||
| Stroke | 1.5 | 2.5 | 0.5 | 0.22 |
| Transient | 1.5 | 2.0 | 1.0 | 0.69 |
| Continuous coma ≥24 h | 1.5 | 2.0 | 1.0 | 0.69 |
| Renal failure requiring dialysis | 3.8 | 5.0 | 2.5 | 0.29 |
| Renal failure | 9.5 | 12.0 | 7.0 | 0.12 |
| Deep sternal wound infection | 1.0 | 1.0 | 1.0 | 1.00 |
| Prolonged ventilation | 11.0 | 15.0 | 7.0 | 0.016 |
| Reoperation for bleeding | 3.5 | 4.0 | 3.0 | 0.79 |
| Perioperative myocardial infarction | 3.0 | 4.5 | 1.5 | 0.14 |
| Atrial fibrillation | 12.5 | 14.0 | 11.0 | 0.45 |
| Heart block | 0.3 | 0.5 | 0.0 | 1.00 |
| Gastrointestinal complication | 2.0 | 2.0 | 2.0 | 1.00 |
| Pneumonia | 3.3 | 4.0 | 2.5 | 0.58 |
| Readmission within 30 days | 3.5 | 4.5 | 2.5 | 0.42 |
| Composite 30-day mortality or major morbidity | 16.3 | 21.5 | 11.0 | 0.0060 |
Values are percentage or mean ± SD values.
| . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Early outcomes | ||||
| 30-day mortality | 5.3 | 7.0 | 3.5 | 0.18 |
| Operative mortality | 7.5 | 9.5 | 5.5 | 0.18 |
| Blood transfusion | 82.8 | 94.0 | 71.5 | <0.00010 |
| Initial ventilator time (h) | 47.8 ± 146.08 | 58.9 ± 137.5 | 36.8 ± 153.7 | 0.13 |
| Intensive care unit stay ≥8 days | 10.8 | 14.5 | 7.0 | 0.023 |
| Cause of all death | 0.59 | |||
| Arrhythmia | 1.0 | 1.0 | 1.0 | |
| Bleeding | 0.3 | 0.5 | 0.0 | |
| Infection | 0.8 | 0.5 | 1.0 | |
| Low output syndrome | 4.0 | 5.0 | 3.0 | |
| Pulmonary | 0.5 | 0.5 | 0.5 | |
| Renal | 0.3 | 0.5 | 0.0 | |
| Others | 2.0 | 3.0 | 1.0 | |
| Postoperative morbidity | ||||
| Stroke | 1.5 | 2.5 | 0.5 | 0.22 |
| Transient | 1.5 | 2.0 | 1.0 | 0.69 |
| Continuous coma ≥24 h | 1.5 | 2.0 | 1.0 | 0.69 |
| Renal failure requiring dialysis | 3.8 | 5.0 | 2.5 | 0.29 |
| Renal failure | 9.5 | 12.0 | 7.0 | 0.12 |
| Deep sternal wound infection | 1.0 | 1.0 | 1.0 | 1.00 |
| Prolonged ventilation | 11.0 | 15.0 | 7.0 | 0.016 |
| Reoperation for bleeding | 3.5 | 4.0 | 3.0 | 0.79 |
| Perioperative myocardial infarction | 3.0 | 4.5 | 1.5 | 0.14 |
| Atrial fibrillation | 12.5 | 14.0 | 11.0 | 0.45 |
| Heart block | 0.3 | 0.5 | 0.0 | 1.00 |
| Gastrointestinal complication | 2.0 | 2.0 | 2.0 | 1.00 |
| Pneumonia | 3.3 | 4.0 | 2.5 | 0.58 |
| Readmission within 30 days | 3.5 | 4.5 | 2.5 | 0.42 |
| Composite 30-day mortality or major morbidity | 16.3 | 21.5 | 11.0 | 0.0060 |
| . | Total redo CABG . | On-pump redo CABG . | Redo OPCAB . | P-value . |
|---|---|---|---|---|
| Early outcomes | ||||
| 30-day mortality | 5.3 | 7.0 | 3.5 | 0.18 |
| Operative mortality | 7.5 | 9.5 | 5.5 | 0.18 |
| Blood transfusion | 82.8 | 94.0 | 71.5 | <0.00010 |
| Initial ventilator time (h) | 47.8 ± 146.08 | 58.9 ± 137.5 | 36.8 ± 153.7 | 0.13 |
| Intensive care unit stay ≥8 days | 10.8 | 14.5 | 7.0 | 0.023 |
| Cause of all death | 0.59 | |||
| Arrhythmia | 1.0 | 1.0 | 1.0 | |
| Bleeding | 0.3 | 0.5 | 0.0 | |
| Infection | 0.8 | 0.5 | 1.0 | |
| Low output syndrome | 4.0 | 5.0 | 3.0 | |
| Pulmonary | 0.5 | 0.5 | 0.5 | |
| Renal | 0.3 | 0.5 | 0.0 | |
| Others | 2.0 | 3.0 | 1.0 | |
| Postoperative morbidity | ||||
| Stroke | 1.5 | 2.5 | 0.5 | 0.22 |
| Transient | 1.5 | 2.0 | 1.0 | 0.69 |
| Continuous coma ≥24 h | 1.5 | 2.0 | 1.0 | 0.69 |
| Renal failure requiring dialysis | 3.8 | 5.0 | 2.5 | 0.29 |
| Renal failure | 9.5 | 12.0 | 7.0 | 0.12 |
| Deep sternal wound infection | 1.0 | 1.0 | 1.0 | 1.00 |
| Prolonged ventilation | 11.0 | 15.0 | 7.0 | 0.016 |
| Reoperation for bleeding | 3.5 | 4.0 | 3.0 | 0.79 |
| Perioperative myocardial infarction | 3.0 | 4.5 | 1.5 | 0.14 |
| Atrial fibrillation | 12.5 | 14.0 | 11.0 | 0.45 |
| Heart block | 0.3 | 0.5 | 0.0 | 1.00 |
| Gastrointestinal complication | 2.0 | 2.0 | 2.0 | 1.00 |
| Pneumonia | 3.3 | 4.0 | 2.5 | 0.58 |
| Readmission within 30 days | 3.5 | 4.5 | 2.5 | 0.42 |
| Composite 30-day mortality or major morbidity | 16.3 | 21.5 | 11.0 | 0.0060 |
Values are percentage or mean ± SD values.
Operation time was significantly longer in the on-pump redo CABG group than in the redo OPCAB group (441.3 ± 146.3 vs 353.7 ± 136.9 min, P < 0.00010) (Table 5).
Regarding early outcomes (Table 6), the patients in the redo OPCAB group had a lower 30-day mortality rate (3.5 vs 7.0%, P = 0.18), a lower initial ventilator time (36.8 ± 153.7 vs 58.9 ± 137.5 h, P = 0.13), significantly shorter ICU duration (7.0 vs 14.5%, P = 0.023) and significantly decreased need for blood transfusions (71.5 vs 94.0%, P < 0.00010).
As for postoperative morbidities (Table 6), the redo OPCAB group had a significantly lower rate of prolonged ventilation (>24 h) (7.0 vs 15.0%, P = 0.016) and lower rates of composite mortality or major morbidities (11.0 vs 21.5%, P = 0.0060) than the on-pump redo CABG group. Although there were no significant differences in other morbidities, the redo OPCAB group had a lower rate in many postoperative morbidities.
DISCUSSION
In the present study, we present the contemporary Japanese results for redo CABG obtained from the JCVSD, which currently contains the clinical data from almost all Japanese institutions performing cardiovascular surgery.
Off-pump coronary artery grafting
OPCAB emerged in the mid-1980s and has since become increasingly popular worldwide. In Japan, OPCAB has become the standard management strategy for surgical coronary revascularization. The ratio of OPCAB in CABG has been increasing, especially since the year 2000; the annual rate of performing OPCAB is >60% [20, 21]. Several studies, including those conducted in Japan, have reported the effects of OPCAB and have compared OPCAB with conventional CABG [1–3], especially in patients with high comorbidity [4, 5].
Redo coronary artery bypass grafting
As described in several previous reports, the prevalence of redo CABG has recently reached a plateau [22, 23]. The Japanese Association for Thoracic Surgery (JATS) has been maintaining a registry of cardiovascular procedures in Japan since 1986; the number of isolated redo CABG in Japan has been basically decreasing, and its prevalence reached a plateau over a 10-year period. The latest version of the JATS Annual Report in 2011 showed that 223 patients underwent redo isolated CABG, and the prevalence of reoperation cases was 1.56% of total isolated CABG cases (14 256 patients) in 2011 [20]. This trend is apparently caused by improved medication for post-CABG patients, more frequent use of the internal mammary artery, more complete revascularization, older patient age at the time of primary surgery and increased use of percutaneous coronary intervention (PCI) for recurrent coronary disease after CABG in patients. Compared with Western countries, in Japan, PCI is used for patients with more severe coronary artery disease, and indications for PCI have expanded to include more indications than those included in its early stages.
Redo CABG can be performed safely in certain patients because of growing surgical experience, new technical strategies and better management of patient comorbidities. However, even in recent reports, the morbidity and mortality of such procedures remain higher (in the range of 4.2–6.8%) than those of primary procedures [20, 22–24].
Our data showed that the 30-day mortality in the redo CABG was significantly higher than in the primary CABG group (5.63 vs 1.46%, P < 0.00010). We hypothesize that the different characteristics inherent in the redo CABG patients may explain the higher mortality or morbidity rate in this group of patients compared with the primary CABG group. Redo patients are generally older, with a lower ejection fraction, and have multiple risk factors. In addition, their general physical condition is usually worse. Additionally, redo CABG tends to be more technically demanding, e.g. successful re-entry into the chest, management of patent bypass grafts or diseased patent grafts with adhesions, ischaemia caused by embolization of atheromatous debris from venous conduits, location of suitable conduits, aortic atherosclerotic disease that may prevent cross-clamping or aortic cannulation and/or inadequate myocardial protection caused by difficulties in delivering cardioplegia in the face of native and conduit stenoses [23]. The last two factors are directly associated with disadvantages of the on-pump procedure.
Concordantly, many reports have mentioned the benefits of OPCAB, especially in patients with several comorbidities, such as those undergoing redo CABG [4, 5]. Several recent reports of off-pump redo CABG have stated the advantage of morbidity and mortality [6–11]. Conversely, some reports have stated that these benefits may be limited to a selected group of patients because of the higher rate of incomplete revascularization in off-pump CABG. However, the mean number of distal anastomoses in these reports was 2 (Czerny et al. [12]: 1.3 ± 0.5; Tugtekin et al. [13]: 1.6 ± 0.60 and Kara et al. [14]: 1.15 ± 0.41). The present study data indicated that single-vessel disease had a frequency of only 13.9% (Table 1), which was reduced to only 5.8% after matching data were included. Most data (94.2%) were for multivessel disease (Table 4), and the mean number of distal anastomoses was no less than 2 in the redo OPCAB group (Tables 2 and 5). These reports were from many countries that have various circumstances concerning the management of CABG procedures (OPCAB is used only in 30% of cases in Western countries and in other Asian countries). Further, most of these results were reported single-institution studies [6, 7, 10–12, 14]. Therefore, these data might not clarify the specific benefits of off-pump surgery in redo CABG.
The present study was a comparative clinical study utilizing a large nationwide database (JCVSD) to achieve a higher evidence level and to obtain up-to-date clinical outcomes. To the best of our knowledge, thus far, similar comparative clinical studies aiming to clarify the superiority of each variable of the off-pump technique in redo CABG have not been conducted. In addition, Japan is the only country in which >60% of isolated CABGs are performed without a CPB, and our results may be very informative.
Data from the JCVSD in redo CABG
The present retrospective comparison before matching data is presented in Tables 1 and 2.
These data suggest that surgeons in Japan tend to perform off-pump procedures for patients requiring fewer grafts in stable condition and perform on-pump procedures for patients requiring a greater number of grafts in unstable conditions for redo CABG. Thus, longer operative times are observed in the on-pump redo CABG group than in the redo OPCAB group (427.61 ± 151.23 vs 339.06 ± 132.61 min, P < 0.00010). Early outcomes and most morbidities in the on-pump redo CABG group were worse than in the redo OPCAB. Thus, off-pump surgery seems to offer certain advantages in cases of redo CABG surgery. However, the indications for off-pump or on-pump reoperations are based on individual surgeon and institutional preferences. In addition, the two groups had unequal patient characteristics and significantly different severities in patient preoperative backgrounds, according to the Japan SCORE. Based on the unadjusted results, we selected 13 preoperative risk factors to eliminate the differences between the two groups; these included the number of diseased vessels, preoperative status and the circumstances under which surgeons in Japan generally tend to avoid or prefer to use CPB (Table 3). After matching the data of these patients with the data of those in the JCVSD, no significant differences were observed in expected mortality and expected 30-day mortality or major composite morbidities between the two groups. Therefore, the severity of patient preoperative backgrounds and the overall picture of the entire patient group profile were adequately adjusted between the two groups (Table 4).
In addition, there were no significant differences in the distribution of grafts (Table 5). Therefore, these factors, including severity of patient preoperative backgrounds, did not influence the results of our study when comparing the two groups after matching. In the present retrospective comparison, the results after propensity matching data from the JCVSD demonstrated that redo OPCAB was safe and effective when each variable was considered.
The most important result of the adjusted portion of the study was the absence of significant differences in the mean number of distal anastomoses after matching. In addition, there were no significant differences in the distribution of grafts. Nevertheless, the mean operation time was significantly shorter in the redo OPCAB group than in the on-pump redo CABG group (353.7 vs 441.3 min, P < 0.00010) (Table 5). These results suggests that redo OPCAB reduced the operation time in the equivalent number of distal anastomoses performed by the on-pump procedure, even for over 90% of multivessel disease cases (Table 4). We considered that the operation time increased in the on-pump redo CABG group because of the difficulty in achieving haemostasis during adhesion dissection in patients who developed coagulation abnormalities, a potential side-effect of CPB [25]. This may have led to higher intraoperative and postoperative blood loss and need for blood transfusions. Moreover, patients who developed pulmonary dysfunction secondary to CPB may have also presented longer operation times, contributing to worsening of respiratory insufficiency, prolonged mechanical ventilation and ICU stay (Table 6). These increased operation times emphasize several adverse occurrences associated with potential side-effects of CPB, such as a coagulopathy or pulmonary dysfunction [25], redo CABG procedure such as cardioplegia, arterial cannulation or aortic cross-clamping and the multiple risk factors usually present in redo CABG patients. In other words, by avoiding CPB, the operation time could be decreased, and the complications associated with on-pump redo CABG were reduced. As a result, the incidence of composite mortality or major complication in redo OPCAB was significantly lower than in the on-pump redo CABG. Most morbidities tend to be lower in redo OPCAB (Table 6).
Our findings from the JCVSD indicate that the off-pump surgical technique for redo CABG offers certain advantages, that is, it prevents potential side-effects of CPB and prevents complications of surgical procedures.
Despite these important findings, there were several limitations in the present study regarding data interpretation. It was a retrospective clinical study based on a large-scale database, and it provides weaker clinical evidence than a randomized prospective study and we did not have medium-term or long-term outcomes. In this study, a 30-day mortality rate of 7.00% and an operative mortality rate of 9.5% were observed (Table 6); our observation interval was 30 days, but early risk extends beyond this period. The JCVSD does not have data to account for this.
Nonetheless, our data suggest that the off-pump technique for redo CABG reduces operation time for an equivalent number of distal anastomoses performed using the on-pump procedure for multivessel diseases. As a result, the off-pump procedures had significantly lower rates of prolonged ventilation, blood transfusion and shorter duration of ICU stay and lower rates of composite mortality and major morbidities compared with the on-pump procedure.
In conclusion, the off-pump technique reduced operative mortality and reduced the incidence of major complications in redo CABG.
ACKNOWLEDGMENTS
We thank all members of the Japanese Cardiovascular Surgery Database for their tireless efforts to improve the quality of cardiovascular surgery.
Funding
This work was supported by The Japanese Society for Cardiovascular Surgery, The Japanese Association for Thoracic Surgery and Japan Heart Foundation.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr E. Wolner(Vienna, Austria): This is a very interesting, very good paper and, for me, an unexpectedly greater difference in the mortality of the off-pump versus the on-pump group, in favour of the OPCAB group. This difference results from a matched group of patients. However, if you look at the unadjusted data in your slide No. 2, you see that you have more hypertension and more cardiovascular disease in the on-pump group. It is not statistically significant, but there are differences. And if you perhaps count all this data together (you have higher New York Heart Association class, you have more heart failure, you have, as you said, more unstable angina and more stroke), my conclusion is that the on-pump group is sicker than the OPCAB group.
So my first question is: Are you sure that your statistical analysis, this propensity score matching, reflects the real data between these two groups? Secondly, for me it is very surprising that in your country you have such a high number, I mean 60% or so, of off-pump versus on-pump coronary surgery. Usually in Germany it's 20%. In the STS database, it is also I believe a little bit more than 20%. What is the explanation that you have for such a high percentage of off-pump surgery in your country?
Dr Dohi: Thank you, a good question about an important point. In fact, before matching data were available, Japanese surgeons performed off-pump procedures for patients requiring fewer grafts and, on other hand, they performed on-pump procedures for patients requiring a greater number of grafts and who were in an unstable condition. Thus, the results are significantly worse in the on-pump group compared to those in the off-pump group. Could you repeat the other question?
Dr Wolner: Percentage of off- versus on-pump as a difference between your country and others.
Dr Dohi: In Japan almost 60% of isolated CABGs are performed as off-pump procedures. Our data indicate that approximately 70% off-pump procedures are performed, and OPCAB has become the standard CABG procedure in Japan. PCI is used for patients with more severe coronary artery disease in Japan compared with Western countries, in some cases in patients with LM lesions, 3-vessel disease or chronic total occlusion. This increased utilization of PCI has led Japanese surgeons to perform less invasive surgical procedures such as OPCAB.
Dr D. Taggart(Oxford, United Kingdom): I think in many Asian countries it's very high. And India is probably even higher, somewhere in the region of 80% to 90%. In Japan the figures I saw were even higher than 60%. So in the Far East and South East Asia, off-pump surgery is the standard.
Could I just ask the audience, on this basis, not for redos but for a first time coronary, which of you five years ago would have been routinely using off-pump surgery in, say, at least 30% or 40% of patients?
(Show of hands.)
Who is still using off-pump surgery in at least 30% to 40% of patients?
(Show of hands.)
It's quite interesting, just looking at this audience indicates that most of the people who five years ago were doing a significant proportion of off-pump surgery are still doing it. In my own practice it was 90% 15 years ago and it's still 90% today. But I think what's happened with recent publications is that people who were a bit less sure about off-pump surgery have now dropped off, because we're seeing the figures for off-pump surgery in the United Kingdom have dropped from around 20% to 14% now in the last year. And I think the recent publications of Coronary and GOPCABE, the big German trial, have had a negative impact on off-pump surgery.
Dr Dohi: Japanese surgeons are familiar with the off-pump procedure. Therefore, even for redo CABG, Japanese surgeons perform the off-pump procedure quite well.
Author notes
Presented at the 27th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 5–9 October 2013.




