-
PDF
- Split View
-
Views
-
Cite
Cite
Xin Xu, Hanzhang Chen, Weiqiang Yin, Wenlong Shao, Wei Wang, Guilin Peng, Jun Huang, Jianxing He, Initial experience of thoracoscopic lobectomy with partial removal of the superior vena cava for lung cancers, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 1, January 2015, Pages e8–e12, https://doi.org/10.1093/ejcts/ezu416
Close - Share Icon Share
Abstract
The objectives of this study were to report the surgical techniques and clinical outcome of thoracoscopic lobectomy with partial removal of the superior vena cava for lung carcinomas.
Between January 2010 and November 2013, 1132 patients with lung cancer underwent radical surgery by thoracoscopy; 5 (0.4%) underwent thoracoscopic lobectomy with partial removal of the superior vena cava. Perioperative variables and postoperative outcomes of these cases were analysed to evaluate the technical feasibility and safety of this operation.
For all cases, a right upper lobectomy was performed. The average time of surgery was 260 min (range, 170–380, 260 ± 90 min).The intraoperative blood loss averaged 160 ml (range, 50–300, 160 ± 90 ml). The median postoperative hospital stay was 11 days (interquartile range, 7–15 days). Postoperatively, tracheal extubation was achieved in the recovery room without further need for mechanical ventilation. In 1 case, the patient experienced postoperative superior vena cava thrombosis; he recovered after administration of anticoagulation drugs. None of the patients developed active blood leakage postoperatively. Perioperative mortality was not observed.
Thoracoscopic lobectomy with partial removal of the superior vena cava can be considered a feasible and safe operation for selected patients with lung cancer.
INTRODUCTION
Lung cancer is one of the leading causes of death worldwide. Surgery is still the first choice for selected patients with the most common type: non-small-cell lung cancer (NSCLC) [1]. NSCLC with invasion of the superior vena cava (SVC) is locally advanced lung cancer, and is generally considered a contraindication for surgery. However, several reports showed encouraging surgical results in select cases [2–4]. Generally, open thoracotomy was the surgery of choice in these cases.
Advances in the field of minimally invasive surgery have radically transformed thoracic surgical practice. In 1992, Lewis et al. demonstrated the technical feasibility of thoracoscopic lobectomy; however, whether thoracoscopy was a suitable approach for lung cancer surgery was still controversial [5]. McKenna et al. widely applied the combination of thoracoscopic lobectomy and lymph node dissection to surgical treatment of lung cancer, and demonstrated its efficacy and safety by achieving complete removal of the tumour [6].
Despite the reported advantages of thoracoscopic techniques (e.g. smaller incision, shorter hospital stay, reduced postoperative pain and bleeding, and less damage to lung function) [7–9], there was still a question as to whether video-assisted thoracoscopic surgery (VATS) was also feasible in NSCLC patients with invasion of the SVC. In the reported articles, VATS is most commonly used for peripheral lesions [10, 11]. For more complicated cases, such as lobectomy with bronchoplasty and/or removal of the pulmonary artery, the thoracoscopic approach has only been reported in a limited number of studies [12–15]. To the best of our knowledge, there are no previous reports of VATS for cases of NSCLC with invasion of the SVC.
From January 2010 to November 2013, we conducted VATS lobectomy with partial removal of the SVC for 5 patients with NSCLC. The objectives of this study were to report the surgical techniques and clinical outcome.
MATERIALS AND METHODS
Between January 2010 and November 2013, 1132 patients with lung cancer underwent radical surgery by thoracoscopy; 5 (0.4%) underwent thoracoscopic lobectomy with partial removal of the SVC. The surgical procedures were routinely recorded, and the analysis of the surgical data was based on the reviewing of these video clips.
Inclusion criteria
The inclusion criteria were as follows: (i) good general condition and generally normal function of organs (so they could tolerate surgery); (ii) thorough preoperative examinations including head, chest and abdominal CT and bone scintigraphy confirmed that there was no distant metastasis; non-N3-positive patients; N2-positive patients without obvious fusion of N2 lymph nodes or multi-site metastasis; limited local lymph node metastasis, which was surgically removable; (iii) involvement of the SVC did not exceed one-sixth of the perimeter; (iv) no surgical resection or reconstruction was needed due to the involvement of other organs.
Operative procedure
Combined intravenous anaesthesia was achieved using double-lumen tube intubation. A bronchoscope was used to ensure proper positioning of the endobronchial tubes in order to assure proper lung ventilation. Patients were positioned on the contralateral side with their waist elevated to allow for maximum width of the intercostal spaces. The surgical operating table was rotated to facilitate the surgery.
Overall, three incisions were made. The 1.2-cm-long camera incision was placed in the sixth or seventh intercostal space at the mid-axillary line. The 1.2-cm-long auxiliary incision was located in the same intercostal space at the posterior axillary line to minimize damage to the intercostal nerves. The main incision, 4–6 cm in length, was located in the fourth or fifth intercostal space between the anterior and posterior auxiliary line, and retracted with a soft incision protector (Fig. 1).
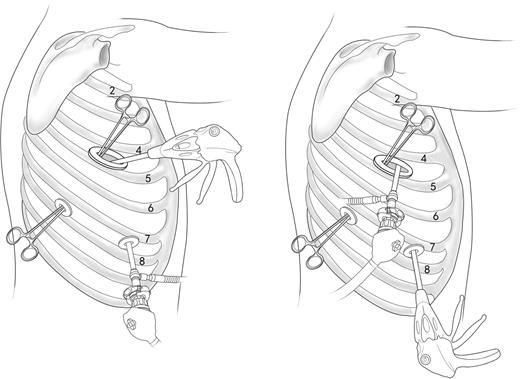
Incisions for the operation. A soft incision protector was used for the main incision which was located in the fourth or fifth intercostal space between the anterior and posterior auxiliary line. Two 1.2-cm-long incisions were placed in the sixth or seventh intercostal space at the middle auxiliary line and posterior auxiliary line individually. Position of the camera and endo-stapler could exchange positions depending on convenience of operation.
The pulmonary hilar structures were first dissected. The lobar veins, pulmonary arteries and bronchia were then dissected and cut using an endo-stapler (Echelon® 60 stapler, Ethicon Endo-Surgery, Inc., Blue Ash, OH, USA). The proximal and distal ends of the SVC were fully separated to accommodate an endo-stapler, so as to cut off the side wall of the vena cava (Fig. 2). A sealant, buttress or manual suture was not used to reinforce the stapler line over the SVC. The resected lobe was then extracted with a bag. Next, hilar and mediastinal lymph node dissection was conducted. A negative margin was confirmed by frozen-section diagnosis.
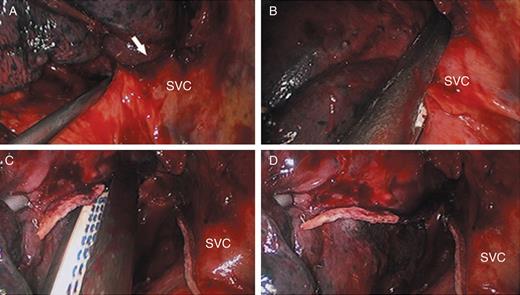
Details for removal of the partial wall of SVC. (A) the lesion invading the SVC; (B) the side wall of the SVC clamped by the endo-stapler; (C and D) the side wall of SVC cut out; SVC: superior vena cava; arrow: the SVC invaded by lung cancer.
Intraoperative and postoperative management
In all cases right upper lobectomy was performed. Two 28-Fr chest tubes were placed after the main procedure. One tube was placed through the observation incision with the tip above the costophrenic angle. Another tube was placed through the auxiliary operation incision, with the tip at the apex. The drainage tubes were removed once no air leak was observed when coughing, and the 24-h drainage volume was less than 150 ml. Before removal of the drainage tube, chest radiography was performed to confirm proper lung expansion. Postoperative anticoagulation drugs were not routinely administered.
RESULTS
The intra- and postoperative conditions are summarized in Table 1. All these patients were diagnosed with adenocarcinoma of the right upper lung, which was treated by right upper lobectomy. The average time of surgery was 260 min (range, 170–380, 260 ± 90 min). The average intraoperative blood loss was 160 ml (range: 50–300 ml), and the average hospital stay was 11 days (range: 7–15 days). Tracheal extubation was achieved in the recovery room. No patient underwent postoperative mechanical ventilation or further intubation. However, Case 2 suffered venous thrombosis of the SVC and its distal veins, which required anticoagulation therapy, and it ultimately subsided. There was no surgical active bleeding that required a second surgery. Also, no perioperative death was noted.
| Case no. . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Age | 74 | 62 | 65 | 62 | 70 |
| Sex | M | F | M | M | F |
| Clinical TNM | T1N2M0 | T1N2M0 | T4N0M0 | T4N2M0 | T4N0M0 |
| Disease | PLC | PLC | PLC | PLC | PLC |
| Histological type | Ad | Ad | Ad | Ad | Ad |
| Tumour location | RUL | RUL | RUL | RUL | RUL |
| Lung resection | Lobectomy | Lobectomy | Lobectomy | Lobectomy | Lobectomy |
| Cause of SVC partial resection | Lymph node invasion to SVC | Lymph node invasion to SVC | Direct tumour invasion to SVC | Direct tumour invasion to SVC | Direct tumour invasion to SVC |
| Pathological TNM | T1N2M0 | T2N2M0 | T4N0M0 | T4N2M0 | T4N0M0 |
| Time (min) | 330 | 200 | 380 | 170 | 220 |
| Blood loss (ml) | 50 | 120 | 300 | 180 | 170 |
| Outcome (month) | Dead, 33 | Alive, 25 | Alive, 40 | Alive, 7 | Alive, 11 |
| Case no. . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Age | 74 | 62 | 65 | 62 | 70 |
| Sex | M | F | M | M | F |
| Clinical TNM | T1N2M0 | T1N2M0 | T4N0M0 | T4N2M0 | T4N0M0 |
| Disease | PLC | PLC | PLC | PLC | PLC |
| Histological type | Ad | Ad | Ad | Ad | Ad |
| Tumour location | RUL | RUL | RUL | RUL | RUL |
| Lung resection | Lobectomy | Lobectomy | Lobectomy | Lobectomy | Lobectomy |
| Cause of SVC partial resection | Lymph node invasion to SVC | Lymph node invasion to SVC | Direct tumour invasion to SVC | Direct tumour invasion to SVC | Direct tumour invasion to SVC |
| Pathological TNM | T1N2M0 | T2N2M0 | T4N0M0 | T4N2M0 | T4N0M0 |
| Time (min) | 330 | 200 | 380 | 170 | 220 |
| Blood loss (ml) | 50 | 120 | 300 | 180 | 170 |
| Outcome (month) | Dead, 33 | Alive, 25 | Alive, 40 | Alive, 7 | Alive, 11 |
PLC: primary lung cancer; Ad: adenocarcinomas; RUL: right upper lobe.
| Case no. . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Age | 74 | 62 | 65 | 62 | 70 |
| Sex | M | F | M | M | F |
| Clinical TNM | T1N2M0 | T1N2M0 | T4N0M0 | T4N2M0 | T4N0M0 |
| Disease | PLC | PLC | PLC | PLC | PLC |
| Histological type | Ad | Ad | Ad | Ad | Ad |
| Tumour location | RUL | RUL | RUL | RUL | RUL |
| Lung resection | Lobectomy | Lobectomy | Lobectomy | Lobectomy | Lobectomy |
| Cause of SVC partial resection | Lymph node invasion to SVC | Lymph node invasion to SVC | Direct tumour invasion to SVC | Direct tumour invasion to SVC | Direct tumour invasion to SVC |
| Pathological TNM | T1N2M0 | T2N2M0 | T4N0M0 | T4N2M0 | T4N0M0 |
| Time (min) | 330 | 200 | 380 | 170 | 220 |
| Blood loss (ml) | 50 | 120 | 300 | 180 | 170 |
| Outcome (month) | Dead, 33 | Alive, 25 | Alive, 40 | Alive, 7 | Alive, 11 |
| Case no. . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Age | 74 | 62 | 65 | 62 | 70 |
| Sex | M | F | M | M | F |
| Clinical TNM | T1N2M0 | T1N2M0 | T4N0M0 | T4N2M0 | T4N0M0 |
| Disease | PLC | PLC | PLC | PLC | PLC |
| Histological type | Ad | Ad | Ad | Ad | Ad |
| Tumour location | RUL | RUL | RUL | RUL | RUL |
| Lung resection | Lobectomy | Lobectomy | Lobectomy | Lobectomy | Lobectomy |
| Cause of SVC partial resection | Lymph node invasion to SVC | Lymph node invasion to SVC | Direct tumour invasion to SVC | Direct tumour invasion to SVC | Direct tumour invasion to SVC |
| Pathological TNM | T1N2M0 | T2N2M0 | T4N0M0 | T4N2M0 | T4N0M0 |
| Time (min) | 330 | 200 | 380 | 170 | 220 |
| Blood loss (ml) | 50 | 120 | 300 | 180 | 170 |
| Outcome (month) | Dead, 33 | Alive, 25 | Alive, 40 | Alive, 7 | Alive, 11 |
PLC: primary lung cancer; Ad: adenocarcinomas; RUL: right upper lobe.
DISCUSSION
Lung cancer invading the SVC is a locally advanced condition, for which poor prognosis is expected with conservative treatment alone. Combined treatment with surgery can rapidly relieve the symptoms and significantly improve survival for some patients, though long-term survivors are not uncommon. Surgeons are devoted to pursuing approaches to better disease management while minimizing trauma to the patient, i.e. smaller incisions. To the best of our knowledge, this is the first report of treatment for invasion of the SVC under complete thoracoscopy. Since there have been few surgical procedures of this kind, the methods and techniques are yet to be explored.
In the past, for cases of lung cancer invading the SVC, resection and replacement of the SVC (including simple removal and replacement of the SVC, and replacement of bilateral innominate veins), repair of the SVC and partial resection of the SVC wall followed by direct closure via traditional open surgery has been the method of choice [16]. When removing the invaded section of the SVC, if the diameter is not less than half of the original after closure, a direct suture can be applied. Otherwise, the local vessel may be too narrow for blood flow following direct suture and therefore be prone to the formation of blood clots. In this case, repair with autologous pericardial patches is preferred. For patients with a larger section needing removal, vessel replacement should be considered. In this study, another type of direct suture, a mechanical suture, was used for the removal. Traditionally, the removal of the SVC requires occlusion of a part of the side wall by a clamp or complete occlusion of the vessel from the proximal to distal ends. With thoracoscopic suturing, a number of instruments go through a smaller incision, and suturing is completed using a needle; the combination of these factors makes it extremely difficult to complete the operation. The most important feature of this surgery is the use of a mechanical automatic suture for the side wall of the SVC to avoid blocking and hand stitching of the vessel—this is the important factor hindering reconstruction under thoracoscopy even though the difficulty of the surgery is reduced.
The method used in this study has two key technical concerns: first, how to ensure safe resection without bleeding margins of the side wall, and second, how to ensure thoracoscopic resection with negative margins while maintaining sufficient blood flow in the residual vessel.
Regarding the first technical issue, in thoracoscopic lung surgery, cutting blood vessels of different diameters and thicknesses with a stapler, such as the pulmonary artery, pulmonary vein or even azygos vein, is a common operation. Therefore, we do not worry about leakage of blood from cut margins if they can be properly stapled. However, in our cases, we often select lesions that can be removed with only one staple cartridge, because of the concern that the use of more cartridges may result in bleeding if the connection of any two cartridges is not well positioned. Because of the exploratory nature of this surgery, we found it prudent to select patients with a smaller length of SVC invasion. From a technical point of view, resection of the side wall of the SVC with a stapler is essentially the same as handling the pulmonary artery, pulmonary vein or even azygos vein.
In view of our experience with operations on the latter, we did not administer systemic heparin during the surgery or routine anticoagulation after it. Case 2 suffered venous thrombosis of the SVC and its distal veins, which required anticoagulation therapy, and ultimately subsided, after which we administered a subcutaneous injection of low molecular weight heparin daily postoperatively until discharge, but no routine warfarin or aspirin was prescribed afterwards.
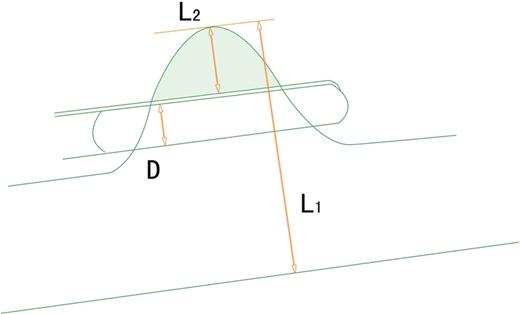
Relation between the lesion, SVC and endo-stapler. L1 = 1/2 circumference = 1/2 (π × diameter of superior the vena cava); L2 = 1/2 width of the invaded SVC; D = width of the endo-stapler, 1/2 ≥ (D + L2)/L1, 1/2 − D/L1 ≥ L2/L1, 1/2 − D/πr ≥ L2/L1. The width of the endo-stapler is ∼1 cm and the diameter of the SVC of the Chinese population is ∼2 cm (r ≈ 1 cm), so L2/L1 is simplifies to less than 1/6; SVC: superior vena cava.
The stapler width is ∼1 cm; in the Chinese population, the diameter of the SVC is ∼2 cm. Therefore, invasion of the vessel should generally be less than one-sixth of the diameter for a patient to be eligible for the operation. This range can be further expanded if a more delicate stapler is used or a patient has a larger body size.
Thoracoscopic resection of SVC lesions is indeed a difficult surgical procedure. However, as can be seen from the evolution of thoracoscopic lobectomy and lymph node dissection for the treatment of lung cancer, the initiation of a certain procedure has always been accompanied by difficulty. With intensified training and increased number of cases, they have become widely and commonly performed procedures. Despite difficulty, thoracoscopic resection of the invaded vessel can be performed safely following a certain amount of training.
Needless to say, this method does have its limitations. As previously described, the selection of patients is associated with vessel thickness and stapler width. In the event of a small SVC with extensive invasion, the procedure is not applicable; then the retained SVC will be smaller than half of the original diameter.
In summary, we believe that complete thoracoscopic resection of the SVC side wall is feasible in selected cases of lung cancer with invasion of the SVC. Mechanical stitching is a safe and easy option for thoracoscopic resection of the vessel.
Conflict of interest: none declared.
REFERENCES
- anticoagulants
- thrombosis
- superior vena cava
- objective (goal)
- postoperative anesthesia care unit
- safety
- surgical procedures, operative
- thoracoscopy
- treatment outcome
- lung cancer
- lobectomy
- extubation
- mechanical ventilation
- carcinoma of lung
- intraoperative hemorrhage
- right upper lung lobectomy
- surgical mortality
- surgical outcome




