-
PDF
- Split View
-
Views
-
Cite
Cite
Thorsten Drews, Miralem Pasic, Semih Buz, Stephan Dreysse, Christoph Klein, Marian Kukucka, Alexander Mladenow, Roland Hetzer, Axel Unbehaun, Elective use of femoro-femoral cardiopulmonary bypass during transcatheter aortic valve implantation, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 1, January 2015, Pages 24–30, https://doi.org/10.1093/ejcts/ezu088
Close - Share Icon Share
Abstract
Elective use of normothermic cardiopulmonary bypass (CPB) may reduce the risks associated with the transcatheter aortic valve implantation (TAVI) procedure in selected high-risk TAVI patients.
Between April 2008 and August 2013, 1177 consecutive patients underwent TAVI. Elective normothermic femoro-femoral CPB was used in 3.7% of patients (n = 43, 27 males, 16 females; mean age 75 ± 10 [range 38–90] years). The EuroSCORE I was 65 ± 23%, the EuroSCORE II was 39 ± 24% and the Society of Thoracic Surgeons Predicted Risk of Mortality score was 31 ± 24%. The mean left ventricular ejection fraction (LVEF) was 24 ± 12% (range 5–50%).
The device success rate (Valve Academic Research Consortium-2 criteria) was 98% in this study group. The median duration of CPB was 20 (range 5–297) min. In 20 patients with pulmonary hypertension combined with an enlarged right ventricle (RV), or with poor RV ejection fraction or LVEF (mean LVEF: 18 ± 3% [range 10–20%]), CPB was used to prevent haemodynamic instability during valve deployment and to eliminate the adverse effects of possible ventricular fibrillation. Additionally, it was used to promote cardiac recovery by unloaded failing hearts in 23 patients (53%) with cardiogenic shock. Whereas the 30-day mortality rate in the group of patients in cardiogenic shock was 28.6%, no patient in the other group died. The 1-year survival rate was 36 ± 11 and 86 ± 9.5%, respectively.
The use of preoperatively planned CPB may increase the safety of the TAVI procedure in patients with severely reduced heart function or in cardiogenic shock.
INTRODUCTION
As shown in an earlier publication in 2013 [1], elective femoro-femoral cardiopulmonary bypass (CPB) during transcatheter aortic valve implantation (TAVI) may make this procedure safe and reproducible in patients with severely depressed myocardial function (ejection fraction [EF] <20%), in cardiogenic shock or with concomitant heart disease.
Although Walther et al. [2] already showed in 2007 that transapical aortic valve implantation might reduce the risk of conventional surgical aortic valve replacement in very high-risk patients, some authors recommend performing this new procedure only in patients with an Society of Thoracic Surgeons (STS) mortality score <20% and a EuroSCORE <40% and in those with an expected survival of over 1 year [3]. Therefore, some authors recommend balloon aortic valvuloplasty [4] mostly as a bridge to TAVI or as palliative treatment. However, the prognosis of these patients is grave with an in-hospital mortality rate of up to 56.5% [4, 5]. Other authors recommend performing TAVI in patients in cardiogenic shock, reporting a possible reduction in the 30-day mortality rate of up to 19% [6].
The use of CPB may represent a feasible therapeutic option for very high-risk patients with cardiogenic shock, depressed left (LV) or right ventricular (RV) function or severe concomitant disease. In this study, we examine the surgical procedure and the postoperative course of patients undergoing TAVI on CPB.
PATIENTS AND METHODS
Patients
TAVI was performed in 1177 patients between April 2008 and August 2011 at the Deutsches Herzzentrum Berlin, Germany. In 792 patients, an Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) and in 385 patients a CoreValve (Medtronic, Minneapolis, MN, USA) was implanted. The approach was chosen on the basis of the individual patient's risk profile, being either transapical (63%), transfemoral (35%), transaxillary, transaortic or through the apical descending conduit (2%). For patients undergoing reoperation, the transapical route was primarily chosen because it enables more precise handling and positioning of the valve.
Patient cohort
A total of 63 patients received elective CPB during the TAVI procedure due to preoperative cardiogenic shock (25 patients), severely depressed LV (17 patients) or RV function (2 patients), or pulmonary hypertension with an enlarged RV (2 patients). Additionally, due to the absolute need for CPB during open heart surgery, its use was required in 11 patients during valve implantation through aortotomy (due to severe calcification of the aortic root and leaflets, which makes fixation of the prosthesis with stitches difficult and carries a high risk for annulus rupture during conventional TAVI), and in 6 patients due to a concomitant surgical procedure (e.g. mitral and tricuspid valve reconstruction, coronary artery bypass grafting). To ensure that only patients with elective use of CPB due to cardiogenic shock and/or depressed LV or RV function were included in the study, our study cohort consisted of 43 patients with the following features: preoperative cardiogenic shock (n = 23, 53%), severely depressed LV (n = 16, 37%) or RV (n = 2, 5%) function and pulmonary hypertension with enlarged RV (n = 2, 5%). The cohort consisted of 27 males and 16 females with a mean age of 75 ± 10 (range 38–90) years. The mean LVEF was 24 ± 12% (range 5–50%). The EuroSCORE I was 65 ± 23%, the EuroSCORE II 39 ± 24% and the Society of Thoracic Surgeons Predicted Risk of Mortality score 31 ± 24% (Table 1).
| . | Patients . |
|---|---|
| Number of patients (n) | 43 |
| Cardiogenic shock | 23 (53%) |
| Sex (male/female) | 27/16 |
| Age (years) | 75 ± 10 (38–90) |
| Weight (kg) | 77 ± 15 (53–115) |
| Body mass index | 27 ± 4 (19–43) |
| Comorbidity | |
| Pulmonary hypertension (systolic >60 mmHg) | 31 (72%) |
| Coronary heart disease | 30 (70%) |
| Peripheral vascular disease | 23 (53%) |
| Chronic obstructive pulmonary diseasea | 19 (44%) |
| Diabetes mellitus | 17 (40%) |
| Previous surgery | |
| Coronary artery bypass grafting | 11 (26%) |
| Other | 6 (14%) |
| Mitral valve surgery | 5 (12%) |
| Aortic valve replacement | 4 (9%) |
| Laboratory data | |
| Creatinine (mg/dl) | 1.4 (0.7–8.5) |
| Pro-brain natriuretic peptide (pg/ml) | 10 576 (2159–112 716) |
| Preoperative risk score | |
| EuroSCORE I (logistic, %) | 65 ± 23 (4–97) |
| EuroSCORE II (%) | 39 ± 24 (6–88) |
| STS mortality score (%) | 31 ± 24 (5–90) |
| . | Patients . |
|---|---|
| Number of patients (n) | 43 |
| Cardiogenic shock | 23 (53%) |
| Sex (male/female) | 27/16 |
| Age (years) | 75 ± 10 (38–90) |
| Weight (kg) | 77 ± 15 (53–115) |
| Body mass index | 27 ± 4 (19–43) |
| Comorbidity | |
| Pulmonary hypertension (systolic >60 mmHg) | 31 (72%) |
| Coronary heart disease | 30 (70%) |
| Peripheral vascular disease | 23 (53%) |
| Chronic obstructive pulmonary diseasea | 19 (44%) |
| Diabetes mellitus | 17 (40%) |
| Previous surgery | |
| Coronary artery bypass grafting | 11 (26%) |
| Other | 6 (14%) |
| Mitral valve surgery | 5 (12%) |
| Aortic valve replacement | 4 (9%) |
| Laboratory data | |
| Creatinine (mg/dl) | 1.4 (0.7–8.5) |
| Pro-brain natriuretic peptide (pg/ml) | 10 576 (2159–112 716) |
| Preoperative risk score | |
| EuroSCORE I (logistic, %) | 65 ± 23 (4–97) |
| EuroSCORE II (%) | 39 ± 24 (6–88) |
| STS mortality score (%) | 31 ± 24 (5–90) |
Data are presented as [n]/% or mean ± SD, range or median, range.
SD: standard deviation.
aLong-term use of bronchodilators or steroids for lung disease.
| . | Patients . |
|---|---|
| Number of patients (n) | 43 |
| Cardiogenic shock | 23 (53%) |
| Sex (male/female) | 27/16 |
| Age (years) | 75 ± 10 (38–90) |
| Weight (kg) | 77 ± 15 (53–115) |
| Body mass index | 27 ± 4 (19–43) |
| Comorbidity | |
| Pulmonary hypertension (systolic >60 mmHg) | 31 (72%) |
| Coronary heart disease | 30 (70%) |
| Peripheral vascular disease | 23 (53%) |
| Chronic obstructive pulmonary diseasea | 19 (44%) |
| Diabetes mellitus | 17 (40%) |
| Previous surgery | |
| Coronary artery bypass grafting | 11 (26%) |
| Other | 6 (14%) |
| Mitral valve surgery | 5 (12%) |
| Aortic valve replacement | 4 (9%) |
| Laboratory data | |
| Creatinine (mg/dl) | 1.4 (0.7–8.5) |
| Pro-brain natriuretic peptide (pg/ml) | 10 576 (2159–112 716) |
| Preoperative risk score | |
| EuroSCORE I (logistic, %) | 65 ± 23 (4–97) |
| EuroSCORE II (%) | 39 ± 24 (6–88) |
| STS mortality score (%) | 31 ± 24 (5–90) |
| . | Patients . |
|---|---|
| Number of patients (n) | 43 |
| Cardiogenic shock | 23 (53%) |
| Sex (male/female) | 27/16 |
| Age (years) | 75 ± 10 (38–90) |
| Weight (kg) | 77 ± 15 (53–115) |
| Body mass index | 27 ± 4 (19–43) |
| Comorbidity | |
| Pulmonary hypertension (systolic >60 mmHg) | 31 (72%) |
| Coronary heart disease | 30 (70%) |
| Peripheral vascular disease | 23 (53%) |
| Chronic obstructive pulmonary diseasea | 19 (44%) |
| Diabetes mellitus | 17 (40%) |
| Previous surgery | |
| Coronary artery bypass grafting | 11 (26%) |
| Other | 6 (14%) |
| Mitral valve surgery | 5 (12%) |
| Aortic valve replacement | 4 (9%) |
| Laboratory data | |
| Creatinine (mg/dl) | 1.4 (0.7–8.5) |
| Pro-brain natriuretic peptide (pg/ml) | 10 576 (2159–112 716) |
| Preoperative risk score | |
| EuroSCORE I (logistic, %) | 65 ± 23 (4–97) |
| EuroSCORE II (%) | 39 ± 24 (6–88) |
| STS mortality score (%) | 31 ± 24 (5–90) |
Data are presented as [n]/% or mean ± SD, range or median, range.
SD: standard deviation.
aLong-term use of bronchodilators or steroids for lung disease.
Pre- and postoperative evaluation
The pre- and postoperative examinations included clinical and laboratory examinations, electrocardiography (ECG), chest X-ray, transoesophageal echocardiography (TEE) and multislice computed tomography of the chest and pelvis followed by vascular 3D reconstruction. Preoperative coronary angiography and ultrasound examinations (Doppler) of the arteries and veins of the lower extremities and of the carotid arteries were performed. The Doppler examination allowed the detection of severe peripheral vascular disease. The echocardiographic data, the postoperative course, potential complications and the late outcome were followed.
Aims of the elective use of cardiopulmonary bypass for transcatheter aortic valve implantation
As shown in 2013 [1], we consider the heart–lung machine to be a useful tool during TAVI. Therefore, our first-line strategy in patients with a small distance between the coronary ostia and the aortic annulus or in patients with severe pulmonary hypertension is to place the connecting tubes on the table to save time should complications occur. In patients with markedly reduced LV function (i.e. LVEF <25%) or severe regurgitation of the atrioventricular valves, we prefer prophylactic cannulation of the femoral vessels without starting up CPB. If haemodynamic instability (which is refractory to high-dose catecholamine administration) occurs, the heart–lung machine can be started within 1 s by simply removing the clamps on the connecting tubes. Our third strategy with valvuloplasty and valve deployment under a short duration of CPB is considered at our institution in patients with poor LV performance (LVEF 10–20%), in cardiogenic shock and with decompensated right-sided heart failure with enlarged RV. Primary use of CPB instead of its secondary use on an emergency basis may provide superior results, especially due to the fact that the progression of rapid pacing into ventricular fibrillation is often difficult to manage.
The aim of the CPB is to minimize potential complications. Therefore, it is used to stabilize the patients' condition, to achieve haemodynamic stability during TAVI, and to avoid the possible need for manual cardiopulmonary resuscitation if ventricular fibrillation should occur during rapid pacing for balloon valvuloplasty and during valve deployment in patients with severely depressed myocardial function. In patients with cardiogenic shock, CPB is used for additional myocardial recovery of the unloaded heart after the new transcatheter valve is deployed [7].
Procedural and technical considerations
Immediately before the procedure, the whole team analysed the diagnostic work-up and discussed possible technical difficulties and complications and the means to prevent them. Elective coronary artery stent implantation was considered in patients with concomitant coronary artery disease. Only the most relevant coronary artery stenosis was considered to be a target for stent implantation [8, 9].
The surgical technique of transcatheter valve implantation was based on the procedure described by several authors [2, 7, 10] with the modification of transcatheter valve positioning and liberation under simultaneous angiography with contrast medium in order to find the optimal valve position and to reduce the risk of paravalvular leakage [11]. The procedure was monitored by fluoroscopy, angiography and intraoperative TEE.
Cannulation for elective cardiopulmonary bypass
The use of normothermic femoro-femoral CPB was routinely considered to provide a higher safety level during the procedure, and especially to obviate manual cardiopulmonary resuscitation if ventricular fibrillation occurs during TAVI. We routinely used an open surgical approach to the femoral vessels, using for cannulation a 15 French cannula for the common femoral artery and a 23 French cannula for the femoral vein (‘as small as possible’) in order to neutralize the risk of vascular complications [1]. In the case of severe calcification, the axillary artery was cannulated. A small (2–3 cm) incision in the groin, parallel to the ligamentum inguinale Pouparti, was chosen (and not a classical vascular access to the femoral vessels). This incision enables easier identification and faster and only limited dissection of the vessels, but special attention was given to the ligation (by metallic clips) of the small lymphatic tracts in order to prevent postoperative lymphatic fistulas. In the case of the transfemoral approach for TAVI, the artery of better quality was used for the TAVI procedure and the other site for CPB cannulation. A fully percutaneous approach was not used in order to exclude possible negative aspects of the procedure itself and to achieve optimal access and haemostasis [1].
Technical consideration of the transcatheter aortic valve implantation procedure during elective cardiopulmonary bypass
The introduction of the guide-wires and introducers through the native valve or through the LV apex (in the case of transapical TAVI) into the LV was performed while a slight filling of the heart was achieved by reducing the CPB drainage to maintain LV ejection and facilitate initial passage of the guide-wire through the stenotic aortic valve. If complete unloading of the heart was possible, balloon dilatation of the native valve and valve deployment were performed without rapid pacing in order to avoid possible ventricular fibrillation. If complete drainage and unloading of the heart was not possible, rapid pacing was used for balloon valvuloplasty and valve release, and the LV was drained as much as possible to prevent heart distension and ventricular fibrillation. Once adequate deployment and function of the prosthesis had been achieved, in the case of transapical TAVI, the LV purse strings were tied and haemostasis was achieved. The prophylactic trans-femoral placement of an intra-aortic balloon pump (IABP) was considered in patients with poor LVEF and/or in cardiogenic shock to secure greater safety and haemodynamic stability during the immediate postoperative course [1, 10]. Then the patient was weaned off CPB.
Statistical evaluation
All data analyses were performed using Excel for Office 2010 and PASW Statistics, version 18.0 (SPSS, Inc., Chicago, IL, USA). The mean, median, standard deviation, and the maximum and minimum were calculated.
RESULTS
In all patients the technical procedure was performed using the standard technique [12]; in total, 3 patients received a CoreValve (1 patient a 26 mm and 2 patients a 29 mm valve) and 40 patients an Edwards SAPIEN valve (6 patients a 23 mm, 18 patients a 26 mm and 16 patients a 29 mm valve) (Table 2).
| . | Patients . |
|---|---|
| Number of patients (n) | 43 |
| CoreValve | 3 |
| Size: 26/29/31 mm | 1/2/- |
| Edwards SAPIEN valve | 40 |
| Size: 23/26/29 mm | 6/18/16 |
| Intra-aortic balloon pump | |
| Preoperative | 2 (4.7%) |
| Postoperative | 7 (16%) |
| Pacemaker implantation | |
| DDD/VVI | 1/2 (7%) |
| Reason for CPB | |
| Cardiogenic shock | 23 (53%) |
| Impaired LV function | 16 (37%) |
| Impaired RV function | 2 (5%) |
| Pulmonary hypertension and enlarged RV | 2 (5%) |
| CPB bypass time (min) | |
| All patients | 20 (5–297) |
| Patients with cardiogenic shock | 41 (11–297) |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 14 (6–40) |
| . | Patients . |
|---|---|
| Number of patients (n) | 43 |
| CoreValve | 3 |
| Size: 26/29/31 mm | 1/2/- |
| Edwards SAPIEN valve | 40 |
| Size: 23/26/29 mm | 6/18/16 |
| Intra-aortic balloon pump | |
| Preoperative | 2 (4.7%) |
| Postoperative | 7 (16%) |
| Pacemaker implantation | |
| DDD/VVI | 1/2 (7%) |
| Reason for CPB | |
| Cardiogenic shock | 23 (53%) |
| Impaired LV function | 16 (37%) |
| Impaired RV function | 2 (5%) |
| Pulmonary hypertension and enlarged RV | 2 (5%) |
| CPB bypass time (min) | |
| All patients | 20 (5–297) |
| Patients with cardiogenic shock | 41 (11–297) |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 14 (6–40) |
Data are presented as [n]/% or mean ± SD, range or median, range.
CPB: cardiopulmonary bypass; DDD: dual chamber pacemaker; LV: left ventricular; RV: right ventricular; VVI: ventricular pacemaker.
| . | Patients . |
|---|---|
| Number of patients (n) | 43 |
| CoreValve | 3 |
| Size: 26/29/31 mm | 1/2/- |
| Edwards SAPIEN valve | 40 |
| Size: 23/26/29 mm | 6/18/16 |
| Intra-aortic balloon pump | |
| Preoperative | 2 (4.7%) |
| Postoperative | 7 (16%) |
| Pacemaker implantation | |
| DDD/VVI | 1/2 (7%) |
| Reason for CPB | |
| Cardiogenic shock | 23 (53%) |
| Impaired LV function | 16 (37%) |
| Impaired RV function | 2 (5%) |
| Pulmonary hypertension and enlarged RV | 2 (5%) |
| CPB bypass time (min) | |
| All patients | 20 (5–297) |
| Patients with cardiogenic shock | 41 (11–297) |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 14 (6–40) |
| . | Patients . |
|---|---|
| Number of patients (n) | 43 |
| CoreValve | 3 |
| Size: 26/29/31 mm | 1/2/- |
| Edwards SAPIEN valve | 40 |
| Size: 23/26/29 mm | 6/18/16 |
| Intra-aortic balloon pump | |
| Preoperative | 2 (4.7%) |
| Postoperative | 7 (16%) |
| Pacemaker implantation | |
| DDD/VVI | 1/2 (7%) |
| Reason for CPB | |
| Cardiogenic shock | 23 (53%) |
| Impaired LV function | 16 (37%) |
| Impaired RV function | 2 (5%) |
| Pulmonary hypertension and enlarged RV | 2 (5%) |
| CPB bypass time (min) | |
| All patients | 20 (5–297) |
| Patients with cardiogenic shock | 41 (11–297) |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 14 (6–40) |
Data are presented as [n]/% or mean ± SD, range or median, range.
CPB: cardiopulmonary bypass; DDD: dual chamber pacemaker; LV: left ventricular; RV: right ventricular; VVI: ventricular pacemaker.
Reason for cardiopulmonary bypass
In all 43 cases, CPB was used: in 23 patients (53%) due to preoperative cardiogenic shock, in 16 (37%) due to impaired LV function, in 2 patients (5%) because of severely impaired RV function and in 2 patients (5%) because of pulmonary hypertension (Table 2).
The total median bypass time was 20 min (range 5–297). In patients with cardiogenic shock, the median bypass time was 42 min (range 5–297), whereas the median bypass time in patients with impaired LV or RV function or with severe pulmonary hypertension combined with an enlarged ventricle was significantly shorter at 14 min (range 6–40). An IABP was placed in 2 patients before and in 7 patients (16%) after the procedure (Table 2).
Echocardiographic data
Preoperatively, the echocardiographic data showed that the LV function was significantly reduced (EF 24 ± 12% [range 5–50%] and that the LV end-diastolic diameter was high (59 ± 9 mm [range 38–80 mm]). In all patients with an EF ≤20%, the procedure was performed with elective use of CPB (n = 16). In these patients the mean LVEF was 18 ± 3% (range 10–20). In patients presenting with catecholamine-dependent cardiogenic shock (n = 23), the LVEF was 24 ± 14% (range 5–50). In all patients the aortic valve orifice area was significantly reduced (0.7 ± 0.2 cm2 [range 0.3–1.8 cm2]), except in 1 patient with homograft degeneration (orifice area 1.8 cm2) and severe valve insufficiency (Table 3).
| . | Patients . |
|---|---|
| Preoperative | |
| LV end-diastolic diameter (mm) | 59 ± 9 (38–80) |
| LV ejection fraction (%) | 24 ± 12 (5–50) |
| Aortic valve | |
| Insufficiency (degree) | 1.2 ± 0.9 (0–4) |
| Orifice area (cm2) | 0.7 ± 0.3 (0.3–1.8) |
| Diameter of aortic annulus (mm) | 24 ± 2 (19–28) |
| Gradient max (mmHg) | 50 ± 21 (10–90) |
| Gradient mean (mmHg) | 33 ± 16 (8–70) |
| Mitral valve | |
| Insufficiency (degree) | 1.3 ± 0.7 (0–3) |
| Tricuspid valve | |
| Insufficiency (degree) | 1.3 ± 0.9 (0–4) |
| Postoperative | |
| Central aortic valve insufficiency | 2 (5%) |
| Grade 0–I | 1 |
| Grade I | 1 |
| Grade I–II | |
| Grade II | |
| Paravalvular aortic valve insufficiency | 22 (51%) |
| Grade 0–I | 9 |
| Grade I | 7 |
| Grade I–II | 5 |
| Grade II | 1 |
| Aortic valve gradient | |
| Max (mmHg) | 8.8 ± 4.3 (3–26) |
| Mean (mmHg) | 4.4 ± 2.5 (1–15) |
| . | Patients . |
|---|---|
| Preoperative | |
| LV end-diastolic diameter (mm) | 59 ± 9 (38–80) |
| LV ejection fraction (%) | 24 ± 12 (5–50) |
| Aortic valve | |
| Insufficiency (degree) | 1.2 ± 0.9 (0–4) |
| Orifice area (cm2) | 0.7 ± 0.3 (0.3–1.8) |
| Diameter of aortic annulus (mm) | 24 ± 2 (19–28) |
| Gradient max (mmHg) | 50 ± 21 (10–90) |
| Gradient mean (mmHg) | 33 ± 16 (8–70) |
| Mitral valve | |
| Insufficiency (degree) | 1.3 ± 0.7 (0–3) |
| Tricuspid valve | |
| Insufficiency (degree) | 1.3 ± 0.9 (0–4) |
| Postoperative | |
| Central aortic valve insufficiency | 2 (5%) |
| Grade 0–I | 1 |
| Grade I | 1 |
| Grade I–II | |
| Grade II | |
| Paravalvular aortic valve insufficiency | 22 (51%) |
| Grade 0–I | 9 |
| Grade I | 7 |
| Grade I–II | 5 |
| Grade II | 1 |
| Aortic valve gradient | |
| Max (mmHg) | 8.8 ± 4.3 (3–26) |
| Mean (mmHg) | 4.4 ± 2.5 (1–15) |
Data are presented as mean ± SD, range.
LV: left ventricular.
| . | Patients . |
|---|---|
| Preoperative | |
| LV end-diastolic diameter (mm) | 59 ± 9 (38–80) |
| LV ejection fraction (%) | 24 ± 12 (5–50) |
| Aortic valve | |
| Insufficiency (degree) | 1.2 ± 0.9 (0–4) |
| Orifice area (cm2) | 0.7 ± 0.3 (0.3–1.8) |
| Diameter of aortic annulus (mm) | 24 ± 2 (19–28) |
| Gradient max (mmHg) | 50 ± 21 (10–90) |
| Gradient mean (mmHg) | 33 ± 16 (8–70) |
| Mitral valve | |
| Insufficiency (degree) | 1.3 ± 0.7 (0–3) |
| Tricuspid valve | |
| Insufficiency (degree) | 1.3 ± 0.9 (0–4) |
| Postoperative | |
| Central aortic valve insufficiency | 2 (5%) |
| Grade 0–I | 1 |
| Grade I | 1 |
| Grade I–II | |
| Grade II | |
| Paravalvular aortic valve insufficiency | 22 (51%) |
| Grade 0–I | 9 |
| Grade I | 7 |
| Grade I–II | 5 |
| Grade II | 1 |
| Aortic valve gradient | |
| Max (mmHg) | 8.8 ± 4.3 (3–26) |
| Mean (mmHg) | 4.4 ± 2.5 (1–15) |
| . | Patients . |
|---|---|
| Preoperative | |
| LV end-diastolic diameter (mm) | 59 ± 9 (38–80) |
| LV ejection fraction (%) | 24 ± 12 (5–50) |
| Aortic valve | |
| Insufficiency (degree) | 1.2 ± 0.9 (0–4) |
| Orifice area (cm2) | 0.7 ± 0.3 (0.3–1.8) |
| Diameter of aortic annulus (mm) | 24 ± 2 (19–28) |
| Gradient max (mmHg) | 50 ± 21 (10–90) |
| Gradient mean (mmHg) | 33 ± 16 (8–70) |
| Mitral valve | |
| Insufficiency (degree) | 1.3 ± 0.7 (0–3) |
| Tricuspid valve | |
| Insufficiency (degree) | 1.3 ± 0.9 (0–4) |
| Postoperative | |
| Central aortic valve insufficiency | 2 (5%) |
| Grade 0–I | 1 |
| Grade I | 1 |
| Grade I–II | |
| Grade II | |
| Paravalvular aortic valve insufficiency | 22 (51%) |
| Grade 0–I | 9 |
| Grade I | 7 |
| Grade I–II | 5 |
| Grade II | 1 |
| Aortic valve gradient | |
| Max (mmHg) | 8.8 ± 4.3 (3–26) |
| Mean (mmHg) | 4.4 ± 2.5 (1–15) |
Data are presented as mean ± SD, range.
LV: left ventricular.
Postoperatively, the transvalvular gradient was low, due to the special design of the valve. Central and paravalvular regurgitation is a common feature of transcatheter valve implantation. Therefore, it is not surprising that postoperative echocardiographic examinations showed slight central incompetence in 2 patients in the group (5%) and minimal paravalvular leakage in 22 (41%). The paravalvular regurgitation was mostly of Grade I or less; only in a small number of patients was it between Grades I and II. One patient had Grade II insufficiency. This was a patient in cardiogenic shock with multiorgan failure. TAVI was performed as an ultima ratio procedure. Preoperatively, his aortic valve had shown severe calcification with high risk for annular rupture.
Device success rate (Valve Academic Research Consortium-2)
The Valve Academic Research Consortium (VARC-2) criteria were used to provide standardized endpoints for TAVI procedures [13]. The technical success rate was 98% (42/43). One patient who presented with preoperative cardiogenic shock could not be weaned from CPB, although intraoperatively a stenosis of the right coronary ostium was identified and treated with stent implantation. Finally, a perioperative myocardial infarction was assumed (Table 4).
| . | Patients . |
|---|---|
| Standardized endpoints (VARC-2) | |
| Device success | |
| All patients | 42/43 (98%) |
| Patients with cardiogenic shock | 22/23 (98%) |
| Patients with impaired LV or RV function, pulmonary hypertension with enlarged RV | 20/20 (100%) |
| Safety <30 days | |
| All patients | 34/43 (79%) |
| Patients with cardiogenic shock | 15/23 (65%) |
| Patients with impaired LV or RV function, pulmonary hypertension with enlarged RV | 19/20 (95%) |
| Time of hospitalization (days) | |
| Length of stay in intensive care unit | 3.2 (0.3–66) |
| Total length of stay | 11.8 (2.7–103) |
| Thirty-day mortality | |
| All patients | 6 (14%) |
| Patients with cardiogenic shock | 6 (28.6%) |
| Patients with impaired LV or RV function, pulmonary hypertension with enlarged RV | No death |
| Survival | |
| Six-month survival | 60 ± 8% |
| Patients with cardiogenic shock | 41 ± 11% |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 86 ± 9.5% |
| One-year survival | 57 ± 8.4% |
| Patients with cardiogenic shock | 36 ± 11% |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 86 ± 9.5% |
| Two-year survival | 49 ± 10% |
| Patients with cardiogenic shock | 36 ± 11% |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 68 ± 17% |
| . | Patients . |
|---|---|
| Standardized endpoints (VARC-2) | |
| Device success | |
| All patients | 42/43 (98%) |
| Patients with cardiogenic shock | 22/23 (98%) |
| Patients with impaired LV or RV function, pulmonary hypertension with enlarged RV | 20/20 (100%) |
| Safety <30 days | |
| All patients | 34/43 (79%) |
| Patients with cardiogenic shock | 15/23 (65%) |
| Patients with impaired LV or RV function, pulmonary hypertension with enlarged RV | 19/20 (95%) |
| Time of hospitalization (days) | |
| Length of stay in intensive care unit | 3.2 (0.3–66) |
| Total length of stay | 11.8 (2.7–103) |
| Thirty-day mortality | |
| All patients | 6 (14%) |
| Patients with cardiogenic shock | 6 (28.6%) |
| Patients with impaired LV or RV function, pulmonary hypertension with enlarged RV | No death |
| Survival | |
| Six-month survival | 60 ± 8% |
| Patients with cardiogenic shock | 41 ± 11% |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 86 ± 9.5% |
| One-year survival | 57 ± 8.4% |
| Patients with cardiogenic shock | 36 ± 11% |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 86 ± 9.5% |
| Two-year survival | 49 ± 10% |
| Patients with cardiogenic shock | 36 ± 11% |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 68 ± 17% |
Data are presented as n/n, %, or estimate, standard error.
LV: left ventricular; RV: right ventricular.
| . | Patients . |
|---|---|
| Standardized endpoints (VARC-2) | |
| Device success | |
| All patients | 42/43 (98%) |
| Patients with cardiogenic shock | 22/23 (98%) |
| Patients with impaired LV or RV function, pulmonary hypertension with enlarged RV | 20/20 (100%) |
| Safety <30 days | |
| All patients | 34/43 (79%) |
| Patients with cardiogenic shock | 15/23 (65%) |
| Patients with impaired LV or RV function, pulmonary hypertension with enlarged RV | 19/20 (95%) |
| Time of hospitalization (days) | |
| Length of stay in intensive care unit | 3.2 (0.3–66) |
| Total length of stay | 11.8 (2.7–103) |
| Thirty-day mortality | |
| All patients | 6 (14%) |
| Patients with cardiogenic shock | 6 (28.6%) |
| Patients with impaired LV or RV function, pulmonary hypertension with enlarged RV | No death |
| Survival | |
| Six-month survival | 60 ± 8% |
| Patients with cardiogenic shock | 41 ± 11% |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 86 ± 9.5% |
| One-year survival | 57 ± 8.4% |
| Patients with cardiogenic shock | 36 ± 11% |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 86 ± 9.5% |
| Two-year survival | 49 ± 10% |
| Patients with cardiogenic shock | 36 ± 11% |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 68 ± 17% |
| . | Patients . |
|---|---|
| Standardized endpoints (VARC-2) | |
| Device success | |
| All patients | 42/43 (98%) |
| Patients with cardiogenic shock | 22/23 (98%) |
| Patients with impaired LV or RV function, pulmonary hypertension with enlarged RV | 20/20 (100%) |
| Safety <30 days | |
| All patients | 34/43 (79%) |
| Patients with cardiogenic shock | 15/23 (65%) |
| Patients with impaired LV or RV function, pulmonary hypertension with enlarged RV | 19/20 (95%) |
| Time of hospitalization (days) | |
| Length of stay in intensive care unit | 3.2 (0.3–66) |
| Total length of stay | 11.8 (2.7–103) |
| Thirty-day mortality | |
| All patients | 6 (14%) |
| Patients with cardiogenic shock | 6 (28.6%) |
| Patients with impaired LV or RV function, pulmonary hypertension with enlarged RV | No death |
| Survival | |
| Six-month survival | 60 ± 8% |
| Patients with cardiogenic shock | 41 ± 11% |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 86 ± 9.5% |
| One-year survival | 57 ± 8.4% |
| Patients with cardiogenic shock | 36 ± 11% |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 86 ± 9.5% |
| Two-year survival | 49 ± 10% |
| Patients with cardiogenic shock | 36 ± 11% |
| Patients with impaired LV or RV function, or pulmonary hypertension with enlarged RV | 68 ± 17% |
Data are presented as n/n, %, or estimate, standard error.
LV: left ventricular; RV: right ventricular.
Early safety at 30 days (Valve Academic Research Consortium-2)
Following the VARC-2 criteria [14], the early safety rate at 30 days was 79% (34/55). Six patients with preoperative shock died postoperatively due to multiorgan failure. No patient died with elective CPB due to impaired LV or RV function or with severe pulmonary hypertension combined with an enlarged ventricle (Table 4).
Length of stay
Owing to the multimorbidity of the patients, with 53% in preoperative cardiogenic shock, they needed a long postoperative in-hospital stay. The median length of stay at the intensive care unit was 3.2 days (range 0.3–66). The median length of postoperative in-hospital stay was 11.8 days (range 2.7–103).
Cardiac pacemaker
In the case of postoperative third degree atrioventricular block for more than 3 days, a cardiac pacemaker was implanted. Three patients (5%) needed pacemaker implantation postoperatively (1 patient with an Edwards SAPIEN valve, 26 mm, and 2 patients with an Edwards SAPIEN valve, 29 mm). Since 2 of them had preoperative atrial fibrillation, a VVI pacemaker was chosen and the other patient received a DDD pacemaker.
Survival
The 30-day mortality rate was 14% (6/43). It must be taken into account that all of these patients were in preoperative cardiogenic shock. Additionally, no patient with severely depressed LV or RV function or with severe pulmonary hypertension combined with an enlarged ventricle died during the first 30 days (Table 4).
The 1- and 2-year survival rate was 57 ± 8.4 and 49 ± 10%, respectively (Fig. 1), whereas the survival was significantly better in patients without preoperative cardiogenic shock (1- and 2-year survival rate: 86 ± 9.5 and 68 ± 17%, respectively) (P = 0.005, Fig. 2).
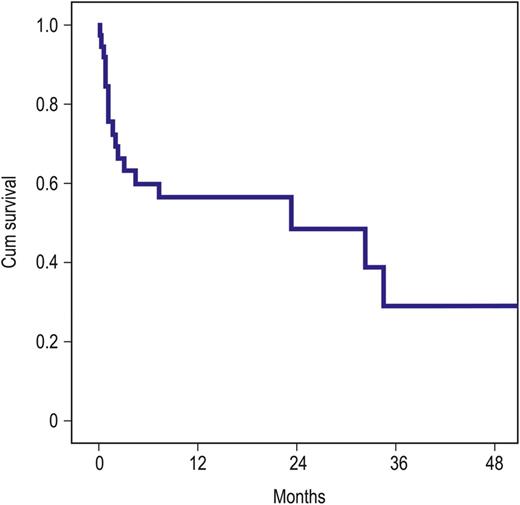
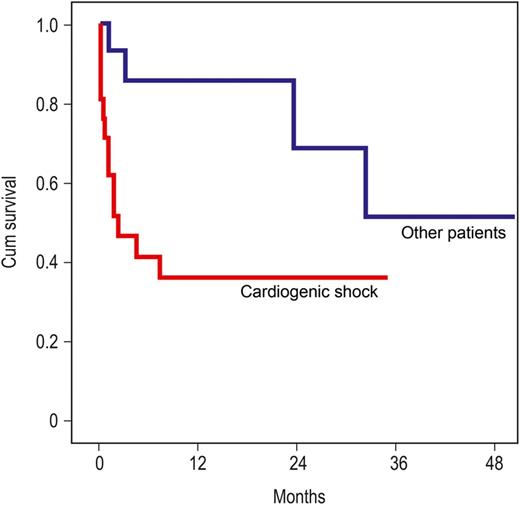
Survival (Kaplan–Meier curve): red: patients in cardiogenic shock (n = 23), blue: patients with impaired left ventricular or right ventricular function, or with pulmonary hypertension and enlarged right ventricular (n = 20) (P = 0.005).
DISCUSSION
TAVI was performed with elective CPB for greater safety and haemodynamic stability during the procedure. In particular, CPB allowed myocardial recovery of the unloaded heart in shock patients and prevented manual cardiopulmonary resuscitation if ventricular fibrillation occurred during the TAVI procedure (especially in patients with poor LV or RV function, enlarged RV with pulmonary hypertension, cardiogenic shock). The TAVI procedure allows replacement of the native aortic valve without the use of cardioplegia. This is a great advantage for these high-risk patients.
In patients with advanced heart failure, the operative risk is elevated during conventional aortic valve replacement. The mortality rate ranges from 3 to 30% [8, 12, 15, 16]. TAVI has been introduced to reduce the surgical risk in patients of advanced age. As shown in an earlier publication [1], the use of CPB during TAVI allows the procedure to be performed with greater safety in patients with advanced heart failure.
As reported in an earlier publication, TAVI can be performed successfully in patients presenting with cardiogenic shock [6]. Although the mortality rate in this patient group is very high, TAVI is a real option for these patients who would otherwise face certain death. D'Ancona et al. [6] showed a 30-day mortality rate of 19% in these patients and Unbehaun et al. [8] showed that, in patients with severely depressed LV function (below 25%), the postoperative course is prolonged, but mid-term survival is not influenced. In his cohort, elective femoro-femoral bypass was used in 43% of patients.
As already reported in 2013, our standard institutional policy is to consider the use of elective normothermic femoro-femoral CPB in patients with low LVEF (10–20%) or cardiogenic shock, or as a rescue procedure in the case of a significantly enlarged RV with poor RVEF and severe pulmonary hypertension [1]. CPB is used to stabilize the patients' condition, to achieve haemodynamic stability during TAVI and to prevent possible manual cardiopulmonary resuscitation if ventricular fibrillation should occur during rapid pacing for balloon valvuloplasty and during valve deployment. In patients with cardiogenic shock, CPB is used to allow additional myocardial recovery of the unloaded heart after the new transcatheter valve is deployed [7].
Until today, the literature on elective or urgent use of CPB during TAVI is still limited. Maeda et al. [14] reported 2 cases of successful TAVI in patients with poor LV function. Pasic et al. [17] published a case of a patient in profound cardiogenic shock with multiorgan failure with a successful TAVI procedure using CPB.
The superiority of valve implantation over balloon valvuloplasty has been demonstrated by several authors [18, 19]. Nevertheless, some authors prefer valvuloplasty as an alternative [20, 21]. An analysis of their results shows that they have observed the 30-day mortality rate of symptomatic patients with high surgical risk and without cardiogenic shock to be 13%. Our study reports 37 patients with severely depressed ventricular function without cardiogenic shock with no death at all during the 30 postoperative days. A two-step procedure with valvuloplasty several days prior to TAVI may have financial advantages, but we believe that the one-step procedure with immediate TAVI and the resulting optimal valve function provides superior results.
The heart–lung machine is rarely necessary for TAVI, but it must be kept on standby in the operation theatre and it is an important part of the safety net [1, 22], although not all patients placed on CPB during the procedure will need the support. Nevertheless, its elective use increases safety for critically ill patients by maintaining haemodynamic stability during rapid pacing phases and by avoiding manual cardiopulmonary resuscitation, given that the postoperative course of such patients is unfavourable.
As shown in our 2013 study [1], the elective use of extracorporeal circulation—in some cases for several minutes only—allows TAVI to be performed in patients in cardiogenic shock and in patients with severely depressed myocardial function (EF <20%). Additionally, patients with concomitant heart disease can be operated upon safely. The following are our guidelines on how to act in specific situations with regard to patient selection, and the use of CPB and the IABP [1]:
‘No exclusion’ policy: all patients with a EuroSCORE of 40% or an STS score of 10 or higher are evaluated as candidates for treatment regardless of comorbidities and clinical status, if it is technically possible to perform the procedure in terms of annular size (except in patients with active endocarditis).
Elective femoro-femoral CPB is considered in patients with severe cardiogenic shock, poor LV function (LVEF 10–20%) or an enlarged RV with severe pulmonary hypertension.
The IABP is considered in very high-risk patients with a depressed haemodynamic condition.
TAVI has been developed as a minimally invasive procedure for high-risk patients without the use of CPB. However, some patients may develop ventricular fibrillation during or immediately after cessation of rapid pacing for balloon dilatation of the native valve or valve deployment. Therefore, for patients with unstable haemodynamics, severely depressed heart function or concomitant heart disease, TAVI combined with the use of CPB seems to provide much better results than medical treatment or conventional aortic valve replacement, which is associated with relatively high operative mortality in these patients [8, 12, 15, 16, 18, 23, 24]. Although the heart–lung machine is rarely necessary in these patients, its use may increase safety and yield acceptable outcomes in this very high-risk patient group.
Conflict of interest: Miralem Pasic, Thorsten Drews, Semih Buz, Axel Unbehaun and Stephan Dreysse served as proctors to Edwards Lifesciences from July 2009 to June 2012. There are no other disclosures.
ACKNOWLEDGEMENTS
Other members of the team are Stephan Dreysse, Marian Kukucka, Tom Gromann, Guna Tetere Ekatarina Ivanitskaja-Kühn, Natalia Solowjowa, Christoph Klein and Katrin Schäfer. We thank Anne Carney for editorial assistance.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr P. Kiefer(Leipzig, Germany): The work and expertise of the authors in transcatheter aortic valve implantation over the past years has influenced our practice, particularly with regard to transapical access.
However, I would like to address some questions regarding your current work. The authors state that they used cardiopulmonary bypass in very high-risk patients to prevent ventricular fibrillation and haemodynamic instability during the procedure. Is that approach based on any negative events in the past? Have you experienced some negative events that prompted you to use this approach?
Secondly, I need some clarification because you stated at the beginning that you enrolled 60 patients in whom the procedure was performed with cardiopulmonary bypass. Eleven were excluded due to open surgery with valve implantation through aortotomy, as stated in the paper. Did these patients require any conversion to sternotomy, and why were they excluded? That was not really clear to me. And the third question is about the access approaches that you used. In your paper, you talked about the device that you used but not the exact transfemoral or transapical access for this patient cohort. Could you please comment on what devices you used for this specific patient population, which access you chose, and why you chose the access?
Dr Drews: I will begin with your first question as to why we started using cardiopulmonary bypass. Some patients present in a very poor condition with a very low ejection fraction and so we are faced with the choice of doing nothing or performing TAVI using the heart-lung machine, and in such cases we are convinced that the heart-lung machine is helpful. We are cardiac surgeon and for us it's not a problem to put the patient on a heart-lung machine. When we put the patient on a heart-lung machine, the procedure is easy and very safe and we have enough time to deploy the valve in the right position. We have found the method to be extremely helpful, and we would like to encourage everybody treating these high-risk patients to use the heart-lung machine. In fact, we have also had patients in whom we used the heart-lung machine due to complications. Last year we published a paper in Circulation on how to manage patients with annulus rupture. We had a rate of six patients in more than 600 implantations. But this is a complication. Everybody knows it can happen. In these cases we converted to conventional surgery, and we had a survival of more than 50%. But this is not the subject of the current paper on the elective use of the heart-lung machine.
Moving on to your next question: why do we use a transapical access? Most of these interventions used the transapical approach I think that transapical access is very safe. The route is short and deployment of the valve is easy. And when you have these high-risk patients, you should use the easiest route and the simplest way of implanting the valves.
Dr N. Moat(London, UK): I think the question that was asked was: what proportion of patients were by a transapical and what proportion by a transfemoral approach?
Dr Drews: There were more patients with transapical.
Dr Moat: So were they all transapical?
Dr Drews: No, they were not all transapical. There were some patients with transapical and some patients with transfemoral implantation of the Edwards valve. In total, 38 of the 43 patients received transapical TAVI.
And finally, in some patients we implanted the Edwards valve through aortotomy. These patients had severe calcification of the native valve, and there were patients who had undergone previous homograft implantation with severe calcification, where we excised the leaflets. In these cases, the calcification was too severe to perform conventional surgery. Conventional suture of the valve would have been too difficult, so we excised the native leaflets and put in the Edwards Sapien valve; it fitted perfectly.
Dr M. Romano(Massy, France): These are extremely sick patients with a very short life expectancy, extremely depressed left ventricular function, patients in cardiogenic shock. And we also have to evaluate and to balance between the utility and the futility of this technique. Why don't you propose to the patient a bridge balloon valvuloplasty, re-evaluate the left ventricular function and, if any residual contractility remains, you propose an implantation of the valve? Or, otherwise, you turn the patient down?
Dr Drews: Our policy is that when we do a balloon valvuloplasty, we implant the valve during the same procedure. Whether the patient has to go down to the lab or to the hybrid room, it is the same for us. We prefer to put the patient on the heart-lung machine. It is fast, safe and easy. Maybe for you, it is a question of cost.
Dr Romano: I think it is not the same thing to just do a balloon valvuloplasty, re-evaluate the patient, or to put the patient on bypass and assist the patient if something goes wrong.
Dr Moat: I think although this is a European meeting, it may have a worldwide message. I think you exist in a unique situation in Germany. In most of the world, we have limited access to TAVI. If you look at your one-year survival, it is worse than the one-year survival in the medical arm of PARTNER B. So in the worldwide context, or in other countries, I think I would agree with Dr Romano. You really have to be very careful about this sort of strategy, and I think most units would adopt a ‘BAV-first’ type of strategy.
Dr G. Dahle(Oslo, Norway): Did any of the patients need an aortic balloon pump afterwards, or did you maybe implant an aortic balloon pump afterwards in any of them to avoid going on bypass?
Dr Drews: Yes, sure. We had 11 patients in whom we implanted an intra-aortic balloon pump. The intra-aortic balloon pump is a really useful tool, and we like it. And also, we had two patients who came into the operating room with an intra-aortic balloon pump.
Dr Dahle: And could you avoid using bypass by implanting an aortic balloon pump in any of the patients?
Dr Drews: At the end of the procedure, we put the intra-aortic balloon pump in and we were able to wean them from the bypass. But we did not avoid the bypass by using the aortic balloon pump.
Dr Moat: Is there a cohort of patients that are between those that you do without any support and those that you do on bypass; so a cohort that you could do with balloon pump support rather than with bypass?
Dr Drews: No. I think it is hard to achieve optimal unloading during the procedure, so bypass is much better than the balloon pump alone. Putting the patient on the bypass machine means you achieve unloading, and it is easy to do. You have so much time. It is much better.
Author notes
Presented at the 27th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 5–9 October 2013.




