-
PDF
- Split View
-
Views
-
Cite
Cite
Motoki Yano, Junji Yoshida, Terumoto Koike, Kotaro Kameyama, Akira Shimamoto, Wataru Nishio, Kentaro Yoshimoto, Tomoki Utsumi, Takayuki Shiina, Atsushi Watanabe, Yasushi Yamato, Takehiro Watanabe, Yusuke Takahashi, Makoto Sonobe, Hiroaki Kuroda, Makoto Oda, Masayoshi Inoue, Masayuki Tanahashi, Hirofumi Adachi, Masao Saito, Masataro Hayashi, Hajime Otsuka, Teruaki Mizobuchi, Yasumitsu Moriya, Mamoru Takahashi, Shigeto Nishikawa, Yuki Matsumura, Satoru Moriyama, Takeshi Nishiyama, Yoshitaka Fujii, on behalf of the Japanese Association for Chest Surgery, Survival of 1737 lobectomy-tolerable patients who underwent limited resection for cStage IA non-small-cell lung cancer, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 1, January 2015, Pages 135–142, https://doi.org/10.1093/ejcts/ezu138
Close - Share Icon Share
Abstract
A precise preoperative diagnosis of ‘very early’ lung carcinoma may identify patients who can undergo curative surgery with limited resections.
Data from a multi-institutional project were collected on 1737 patients who had undergone limited resections (segmentectomy or wedge resection) for T1N0M0 non-small-cell carcinomas. As it was expected, this study was predominantly including ground glass nodules. Computed tomography was used to obtain the ratio of consolidation to the maximal tumour diameter to determine invasive potential of the tumours. Overall and disease-free survivals and recurrence-free proportions were analysed.
Median age was 64 years. Mean maximal diameter of the tumours was 1.4 ± 0.5 cm. Overall and recurrence-free survivals after limited lung resection were 94.0 and 91.1% at 5 years, respectively. Recurrence-free proportions were 93.7% at 5 years. Unfavourable prognostic factors in overall survival were lymph node metastasis, interstitial pneumonia, male gender, older age, comorbidities (cardiac disease, diabetes etc.) and consolidation/tumour ratio (C/T) ≤ 0.25. C/T ≤ 0.25 predicted good outcomes especially in cT1aN0M0 disease. In a subclass analysis of cT1N0M0 squamous cell carcinomas, wedge resection was the only unfavourable prognostic factor in both overall and disease-free survivals.
If the patient was 75 years old or younger and was judged fit for lobectomy, limited resection for cStage I non-small-cell lung cancer (NSCLC) showed excellent outcomes and was not inferior to the reported results of lobectomy for small-sized NSCLC. The carcinomas with C/T ≤ 0.25 rarely recur and are especially good candidates for limited resection.
INTRODUCTION
In early-stage non-small-cell lung cancer (NSCLC), a curative surgical resection has been considered to be the determinant of prognosis [1, 2]. Lobectomy is the standard surgical procedure for early-stage NSCLC [3]. Limited resection has been associated with a higher risk of local recurrence in a randomized controlled study reported in 1995 [3]. However, excellent survival data for limited resections have been reported in patients with small-sized NSCLC [4–7]. Moreover, a recent evaluation of the surveillance, epidemiology and end results (SEER) registry with a large number of patients suggested that limited resection may be an adequate alternative for stage IA NSCLC patients with tumours ≤1 cm in size [8].
Recent widespread use of computed tomography (CT) for lung cancer screening has led to increased detection of ‘very early’ NSCLC [9]. To predict pathological non-invasiveness by radiological findings, the Japan Clinical Oncology Group (JCOG) conducted a prospective radiological and pathological study of clinical stage IA lung cancers (JCOG0201) [10, 11]. As a result, a consolidation/tumour ratio (C/T) of 0.25 or less in cT1a (≤2.0 cm) was reported to be a good predictor of non-invasive adenocarcinoma [10, 11] in studies of predominantly ground glass nodules (GGNs). Using this criterion, a phase II study was conducted of limited surgical resections for peripheral early lung cancers diagnosed by thin-section CT findings (JCOG0804/WJOG4507L). Although patient enrolment ended in 2011, more time will be needed before the results of the study are available.
In practice, limited resection has been widely used for pure or part-solid GGNs especially for small lesions [12, 13]. The aim of this retrospective study was to compile clinical data of a large number of patients who underwent limited resections for clinical stage I NSCLC. To more accurately determine the efficacy of limited resection, our study was limited to patients 75 years old or younger and who were diagnosed fit for lobectomy.
MATERIALS AND METHODS
The Japanese Association for Chest Surgery collected clinical data in a retrospective multi-institutional analysis of limited resections (segmentectomy or wedge resection) for NSCLC patients who were expected to tolerate lobectomy and were 75 years old or younger at the time of operation. Registration enrolment criteria for the patients were: 30 or more consecutive patients with clinical stage I NSCLC undergoing limited resections (segmentectomy or wedge resection) at each institution, 60 or more months of median follow-up period, age ≤75 years at operation and physically fit for lobectomy as diagnosed by the physician. The newest the Union Internationale Contre le Cancer the tumour, node, and metastases staging classification of version 7 was used in this study.
To grasp the present conditions and genuine prognosis of limited resection in Japan, all clinical stage I disease and all pathological types except small-cell carcinomas were included. In this study, lymph node dissection was not queried. In wedge resection, evaluation of lymph node metastasis was not possible. The cases with incomplete pathological evaluation of lymph nodes were expressed by pNx. Also the criteria for patients to fit lobectomy were not set because they varied between institutions. We aimed to clarify the prognosis of the patients who were thought to be curable by intentional limited resection. As we thought the detail criteria for patients to fit lobectomy were not necessary for the analysis of intentional curable limited resection but palliative limited resection, we did not analyse them in this study.
Twenty-six institutions participated in the present study, and 2212 patients were enrolled. They underwent limited resections between August 1993 and July 2012. Two hundred and forty-nine patients were excluded from analysis for the following reasons: small-cell carcinoma, no data of pulmonary function test, low pulmonary function [e.g. forced expiratory volume in one second (FEV1.0) <40% of predicted level], dissemination found at operation or positive cancer cells at the cut end of limited resection. At first, synchronous and metachronous multiple lung cancers were included in the analysis but then these lesions were excluded. The remaining 1737 patients were analysed.
This study was approved by the Institutional Review Boards of the Nagoya City University Hospital and participating institutions. Patient demographics and lung cancer-related factors including the number of comorbidities (diabetes mellitus, cardiac disease etc.), especially comorbidities of interstitial pneumonia (IP) including idiopathic pulmonary fibrosis, lung function [%VC (vital capacity) and FEV1%], radiological findings on CT, location of the nodule (outer 1/3 or inner 2/3), pathology, clinical and pathological TNM status, tumour size, number of nodules and operation performed (segmentectomy/wedge resection), were registered.
As it was expected, this study predominantly included ground glass nodules. A tumour was radiologically classified into two groups; as an invasive group if the ratio of the consolidation (C) to the maximal tumour diameter (T) (C/T ratio) was more than 0.25 (C/T > 0.25), and a non-invasive group (C/T ratio was ≤ 0.25: C/T ≤ 0.25) [10, 11]. This criterion was in accordance with the definition of radiological noninvasive lung cancer reported by the JCOG in the JCOG0201 study reports [10, 11].
Statistical analysis
The overall survival length was defined as the interval in months from the date of surgery to the date of death from any cause and patients who were alive were censored at the date of the last follow-up. Recurrence-free survival was defined as the interval from the date of surgery to the date of recurrence or death from any cause and alive patients without recurrence were censored at the date of the last follow-up. Recurrence-free proportion was defined as the proportion of patients free from recurrence regardless of survival or death and alive patients without recurrence were censored at the date of the last follow-up. In addition, the patients who died of any cause other than lung cancer were censored at the date of the last follow-up.
The probability of survival or proportion was estimated by the Kaplan–Meier method. The statistical significance of differences in survival between categorized groups was evaluated by the log-rank test. The significance of differences between categorized groups was evaluated using Pearson's χ2 test. Cox regression analysis was used to identify prognostic factors. Statistical significance was defined as P <0.05. The EZR software on a personal computer was used for the analysis [14]. The data were analysed at the Nagoya City University Data Center.
RESULTS
The characteristics of 1737 patients are given in Table 1 and Fig. 1. In brief, the median age was 63 years, and women were slightly predominant. The main population of patients in this study were without comorbidities especially without IP, with small maximal diameters of tumours (≤2.0 cm), and diagnosed as cT1aN0M0. Tumours were located in the outer one-third of the lung parenchyma. Almost half of the tumours were diagnosed radiologically as invasive (C/T > 0.25). Segmentectomy was performed in almost two-third cases. Most of the lung cancers were diagnosed as adenocarcinoma with pT1a N0 or pNx.
| Factors . | Characteristics . | n (%) . |
|---|---|---|
| Age | Median 64 (20–75) | |
| <65 | 893 (51.4) | |
| 65≤ | 844 (48.6) | |
| Gender | Women | 950 (54.7) |
| Men | 787 (45.3) | |
| Number of comorbidity | 0 | 1208 (69.5) |
| 1 | 427 (24.6) | |
| 2 or more | 102 (5.9) | |
| Comorbidity of interstitial pneumonia | − | 1706 (98.2) |
| + | 31 (1.8) | |
| %VC | ≥80% | 1653 (95.2) |
| <80% | 84 (4.8) | |
| FEV1% | ≥70% | 1400 (80.6) |
| <70% | 337 (19.4) | |
| Maximal diameter (MD) | Mean 1.4 ± 0.5 cm | |
| MD ≤ 1.0 cm | 551 (31.7) | |
| 1.0 cm < MD ≤ 2.0 cm | 1026 (59.1) | |
| 2.0 cm < MD | 160 (9.2) | |
| Location | Outer | 1250 (72.0) |
| Inner | 234 (13.5) | |
| unknown | 253 (14.6) | |
| Radiological findings (C/T) | C/T > 0.25 | 915 (52.7) |
| C/T ≤ 0.25 | 810 (46.6) | |
| unknown | 14 (0.8) | |
| cStage | cT1aN0M0 | 1576 (90.7) |
| cT1bNoMo | 161 (9.3) | |
| Performed operation | Segmentectomy | 1094 (63.0) |
| Wedge resection | 643 (37.0) | |
| Pathology | Adenocarcinoma | 1586 (91.3) |
| SqCC | 100 (5.8) | |
| Others | 51 (2.9) | |
| pT factors | pT1a | 1497 (86.2) |
| pT1b | 131 (7.5) | |
| pT2-3 | 109 (6.3) | |
| pN factors | pN0 or Nx | 1715 (98.7) |
| pN1 or 2 | 22 (1.3) |
| Factors . | Characteristics . | n (%) . |
|---|---|---|
| Age | Median 64 (20–75) | |
| <65 | 893 (51.4) | |
| 65≤ | 844 (48.6) | |
| Gender | Women | 950 (54.7) |
| Men | 787 (45.3) | |
| Number of comorbidity | 0 | 1208 (69.5) |
| 1 | 427 (24.6) | |
| 2 or more | 102 (5.9) | |
| Comorbidity of interstitial pneumonia | − | 1706 (98.2) |
| + | 31 (1.8) | |
| %VC | ≥80% | 1653 (95.2) |
| <80% | 84 (4.8) | |
| FEV1% | ≥70% | 1400 (80.6) |
| <70% | 337 (19.4) | |
| Maximal diameter (MD) | Mean 1.4 ± 0.5 cm | |
| MD ≤ 1.0 cm | 551 (31.7) | |
| 1.0 cm < MD ≤ 2.0 cm | 1026 (59.1) | |
| 2.0 cm < MD | 160 (9.2) | |
| Location | Outer | 1250 (72.0) |
| Inner | 234 (13.5) | |
| unknown | 253 (14.6) | |
| Radiological findings (C/T) | C/T > 0.25 | 915 (52.7) |
| C/T ≤ 0.25 | 810 (46.6) | |
| unknown | 14 (0.8) | |
| cStage | cT1aN0M0 | 1576 (90.7) |
| cT1bNoMo | 161 (9.3) | |
| Performed operation | Segmentectomy | 1094 (63.0) |
| Wedge resection | 643 (37.0) | |
| Pathology | Adenocarcinoma | 1586 (91.3) |
| SqCC | 100 (5.8) | |
| Others | 51 (2.9) | |
| pT factors | pT1a | 1497 (86.2) |
| pT1b | 131 (7.5) | |
| pT2-3 | 109 (6.3) | |
| pN factors | pN0 or Nx | 1715 (98.7) |
| pN1 or 2 | 22 (1.3) |
%VC: vital capacity; FEV1%: forced expiratory volume in one second; C/T: the ratio of the consolidation (C) to the maximal tumour diameter (T); HR: hazard ratio; CI: confidence interval.
| Factors . | Characteristics . | n (%) . |
|---|---|---|
| Age | Median 64 (20–75) | |
| <65 | 893 (51.4) | |
| 65≤ | 844 (48.6) | |
| Gender | Women | 950 (54.7) |
| Men | 787 (45.3) | |
| Number of comorbidity | 0 | 1208 (69.5) |
| 1 | 427 (24.6) | |
| 2 or more | 102 (5.9) | |
| Comorbidity of interstitial pneumonia | − | 1706 (98.2) |
| + | 31 (1.8) | |
| %VC | ≥80% | 1653 (95.2) |
| <80% | 84 (4.8) | |
| FEV1% | ≥70% | 1400 (80.6) |
| <70% | 337 (19.4) | |
| Maximal diameter (MD) | Mean 1.4 ± 0.5 cm | |
| MD ≤ 1.0 cm | 551 (31.7) | |
| 1.0 cm < MD ≤ 2.0 cm | 1026 (59.1) | |
| 2.0 cm < MD | 160 (9.2) | |
| Location | Outer | 1250 (72.0) |
| Inner | 234 (13.5) | |
| unknown | 253 (14.6) | |
| Radiological findings (C/T) | C/T > 0.25 | 915 (52.7) |
| C/T ≤ 0.25 | 810 (46.6) | |
| unknown | 14 (0.8) | |
| cStage | cT1aN0M0 | 1576 (90.7) |
| cT1bNoMo | 161 (9.3) | |
| Performed operation | Segmentectomy | 1094 (63.0) |
| Wedge resection | 643 (37.0) | |
| Pathology | Adenocarcinoma | 1586 (91.3) |
| SqCC | 100 (5.8) | |
| Others | 51 (2.9) | |
| pT factors | pT1a | 1497 (86.2) |
| pT1b | 131 (7.5) | |
| pT2-3 | 109 (6.3) | |
| pN factors | pN0 or Nx | 1715 (98.7) |
| pN1 or 2 | 22 (1.3) |
| Factors . | Characteristics . | n (%) . |
|---|---|---|
| Age | Median 64 (20–75) | |
| <65 | 893 (51.4) | |
| 65≤ | 844 (48.6) | |
| Gender | Women | 950 (54.7) |
| Men | 787 (45.3) | |
| Number of comorbidity | 0 | 1208 (69.5) |
| 1 | 427 (24.6) | |
| 2 or more | 102 (5.9) | |
| Comorbidity of interstitial pneumonia | − | 1706 (98.2) |
| + | 31 (1.8) | |
| %VC | ≥80% | 1653 (95.2) |
| <80% | 84 (4.8) | |
| FEV1% | ≥70% | 1400 (80.6) |
| <70% | 337 (19.4) | |
| Maximal diameter (MD) | Mean 1.4 ± 0.5 cm | |
| MD ≤ 1.0 cm | 551 (31.7) | |
| 1.0 cm < MD ≤ 2.0 cm | 1026 (59.1) | |
| 2.0 cm < MD | 160 (9.2) | |
| Location | Outer | 1250 (72.0) |
| Inner | 234 (13.5) | |
| unknown | 253 (14.6) | |
| Radiological findings (C/T) | C/T > 0.25 | 915 (52.7) |
| C/T ≤ 0.25 | 810 (46.6) | |
| unknown | 14 (0.8) | |
| cStage | cT1aN0M0 | 1576 (90.7) |
| cT1bNoMo | 161 (9.3) | |
| Performed operation | Segmentectomy | 1094 (63.0) |
| Wedge resection | 643 (37.0) | |
| Pathology | Adenocarcinoma | 1586 (91.3) |
| SqCC | 100 (5.8) | |
| Others | 51 (2.9) | |
| pT factors | pT1a | 1497 (86.2) |
| pT1b | 131 (7.5) | |
| pT2-3 | 109 (6.3) | |
| pN factors | pN0 or Nx | 1715 (98.7) |
| pN1 or 2 | 22 (1.3) |
%VC: vital capacity; FEV1%: forced expiratory volume in one second; C/T: the ratio of the consolidation (C) to the maximal tumour diameter (T); HR: hazard ratio; CI: confidence interval.
Prognosis
The mean observation period was 71.7 months (median: 65.8 months). During the observation period 141 patients died: 70 patients from lung cancer and 71 patients from other causes. Recurrence occurred in 122 patients. The recurrences were distant metastases in 46 patients (37.7%), local recurrence in 30 (24.6%), lymph node recurrence in 28 (23.0%) and pleural dissemination in 18 (14.8%). This local recurrence meant a recurrence lesion, which could be distinguished from the lymph node recurrence and located near the stamp of the bronchus or the lung parenchyma. The overall survival of the 1737 patients who underwent limited resections was 94.0% at 5 years (95% confidence interval (CI): 92.6–95.1%; Fig. 2A). The disease-free survival was 91.1% at 5 years (95 CI: 89.5–92.4%) (Fig. 2B). The recurrence-free proportion was 93.7% at 5 years (95 CI: 92.4–94.8%) (Fig. 2C). The yearly recurrence rate was calculated as the number of patients with recurrence divided by the number of patients at risk at the beginning of each year (Fig. 3). Most recurrences were diagnosed in the first 3 years of follow-up after the surgery (yearly recurrence rate 1.50–1.64%). From 4 to 7 years, the recurrence proportion was <1%. Then from 8 to 9 years, a small peak of the recurrence may be present. The overall survival in the 122 patients with recurrences was 57.4% at 5 years after surgery (95% CI: 47.7–65.9%).
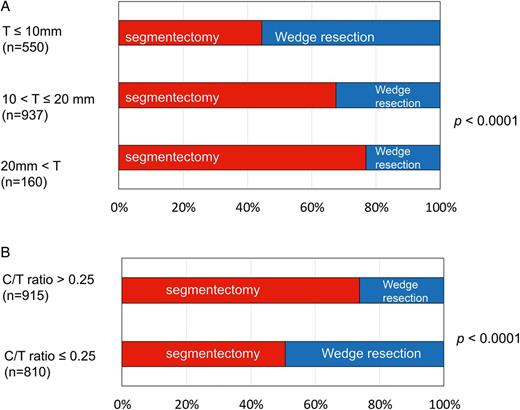
Preoperative tumour factors and differences in surgical procedures, segmentectomy and wedge resection. (A) Maximal diameter of the tumours (T) and differences in surgical procedures. (B) Radiological findings of C/T ratio and differences in surgical procedures. C/T ratio: ratio of the maximal diameters of tumour (T) and consolidation (C).
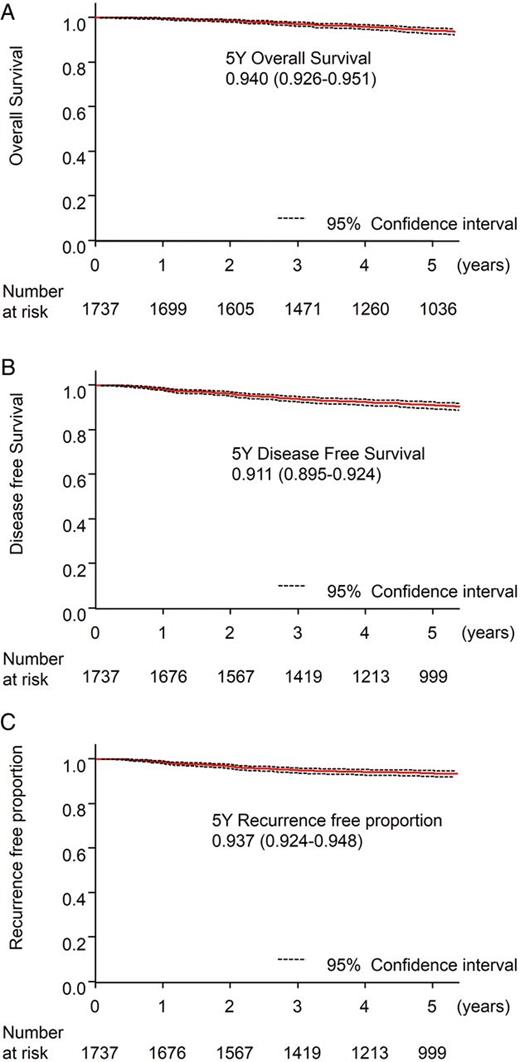
Survival and recurrence data of the entire 1737 patients. (A) Five-year overall survival. (B) Five-year disease-free survival. (C) Five-year recurrence-free proportion.
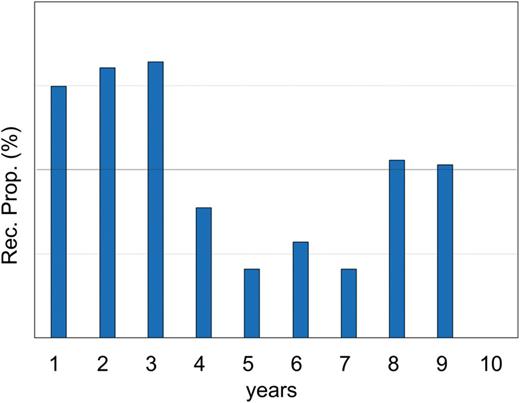
Recurrence proportion for 10 years, representing the ratio of the number of patients with recurrence and the number of patients at risk every year.
Cox's multivariate analysis was performed for prognostic factors for overall survival, disease-free survival, recurrence-free proportion and proportion free from death by other causes (Table 2). In summary, unfavourable prognostic factors in overall survival were pathologically confirmed lymph node metastasis, male gender, comorbidity of IP, older age (≥65), advanced pathological T factor (pT2a-3), C/T > 0.25, and presence of any comorbidities. Unfavourable prognostic factors in disease-free survival were pathologically confirmed lymph node metastasis, male gender, C/T > 0.25, advanced pathological T factor (pT2a-3), low %VC, comorbidity of IP, older age (≥65) and presence of any comorbidities. Unfavourable prognostic factors in recurrence-free proportion were pathologically confirmed lymph node metastasis, C/T > 0.25, comorbidity of IP, advanced pathological T factors (pT2a-3), male gender, low %VC and presence of any comorbidities. Unfavourable prognostic factors in the proportion free of death from other causes were older age (≥65), male gender and squamous cell carcinoma pathology (SqCC).
| Factor . | HRa . | Lower 95% CIb . | Upper 95% CI . | P*** . |
|---|---|---|---|---|
| Overall survival | ||||
| pN+ | 7.07 | 3.63 | 13.8 | <0.001 |
| Male | 2.80 | 1.87 | 4.20 | <0.001 |
| IP + | 2.23 | 1.06 | 4.69 | <0.001 |
| Age ≥ 65 | 2.05 | 1.41 | 2.99 | <0.001 |
| pT2a-3 | 1.86 | 1.15 | 3.02 | 0.011 |
| C/T > 0.25 | 1.77 | 1.14 | 2.74 | 0.011 |
| Comorbidity + | 1.64 | 1.15 | 2.32 | 0.006 |
| Disease-free survival | ||||
| pN+ | 6.71 | 3.65 | 12.4 | <0.001 |
| Male | 2.59 | 1.85 | 3.63 | <0.001 |
| C/T > 0.25 | 2.62 | 1.76 | 3.90 | <0.001 |
| pT2a-3 | 2.11 | 1.40 | 3.17 | <0.001 |
| Low %VC | 2.03 | 1.28 | 3.21 | 0.026 |
| IP+ | 2.02 | 1.03 | 3.97 | 0.041 |
| Age ≥ 65 | 1.79 | 1.31 | 2.46 | <0.001 |
| Comorbidity + | 1.68 | 1.25 | 2.25 | <0.001 |
| Recurrence-free proportion | ||||
| pN+ | 6.95 | 3.54 | 13.7 | <0.001 |
| C/T > 0.25 | 4.72 | 2.52 | 8.85 | <0.001 |
| IP + | 2.78 | 1.33 | 5.83 | <0.001 |
| pT2a-3 | 2.78 | 1.73 | 4.49 | <0.001 |
| Male | 2.35 | 1.54 | 3.58 | <0.001 |
| Low %VC | 2.30 | 1.31 | 4.05 | 0.004 |
| Comorbidity + | 1.72 | 1.19 | 2.50 | 0.004 |
| Free proportion from death by other causes | ||||
| Male | 3.16 | 1.79 | 5.57 | <0.001 |
| Age ≥ 65 | 3.01 | 1.71 | 5.32 | <0.001 |
| SqCC | 2.19 | 1.07 | 4.48 | 0.032 |
| Factor . | HRa . | Lower 95% CIb . | Upper 95% CI . | P*** . |
|---|---|---|---|---|
| Overall survival | ||||
| pN+ | 7.07 | 3.63 | 13.8 | <0.001 |
| Male | 2.80 | 1.87 | 4.20 | <0.001 |
| IP + | 2.23 | 1.06 | 4.69 | <0.001 |
| Age ≥ 65 | 2.05 | 1.41 | 2.99 | <0.001 |
| pT2a-3 | 1.86 | 1.15 | 3.02 | 0.011 |
| C/T > 0.25 | 1.77 | 1.14 | 2.74 | 0.011 |
| Comorbidity + | 1.64 | 1.15 | 2.32 | 0.006 |
| Disease-free survival | ||||
| pN+ | 6.71 | 3.65 | 12.4 | <0.001 |
| Male | 2.59 | 1.85 | 3.63 | <0.001 |
| C/T > 0.25 | 2.62 | 1.76 | 3.90 | <0.001 |
| pT2a-3 | 2.11 | 1.40 | 3.17 | <0.001 |
| Low %VC | 2.03 | 1.28 | 3.21 | 0.026 |
| IP+ | 2.02 | 1.03 | 3.97 | 0.041 |
| Age ≥ 65 | 1.79 | 1.31 | 2.46 | <0.001 |
| Comorbidity + | 1.68 | 1.25 | 2.25 | <0.001 |
| Recurrence-free proportion | ||||
| pN+ | 6.95 | 3.54 | 13.7 | <0.001 |
| C/T > 0.25 | 4.72 | 2.52 | 8.85 | <0.001 |
| IP + | 2.78 | 1.33 | 5.83 | <0.001 |
| pT2a-3 | 2.78 | 1.73 | 4.49 | <0.001 |
| Male | 2.35 | 1.54 | 3.58 | <0.001 |
| Low %VC | 2.30 | 1.31 | 4.05 | 0.004 |
| Comorbidity + | 1.72 | 1.19 | 2.50 | 0.004 |
| Free proportion from death by other causes | ||||
| Male | 3.16 | 1.79 | 5.57 | <0.001 |
| Age ≥ 65 | 3.01 | 1.71 | 5.32 | <0.001 |
| SqCC | 2.19 | 1.07 | 4.48 | 0.032 |
C/T: the ratio of the consolidation (C) to the maximal tumour diameter (T); IP+: comorbidity of interstitial pneumonia; low %VC: %vital capacity <80%; HR: hazard ratio; CI: confidence interval.
***P is shown as P < 0.001 if the actual P-value was <0.001.
| Factor . | HRa . | Lower 95% CIb . | Upper 95% CI . | P*** . |
|---|---|---|---|---|
| Overall survival | ||||
| pN+ | 7.07 | 3.63 | 13.8 | <0.001 |
| Male | 2.80 | 1.87 | 4.20 | <0.001 |
| IP + | 2.23 | 1.06 | 4.69 | <0.001 |
| Age ≥ 65 | 2.05 | 1.41 | 2.99 | <0.001 |
| pT2a-3 | 1.86 | 1.15 | 3.02 | 0.011 |
| C/T > 0.25 | 1.77 | 1.14 | 2.74 | 0.011 |
| Comorbidity + | 1.64 | 1.15 | 2.32 | 0.006 |
| Disease-free survival | ||||
| pN+ | 6.71 | 3.65 | 12.4 | <0.001 |
| Male | 2.59 | 1.85 | 3.63 | <0.001 |
| C/T > 0.25 | 2.62 | 1.76 | 3.90 | <0.001 |
| pT2a-3 | 2.11 | 1.40 | 3.17 | <0.001 |
| Low %VC | 2.03 | 1.28 | 3.21 | 0.026 |
| IP+ | 2.02 | 1.03 | 3.97 | 0.041 |
| Age ≥ 65 | 1.79 | 1.31 | 2.46 | <0.001 |
| Comorbidity + | 1.68 | 1.25 | 2.25 | <0.001 |
| Recurrence-free proportion | ||||
| pN+ | 6.95 | 3.54 | 13.7 | <0.001 |
| C/T > 0.25 | 4.72 | 2.52 | 8.85 | <0.001 |
| IP + | 2.78 | 1.33 | 5.83 | <0.001 |
| pT2a-3 | 2.78 | 1.73 | 4.49 | <0.001 |
| Male | 2.35 | 1.54 | 3.58 | <0.001 |
| Low %VC | 2.30 | 1.31 | 4.05 | 0.004 |
| Comorbidity + | 1.72 | 1.19 | 2.50 | 0.004 |
| Free proportion from death by other causes | ||||
| Male | 3.16 | 1.79 | 5.57 | <0.001 |
| Age ≥ 65 | 3.01 | 1.71 | 5.32 | <0.001 |
| SqCC | 2.19 | 1.07 | 4.48 | 0.032 |
| Factor . | HRa . | Lower 95% CIb . | Upper 95% CI . | P*** . |
|---|---|---|---|---|
| Overall survival | ||||
| pN+ | 7.07 | 3.63 | 13.8 | <0.001 |
| Male | 2.80 | 1.87 | 4.20 | <0.001 |
| IP + | 2.23 | 1.06 | 4.69 | <0.001 |
| Age ≥ 65 | 2.05 | 1.41 | 2.99 | <0.001 |
| pT2a-3 | 1.86 | 1.15 | 3.02 | 0.011 |
| C/T > 0.25 | 1.77 | 1.14 | 2.74 | 0.011 |
| Comorbidity + | 1.64 | 1.15 | 2.32 | 0.006 |
| Disease-free survival | ||||
| pN+ | 6.71 | 3.65 | 12.4 | <0.001 |
| Male | 2.59 | 1.85 | 3.63 | <0.001 |
| C/T > 0.25 | 2.62 | 1.76 | 3.90 | <0.001 |
| pT2a-3 | 2.11 | 1.40 | 3.17 | <0.001 |
| Low %VC | 2.03 | 1.28 | 3.21 | 0.026 |
| IP+ | 2.02 | 1.03 | 3.97 | 0.041 |
| Age ≥ 65 | 1.79 | 1.31 | 2.46 | <0.001 |
| Comorbidity + | 1.68 | 1.25 | 2.25 | <0.001 |
| Recurrence-free proportion | ||||
| pN+ | 6.95 | 3.54 | 13.7 | <0.001 |
| C/T > 0.25 | 4.72 | 2.52 | 8.85 | <0.001 |
| IP + | 2.78 | 1.33 | 5.83 | <0.001 |
| pT2a-3 | 2.78 | 1.73 | 4.49 | <0.001 |
| Male | 2.35 | 1.54 | 3.58 | <0.001 |
| Low %VC | 2.30 | 1.31 | 4.05 | 0.004 |
| Comorbidity + | 1.72 | 1.19 | 2.50 | 0.004 |
| Free proportion from death by other causes | ||||
| Male | 3.16 | 1.79 | 5.57 | <0.001 |
| Age ≥ 65 | 3.01 | 1.71 | 5.32 | <0.001 |
| SqCC | 2.19 | 1.07 | 4.48 | 0.032 |
C/T: the ratio of the consolidation (C) to the maximal tumour diameter (T); IP+: comorbidity of interstitial pneumonia; low %VC: %vital capacity <80%; HR: hazard ratio; CI: confidence interval.
***P is shown as P < 0.001 if the actual P-value was <0.001.
Subclass analysis
To compare the results of the present study to a recent study reporting the results of lobectomy for small-sized NSCLC, subclass analysis was performed in 1538 patients with a cT1aN0M0 disease of a single lesion without IP. The overall survival in 1538 patients was 94.7% at 5 years (95 CI: 93.3–95.8%) (Fig. 4A). The disease-free survival was 92.2% at 5 years (95 CI: 90.7–93.5%) (Fig. 4B). The recurrence-free proportion was 95.0% at 5 years (95 CI: 93.7–96.0%) (Fig. 4C).
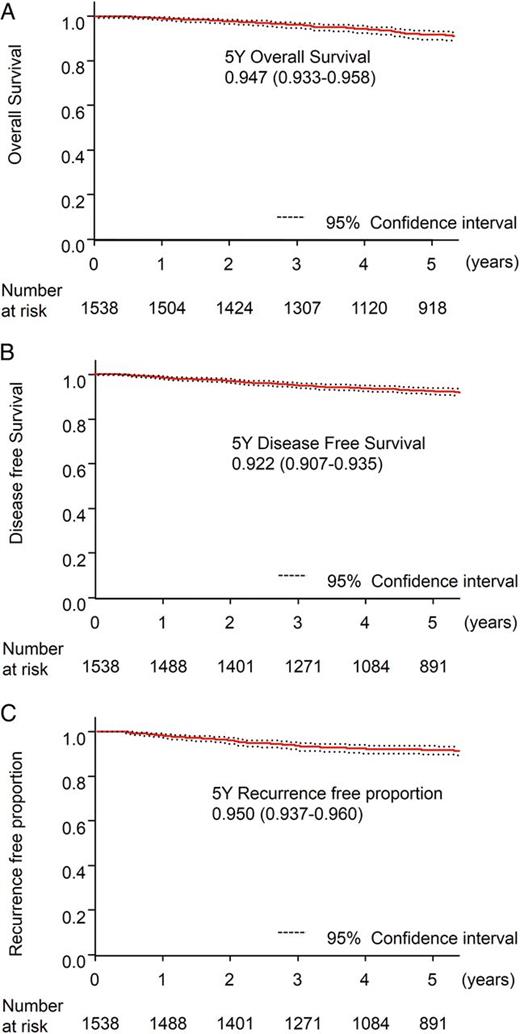
Survival and recurrence data of the 1538 cT1aN0M0 patients with a single lesion and without comorbidity of interstitial pneumonia. (A) Five-year overall survival. (B) Five-year disease-free survival. (C) Five-year recurrence-free proportion.
Among factors available preoperatively, the CT finding according to the C/T ratio was the prognostic factor that had the greatest effect on recurrence (Table 2). The overall survival of patients of C/T > 0.25 (n = 755) and C/T ≤ 0.25 (n = 783) tumours was 92.7 (95 CI: 90.7–94.7%) and 96.7 (95 CI: 95.4–98.2%) at 5 years, respectively (P < 0.0001; Fig. 5A). The disease-free survival of patients of C/T > 0.25 and C/T ≤ 0.25 was 88.2 (95 CI: 85.5–90.4%) and 96.5% at 5 years (95 CI: 94.7–97.7%), respectively (P < 0.0001; Fig. 5B). The recurrence-free proportion in patients of C/T > 0.25 and C/T ≤ 0.25 was 91.6 (95 CI: 89.3–93.4%) and 98.5% at 5 years (95 CI: 97.3–99.2%), respectively (P < 0.0001; Fig. 5C).
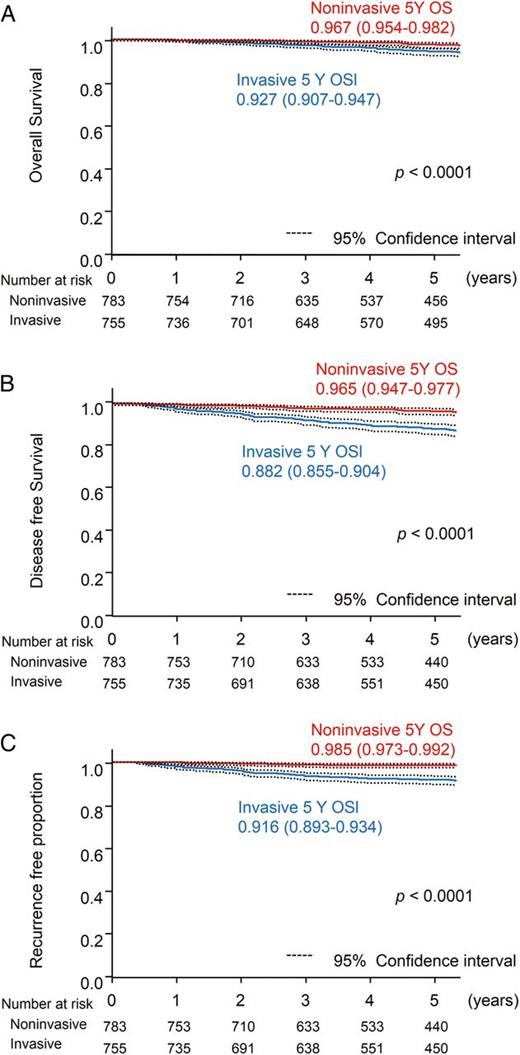
Radiological findings and prognosis of the 1538 cT1aN0M0 patients with a single lesion and without comorbidity of interstitial pneumonia. Comparison between radiological invasive carcinomas (n = 755) and radiological non-invasive carcinomas (n = 783). (A) Five-year overall survival. (B) Five-year disease-free survival. (C) Five-year recurrence-free proportion.
SqCC, the second major pathological type, was analysed separately. One hundred patients with cT1N0M0 SqCC diseases were assessed. The mean tumour size was 1.6 ± 0.6 cm. The overall and disease-free survivals were 83.5 and 76.3% at 5 years, respectively (95 CI: 73.4–90.0 and 65.7–84.0%, respectively). The surgical procedure (segmentectomy or wedge resection), age and comorbidities were prognostic factors in overall survival. In Cox's multivariate analysis, wedge resection was the only unfavourable prognostic factor for overall survival (P = 0.02, HR = 2.8) (Fig. 6A). In disease-free survival, an unfavourable prognostic factor was not apparent (wedge resection: P = 0.09, Fig. 6B).
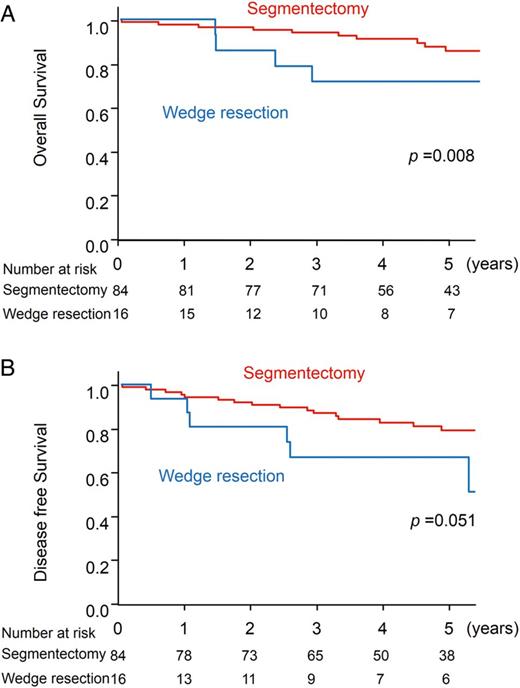
Prognosis of the 100 patients with s cT1N0M0 squamous cell carcinoma disease. (A) Five-year overall survival. (B) Five-year disease-free survival.
DISCUSSION
In the present study, excellent results were seen for patients who had limited resections for clinical stage IA NSCLC and who were diagnosed as able to tolerate lobectomy and were 75 years or younger at the time of operation. This study predominantly included GGNs and part-solid nodules. Unfortunately, we did not know the precise ratio of GGNs; even in radiological invasive carcinomas, many part-solid nodules were included. The 5-year overall survival was 94.0% and was not inferior to the reported survival of lobectomy patients of cStage IA adenocarcinoma [11]. In early-stage NSCLC, curative surgical resection has been considered to be the most important determinant of prognosis [1, 2]. Lobectomy with lymph node dissection remains the standard procedure in tolerable cases. Ginsberg and Rubinstein [3] compared the results of lobectomy and limited resection for stage I lung cancer in 1995. To date, this has been the only randomized trial on limited resection versus lobectomy for lung cancer. Higher death and local recurrence rates were demonstrated in patients undergoing limited resection [3]. However, excellent survival data of limited resection have been reported in patients with small-sized NSCLC [4–8]. Maximum tumour diameter has been discussed as a criterion for an acceptable limited resection. A size of <2 cm was proposed in some reports [4–6], while another [6] suggested <1 cm as the criterion to obtain survival in limited resections equivalent to lobectomy [8]. In this study, the maximum tumour diameter was not a significant prognostic factor. However, pT2 and T3 diseases showed significantly unfavourable prognosis. We could not ignore the maximum tumour diameter.
Stage I lung cancer consists of a heterogeneous population and may include latent systemic diseases. A ‘very early’ lung carcinoma is a good candidate for limited resection if it can be defined by CT images or other biological markers. Recently, the definitions of adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) were introduced by the International Association for the Study of Lung Cancer, American Thoracic Society and European Respiratory Society [15]. Pathologically, AIS and MIA are ‘very early’ and may be good candidates for limited resection. Radiological findings that define these early cancers have been proposed. The JCOG conducted a prospective radiological study of thin-section CT to identify criteria that predict pathological noninvasiveness in clinical stage IA lung cancer (JCOG0201) [10, 11]. A C/T ratio of 0.25 or less among cT1a (≤ 2.0 cm) was shown to be a good radiological indication of histologically non-invasive adenocarcinoma [10, 11]. In the present study, the group with a C/T ratio of 0.25 or less showed better prognosis and this C/T ≤ 0.25 seemed to be a good radiological indicator of noninvasive cancer. Lung cancers that met this criterion rarely recurred after limited resection. The recurrence-free proportion was 98.5% at 5 years (95 CI: 97.3–99.2%), (P < 0.0001, Fig. 5C). A prospective phase II study of limited resection for peripheral early lung cancer using the same criteria with thoracic thin-section CT was conducted (JCOG0804/WJOG4507L). Although patient enrolment ended in 2011, more time will be needed before the results of this trial are available. In practice, limited resection for lung cancer has been widely used, especially as an alternative for patients who will not tolerate lobectomy for functional or other reasons [12, 13]. To be sure, there are tumours that are cured by limited resection, especially when the tumour is small and non-invasive. With the demonstration of the validity of radiological criteria of non-invasive adenocarcinoma, more small GGNs are found by high-resolution CT, and limited resection has been increasing in clinical practice. The present study was conducted in order to investigate the best available long-term results to date of limited resections for lung cancers. Although the study was retrospective and not directly comparable with the prospective study of lobectomy reported for early lung cancer [11], limited resection did not appear to be inferior to lobectomy. In our study, patient fitness for lobectomy was diagnosed by the pulmonary function test result and comorbidities, and the diagnostic criteria may have differed from institution to institution. Even with these limitations, the results on a large cohort of 1737 patients offer a reliable indication that limited resection for selected clinical stage I NSCLC patients is a satisfactory curative surgical procedure. The 95% CIs were extremely narrow. The overall survival in 1737 patients was 94.0% at 5 years (95 CI: 92.6–95.1%), and disease-free survival was 91.1% at 5 years (95 CI: 89.5–92.4%).
Overall, we found that selection of patients by Japanese surgeons was reasonable. Most of the patients were without IP (98.2%) and had none or only one comorbidity (94.1%). The lung cancers presented were cT1aN0M0 diseases in 90.7%. The recurrence rate was low in such patients. However, the prognosis of the patients who had recurrences was unfavourable. The overall survival in all 122 patients with recurrence was 57.4% at 5 years. The median survival time after recurrence of these patients was 34.7 months. This suggests that the recurring lung cancer had high malignant potential and was different from those that did not recur. CT finding of C/T ratio > 0.25 was a significant prognostic factor for recurrence (HR: 4.72, P < 0.001). The recurrence was diagnosed mostly within 3 years of the operation and the second peak was recognized around 8–9 years postoperatively. Nakao et al. [13] reported the necessity of long-term follow-up for the patients who underwent limited resections for lung cancers with ground-glass opacity nodules which usually grow very slowly.
A subclass analysis was performed on 1538 patients with cT1aN0M0 disease excluding those with multiple lesions or with IP, in order to compare our results with those of JCOG0201 [11]. In that study, the 5-year overall and recurrence-free survivals in 289 patients who underwent lobectomy for cT1aN0M0 adenocarcinoma were 93.0 and 88.9%, respectively [11]. Both the 5-year overall and recurrence-free survival rates in 35 patients of C/T ratio ≤0.25 were 97.1% [11]. These data are very similar to our data of limited resections: 94.7 (95 CI: 93.3–95.8%) and 92.2 (95 CI: 90.7–93.5%) for 5-year overall and recurrence-free survivals, respectively, in the JCOG0201; 96.7 (95 CI: 95.4–98.2%) and 96.5 (95 CI: 94.7–97.7%) for the 5-year overall and recurrence-free survivals, respectively, in the 783 patients with tumours of C/T ratio ≤0.25 in our cohort. The lower limit of the 95% CI for the overall survival rate at 5 years in our limited resection patients is higher than that of the lobectomy patients in the JCOG 0201 study. Although it is not possible to directly compare the results of the two studies, these data suggest that the efficacy of limited resection for selected NSCLC patients is not inferior to that of lobectomy. We eagerly await the results of the prospective study (JCOG0804/WJOG4507L), which may finally demonstrate the efficacy of limited resections.
Finally, a subclass analysis of 100 SqCC patients was performed. Unfavourable prognostic factor in overall survival was wedge resection. There was no difference between cT1aN0M0 disease and cT1bN0M0 disease. Prognosis of the SqCC patients who underwent segmentectomy was superior to those who underwent wedge resection. This result was different from the data of all 1737 patients or 1538 patients with cT1aN0M0 adenocarcinoma. Differences in procedures of limited resection should be partly dependent on the differences of pathology. The result appears to suggest that for SqCC nodules segmentectomy or larger resection should be selected.
It should be noted that segmentectomy in this study did not provide better protection from recurrence. There were no prognostic differences between segmentectomy and wedge resection in overall survival. In small-sized NSCLCs, segmentectomy has been considered an alternative to lobectomy [4, 5] and to be superior to wedge resections in prognosis of early-stage NSCLCs [4, 5, 16–18]. Smith et al. [18] recently reported that overall and lung cancer-specific survivals were significantly better among patients who underwent segmentectomy than with wedge resection in a large number of stage IA NSCLC patients registered in the SEER registry. In the present study, segmentectomy was selected more frequently than wedge resection in larger tumours (P < 0.0001) and a candidate of radiological invasive tumours (C/T > 0.25) (P < 0.0001). As a result of a subgroup analysis, we found the operative procedure-influenced survival in tumours with SqCC pathology. Segmentectomy should be recommended for SqCC even for small-sized nodules.
We emphasize that our data do not recommend limited resection for solid carcinomas, although the prognosis of limited resection for tumours with C/T ≤ 0.25 was excellent. Those carcinomas with a C/T ratio >0.25 include pure solid nodules which may be aggressive and part-solid nodules which may be less aggressive. We reserve our recommendation of limited resection only for carcinomas with C/T ≤ 0.25.
In conclusion, using the data of 1963 patients, excellent results have been shown of limited resections for small-sized NSCLC in patients who would tolerate lobectomy. The prognosis of these patients was no worse than that of the similarly carefully selected lobectomy patients. The carcinomas with C/T ≤ 0.25 are good candidates for limited resection.
ACKNOWLEDGEMENTS
The authors acknowledge the important contribution of the members of the Japanese Association for Chest Surgery and statistical collaboration by Takeshi Nishiyama.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 30th Annual Meeting of the Japanese Association for Chest Surgery, Nagoya, Japan, 9–10 May 2013.
- diabetes mellitus
- pneumonitis, interstitial
- computed tomography
- heart diseases
- carcinoma
- squamous cell carcinoma
- non-small-cell lung carcinoma
- comorbidity
- glass
- objective (goal)
- male
- mastectomy, segmental
- preoperative care
- diagnosis
- neoplasms
- lung volume reduction
- lobectomy
- wedge resection
- lymph node metastasis
- carcinoma of lung
- surgery, curative
- prognostic factors
- diameter




