-
PDF
- Split View
-
Views
-
Cite
Cite
Takayoshi Yamamoto, Yuichi Sakairi, Takahiro Nakajima, Hidemi Suzuki, Tetsuzo Tagawa, Takekazu Iwata, Teruaki Mizobuchi, Shigetoshi Yoshida, Yukio Nakatani, Ichiro Yoshino, Comparison between endobronchial ultrasound-guided transbronchial needle aspiration and 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of postoperative nodal recurrence in patients with lung cancer, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 2, February 2015, Pages 234–238, https://doi.org/10.1093/ejcts/ezu214
Close - Share Icon Share
Abstract
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has a high diagnostic value for preoperative mediastinal staging in patients with lung cancer. In this study, the utility of EBUS-TBNA for the pathological diagnosis of postoperative lymph node recurrence was investigated and compared with that of 18F-fluorodeoxyglucose positron emission tomography (FDG-PET).
Patients who received both EBUS-TBNA and FDG-PET for the diagnosis of postoperative lymph node recurrence were retrospectively investigated. They underwent routine chest computed tomography (CT) follow-up after thoracotomy, and when hilar or mediastinal lymph nodes showed enlargement on CT compared with the previous chest CT, they were referred for FDG-PET and EBUS-TBNA. We compared the diagnostic performance of these two modalities. In addition, pathological findings of the biopsied sample were evaluated precisely and compared with the results of FDG-PET. Positivity for hypermetabolism on FDG-PET was defined as a standardized uptake value (SUV) greater than 2.5.
A total of 40 patients were retrospectively reviewed. The sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy of EBUS-TBNA were 100% for each parameter, whereas those of FDG-PET were 95.8, 12.5, 62.2, 66.7 and 62.5%, respectively. The SUV of true-positive nodes was significantly higher than that of false-positive nodes (P = 0.001). Twenty-two of 24 patients who were confirmed for recurrence by EBUS-TBNA underwent anticancer treatment. The pathological diagnoses of 14 false-positive cases by FDG-PET were chronic inflammation in 12 and non-specific granuloma in 2.
The diagnostic yield of EBUS-TBNA is higher than that of FDG-PET when postoperative lymph node recurrence is suspected.
INTRODUCTION
Surgery is the most effective curative treatment for early stage non-small-cell lung cancer (NSCLC). Although 5-year survival rates of greater than 60% can be achieved for early stage NSCLC patients, ∼50% of those who undergo resection die of a recurrence [1]. The most commonly involved organ is the contralateral lung and regional lymph nodes, followed by the ipsilateral lung and lymph nodes [2]. Accurate diagnosis of recurrent disease is critical to determine the proper use of anticancer treatments, including salvage surgery [3, 4]. Recently, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) has been used as a diagnostic tool for recurrence, with a high sensitivity [5]. However, its high false-positive rates cannot be overlooked [6], and tissue confirmation is recommended even when FDG-PET/computed tomography (CT) indicates positive findings in regional nodes [7]. Therefore, the utility of FDG-PET is still controversial for the diagnosis of regional nodal recurrence. Furthermore, lymph node sampling by mediastinoscopy is usually technically difficult because of previous lymph node dissection.
The usefulness of endobronchial ultrasound-guided trans-bronchial needle aspiration (EBUS-TBNA) for preoperative lymph node staging in patients with lung cancer is widely accepted, with a high sensitivity (∼90%) and specificity (∼100%) [8, 9]. EBUS-TBNA can also be used during and after the treatment of lung cancer [4, 10]. The usefulness of EBUS-TBNA for restaging after induction treatment has been reported previously [11]. However, the efficacy of EBUS-TBNA for the diagnosis of postoperative lymph node recurrence is still unclear. Postoperative mediastinal and hilar adenopathy are observed in ∼14% of patients with lung cancer, although it does not always indicate recurrent disease [12]. The negative predictive value (NPV) of CT scan for postoperative adenopathy is high; however, the sensitivity and positive predictive value are lower than the NPV [12]. In this study, we retrospectively compared the diagnostic performance between FDG-PET and EBUS-TNBA for postoperative lymph node recurrence after radical surgery and compared the pathological findings with the results of FDG-PET.
MATERIALS AND METHODS
Patients
From January 2007 to June 2012, the records of patients who were suspected to have hilar or mediastinal lymph node recurrence by CT during postoperative routine follow-up of NSCLC in the Chiba University Hospital, Chiba, Japan, were retrospectively reviewed. A total of 639 patients underwent thoracotomy during this period. Patients who underwent both EBUS-TBNA and FDG-PET were eligible for this study. We retrospectively compared the diagnostic performance of these two modalities. There were no exclusion criteria used in this study. The study was approved by the ethical committee of our institute. Primary lesions were classified according to the international TNM Classification of Malignant Tumours (TNM) staging system for the postoperative pathology [13], and WHO histopathological classification [14].
The Ethics Committee at Chiba University approved this study (No. 1574) and the written consent was waived due to retrospective chart review.
Clinical follow-up after surgery
All patients had undergone a curative resection, including systematic nodal dissection (the area of nodal dissection was defined by the criteria of the Japan Lung Cancer Society), and were subjected to the routine follow-up protocol in our outpatient clinic. The physical examination included: clinical blood tests, including blood cell counts, hepatic enzymes, creatinine, total protein and tumour markers (carcinoembryonic antigen, cytokeratin 19 fragment and pro-gastrin-releasing peptide); and chest X-rays were performed every 3 months for the first 2 years and every 6 months thereafter until 5 years after surgery. A thoraco-abdominal CT scan, bone scan and brain magnetic resonance imaging were performed every 6 months during the follow-up period. In cases of pathological stage IA, bone scans and brain magnetic resonance imaging were not routinely performed. When hilar or mediastinal lymph nodes showed enlargement on CT in comparison with the previous follow-up CT or preoperative CT, FDG-PET and EBUS-TBNA were performed for the diagnosis of lymph node recurrence.
18F-fluorodeoxyglucose positron emission tomography and endobronchial ultrasound-guided transbronchial needle aspiration
Positivity for hypermetabolism on FDG-PET was defined as a standardized uptake value (SUV) greater than 2.5. EBUS-TBNA was performed for the lymph nodes of interest on CT and/or FDG-PET. The lymph nodes that were assessable by EBUS-TBNA were analysed in this study. The lymph nodes that were not accessible by EBUS-TBNA were excluded. A dedicated 22-gauge needle was used for TBNA. Rapid on-site cytological evaluation was performed during EBUS-TBNA, and TBNA was repeatedly performed until a sufficient sample was obtained to determine a diagnosis. Pathological diagnoses for both cytological and histological samples regarding the presence or absence of cancer cells in the EBUS-TBNA samples were made by independent pathologists.
Gold standard of recurrence
The gold standard of diagnosis was defined according to the following criteria: (i) the presence of cancer cells at EBUS-TBNA, (ii) the pathological confirmation of a negative result after lymph node resection, (iii) the lack of substantial changes or decrease in size of the referred nodes, according to CT, during more than 6 months clinical follow-up without any cancer treatment and (iv) the stability or decrease of SUV mediastinal values during more than 6 months clinical follow-up without any cancer treatment. A negative result was initially determined by pathological examination of EBUS-TBNA for both the absence of tumour cells and the presence of enough lymphocytes or macrophages or lymph node tissue. Especially, the negative results of EBUS-TBNA were intensively followed by repeated chest CT scan and repeated FDG-PET if needed.
Statistical analysis
Survival duration was defined as the time from the date of EBUS-TBNA examination to the date of the last follow-up or death. Survival curves were estimated by the Kaplan–Meier method. Differences in survival were assessed by the log-rank test. The differences between the two groups were examined with the Mann–Whitney U-test for continuous variables. The hypothesis tests used were two-sided, and a P-value of <0.05 was used to define statistical significance. Statistical analyses were performed with JMP version 7.0 (SAS Institute Japan, Tokyo, Japan).
RESULTS
Forty patients were eligible for this study, and their characteristics are listed in Table 1. There were 5 patients who underwent EBUS-TBNA without FDG-PET, and they were excluded from this study. Two patients received induction chemotherapy prior to the thoracotomy, and 10 patients had adjuvant chemotherapy after the surgery. The mean duration between thoracotomy and EBUS-TBNA was 23.5 months, and the median follow-up period after EBUS-TBNA was 43.5 (range: 12–52) months. The evaluated lymph nodes (n = 70) are shown in Table 2, and the mean number of punctured lymph nodes was 1.75 per patient. The median size of the punctured lymph nodes was 10 mm (IQR: 8–13 mm) on chest CT and 9.5 mm (IQR: 7–12 mm) on EBUS. The median SUV of these lymph nodes was 4.05 (1.9–43.9). Thirty-seven patients showed positive metabolic activity on FDG-PET. EBUS-TBNA was successfully performed in all 40 patients without any complications. EBUS-TBNA identified lymph node recurrence of lung cancer at 36 stations in 24 patients.
| Patients . | n = 40 . |
|---|---|
| Sex, n | |
| Male | 31 |
| Female | 9 |
| Mean age, years (range) | 71.0 (57–82) |
| Pathological diagnosis, n | |
| Adenocarcinoma | 29 |
| Squamous cell carcinoma | 9 |
| Large cell neuroendocrine carcinoma | 2 |
| Pathological stage, n | |
| I | 23 |
| II | 8 |
| III | 8 |
| IV | 1 |
| Patients . | n = 40 . |
|---|---|
| Sex, n | |
| Male | 31 |
| Female | 9 |
| Mean age, years (range) | 71.0 (57–82) |
| Pathological diagnosis, n | |
| Adenocarcinoma | 29 |
| Squamous cell carcinoma | 9 |
| Large cell neuroendocrine carcinoma | 2 |
| Pathological stage, n | |
| I | 23 |
| II | 8 |
| III | 8 |
| IV | 1 |
| Patients . | n = 40 . |
|---|---|
| Sex, n | |
| Male | 31 |
| Female | 9 |
| Mean age, years (range) | 71.0 (57–82) |
| Pathological diagnosis, n | |
| Adenocarcinoma | 29 |
| Squamous cell carcinoma | 9 |
| Large cell neuroendocrine carcinoma | 2 |
| Pathological stage, n | |
| I | 23 |
| II | 8 |
| III | 8 |
| IV | 1 |
| Patients . | n = 40 . |
|---|---|
| Sex, n | |
| Male | 31 |
| Female | 9 |
| Mean age, years (range) | 71.0 (57–82) |
| Pathological diagnosis, n | |
| Adenocarcinoma | 29 |
| Squamous cell carcinoma | 9 |
| Large cell neuroendocrine carcinoma | 2 |
| Pathological stage, n | |
| I | 23 |
| II | 8 |
| III | 8 |
| IV | 1 |
| Punctured lymph node . | n = 70 . |
|---|---|
| Mediastinal lymph node, n | (54) |
| #2R | 5 |
| #4R | 17 |
| #4L | 13 |
| #7 | 19 |
| Hilar lymph nodes, n | (16) |
| #10 | 3 |
| #11 | 10 |
| #12 | 3 |
| Median size of lymph nodes | |
| Chest CT | 10 mm (IQR: 8–13 mm) |
| EBUS | 9.5 mm (IQR: 7–12 mm) |
| Positive lymph nodes | |
| Chest CT | 11 mm (IQR: 8–15 mm) |
| EBUS | 11 mm (IQR: 7–16 mm) |
| Negative lymph nodes | |
| Chest CT | 10 mm (IQR: 8–12 mm) |
| EBUS | 9 mm (IQR: 7–11 mm) |
| Median SUV for | 5.1 (IQR: 3.7–7.1) |
| Positive nodes | 6.7 (IQR: 5.4–9.1) |
| Negative nodes | 4.0 (IQR: 3.4–4.9) |
| Punctured lymph node . | n = 70 . |
|---|---|
| Mediastinal lymph node, n | (54) |
| #2R | 5 |
| #4R | 17 |
| #4L | 13 |
| #7 | 19 |
| Hilar lymph nodes, n | (16) |
| #10 | 3 |
| #11 | 10 |
| #12 | 3 |
| Median size of lymph nodes | |
| Chest CT | 10 mm (IQR: 8–13 mm) |
| EBUS | 9.5 mm (IQR: 7–12 mm) |
| Positive lymph nodes | |
| Chest CT | 11 mm (IQR: 8–15 mm) |
| EBUS | 11 mm (IQR: 7–16 mm) |
| Negative lymph nodes | |
| Chest CT | 10 mm (IQR: 8–12 mm) |
| EBUS | 9 mm (IQR: 7–11 mm) |
| Median SUV for | 5.1 (IQR: 3.7–7.1) |
| Positive nodes | 6.7 (IQR: 5.4–9.1) |
| Negative nodes | 4.0 (IQR: 3.4–4.9) |
| Punctured lymph node . | n = 70 . |
|---|---|
| Mediastinal lymph node, n | (54) |
| #2R | 5 |
| #4R | 17 |
| #4L | 13 |
| #7 | 19 |
| Hilar lymph nodes, n | (16) |
| #10 | 3 |
| #11 | 10 |
| #12 | 3 |
| Median size of lymph nodes | |
| Chest CT | 10 mm (IQR: 8–13 mm) |
| EBUS | 9.5 mm (IQR: 7–12 mm) |
| Positive lymph nodes | |
| Chest CT | 11 mm (IQR: 8–15 mm) |
| EBUS | 11 mm (IQR: 7–16 mm) |
| Negative lymph nodes | |
| Chest CT | 10 mm (IQR: 8–12 mm) |
| EBUS | 9 mm (IQR: 7–11 mm) |
| Median SUV for | 5.1 (IQR: 3.7–7.1) |
| Positive nodes | 6.7 (IQR: 5.4–9.1) |
| Negative nodes | 4.0 (IQR: 3.4–4.9) |
| Punctured lymph node . | n = 70 . |
|---|---|
| Mediastinal lymph node, n | (54) |
| #2R | 5 |
| #4R | 17 |
| #4L | 13 |
| #7 | 19 |
| Hilar lymph nodes, n | (16) |
| #10 | 3 |
| #11 | 10 |
| #12 | 3 |
| Median size of lymph nodes | |
| Chest CT | 10 mm (IQR: 8–13 mm) |
| EBUS | 9.5 mm (IQR: 7–12 mm) |
| Positive lymph nodes | |
| Chest CT | 11 mm (IQR: 8–15 mm) |
| EBUS | 11 mm (IQR: 7–16 mm) |
| Negative lymph nodes | |
| Chest CT | 10 mm (IQR: 8–12 mm) |
| EBUS | 9 mm (IQR: 7–11 mm) |
| Median SUV for | 5.1 (IQR: 3.7–7.1) |
| Positive nodes | 6.7 (IQR: 5.4–9.1) |
| Negative nodes | 4.0 (IQR: 3.4–4.9) |
The diagnostic parameters of EBUS-TBNA and FDG-PET are summarized in Table 3. The sensitivity, specificity, positive predictive value, NPV and diagnostic accuracy of EBUS-TBNA were each 100%, whereas those of FDG-PET were 95.8, 12.5, 62.2, 66.7 and 62.5%, respectively. The median size of the negative lymph nodes was 10 mm (IQR: 8–12 mm) on CT and 9 mm (IQR: 7–11 mm) on EBUS. The median size of the positive lymph nodes was 11 mm (IQR: 8–15 mm) on CT and 11 mm (IQR: 7–16 mm) on EBUS. There was no significant difference in size between the negative and positive lymph nodes on CT (P = 0.07) and EBUS (P = 0.18). The median SUV of the negative lymph nodes [4.0 (IQR: 3.4–4.9)] was significantly lower than that of the positive lymph nodes [6.7 (IQR: 5.4–9.1)] (P = 0.001). The cut-off value of the SUV for predicting positive lymph nodes was 2.5, with a sensitivity of 76.9% and specificity of 59.1%.
| n = 40 . | EBUS-TBNA (+) . | EBUS-TBNA (−) . |
|---|---|---|
| FDG-PET (+) | 23 | 14 |
| FDG-PET (−) | 1 | 2 |
| n = 40 . | EBUS-TBNA (+) . | EBUS-TBNA (−) . |
|---|---|---|
| FDG-PET (+) | 23 | 14 |
| FDG-PET (−) | 1 | 2 |
| n = 40 . | EBUS-TBNA (+) . | EBUS-TBNA (−) . |
|---|---|---|
| FDG-PET (+) | 23 | 14 |
| FDG-PET (−) | 1 | 2 |
| n = 40 . | EBUS-TBNA (+) . | EBUS-TBNA (−) . |
|---|---|---|
| FDG-PET (+) | 23 | 14 |
| FDG-PET (−) | 1 | 2 |
Of the 24 EBUS-TBNA-positive cases, there were 23 FDG-PET true-positive cases and 1 FDG-PET false-negative case. On the other hand, of the 16 EBUS-TBNA negative cases, there were 14 FDG-PET false-positive cases and 2 FDG-PET true-negative cases. Of the 14 FDG-PET false-positive cases, the pathological findings within their EBUS-TBNA samples included chronic inflammation manifested by histiocyte phagocytosis of coal dust in 12 (Fig. 1A) and epithelioid cell granulomas in 2 cases (Fig. 1B).
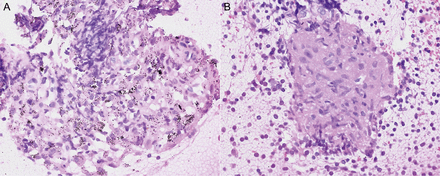
Representative pathological findings of PET false-positive lymph nodes. Pathological findings of false-positive cases by FDG-PET showed (A) chronic inflammation manifested by histiocytes phagocytizing coal dust and (B) epithelioid cell granulomas.
Twenty-four patients with a confirmation of cancer recurrence received non-surgical therapy, including 8 who received systemic chemotherapy, 7 who received conventional radiotherapy, 5 who received chemo-radiotherapy, 1 who received heavy ion radiotherapy, 1 who received clinical trial immunotherapy and 2 who received best supportive care. Two of 16 patients without cancer recurrence in the lymph nodes underwent surgery due to the chest CT finding of a tumour in the lung parenchyma. One patient underwent a wedge resection of the right lower lobe; another patient received a right S6 segmentectomy and wedge resection of the upper lobe. Both were diagnosed as a second primary lung cancer. The referenced lymph nodes were simultaneously resected, but no metastasis was found in the pathology. There were no cases with local recurrence.
DISCUSSION
We retrospectively compared the diagnostic performance of EBUS-TBNA and FDG-PET for the diagnosis of lymph node recurrence after radical surgery in patients with lung cancer. The diagnostic accuracy of EBUS-TBNA for this purpose was significantly higher than that of FDG-PET. There were 14 false-positive cases by FDG-PET, and the positive predictive value was 62.2%. We reviewed the pathological findings of PET false-positive lymph nodes sampled by EBUS-TBNA, and determined that they were chronic inflammation or unspecific granuloma. Interestingly, there was no statistical difference in size between negative and positive nodes. On the other hand, false-positive nodes had significantly lower SUVs.
There is no doubt that tissue confirmation of cancer recurrence leads to the selection of proper treatments and reduces futile cancer therapy. Mediastinoscopy showed high diagnostic performance for the pathological confirmation of lymph node recurrence after surgery [15]; however, it is often technically difficult to carry out mediastinoscopy after systematic lymph node dissection due to adhesion, the same as with re-mediastinoscopy [16, 17]. As for the other restaging procedures, restaging using video-assisted thoracoscopic surgery (VATS) was considered to be feasible; however, the sensitivity and NPV were 67 and 73%, respectively [18]. Restaging using VATS has been recommended for EBUS/EUS-negative cases [18]. Anterior mediastinotomy is another modality for restaging; however, the assessable area of the anterior mediastinum is limited [19]. The utility of transcervical extended mediastinal lymphadenectomy (TEMLA) has been reported, and the diagnostic yield is superior to that of EBUS/EUS [20]. However, the results were only reported by specific specialists, and TEMLA is not commonly performed. Recently, the results of restaging using combined EBUS and EUS were reported, and the sensitivity and NPV were 67.3 and 73.0%, respectively [21]. This result was reasonable, and EBUS and EUS are considered safe procedures, but it is recommended that the negative results be confirmed by surgical procedures [21].
EBUS-TBNA is known as a less invasive modality for tissue diagnosis of mediastinal and hilar lymph nodes. It is recommended as an initial preoperative staging tool for patients with lung cancer [22]. We conclude that EBUS-TBNA is also a useful modality for the diagnosis of lymph node recurrence after radical surgery for lung cancer. EBUS-TBNA can access N1 nodes in addition to N2 nodes, which are not accessible to mediastinoscopy.
An additional advantage of EBUS-TBNA was the ability to perform multiple tissue sampling. The samples obtained by EBUS-TBNA can be used for biomarker testing [23, 24], which is now crucial in the era of molecular-targeted therapy for lung cancer.
Integrated PET/CT can be a useful diagnostic tool to detect cancer recurrence in NSCLC [2]; however, the issue of its low positive predictive value was discussed. In addition to the postsurgical condition, patients with lung cancer often have chronic obstructive pulmonary disease or a smoking habit, which may lead to false-positive results of FDG-PET due to chronic airway inflammation. Therefore, the pathological confirmation of PET-positive lesions is recommended [6].
There were 14 false-positive PET cases (37.5%) in this study. The pathological findings of these 14 cases revealed inflammatory lesions manifested as histiocytes phagocytizing coal dust or non-specific epithelioid granulomas. After pulmonary resection and nodal dissection, such inflammatory changes may occur in the lesion. These findings would lead to false-positive PET results, which would have resulted in patients receiving unnecessary or harmful therapy if EBUS-TBNA had not been applied for the lesions [25].
A limitation of this study was the lack of a method to confirm the negative EBUS-TBNA results pathologically. In this study, the final negative result was defined as no radiological change observed over more than 6 months of follow-up or surgical pathology if the case underwent surgery. It is unknown whether any cases developed metastatic nodes after 6 months of follow-up since undergoing EBUS-TBNA. However, the minimum follow-up period of this study population was 12 months and the possibility of a false-positive result by EBUS-TBNA would be very low. In addition, we compared the different modalities, radiology versus pathology, which cannot be evaluated equally, especially for the assessable area. We excluded those lymph nodes that cannot be assessed by EBUS-TBNA; therefore, the results of this study are only relevant to the lymph nodes located within the areas accessible to the EBUS scope.
In conclusion, when lymph node recurrence is suspected during follow-up after radical surgery in patients with lung cancer, tissue confirmation is mandatory, since false-positive results are frequently observed by CT and/or FDG-PET. EBUS-TBNA can be considered an accurate and safe procedure for the confirmation of abnormal radiological findings in the postoperative condition.
ACKNOWLEDGEMENTS
We thank Yositaka Uchida (Sannou Hospital Medical Center) and Yoshihiro Ozaki (radiology technician at the Chiba Ryogo Center) for providing support in the calculation of the SUV.
Conflict of interest: Dr Nakajima received honoraria and lecture fee for the seminar and hands-on training course of EBUS-TBNA from Olympus Medical Systems.




