-
PDF
- Split View
-
Views
-
Cite
Cite
Arturo Evangelista, Martin Czerny, Christoph Nienaber, Marc Schepens, Hervé Rousseau, Piergiorgio Cao, Sergio Moral, Rossella Fattori, Interdisciplinary expert consensus on management of type B intramural haematoma and penetrating aortic ulcer, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 2, February 2015, Pages 209–217, https://doi.org/10.1093/ejcts/ezu386
Close - Share Icon Share
An expert panel on the treatment of type B intramural haematoma (IMH) and penetrating atherosclerotic ulcer (PAU) consisting of cardiologists, cardiothoracic surgeons, vascular surgeons and interventional radiologists reviewed the literature to develop treatment algorithms using a consensus method. Data from 46 studies considered relevant were retrieved for a total of 1386 patients consisting of 925 with IMH, and 461 with PAU. The weighted mean 30-day mortality from IMH was 3.9%, 3-year aortic event-related mortality with medical treatment 5.4%, open surgery 23.2% and endovascular therapy 7.1%. In patients with PAU early and 3-year aortic event-mortality rates with open surgery were 15.9 and 25.0%, respectively, and with TEVAR were 7.2 and 10.4%, respectively. According to panel consensus statements, haemodynamic instability, persistent pain, signs of impending rupture and progressive periaortic haemorrhage in two successive imaging studies require immediate surgical or endovascular treatment. In the absence of these complications, medical treatment is warranted, with imaging control at 7 days, 3 and 6 months and annually thereafter. In the chronic phase, aortic diameter >55 mm or a yearly increase ≥5 mm should be considered indications for open surgery or thoracic endovascular treatment, with the latter being preferred. In complicated type B aortic PAU and IMH, endovascular repair is the best treatment option in the presence of suitable anatomy.
INTRODUCTION
Aortic intramural haematoma (IMH) is an entity belonging to the spectrum of acute aortic syndrome (AAS) in which haemorrhage occurs in the media of the aortic wall in the absence of a demonstrable two-lumen flow and primary intimal tear [1]. IMH is diagnosed in the presence of circular or crescentic thickening >5 mm of the aortic wall in the absence of detectable blood flow in the vessel wall. The term penetrating aortic ulcer (PAU) describes the condition in which ulceration of an atherosclerotic lesion penetrates the internal elastic lamina into the media [1]. PAU is considered to be a disease of the intima (i.e. atherosclerosis), whereas aortic dissection and its variant (IMH) are fundamentally diseases of the media. Approximately 5–15% of AAS are diagnosed as IMH [2, 3] and 5% as PAU. Symptoms of these entities are similar to those of aortic dissection and may be indistinguishable, although patients are less likely to suffer from malperfusion syndrome. The main objective of IMH and PAU treatment is to prevent aortic rupture or progression to classic dissection. As type A IMH and PAU have a high, early risk of complications and death with medical treatment alone, surgery is usually indicated [4]. A conservative approach to uncomplicated type B IMH such as antihypertensive treatment and watchful monitoring is currently preferred as it appears to be a safer strategy. However, in some cases, the disease may still progress despite optimal medical treatment. Notwithstanding some recommendations for endovascular repair of AAS [4–6], the indications in IMH and PAU remain controversial, and the general approach is to treat them like aortic dissection, even without scientific data. In the American Heart Association guidelines published in 2010, these entities were briefly discussed in the context of three overlapping aortic lesions: intimal defect without IMH, intimal defect with IMH and IMH without an intimal defect. The course, morbidity and mortality rates of each treatment remain unknown owing to the lack of published large series. Although recent studies reported the benefit of thoracic endovascular aortic repair (TEVAR) in the treatment of distal ascending aortic diseases [7], the present consensus will focus only on type B lesions.
This review of the literature was undertaken to identify current morbidity and mortality rates in the medical, surgical and endovascular treatment of IMH and PAU and, based on the results, develop treatment algorithms using a consensus method.
METHODS
Literature search
The review of the literature was planned in accordance with current guidelines for conducting comprehensive systematic reviews. The literature search was implemented to identify studies in peer-reviewed journals through a comprehensive search of computerized databases including PubMed and Ovid Medline. The search was inclusive up to February 2014 and limited to the past 20 years. Search strings included ‘intramural hematoma and/or penetrating aortic ulcer’ combined with the terms ‘endovascular treatment’, ‘surgical treatment’ and ‘medical treatment’. The search was limited to articles on humans only with an abstract available in English. After relevant studies were identified, additional tangential searches were conducted using related article links within PubMed. The assessment of studies for inclusion and data extraction was conducted by one independent reviewer, and validated by the panelists during the first meeting. For clinical outcomes, definitions provided by authors of the studies were generally used. Only studies with specific analyses of type B involvement were included. Early mortality and morbidity rates were calculated perioperatively and 30 days postoperatively; late mortality and morbidity rates were calculated as death or events that occurred from Day 31 and beyond. Mortality related to aortic events during the first 3 years was specifically analysed to establish a similar follow-up period among treatment groups.
Expert panel
An expert panel of seven leaders in the treatment of type B IMH and PAU consisting of cardiologists, cardiothoracic surgeons, vascular surgeons and interventional radiologists was organized to participate in the consensus. All members represented the Western European geographical area and were from referral centres of aortic pathology. The members of the panel reviewed the available literature and provided a consensus for the treatment of these pathologies and tried to standardize definitions.
Consensus method
Current topics of debate in relation with the definition, predictive factors of complicated course and treatment of both entities were discussed from the results of published literature. Treatment algorithms were created when general agreement among members was reached. Focalized meetings were organized for the evaluation of the initial disagreement issues to achieve unanimous approval after a re-review of the medical literature.
Statistical analysis
Literature data were stratified by pathology and type of treatment (medical, TEVAR or open surgery). Number of cases, event rates and weighted averages, obtained from the total of deaths with respect to the total number of cases, are specified in each table. Only comparisons of different treatment outcomes were combined when treatments were applied in similar populations (TEVAR vs open surgery).
SEARCH RESULTS
Aortic intramural haematoma
The literature search identified 157 potential publications. Of these, 30 were considered relevant for the purposes of this review. Several publications included clinical data on more than one treatment modality. The majority of the publications with clinical data were retrospective analyses. The 30 publications summarized included a total of over 900 patients who suffered from type B IMH; 731 patients underwent conservative medical management, 108 surgical treatment and 86 endovascular repair of the thoracic aorta (TEVAR) with different commercial and homemade stent grafts. Follow-up of patients was extensive and ranged up to 3 years.
Mortality in type B intramural haematoma
The weighted average 30-day mortality rate in patients with type B IMH was 3.9% and the overall late mortality rate in a mean follow-up of 36 months was 14.3% [8–26]. Predictors of mortality in the acute phase, i.e. persistent pain, haemodynamic instability, maximum aortic diameter (MAD) and periaortic haemorrhage were reported in only a few articles [18, 27]. Late mortality in type B IMH was due to aortic complications in at least 50% of cases; other causes were cancer, infections or other cardiovascular diseases [15, 17, 23].
Evolution of intramural haematoma
Sixteen of the selected publications contained data on IMH progression [10, 14–17, 19, 22, 23, 28–35]. Progression was defined as classical or localized dissection, impending rupture or aneurysm formation of the aorta. In general, IMH is more likely to stabilize or regress than to progress (58.5 vs 48.7%) at 1 year. The mean rate for progression to classic dissection was 5.3%, to localized dissection or ulcer-like projection (ULP) 25.3%, to rupture 3.9% and to aneurysm 26.6%. Mean rates for stabilization, regression and resolution were: 11.1, 32.1 and 59.9%, respectively. The morphological changes in IMH, particularly in the first 6 months, are very dynamic. Only nine studies distinguished between classic and localized dissection; this difference is significant since the latter causes most of the ULP images.
Predictors of complications
In the acute phase, persistent pain, haemodynamic instability, MAD, IMH wall thickness, presence of ULPs, pleural effusion or haemomediastinum and periaortic haemorrhage have been identified as predictors of complications (Table 1). Most of these predictors may be defined by imaging techniques:
MAD in the acute phase is one of the major predictors of progression in type B IMH [10, 27, 30, 36]. Patients with a MAD >45 mm have a higher risk of dissection, regardless of the location [17, 36].
Wall thickness has been described as a predictor of progression [17, 29, 30, 33]; however, this issue is controversial [23]. Sueyoshi et al. [30] proposed a cut-off ≥10 mm, although this value varied considerably in the different series published from 10 to 15 mm.
The incidence of periaortic haemorrhage or pleural effusion is higher in IMH than in aortic dissection; in some studies, this incidence rate rose to 40% [23]. Some series-related pleural effusion led to unfavourable prognosis in IMH [27, 29, 33, 37]. However, there are at least two mechanisms to explain this finding, a leakage of blood from the aorta through microperforations, or a non-haemorrhagic exudate from aortic wall inflammatory reaction [38, 39] owing to the proximity of the IMH to the adventitia. The difference between the prognostic value of each type of pleural effusion may explain the discordance in the medical literature.
ULP is a frequent finding in type B IMH, and its incidence rate ranges from 20–60% of cases [15, 26, 32, 33, 37, 40–42]. It is defined as a localized blood-filled pouch protruding into the haematoma of the aortic wall [32], with a wide communicating orifice of more than 3 mm [43]. In most cases, ULPs result from a localized dissection. The prognostic significance of ULP is unclear, and a discrepancy exists as to its real meaning in the context of type B IMH. This specific complication will be discussed in the treatment section.
| High-risk feature . | Cut-off or sign of complicated evolution . |
|---|---|
| Age (years) | >70 [15, 36] |
| Initial aortic diameter (mm) | >45 [17, 36] |
| Mean aortic diameter growth rate (mm/year) | ≥5 [4, 6] |
| Wall thickness of involved segment (mm) | ≥10 [30] |
| Pleural effusion | Presence [27, 29] |
| Aortic ulcer | Presence [15, 26] |
| Ulcer-like projection |
| High-risk feature . | Cut-off or sign of complicated evolution . |
|---|---|
| Age (years) | >70 [15, 36] |
| Initial aortic diameter (mm) | >45 [17, 36] |
| Mean aortic diameter growth rate (mm/year) | ≥5 [4, 6] |
| Wall thickness of involved segment (mm) | ≥10 [30] |
| Pleural effusion | Presence [27, 29] |
| Aortic ulcer | Presence [15, 26] |
| Ulcer-like projection |
IMH: intramural haematoma.
| High-risk feature . | Cut-off or sign of complicated evolution . |
|---|---|
| Age (years) | >70 [15, 36] |
| Initial aortic diameter (mm) | >45 [17, 36] |
| Mean aortic diameter growth rate (mm/year) | ≥5 [4, 6] |
| Wall thickness of involved segment (mm) | ≥10 [30] |
| Pleural effusion | Presence [27, 29] |
| Aortic ulcer | Presence [15, 26] |
| Ulcer-like projection |
| High-risk feature . | Cut-off or sign of complicated evolution . |
|---|---|
| Age (years) | >70 [15, 36] |
| Initial aortic diameter (mm) | >45 [17, 36] |
| Mean aortic diameter growth rate (mm/year) | ≥5 [4, 6] |
| Wall thickness of involved segment (mm) | ≥10 [30] |
| Pleural effusion | Presence [27, 29] |
| Aortic ulcer | Presence [15, 26] |
| Ulcer-like projection |
IMH: intramural haematoma.
Mortality and treatment strategies
Medical treatment
Mortality rates for patients with type B IMH who received medical treatment were identified in 18 publications [2, 9, 11–20, 22–26, 31]. Patients diagnosed with type B IMH were initially treated medically with beta blockers and other antihypertensive therapies. The mean mortality rate in the acute phase for these patients was 3.4% and within 3 years that related to aortic events was 5.4%.
Surgical treatment
Sixteen publications contained data regarding the mortality rates of surgical treatment in IMH [2, 9, 11–14, 16–20, 23–26, 31]. Patients with type B IMH were treated with open surgery when medical treatment failed and/or when the IMH progressed to dissection, aneurysm or rupture. The mortality rate in the acute phase was 16% and within 3 years that for surgical treatment in type B IMH was 23.2%.
Endovascular treatment
Data on endovascular treatment for IMH are scant. Mortality rates could be assessed from only nine articles [2, 17, 21–23, 31, 44–46]. The mean mortality rate in patients treated with TEVAR in the acute phase was 4.6% and within 3 years of follow-up was 7.1%. Indications for endovascular treatment are not well established; however, larger series accepted invasive intervention in patients who showed signs of aortic rupture or aortic enlargement (MAD ≥55 mm or rapid enlargement of the affected aorta or ULP) during follow-up [15, 26]. In comparison with open surgery, fewer cases were treated in the acute phase (84.6%).
Complications in TEVAR
Complications in the use of TEVAR in IMH treatment were similar to those in other aortic diseases; however, endoleaks in the acute phase might be more frequent than in other TEVAR indications [47, 48]. Endoleaks and lesions of the intimal layer at the ends of the device frequently necessitated reintervention [21, 44, 49]. Some groups pointed out the presence of complications secondary to lesions caused in the intimal layer when the ends of the stent were placed in the aortic wall affected by the IMH [50]. Most of these cases initiated the formation of pseudoaneurysms, which required a new TEVAR [21, 50–53].
Penetrating aortic ulcer
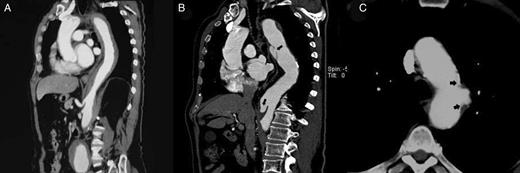
(A) CT image of an intramural haematoma in the descending thoracic aorta at the diagnosis of the acute aortic syndrome. (B) Development of two ulcer-like projections in the same patient at sixth months CT control (black arrows). (C) Penetrating atherosclerotic ulcer in the aortic arch. Significant atherosclerosis and calcification of the borders of the lesion (black arrows) with deformation of the external morphology of the vessel.
Mortality in penetrating atherosclerotic ulcer
Little is known of the mortality risk of PAU. Some authors considered it to pose a higher risk than classic aortic dissection. However, others reported that disease progression is slow, with a low prevalence of acute rupture. These discrepancies can be explained by differences in patient selection, particularly when cases have an incidental diagnosis or are secondary to AAS.
Predictors of complications
Spontaneous rupture of the aorta in PAU is a rare condition in the absence of AAS or severe progressive dilatation. However, in symptomatic patients, the risk of complications may be high. Significant predictors of aortic rupture have been considered to be recurrent or refractory pain despite medical treatment [31, 57–59], haemodynamic instability, periaortic bleeding or significant/progressive pleural effusion [31, 47], association with IMH [31, 60] and large ulcer size. In some cases, PAUs evolve with surrounding IMHs and in others with saccular aneurysm formation [17, 61]. There is no consensus on ulcer size cut-off values; however, growth rate and MAD at the site of the lesion have been considered, as in other aortic entities [4, 47].
Treatment of PAU
Considerable controversy exists regarding the natural history of penetrating ulcers and, accordingly, the indications for open surgical or endovascular treatment. A number of authors reported satisfactory results with a conservative approach to PAU [11, 62]. However, in most of those reports, PAU were ULP in the context of IMH [48, 60]. Nevertheless, most authors suggested that surgical intervention with grafting of the affected area was the treatment of choice owing to a possible malignant course [31]. Most patients presenting PAU may not be candidates for conventional surgery owing to their general status or significant comorbidities [52]. Conventional surgery for PAU was associated with a mortality rate of 15.9% [31, 55, 60–65]. It is obvious that patients undergoing surgery for PAU are the ones with a negative selection bias as the natural course of their disease warrants treatment but endovascular therapy is not feasible. As patients develop a PAU as a result of a severe systemic obliterative process, they are usually affected by any of several kinds of symptomatic obliterative arteriopathy limiting the success of surgical therapy. It remains to the individual clinical situation as well as to the estimation of the treating physician to determine a conservative option if the operative risk is deemed unacceptably high.
As PAU is commonly observed as a segmental localized wall lesion, it is an ideal target for endovascular stent grafting. Early mortality in TEVAR is estimated to be 7.2% [48, 66–74]. The presence of associated IMH may increase the risk of treatment failure, aortic rupture or aorta-related death [75], thereby highlighting the need for careful planning, prudent balancing of the benefits of a possible delayed treatment to avoid fragility of the affected aortic wall and other complications such as leaks, strokes etc. Moreover, in one of the larger series published, Geisbüsch et al. [48] reported a total of nine primary (19%: 4% of type I endoleak, 13% of type II endoleak and 2% of type IV endoleak) and two secondary (4%: 2% of type I endoleak and 2% of type IV endoleak) endoleaks among 48 patients. Reintervention was necessary in 4 of these 11 endoleaks (36%). Owing to the occurrence of secondary endoleaks, close life-long follow-up was recommended in these patients [47]. In Table 2, the suggested indications for stent-graft repair are defined, based on the medical literature reviewed.
High-risk features of type B PAU based on the medical literature reviewed and recommendations for invasive treatment
| High-risk feature . | Indication . |
|---|---|
| Symptomatic patient | Symptoms despite medical treatment [31, 57–59] |
| Asymptomatic patient | |
| Pleural effusion | Increase in pleural effusion [31, 47] |
| IMH-associated | Presence of IMH [31, 47] |
| Initial PAU depth and diametera | Large initial PAU depth (>10 mm) and diameter (>20 mm) or high growth rate size [31] |
| High-risk feature . | Indication . |
|---|---|
| Symptomatic patient | Symptoms despite medical treatment [31, 57–59] |
| Asymptomatic patient | |
| Pleural effusion | Increase in pleural effusion [31, 47] |
| IMH-associated | Presence of IMH [31, 47] |
| Initial PAU depth and diametera | Large initial PAU depth (>10 mm) and diameter (>20 mm) or high growth rate size [31] |
aControversial: not fully accepted, and cut-off value unclear.
IMH: intramural haematoma; PAU: penetrating atherosclerotic ulcer.
High-risk features of type B PAU based on the medical literature reviewed and recommendations for invasive treatment
| High-risk feature . | Indication . |
|---|---|
| Symptomatic patient | Symptoms despite medical treatment [31, 57–59] |
| Asymptomatic patient | |
| Pleural effusion | Increase in pleural effusion [31, 47] |
| IMH-associated | Presence of IMH [31, 47] |
| Initial PAU depth and diametera | Large initial PAU depth (>10 mm) and diameter (>20 mm) or high growth rate size [31] |
| High-risk feature . | Indication . |
|---|---|
| Symptomatic patient | Symptoms despite medical treatment [31, 57–59] |
| Asymptomatic patient | |
| Pleural effusion | Increase in pleural effusion [31, 47] |
| IMH-associated | Presence of IMH [31, 47] |
| Initial PAU depth and diametera | Large initial PAU depth (>10 mm) and diameter (>20 mm) or high growth rate size [31] |
aControversial: not fully accepted, and cut-off value unclear.
IMH: intramural haematoma; PAU: penetrating atherosclerotic ulcer.
DISCUSSION AND PANEL CONSENSUS
Considerable controversy exists regarding the natural history of these diseases and, consequently, the indication for open surgery or TEVAR. Recent published guidelines [4] and a task force [6] discussed the three overlapping aortic lesions: intimal defect without IMH, intimal defect with IMH and IMH without an intimal defect. Thus, no specific recommendations were provided for the management in acute, subacute or chronic phases of these different entities or, more specifically, IMH complicated by ULP versus PAU surrounded by IMH.
Medical treatment was indicated in patients with an uncomplicated course, whereas endovascular or surgical therapy was generally considered in complicated cases. This issue is particularly important for understanding the obtained results and the subsequent recommendations of the expert panel. The strength of proposals provided by this document is limited because of the large heterogeneity among studies and the absence of randomized trials comparing TEVAR, with open surgery and medical therapy.
Aortic intramural haematoma
Results of the literature review showed that type B IMH presented a low mortality rate in the acute phase. However, persistent pain despite medical treatment, haemodynamic instability, MAD >55 mm and significant periaortic haemorrhage are predictors of acute-phase mortality. In these cases, invasive treatment is indicated. The mortality rate in acute phase in complicated cases treated with open surgery was 16% and with TEVAR 4.6%. However, these results may be biased owing to a tendency to report positive results of a novel technique. The main limitation of TEVAR in the acute phase is the high risk of secondary endoleaks and intimal ruptures or pseudoaneurysm formation, when the ends of the device are placed in the aortic wall affected by the IMH [21, 44, 49], secondary to mechanical stress and the pulsatile force acting on the stent ends [21, 50]. Sufficient landing zones, a minimum of 15 mm from the affected zone [60], are necessary and, in some cases, a considerable portion of the proximal and distal aorta needs to be covered [76]. Nevertheless, as aortic coverage length represents an independent predictor for spinal cord ischaemia, the risk of occlusion of segmental arteries is increased and should be prevented whenever possible. Usually, 10–20% oversizing with respect to the aortic diameter is recommended in order to promote aneurysm sac exclusion and avoid stent-graft migration. However, in a fragile aortic wall, such as in an IMH, a compromise between stent-graft fixation and the risk of iatrogenic dissection may indicate the use of inferior oversizing (not more than 10%) and the choice of the most flexible device. This concept is crucial when the haemorrhage is extended to the proximal aortic neck. Furthermore, caution has been advised against aggressive attempts to balloon-dilate landing zones to avoid stent-graft erosion into the aortic wall. When treatment of IMH in an acute stage is clinically necessary, e.g. when there is persistent pain with medical management, severe expansion >5 mm or signs of impending rupture, anchorage of the endograft in the noninvolved aortic wall is required. Thus, in these cases, open surgery performed by expert groups may remain preferable.
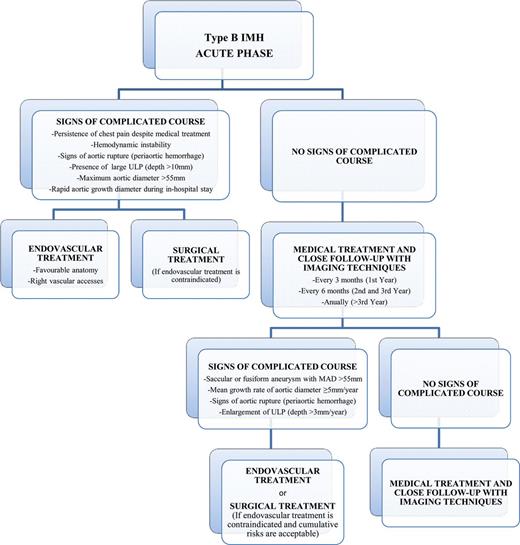
Acute and chronic management pathway for type B IMH. ULP: ulcer-like projection; MAD: maximum aortic diameter; IMH: intramural haematoma.
Penetrating aortic ulcer
The first step in choosing the correct management of an aortic ulcer is to distinguish PAU from ULP. Usually, in the context of an AAS, ULP is not detected in the first imaging study, since this lesion appears some days or weeks after of acute IMH. In contrast, when a PAU is the cause of AAS, it must be diagnosed in the first study. Some imaging findings could aid the differential diagnosis, e.g. the presence of atherosclerotic plaque and some morphological characteristics defined by multidetector CT or transoesophageal echocardiography (TEE) .
Generally, a PAU presents many irregularities in the intimal layer, with calcification of the ulcer edges, typical of atherosclerotic plaques, and could be accompanied by a haematoma localized around the lesion. On the other hand, a ULP is detected during the course of an IMH, and frequently appears as an image of intimal rupture with a small intimal flap. Unfortunately, differentiation of the two entities is not always possible owing to the rapid tempo of morphological evolution. Thus, depending on exactly when an imaging ‘snapshot’ is taken after the onset of symptoms, a ULP may be erroneously interpreted as a PAU.
Differentiation of both entities is crucial since a PAU surrounded by an IMH has a higher risk of aortic rupture than an IMH complicated with a ULP or localized dissection. A PAU with persistent pain, with an IMH or periaortic haemorrhage must be treated surgically or with TEVAR. In these cases, the lesion is frequently localized and permits endovascular treatment without the risk of intimal ruptures at the ends of the device. The mortality rate in the acute phase of open surgical treatment was 15.9 vs 7.2% of TEVAR. Considering that many patients are inoperable or run a prohibitively high open surgery risk, TEVAR may be an excellent therapeutic option.
In asymptomatic patients with a non-complicated PAU, general treatment recommendations have yet to be defined owing to the lack of reliable data concerning the natural course of the disease. Patients with PAU often have extensive arteriosclerotic disease, possibly including peripheral occlusive disease, which renders a suitable TEVAR access challenging. Therefore, meticulous examination of the access vessels is mandatory to achieve safe access. Although TEVAR has yielded favourable perioperative results, the available mid-term outcomes underline the significance of comorbidities: the 5-year survival rate is around 65% [48, 72]. Coronary artery disease, neurological complications and the risk of endoleak should be considered. Severe coronary disease is a common finding in patients with PAU, and cardiac complications are frequently observed after TEVAR. Thus, meticulous preoperative cardiological evaluation is warranted. Patients with thoracic aortic disease who undergo surgical or endovascular intervention and present symptoms or findings of myocardial ischaemia should be studied to determine whether significant coronary artery disease is present. Multidetector computed tomography coronarography or cardiac catheterization is advisable in preoperative evaluation of these patients. In cases of unstable coronary syndromes, revascularization prior to or at the time of surgical or endovascular aortic treatment is recommended [4]. The reported risk of stroke is similar for TEVAR and open surgery in the reviewed literature (4 vs 7%, respectively) [47, 60]. The use of TEE for monitoring the procedure may minimize the risk of embolic stroke caused by guide-wire manipulation in the presence of high-grade atheroma of the aortic arch and prevent secondary endoleaks due to laminated thrombi in stent-graft landing zones.
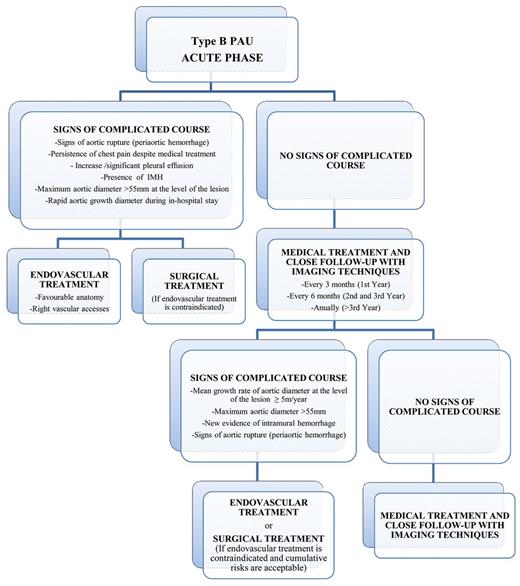
Acute and chronic management pathway for type B PAU. IMH: intramural haematoma; PAU: penetrating atherosclerotic ulcer.
CONCLUSIONS
Endovascular repair has become the first option in the treatment of complicated type B aortic PAU and IMH, mainly when lesions are localized and there are no constraints for endograft technology. The low mortality rate in published series and the high percentage of good results of stent-graft repair have been the keystones of this success. In comparison with open surgery results at centres of excellence, TEVAR seems to be a more accessible and less demanding treatment at centres where this technique is usually carried out. However, owing to the lack of randomized, controlled trials, some doubts remain, which may be resolved with the increasing use of these techniques in coming years.
Funding
The literature search was partially sponsored by an educational grant provided by Medtronic, Inc.
Conflict of interest: none declared.
REFERENCES
Author notes
The first two authors equally contributed to this work.
Sergio Moral was not a member of the Expert Panel, but participated in the literature review, results analysis and editing.




