-
PDF
- Split View
-
Views
-
Cite
Cite
Denis R. Merk, Sven Lehmann, David M. Holzhey, Pascal Dohmen, Pascal Candolfi, Martin Misfeld, Friedrich W. Mohr, Michael A. Borger, Minimal invasive aortic valve replacement surgery is associated with improved survival: a propensity-matched comparison, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 1, January 2015, Pages 11–17, https://doi.org/10.1093/ejcts/ezu068
Close - Share Icon Share
Abstract
To compare early and long-term outcomes of minimally invasive surgery (MIS) versus full sternotomy (FS) isolated aortic valve replacement (AVR).
We retrospectively analysed all patients who underwent isolated bioprosthetic AVR between 2003 and March 2012 at our institution. Matching was performed based on a propensity score, which was obtained using the output of a logistic regression on relevant preoperative risk factors. Mean follow-up was 3.1 ± 2.7 years (range 0–9.0 years) and was 99.8% complete.
A total of 2051 patients (FS, 1572; MIS, 479) underwent isolated bioprosthetic AVR during the study period. MIS patients were significantly younger (67.8 ± 11.2 vs 70.4 ± 9.4 years) and had a lower logistic EuroSCORE (6.6 ± 6.4 vs 11.2 ± 13.4%, both P < 0.001). Propensity matching resulted in 477 matched patients from each group, with no significant differences in any of the preoperative variables. Aortic cross-clamp times were significantly longer in MIS patients (59.4 ± 16.0 vs 56.9 ± 14.6 min, P = 0.008). Nonetheless, MIS AVR was associated with a significantly lower incidence of intra-aortic balloon pump usage (0.4 vs 2.1%, P = 0.042) and in-hospital mortality (0.4 vs 2.3%, P = 0.013), while FS patients had a lower rate of re-exploration for bleeding (1.5 vs 4.2%, P = 0.019). Five- and 8-year survival post-AVR was significantly higher in MIS patients (89.3 ± 2.4% and 77.7 ± 4.7% vs 81.8 ± 2.2% and 72.8 ± 3.1%, respectively, P = 0.034). Cox regression analysis revealed MIS (hazard ratio: 0.47, 95% confidence interval: 0.26–0.87) as an independent predictor of long-term survival.
MIS AVR is associated with very good early and long-term survival, despite longer myocardial ischaemic times. MIS AVR can be performed safely with results that are at least equivalent to those achieved through an FS.
INTRODUCTION
Aortic valve replacement (AVR) has been the gold standard for treatment of severe aortic stenosis for the last 40 years. AVR was performed for decades via a median sternotomy with direct aortic and right atrial cannulation for cardiopulmonary bypass (CPB). A significant change in surgical techniques was heralded by the first minimally invasive surgery (MIS) AVR performed by Cosgrove and Sabik [1] in 1996. MIS AVR has been reported to offer several benefits over conventional full sternotomy (FS) procedures such as better cosmesis, reduced pain, reduced surgical trauma, decreased blood loss, earlier functional recovery and shorter hospital stay [2]. However, not all studies have confirmed a beneficial effect for MIS surgery [3].
Various surgical approaches have been developed for MIS AVR surgery. Currently, the most commonly performed MIS access route is via a partial upper sternotomy that extends into the third or fourth intercostal space, referred to as a ‘J’ sternotomy or an inverted ‘T’ sternotomy [4, 5]. However, a right anterior lateral minithoracotomy approach has been successfully employed in select centres [6].
The aim of this study was to compare early and long-term outcomes of MIS vs FS surgery in a contemporary cohort of patients undergoing isolated AVR. In order to control for patient selection, we also performed a propensity-matched comparison of outcomes.
MATERIALS AND METHODS
Patients
We performed a database search and identified 2364 consecutive patients who underwent isolated AVR at our institution between 2003 and March 2012. We excluded patients who underwent isolated mechanical AVR (n = 313) in order to minimize the effect of age on patient selection. We did not exclude any patients on the basis of surgical timing or aortic valve pathology. Patients requiring concomitant procedures such as coronary artery bypass grafting, mitral or other valve surgery, replacement of the ascending aorta or atrial fibrillation ablation were excluded. Patients undergoing aortic valve repair were also excluded.
The final analysis included two groups of patients who received a bioprosthesis during isolated AVR via either an FS (n = 1572) or an MIS procedure (n = 479). Emergency conversion to sternotomy was required in 4 MIS patients (0.8%) due to severe bleeding (2 patients) or poor exposure of the aortic root (2 patients), but these patients remained in the MIS group for all subsequent comparisons in accordance with the ‘intention-to-treat’ principle.
Patient selection for minimally invasive surgery
The decision of whether patients underwent an FS or MIS approach was predominantly surgeon-specific. Some surgeons used the MIS approach in virtually all patients, while others used an FS approach in virtually all patients. Other surgeons selectively applied MIS to those patients with a normal body mass index (BMI) or a high risk of postoperative deep sternal wound infection, to younger patients or to those patients who explicitly requested an MIS approach.
Surgical technique
Conventional AVR was performed via a full median sternotomy and direct aortic and right atrial cannulation for CPB. Myocardial protection consisted of antegrade or retrograde administration of blood cardioplegia with mild hypothermia, or antegrade administration of crystalloid cardioplegia (Bretschneider; Dr Franz Kohler, Chemie GmbH, Bensheim, Germany). Standard techniques were used to remove the native aortic valve and surrounding calcium, followed by standard insertion of a biological prosthesis.
The MIS procedure was performed with either a ‘J’ or an inverted ‘T’ upper hemisternotomy, using an oscillating saw with extension into the third or preferably fourth intercostal space. Standard cannulation of the ascending aorta was performed directly through the incision. Venous cannulation was performed via the right atrial appendage in the vast majority of patients, with percutaneous femoral vein cannulation being reserved for those patients with poor visualization of the right atrial appendage.
Depending of the surgeon's preference, the left heart was vented through the right superior pulmonary vein or the pulmonary artery. Myocardial protection, removal of the native aortic valve and insertion of the new aortic valve prosthesis were performed as described above for conventional FS surgery.
Data collection and follow-up
All preoperative data and in-hospital outcomes were analysed from our prospectively gathered medical records database. Our database results from an in-house computer system where patient variables and outcomes are entered prospectively by active clinicians. The patient follow-up was performed annually by our in-house study centre and consisted of a mailed paper questionnaire or phone call to the patient or family members, or by contacting the family physician. The mean follow-up time was 3.1 ± 2.7 years (range 0–9.0 years) for a total of 6367 patient-years, and was complete in 2047 patients (99.8%).
Statistics
Quantitative continuous variables are described with means ± standard deviation and quantitative discrete variables with absolute and relative frequencies throughout the manuscript. Inference statistics comparing continuous variables are made using the Wilcoxon rank-sum test. To compare discrete variables, Pearson's χ2 test was applied. Two-sided tests were utilized and a type I error significance level of 0.05 was considered. Early events (≤30 days post implantation) were calculated as simple percentages (number of complications divided by number of patients). Kaplan–Meier actuarial analyses, including both early and late events, were performed with the Greenwood formula for variance. Testing differences between two or more survival curves was done with the use of log-rank. Multivariate Cox proportional hazards regression was performed to estimate the risk factor hazard ratio’s (HRs) effects on mortality.
Propensity score matching was used to reduce the baseline characteristic differences between MIS and FS patients. A multivariable logistic regression model including 12 preoperative risk factors was used to generate the propensity score. Propensity score statistics showed a mean of 0.281 ± 0.091 in the MIS group and a mean of 0.279 ± 0.088 in the FS group. The C-statistic was 0.657, indicating good discrimination. The Cessie–van Houwelingen–Copas–Hosmer unweighted sum of squares test [7] for global goodness-of-fit P-value was 0.229, confirming good calibration and fit of the model. One-to-one matching was performed with the ‘Matching’ library in R using the nearest neighbour matching without replacement algorithm. After matching, comparisons were done using the paired Wilcoxon signed-rank test and the McNemar's test for continuous and discrete variables, respectively. All analyses were performed using the R software, version 2.13.1 [8].
RESULTS
The unmatched comparisons of preoperative variables between MIS and FS patients are listed in Table 1. MIS patients had a significantly lower risk profile as reflected by the fact that they were younger, had a lower predictive logistic EuroSCORE, less diabetes, better left ventricular ejection fraction, higher creatinine clearance and less endocarditis. In addition, MIS patients were less likely to undergo urgent or emergent surgery.
| Variables . | MIS (n = 479) . | FS (n = 1572) . | P-value . |
|---|---|---|---|
| Age, year | 67.8 ± 11.2 | 70.4 ± 9.4 | <0.001 |
| Male sex (male), no. (%) | 279/479 (41.8%) | 862/1572 (45.2%) | 0.21 |
| Weight, kg | 79.6 ± 13.9 | 79.1 ± 15.5 | 0.16 |
| Height, cm | 169.1 ± 8.9 | 167.5 ± 9.2 | 0.002 |
| Body mass index, kg/m2 | 27.8 ± 4.3 | 28.2 ± 5.1 | 0.55 |
| Logistic EuroSCORE, % | 6.6 ± 6.4 | 11.2 ± 13.4 | <0.001 |
| NYHA, no./total no. (%) | 0.014 | ||
| Class I | 38/363 (10.5%) | 67/852 (7.9%) | |
| Class II | 152/363 (41.9%) | 320/852 (37.6%) | |
| Class III | 160/363 (44.1%) | 398/852 (46.7%) | |
| Class IV | 13/363 (3.6%) | 67/852 (7.9%) | |
| Clinical history, no./total no. (%) | |||
| Diabetes mellitus | 135/478 (28.2%) | 545/1571 (34.7%) | 0.010 |
| Myocardial infarction | 21/478 (4.4%) | 101/1571 (6.4%) | 0.12 |
| Hyperlipidaemia | 234/478 (49.0%) | 784/1568 (50.0%) | 0.73 |
| Hypertension | 400/478 (83.7%) | 1313/1570 (83.6%) | 0.97 |
| Pulmonary hypertension | 18/478 (3.8%) | 104/1565 (6.6%) | 0.027 |
| Liver failure | 12/478 (2.5%) | 72/1570 (4.6%) | 0.061 |
| Cerebrovascular accident | 22/479 (4.6%) | 94/1572 (6.0%) | 0.30 |
| Peripheral vascular disease | 69/479 (14.4%) | 271/1569 (17.3%) | 0.16 |
| Cardiac surgery | 7/479 (1.5%) | 131/1571 (8.3%) | <0.001 |
| Active endocarditis | 9/479 (1.9%) | 155/1572 (9.9%) | <0.001 |
| COPD | 27/479 (5.6%) | 133/1572 (8.5%) | 0.055 |
| Dialysis | 4/478 (0.8%) | 23/1570 (1.5%) | 0.41 |
| Creatinine, mg/dl | 1.01 ± 0.6 | 1.06 ± 1.7 | 0.60 |
| Creatinine clearance, ml/min | 81.9 ± 28.4 | 77.9 ± 28.4 | 0.002 |
| LVEF, % | 59.1 ± 15.7 | 56.2 ± 18.4 | 0.012 |
| Prior PCI, no./total no. (%) | 29/478 (6.1%) | 105/1572 (6.7%) | 0.71 |
| Timing, no./total no. (%) | <0.001 | ||
| Elective | 451/479 (94.2%) | 1257/1572 (80%) | |
| Urgent | 25/479 (5.2%) | 191/1572 (12.2%) | |
| Emergency | 3/479 (0.6%) | 1124/1572 (7.8%) | |
| Variables . | MIS (n = 479) . | FS (n = 1572) . | P-value . |
|---|---|---|---|
| Age, year | 67.8 ± 11.2 | 70.4 ± 9.4 | <0.001 |
| Male sex (male), no. (%) | 279/479 (41.8%) | 862/1572 (45.2%) | 0.21 |
| Weight, kg | 79.6 ± 13.9 | 79.1 ± 15.5 | 0.16 |
| Height, cm | 169.1 ± 8.9 | 167.5 ± 9.2 | 0.002 |
| Body mass index, kg/m2 | 27.8 ± 4.3 | 28.2 ± 5.1 | 0.55 |
| Logistic EuroSCORE, % | 6.6 ± 6.4 | 11.2 ± 13.4 | <0.001 |
| NYHA, no./total no. (%) | 0.014 | ||
| Class I | 38/363 (10.5%) | 67/852 (7.9%) | |
| Class II | 152/363 (41.9%) | 320/852 (37.6%) | |
| Class III | 160/363 (44.1%) | 398/852 (46.7%) | |
| Class IV | 13/363 (3.6%) | 67/852 (7.9%) | |
| Clinical history, no./total no. (%) | |||
| Diabetes mellitus | 135/478 (28.2%) | 545/1571 (34.7%) | 0.010 |
| Myocardial infarction | 21/478 (4.4%) | 101/1571 (6.4%) | 0.12 |
| Hyperlipidaemia | 234/478 (49.0%) | 784/1568 (50.0%) | 0.73 |
| Hypertension | 400/478 (83.7%) | 1313/1570 (83.6%) | 0.97 |
| Pulmonary hypertension | 18/478 (3.8%) | 104/1565 (6.6%) | 0.027 |
| Liver failure | 12/478 (2.5%) | 72/1570 (4.6%) | 0.061 |
| Cerebrovascular accident | 22/479 (4.6%) | 94/1572 (6.0%) | 0.30 |
| Peripheral vascular disease | 69/479 (14.4%) | 271/1569 (17.3%) | 0.16 |
| Cardiac surgery | 7/479 (1.5%) | 131/1571 (8.3%) | <0.001 |
| Active endocarditis | 9/479 (1.9%) | 155/1572 (9.9%) | <0.001 |
| COPD | 27/479 (5.6%) | 133/1572 (8.5%) | 0.055 |
| Dialysis | 4/478 (0.8%) | 23/1570 (1.5%) | 0.41 |
| Creatinine, mg/dl | 1.01 ± 0.6 | 1.06 ± 1.7 | 0.60 |
| Creatinine clearance, ml/min | 81.9 ± 28.4 | 77.9 ± 28.4 | 0.002 |
| LVEF, % | 59.1 ± 15.7 | 56.2 ± 18.4 | 0.012 |
| Prior PCI, no./total no. (%) | 29/478 (6.1%) | 105/1572 (6.7%) | 0.71 |
| Timing, no./total no. (%) | <0.001 | ||
| Elective | 451/479 (94.2%) | 1257/1572 (80%) | |
| Urgent | 25/479 (5.2%) | 191/1572 (12.2%) | |
| Emergency | 3/479 (0.6%) | 1124/1572 (7.8%) | |
NYHA: New York Heart Association; COPD: chronic obstructive lung disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention.
| Variables . | MIS (n = 479) . | FS (n = 1572) . | P-value . |
|---|---|---|---|
| Age, year | 67.8 ± 11.2 | 70.4 ± 9.4 | <0.001 |
| Male sex (male), no. (%) | 279/479 (41.8%) | 862/1572 (45.2%) | 0.21 |
| Weight, kg | 79.6 ± 13.9 | 79.1 ± 15.5 | 0.16 |
| Height, cm | 169.1 ± 8.9 | 167.5 ± 9.2 | 0.002 |
| Body mass index, kg/m2 | 27.8 ± 4.3 | 28.2 ± 5.1 | 0.55 |
| Logistic EuroSCORE, % | 6.6 ± 6.4 | 11.2 ± 13.4 | <0.001 |
| NYHA, no./total no. (%) | 0.014 | ||
| Class I | 38/363 (10.5%) | 67/852 (7.9%) | |
| Class II | 152/363 (41.9%) | 320/852 (37.6%) | |
| Class III | 160/363 (44.1%) | 398/852 (46.7%) | |
| Class IV | 13/363 (3.6%) | 67/852 (7.9%) | |
| Clinical history, no./total no. (%) | |||
| Diabetes mellitus | 135/478 (28.2%) | 545/1571 (34.7%) | 0.010 |
| Myocardial infarction | 21/478 (4.4%) | 101/1571 (6.4%) | 0.12 |
| Hyperlipidaemia | 234/478 (49.0%) | 784/1568 (50.0%) | 0.73 |
| Hypertension | 400/478 (83.7%) | 1313/1570 (83.6%) | 0.97 |
| Pulmonary hypertension | 18/478 (3.8%) | 104/1565 (6.6%) | 0.027 |
| Liver failure | 12/478 (2.5%) | 72/1570 (4.6%) | 0.061 |
| Cerebrovascular accident | 22/479 (4.6%) | 94/1572 (6.0%) | 0.30 |
| Peripheral vascular disease | 69/479 (14.4%) | 271/1569 (17.3%) | 0.16 |
| Cardiac surgery | 7/479 (1.5%) | 131/1571 (8.3%) | <0.001 |
| Active endocarditis | 9/479 (1.9%) | 155/1572 (9.9%) | <0.001 |
| COPD | 27/479 (5.6%) | 133/1572 (8.5%) | 0.055 |
| Dialysis | 4/478 (0.8%) | 23/1570 (1.5%) | 0.41 |
| Creatinine, mg/dl | 1.01 ± 0.6 | 1.06 ± 1.7 | 0.60 |
| Creatinine clearance, ml/min | 81.9 ± 28.4 | 77.9 ± 28.4 | 0.002 |
| LVEF, % | 59.1 ± 15.7 | 56.2 ± 18.4 | 0.012 |
| Prior PCI, no./total no. (%) | 29/478 (6.1%) | 105/1572 (6.7%) | 0.71 |
| Timing, no./total no. (%) | <0.001 | ||
| Elective | 451/479 (94.2%) | 1257/1572 (80%) | |
| Urgent | 25/479 (5.2%) | 191/1572 (12.2%) | |
| Emergency | 3/479 (0.6%) | 1124/1572 (7.8%) | |
| Variables . | MIS (n = 479) . | FS (n = 1572) . | P-value . |
|---|---|---|---|
| Age, year | 67.8 ± 11.2 | 70.4 ± 9.4 | <0.001 |
| Male sex (male), no. (%) | 279/479 (41.8%) | 862/1572 (45.2%) | 0.21 |
| Weight, kg | 79.6 ± 13.9 | 79.1 ± 15.5 | 0.16 |
| Height, cm | 169.1 ± 8.9 | 167.5 ± 9.2 | 0.002 |
| Body mass index, kg/m2 | 27.8 ± 4.3 | 28.2 ± 5.1 | 0.55 |
| Logistic EuroSCORE, % | 6.6 ± 6.4 | 11.2 ± 13.4 | <0.001 |
| NYHA, no./total no. (%) | 0.014 | ||
| Class I | 38/363 (10.5%) | 67/852 (7.9%) | |
| Class II | 152/363 (41.9%) | 320/852 (37.6%) | |
| Class III | 160/363 (44.1%) | 398/852 (46.7%) | |
| Class IV | 13/363 (3.6%) | 67/852 (7.9%) | |
| Clinical history, no./total no. (%) | |||
| Diabetes mellitus | 135/478 (28.2%) | 545/1571 (34.7%) | 0.010 |
| Myocardial infarction | 21/478 (4.4%) | 101/1571 (6.4%) | 0.12 |
| Hyperlipidaemia | 234/478 (49.0%) | 784/1568 (50.0%) | 0.73 |
| Hypertension | 400/478 (83.7%) | 1313/1570 (83.6%) | 0.97 |
| Pulmonary hypertension | 18/478 (3.8%) | 104/1565 (6.6%) | 0.027 |
| Liver failure | 12/478 (2.5%) | 72/1570 (4.6%) | 0.061 |
| Cerebrovascular accident | 22/479 (4.6%) | 94/1572 (6.0%) | 0.30 |
| Peripheral vascular disease | 69/479 (14.4%) | 271/1569 (17.3%) | 0.16 |
| Cardiac surgery | 7/479 (1.5%) | 131/1571 (8.3%) | <0.001 |
| Active endocarditis | 9/479 (1.9%) | 155/1572 (9.9%) | <0.001 |
| COPD | 27/479 (5.6%) | 133/1572 (8.5%) | 0.055 |
| Dialysis | 4/478 (0.8%) | 23/1570 (1.5%) | 0.41 |
| Creatinine, mg/dl | 1.01 ± 0.6 | 1.06 ± 1.7 | 0.60 |
| Creatinine clearance, ml/min | 81.9 ± 28.4 | 77.9 ± 28.4 | 0.002 |
| LVEF, % | 59.1 ± 15.7 | 56.2 ± 18.4 | 0.012 |
| Prior PCI, no./total no. (%) | 29/478 (6.1%) | 105/1572 (6.7%) | 0.71 |
| Timing, no./total no. (%) | <0.001 | ||
| Elective | 451/479 (94.2%) | 1257/1572 (80%) | |
| Urgent | 25/479 (5.2%) | 191/1572 (12.2%) | |
| Emergency | 3/479 (0.6%) | 1124/1572 (7.8%) | |
NYHA: New York Heart Association; COPD: chronic obstructive lung disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention.
Examination of intraoperative variables revealed that FS patients had a significantly shorter aortic cross-clamp time (56.1 ± 17.3 vs 59.0 ± 16.8 min, P < 0.001), but similar CPB times (82.3 ± 21.7 vs 82.2 ± 25.7 min, P = 0.184). The total length of surgery was also significantly shorter for FS patients (148.4 ± 44.3 vs 156.5 ± 33.5 min, P < 0.001).
Examination of early postoperative outcomes revealed that MIS patients had a significantly lower prevalence of low cardiac output syndrome (0.6 vs 2.9% P < 0.001), IABP implantation (0.4 vs 3.2% P < 0.001) and less cerebral vascular accidents (0.9 vs 2.6%, P < 0.001). Postoperative respiratory failure rates were higher for FS patients (10.8 vs 6.7%, P = 0.011), although they had a significantly lower rate of re-exploration for bleeding (1.3 vs 4.2%, P < 0.001).
In-hospital mortality was significantly lower in MIS patients (0.6 vs 4.1%, P < 0.001). Kaplan–Meier analysis revealed a significantly lower survival in FS patients at 5 years (73.6 ± 1.3 vs 88.2 ± 2.5%) and 8 years (59.3 ± 2.2 vs 76.7 ± 4.7%, P < 0.001) postoperatively (Fig. 1).
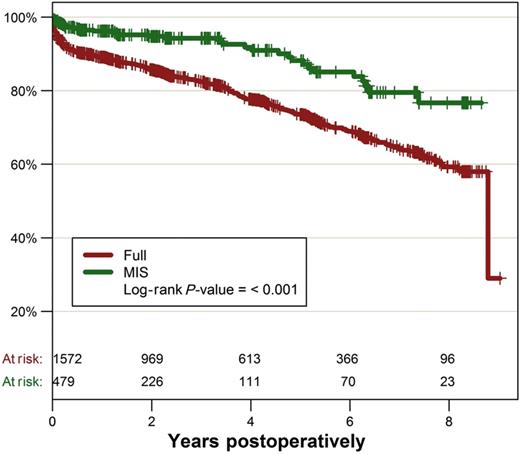
Propensity-matched comparison
We performed a propensity-matched comparison in order to reduce the above-described baseline characteristic differences between MIS and FS patients. The multivariable logistic regression model included 12 risk factors that were used to generate a propensity score (Table 2). Propensity matching resulted in two groups of 477 patients with a similar risk profile. The only differences in preoperative risk factors that persisted after propensity matching were patient height and preoperative serum creatinine, although creatinine clearance was similar between groups (Table 3). Similar to the findings in the unmatched groups, MIS patients had significantly longer aortic cross clamp (59.4 ± 16.0 vs 56.9 ± 14.6 min, P = 0.017) and operative times (156.3 ± 33.4 vs 145.1 ± 30.5 min, P < 0.001) than FS patients, with no significant difference in CPB times (82.2 ± 21.7 vs 81.0 ± 19.9 min, P = 0.641).
Preoperative variables used to generate the logistic regression propensity score
| Variables . | Estimate . | Standard error . | P-value . |
|---|---|---|---|
| Intercept | 1.100 | 0.413 | 0.008 |
| Logistic EuroSCORE | −0.008 | 0.012 | 0.50 |
| Age | −0.028 | 0.006 | <0.001 |
| Diabetes | −0.202 | 0.120 | 0.092 |
| Previous myocardial infarction | 0.097 | 0.264 | 0.71 |
| Pulmonary hypertension | −0.257 | 0.294 | 0.38 |
| Liver failure | −0.441 | 0.329 | 0.18 |
| Cerebrovascular accident | 0.231 | 0.276 | 0.40 |
| Active endocarditis | −0.880 | 0.405 | 0.030 |
| COPD | −0.166 | 0.231 | 0.47 |
| Dialysis | 0.068 | 0.593 | 0.91 |
| Prior cardiac surgery | −1.417 | 0.425 | 0.001 |
| Timing | |||
| Elective | −0.681 | 0.230 | 0.003 |
| Urgent | −2.068 | 0.663 | 0.002 |
| Emergency | −13.032 | 340.880 | 0.97 |
| Variables . | Estimate . | Standard error . | P-value . |
|---|---|---|---|
| Intercept | 1.100 | 0.413 | 0.008 |
| Logistic EuroSCORE | −0.008 | 0.012 | 0.50 |
| Age | −0.028 | 0.006 | <0.001 |
| Diabetes | −0.202 | 0.120 | 0.092 |
| Previous myocardial infarction | 0.097 | 0.264 | 0.71 |
| Pulmonary hypertension | −0.257 | 0.294 | 0.38 |
| Liver failure | −0.441 | 0.329 | 0.18 |
| Cerebrovascular accident | 0.231 | 0.276 | 0.40 |
| Active endocarditis | −0.880 | 0.405 | 0.030 |
| COPD | −0.166 | 0.231 | 0.47 |
| Dialysis | 0.068 | 0.593 | 0.91 |
| Prior cardiac surgery | −1.417 | 0.425 | 0.001 |
| Timing | |||
| Elective | −0.681 | 0.230 | 0.003 |
| Urgent | −2.068 | 0.663 | 0.002 |
| Emergency | −13.032 | 340.880 | 0.97 |
COPD: chronic obstructive lung disease.
Preoperative variables used to generate the logistic regression propensity score
| Variables . | Estimate . | Standard error . | P-value . |
|---|---|---|---|
| Intercept | 1.100 | 0.413 | 0.008 |
| Logistic EuroSCORE | −0.008 | 0.012 | 0.50 |
| Age | −0.028 | 0.006 | <0.001 |
| Diabetes | −0.202 | 0.120 | 0.092 |
| Previous myocardial infarction | 0.097 | 0.264 | 0.71 |
| Pulmonary hypertension | −0.257 | 0.294 | 0.38 |
| Liver failure | −0.441 | 0.329 | 0.18 |
| Cerebrovascular accident | 0.231 | 0.276 | 0.40 |
| Active endocarditis | −0.880 | 0.405 | 0.030 |
| COPD | −0.166 | 0.231 | 0.47 |
| Dialysis | 0.068 | 0.593 | 0.91 |
| Prior cardiac surgery | −1.417 | 0.425 | 0.001 |
| Timing | |||
| Elective | −0.681 | 0.230 | 0.003 |
| Urgent | −2.068 | 0.663 | 0.002 |
| Emergency | −13.032 | 340.880 | 0.97 |
| Variables . | Estimate . | Standard error . | P-value . |
|---|---|---|---|
| Intercept | 1.100 | 0.413 | 0.008 |
| Logistic EuroSCORE | −0.008 | 0.012 | 0.50 |
| Age | −0.028 | 0.006 | <0.001 |
| Diabetes | −0.202 | 0.120 | 0.092 |
| Previous myocardial infarction | 0.097 | 0.264 | 0.71 |
| Pulmonary hypertension | −0.257 | 0.294 | 0.38 |
| Liver failure | −0.441 | 0.329 | 0.18 |
| Cerebrovascular accident | 0.231 | 0.276 | 0.40 |
| Active endocarditis | −0.880 | 0.405 | 0.030 |
| COPD | −0.166 | 0.231 | 0.47 |
| Dialysis | 0.068 | 0.593 | 0.91 |
| Prior cardiac surgery | −1.417 | 0.425 | 0.001 |
| Timing | |||
| Elective | −0.681 | 0.230 | 0.003 |
| Urgent | −2.068 | 0.663 | 0.002 |
| Emergency | −13.032 | 340.880 | 0.97 |
COPD: chronic obstructive lung disease.
| Variables . | MIS (n = 477) . | FS (n = 477) . | P-value . |
|---|---|---|---|
| Age, year | 67.8 ± 11.2 | 67.5 ± 11.0 | 0.32 |
| Male sex (male), no. (%) | 279/477 (58.5%) | 267/477 (56.0%) | 0.43 |
| Weight, km | 79.7 ± 13.9 | 79.5 ± 15.6 | 0.54 |
| Height, cm | 169.2 ± 8.8 | 167.8 ± 9.3 | 0.013 |
| Body mass index, kg/m2 | 27.8 ± 4.3 | 28.2 ± 4.8 | 0.27 |
| Logistic EuroSCORE, % | 6.6 ± 6.4 | 6.6 ± 6.8 | 0.66 |
| NYHA, no./total no. (%) | 0.98 | ||
| Class I | 23/248 (9.3%) | 17/248 (6.9%) | |
| Class II | 108/248 (43.5%) | 114/248 (46%) | |
| Class III | 109/248 (44%) | 106/248 (42.7%) | |
| Class IV | 8/248 (3.2%) | 11/248 (4.4%) | |
| Clinical history, no./total no. (%) | |||
| Diabetes | 135/477 (28.3%) | 119/477 (24.9%) | 0.20 |
| Myocardial infarction | 21/477 (4.4%) | 20/477 (4.2%) | 0.87 |
| Hyperlipidaemia | 234/476 (49.2%) | 217/476 (45.6%) | 0.28 |
| Arterial hypertension | 399/477 (83.6%) | 382/477 (80.1%) | 0.14 |
| Pulmonary hypertension | 18/477 (3.8%) | 22/477 (4.6%) | 0.51 |
| Liver failure | 12/477 (2.5%) | 15/477 (3.1%) | 0.56 |
| Cerebrovascular accident | 22/477 (4.6%) | 17/477 (3.6%) | 0.40 |
| Peripheral vascular disease | 69/476 (14.5%) | 72/476 (15.1%) | 0.79 |
| Previous cardiac surgery | 7/477 (1.5%) | 6/477 (1.3%) | 0.76 |
| Active endocarditis | 9/477 (1.9%) | 9/477 (1.9%) | 1.0 |
| COPD | 27/477 (5.7%) | 32/477 (6.7%) | 0.46 |
| Prior PCI | 29/477 (6.1%) | 32/477 (6.7%) | 0.69 |
| Dialysis | 4/477 (0.8%) | 4/477 (0.8%) | 1.0 |
| Creatinine, mg/dl | 1.0 ± 0.5 | 0.9 ± 0.4 | 0.047 |
| Creatinine clearance, ml/min | 82 ± 28.2 | 83.6 ± 27.6 | 0.31 |
| LVEF, % | 59.1 ± 15.4 | 57.5 ± 18.2 | 0.23 |
| Timing, no./total no. (%) | 0.55 | ||
| Elective | 449/477 (94.1%) | 441/477 (92.5%) | |
| Urgent | 25/477 (5.2%) | 31/477 (6.5%) | |
| Emergency | 3/477 (0.6%) | 5/477 (1%) | |
| Variables . | MIS (n = 477) . | FS (n = 477) . | P-value . |
|---|---|---|---|
| Age, year | 67.8 ± 11.2 | 67.5 ± 11.0 | 0.32 |
| Male sex (male), no. (%) | 279/477 (58.5%) | 267/477 (56.0%) | 0.43 |
| Weight, km | 79.7 ± 13.9 | 79.5 ± 15.6 | 0.54 |
| Height, cm | 169.2 ± 8.8 | 167.8 ± 9.3 | 0.013 |
| Body mass index, kg/m2 | 27.8 ± 4.3 | 28.2 ± 4.8 | 0.27 |
| Logistic EuroSCORE, % | 6.6 ± 6.4 | 6.6 ± 6.8 | 0.66 |
| NYHA, no./total no. (%) | 0.98 | ||
| Class I | 23/248 (9.3%) | 17/248 (6.9%) | |
| Class II | 108/248 (43.5%) | 114/248 (46%) | |
| Class III | 109/248 (44%) | 106/248 (42.7%) | |
| Class IV | 8/248 (3.2%) | 11/248 (4.4%) | |
| Clinical history, no./total no. (%) | |||
| Diabetes | 135/477 (28.3%) | 119/477 (24.9%) | 0.20 |
| Myocardial infarction | 21/477 (4.4%) | 20/477 (4.2%) | 0.87 |
| Hyperlipidaemia | 234/476 (49.2%) | 217/476 (45.6%) | 0.28 |
| Arterial hypertension | 399/477 (83.6%) | 382/477 (80.1%) | 0.14 |
| Pulmonary hypertension | 18/477 (3.8%) | 22/477 (4.6%) | 0.51 |
| Liver failure | 12/477 (2.5%) | 15/477 (3.1%) | 0.56 |
| Cerebrovascular accident | 22/477 (4.6%) | 17/477 (3.6%) | 0.40 |
| Peripheral vascular disease | 69/476 (14.5%) | 72/476 (15.1%) | 0.79 |
| Previous cardiac surgery | 7/477 (1.5%) | 6/477 (1.3%) | 0.76 |
| Active endocarditis | 9/477 (1.9%) | 9/477 (1.9%) | 1.0 |
| COPD | 27/477 (5.7%) | 32/477 (6.7%) | 0.46 |
| Prior PCI | 29/477 (6.1%) | 32/477 (6.7%) | 0.69 |
| Dialysis | 4/477 (0.8%) | 4/477 (0.8%) | 1.0 |
| Creatinine, mg/dl | 1.0 ± 0.5 | 0.9 ± 0.4 | 0.047 |
| Creatinine clearance, ml/min | 82 ± 28.2 | 83.6 ± 27.6 | 0.31 |
| LVEF, % | 59.1 ± 15.4 | 57.5 ± 18.2 | 0.23 |
| Timing, no./total no. (%) | 0.55 | ||
| Elective | 449/477 (94.1%) | 441/477 (92.5%) | |
| Urgent | 25/477 (5.2%) | 31/477 (6.5%) | |
| Emergency | 3/477 (0.6%) | 5/477 (1%) | |
NYHA: New York Heart Association; COPD: chronic obstructive lung disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention.
| Variables . | MIS (n = 477) . | FS (n = 477) . | P-value . |
|---|---|---|---|
| Age, year | 67.8 ± 11.2 | 67.5 ± 11.0 | 0.32 |
| Male sex (male), no. (%) | 279/477 (58.5%) | 267/477 (56.0%) | 0.43 |
| Weight, km | 79.7 ± 13.9 | 79.5 ± 15.6 | 0.54 |
| Height, cm | 169.2 ± 8.8 | 167.8 ± 9.3 | 0.013 |
| Body mass index, kg/m2 | 27.8 ± 4.3 | 28.2 ± 4.8 | 0.27 |
| Logistic EuroSCORE, % | 6.6 ± 6.4 | 6.6 ± 6.8 | 0.66 |
| NYHA, no./total no. (%) | 0.98 | ||
| Class I | 23/248 (9.3%) | 17/248 (6.9%) | |
| Class II | 108/248 (43.5%) | 114/248 (46%) | |
| Class III | 109/248 (44%) | 106/248 (42.7%) | |
| Class IV | 8/248 (3.2%) | 11/248 (4.4%) | |
| Clinical history, no./total no. (%) | |||
| Diabetes | 135/477 (28.3%) | 119/477 (24.9%) | 0.20 |
| Myocardial infarction | 21/477 (4.4%) | 20/477 (4.2%) | 0.87 |
| Hyperlipidaemia | 234/476 (49.2%) | 217/476 (45.6%) | 0.28 |
| Arterial hypertension | 399/477 (83.6%) | 382/477 (80.1%) | 0.14 |
| Pulmonary hypertension | 18/477 (3.8%) | 22/477 (4.6%) | 0.51 |
| Liver failure | 12/477 (2.5%) | 15/477 (3.1%) | 0.56 |
| Cerebrovascular accident | 22/477 (4.6%) | 17/477 (3.6%) | 0.40 |
| Peripheral vascular disease | 69/476 (14.5%) | 72/476 (15.1%) | 0.79 |
| Previous cardiac surgery | 7/477 (1.5%) | 6/477 (1.3%) | 0.76 |
| Active endocarditis | 9/477 (1.9%) | 9/477 (1.9%) | 1.0 |
| COPD | 27/477 (5.7%) | 32/477 (6.7%) | 0.46 |
| Prior PCI | 29/477 (6.1%) | 32/477 (6.7%) | 0.69 |
| Dialysis | 4/477 (0.8%) | 4/477 (0.8%) | 1.0 |
| Creatinine, mg/dl | 1.0 ± 0.5 | 0.9 ± 0.4 | 0.047 |
| Creatinine clearance, ml/min | 82 ± 28.2 | 83.6 ± 27.6 | 0.31 |
| LVEF, % | 59.1 ± 15.4 | 57.5 ± 18.2 | 0.23 |
| Timing, no./total no. (%) | 0.55 | ||
| Elective | 449/477 (94.1%) | 441/477 (92.5%) | |
| Urgent | 25/477 (5.2%) | 31/477 (6.5%) | |
| Emergency | 3/477 (0.6%) | 5/477 (1%) | |
| Variables . | MIS (n = 477) . | FS (n = 477) . | P-value . |
|---|---|---|---|
| Age, year | 67.8 ± 11.2 | 67.5 ± 11.0 | 0.32 |
| Male sex (male), no. (%) | 279/477 (58.5%) | 267/477 (56.0%) | 0.43 |
| Weight, km | 79.7 ± 13.9 | 79.5 ± 15.6 | 0.54 |
| Height, cm | 169.2 ± 8.8 | 167.8 ± 9.3 | 0.013 |
| Body mass index, kg/m2 | 27.8 ± 4.3 | 28.2 ± 4.8 | 0.27 |
| Logistic EuroSCORE, % | 6.6 ± 6.4 | 6.6 ± 6.8 | 0.66 |
| NYHA, no./total no. (%) | 0.98 | ||
| Class I | 23/248 (9.3%) | 17/248 (6.9%) | |
| Class II | 108/248 (43.5%) | 114/248 (46%) | |
| Class III | 109/248 (44%) | 106/248 (42.7%) | |
| Class IV | 8/248 (3.2%) | 11/248 (4.4%) | |
| Clinical history, no./total no. (%) | |||
| Diabetes | 135/477 (28.3%) | 119/477 (24.9%) | 0.20 |
| Myocardial infarction | 21/477 (4.4%) | 20/477 (4.2%) | 0.87 |
| Hyperlipidaemia | 234/476 (49.2%) | 217/476 (45.6%) | 0.28 |
| Arterial hypertension | 399/477 (83.6%) | 382/477 (80.1%) | 0.14 |
| Pulmonary hypertension | 18/477 (3.8%) | 22/477 (4.6%) | 0.51 |
| Liver failure | 12/477 (2.5%) | 15/477 (3.1%) | 0.56 |
| Cerebrovascular accident | 22/477 (4.6%) | 17/477 (3.6%) | 0.40 |
| Peripheral vascular disease | 69/476 (14.5%) | 72/476 (15.1%) | 0.79 |
| Previous cardiac surgery | 7/477 (1.5%) | 6/477 (1.3%) | 0.76 |
| Active endocarditis | 9/477 (1.9%) | 9/477 (1.9%) | 1.0 |
| COPD | 27/477 (5.7%) | 32/477 (6.7%) | 0.46 |
| Prior PCI | 29/477 (6.1%) | 32/477 (6.7%) | 0.69 |
| Dialysis | 4/477 (0.8%) | 4/477 (0.8%) | 1.0 |
| Creatinine, mg/dl | 1.0 ± 0.5 | 0.9 ± 0.4 | 0.047 |
| Creatinine clearance, ml/min | 82 ± 28.2 | 83.6 ± 27.6 | 0.31 |
| LVEF, % | 59.1 ± 15.4 | 57.5 ± 18.2 | 0.23 |
| Timing, no./total no. (%) | 0.55 | ||
| Elective | 449/477 (94.1%) | 441/477 (92.5%) | |
| Urgent | 25/477 (5.2%) | 31/477 (6.5%) | |
| Emergency | 3/477 (0.6%) | 5/477 (1%) | |
NYHA: New York Heart Association; COPD: chronic obstructive lung disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention.
Examination of early postoperative outcomes revealed that the FS group required significantly less major transfusions of red blood cells (defined as more than 3 units) and had a significantly lower re-exploration rate compared with MIS patients (Table 4). In addition, FS patients had a significantly lower incidence of postoperative delirium, although the stroke rate was not different between groups. MIS patients had a significantly lower incidence of IABP implantation, as well as a reduced in-hospital mortality rate (0.4 vs 2.3%, P = 0.013) (Table 4).
| Variables . | MIS (n = 477) . | FS (n = 477) . | P-value . |
|---|---|---|---|
| Low cardiac output | 3/477 (0.6%) | 8/477 (1.7%) | 0.13 |
| Intra-aortic balloon pump | 2/477 (0.4%) | 10/477 (2.1%) | 0.021 |
| Extracorporeal membrane oxygenation | 1/477 (0.2%) | 4/477 (0.8%) | 0.18 |
| Myocardial infarction | 1/477 (0.2%) | 3/477 (0.6%) | 0.32 |
| Cardiac arrhythmia | 159/477 (33.3%) | 171/477 (35.8%) | 0.38 |
| Re-exploration | 20/477 (4.2%) | 7/477 (1.5%) | 0.009 |
| Red blood cell transfusion >3 | 134/477 (28.1%) | 94/477 (19.7%) | 0.002 |
| Delirium | 28/477 (5.9%) | 10/477 (2.1%) | 0.003 |
| Cerebrovascular accident | 3/441 (0.7%) | 9/441 (2.0%) | 0.083 |
| Wound infection | 0/477 (0%) | 0/477 (0%) | – |
| Dialysis | 17/477 (3.6%) | 17/477 (3.6%) | 1.0 |
| Respiratory failure | 32/474 (6.8%) | 33/474 (7.0%) | 0.90 |
| Ventilation >24 h | 30/125 (24.0%) | 24/125 (19.2%) | 0.36 |
| Reintubation | 13/125 (10.4%) | 19/125 (15.2%) | 0.27 |
| Tracheotomy | 7/470 (1.5%) | 10/470 (2.1%) | 0.47 |
| New pacemaker | 20/477 (4.2%) | 20/477 (4.2%) | 1.0 |
| In-hospital mortality | 2/477 (0.4%) | 11/477 (2.3%) | 0.013 |
| Hospital length of stay | 12.9 ± 6.7 | 12.3 ± 5.6 | 0.35 |
| Variables . | MIS (n = 477) . | FS (n = 477) . | P-value . |
|---|---|---|---|
| Low cardiac output | 3/477 (0.6%) | 8/477 (1.7%) | 0.13 |
| Intra-aortic balloon pump | 2/477 (0.4%) | 10/477 (2.1%) | 0.021 |
| Extracorporeal membrane oxygenation | 1/477 (0.2%) | 4/477 (0.8%) | 0.18 |
| Myocardial infarction | 1/477 (0.2%) | 3/477 (0.6%) | 0.32 |
| Cardiac arrhythmia | 159/477 (33.3%) | 171/477 (35.8%) | 0.38 |
| Re-exploration | 20/477 (4.2%) | 7/477 (1.5%) | 0.009 |
| Red blood cell transfusion >3 | 134/477 (28.1%) | 94/477 (19.7%) | 0.002 |
| Delirium | 28/477 (5.9%) | 10/477 (2.1%) | 0.003 |
| Cerebrovascular accident | 3/441 (0.7%) | 9/441 (2.0%) | 0.083 |
| Wound infection | 0/477 (0%) | 0/477 (0%) | – |
| Dialysis | 17/477 (3.6%) | 17/477 (3.6%) | 1.0 |
| Respiratory failure | 32/474 (6.8%) | 33/474 (7.0%) | 0.90 |
| Ventilation >24 h | 30/125 (24.0%) | 24/125 (19.2%) | 0.36 |
| Reintubation | 13/125 (10.4%) | 19/125 (15.2%) | 0.27 |
| Tracheotomy | 7/470 (1.5%) | 10/470 (2.1%) | 0.47 |
| New pacemaker | 20/477 (4.2%) | 20/477 (4.2%) | 1.0 |
| In-hospital mortality | 2/477 (0.4%) | 11/477 (2.3%) | 0.013 |
| Hospital length of stay | 12.9 ± 6.7 | 12.3 ± 5.6 | 0.35 |
| Variables . | MIS (n = 477) . | FS (n = 477) . | P-value . |
|---|---|---|---|
| Low cardiac output | 3/477 (0.6%) | 8/477 (1.7%) | 0.13 |
| Intra-aortic balloon pump | 2/477 (0.4%) | 10/477 (2.1%) | 0.021 |
| Extracorporeal membrane oxygenation | 1/477 (0.2%) | 4/477 (0.8%) | 0.18 |
| Myocardial infarction | 1/477 (0.2%) | 3/477 (0.6%) | 0.32 |
| Cardiac arrhythmia | 159/477 (33.3%) | 171/477 (35.8%) | 0.38 |
| Re-exploration | 20/477 (4.2%) | 7/477 (1.5%) | 0.009 |
| Red blood cell transfusion >3 | 134/477 (28.1%) | 94/477 (19.7%) | 0.002 |
| Delirium | 28/477 (5.9%) | 10/477 (2.1%) | 0.003 |
| Cerebrovascular accident | 3/441 (0.7%) | 9/441 (2.0%) | 0.083 |
| Wound infection | 0/477 (0%) | 0/477 (0%) | – |
| Dialysis | 17/477 (3.6%) | 17/477 (3.6%) | 1.0 |
| Respiratory failure | 32/474 (6.8%) | 33/474 (7.0%) | 0.90 |
| Ventilation >24 h | 30/125 (24.0%) | 24/125 (19.2%) | 0.36 |
| Reintubation | 13/125 (10.4%) | 19/125 (15.2%) | 0.27 |
| Tracheotomy | 7/470 (1.5%) | 10/470 (2.1%) | 0.47 |
| New pacemaker | 20/477 (4.2%) | 20/477 (4.2%) | 1.0 |
| In-hospital mortality | 2/477 (0.4%) | 11/477 (2.3%) | 0.013 |
| Hospital length of stay | 12.9 ± 6.7 | 12.3 ± 5.6 | 0.35 |
| Variables . | MIS (n = 477) . | FS (n = 477) . | P-value . |
|---|---|---|---|
| Low cardiac output | 3/477 (0.6%) | 8/477 (1.7%) | 0.13 |
| Intra-aortic balloon pump | 2/477 (0.4%) | 10/477 (2.1%) | 0.021 |
| Extracorporeal membrane oxygenation | 1/477 (0.2%) | 4/477 (0.8%) | 0.18 |
| Myocardial infarction | 1/477 (0.2%) | 3/477 (0.6%) | 0.32 |
| Cardiac arrhythmia | 159/477 (33.3%) | 171/477 (35.8%) | 0.38 |
| Re-exploration | 20/477 (4.2%) | 7/477 (1.5%) | 0.009 |
| Red blood cell transfusion >3 | 134/477 (28.1%) | 94/477 (19.7%) | 0.002 |
| Delirium | 28/477 (5.9%) | 10/477 (2.1%) | 0.003 |
| Cerebrovascular accident | 3/441 (0.7%) | 9/441 (2.0%) | 0.083 |
| Wound infection | 0/477 (0%) | 0/477 (0%) | – |
| Dialysis | 17/477 (3.6%) | 17/477 (3.6%) | 1.0 |
| Respiratory failure | 32/474 (6.8%) | 33/474 (7.0%) | 0.90 |
| Ventilation >24 h | 30/125 (24.0%) | 24/125 (19.2%) | 0.36 |
| Reintubation | 13/125 (10.4%) | 19/125 (15.2%) | 0.27 |
| Tracheotomy | 7/470 (1.5%) | 10/470 (2.1%) | 0.47 |
| New pacemaker | 20/477 (4.2%) | 20/477 (4.2%) | 1.0 |
| In-hospital mortality | 2/477 (0.4%) | 11/477 (2.3%) | 0.013 |
| Hospital length of stay | 12.9 ± 6.7 | 12.3 ± 5.6 | 0.35 |
Long-term survival for the two groups of matched patients is displayed in Fig. 2. The MIS approach continued to be associated with a significantly better long-term survival (P = 0.034). At 5 years, survival was 89.3 ± 2.4% in the MIS group vs 81.8 ± 2.2% in the FS group, and 77.7 ± 4.7 vs 72.8 ± 3.1% for survival at 8 years.
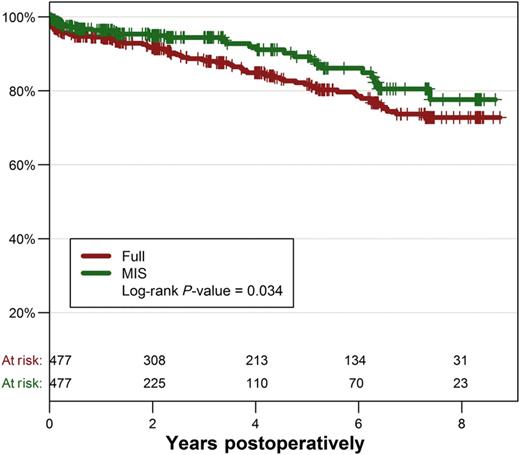
We performed a multivariate Cox regression model to identify independent predictors of long-term mortality in matched patients. Interactions between risk factors and MIS approach were tested and were not significant. The independent risk factor with the highest HR was preoperative dialysis (HR: 7.34, 95% CI: 2.81–19.17), followed by liver failure (HR: 5.03, 95% CI: 2.51–10.08), age (HR: 1.05, 95% CI: 1.02–1.07) and logistic EuroSCORE (HR: 1.03 95% CI: 1.01–1.06). After adjustment for these risk factors, MIS remained significantly protective against long-term mortality (HR: 0.47, 95% CI: 0.26–0.87, P = 0.016).
DISCUSSION
Since the first successful MIS AVR was performed by Cosgrove and Sabik [1] in 1996, MIS procedures have increased in number and evolved in technique. A number of previous publications have shown that MIS is superior to a conventional median sternotomy approach due to shorter hospitalization stay, reduced postoperative ventilation time, less blood loss and lower transfusion rates [9–12]. Although some studies have found contrary results with no obvious benefit for a MIS approach [13, 14], a meta-analysis has confirmed the above-mentioned advantages [2].
Although MIS AVR has several benefits, it is also associated with longer aortic cross clamp, CPB and surgical times [2], probably because of the increased technical difficulty posed by the reduced surgical field. Sutureless and rapid deployment aortic valves have been recently developed in order to facilitate the performance of MIS surgery and thereby reduce operative times [15, 16], but medium- and long-term results with these devices remain unknown.
Glauber et al. [6] recently performed a propensity-matched analysis comparing MIS to conventional AVR. In contrast to our study, these investigators used a right anterior minithoracotomy approach in all MIS patients. They demonstrated a lower incidence of postoperative atrial fibrillation and blood transfusion, as well as shorter ventilation times and hospital stays in MIS patients with no difference in hospital mortality rates. Although these investigators prefer a right anterior minithoracotomy approach, most centres continue to perform MIS AVR surgery via an upper hemisternotomy.
In our retrospective analysis, we compared early and long-term outcomes of MIS with those of conventional sternotomy in patients undergoing isolated bioprosthetic AVR. We excluded patients undergoing mechanical AVR in order to minimize the effect of patient age on outcomes. We performed a propensity-matched analysis to further limit differences in baseline risk factors between groups. After matching, we did not observe any clinically significant differences in preoperative variables.
We found longer myocardial ischaemia times in our MIS patients before and after propensity matching, similar to other reports [2, 6, 9, 11]. Interestingly, we did not observe a statistically significant increase in CPB times. Despite the longer aortic cross-clamp times, we did not observe any increase in related adverse effects such as myocardial infarction, IABP use or low cardiac output syndrome in MIS patients. Such an observation implies that our observed increase in myocardial ischaemic time (i.e. 2.5 min) is not clinically relevant in patients undergoing isolated AVR surgery. It remains to be seen if newer valve technologies, particularly sutureless or rapid deployment aortic valves, can reduce the myocardial ischaemic times associated with MIS surgery.
To our surprise, we found that an FS approach was associated with a lower postoperative bleeding red blood cell transfusion rate than MIS surgery. Several other studies have reported reduced postoperative blood loss and red blood cell transfusion in MIS patients. Murtuza et al. [2] found a markedly lower incidence of red blood cell transfusion in MIS patients in their meta-analysis (46.6 vs 63.5%, P < 0.0001), as well as a decreased amount of blood loss 24 h postoperatively. However, these investigators were unable to demonstrate a statistically significant reduction in the total number of red blood cells transfused or in the re-exploration rate for bleeding. The reason for our higher observed postoperative transfusion and re-exploration rate in MIS patients is not clear. A review of several operative reports from patients requiring re-exploration at our centre, however, revealed that the most common source of bleeding was the sternum in MIS patients. Closure of the sternal wound is more technically challenging in MIS than in conventional sternotomy surgery, and should probably be performed with increased vigilance in order to avoid bleeding complications.
We observed a low conversion rate to FS in MIS AVR patients (0.8%) in the current study, comparing favourably with other reports, wherein this complication occurs in 2–2.6% of patients [11, 17]. We believe that detailed preoperative planning and a relatively large clinical experience may have contributed to our ability to avoid an FS in MIS patients.
We routinely insufflate CO2 into the pericardium during all aortic valve procedures (i.e. FS and MIS) at our institution. The reason for our observed lower incidence of postoperative delirium in patients undergoing FS AVR is not known, but may be related to technical difficulties in deairing the left ventricle through a ministernotomy approach.
We failed to find a significant effect of MIS AVR on hospital stay in the current study, despite numerous reports of shorter hospitalization in MIS patients in the literature [2, 6, 9, 17]. Our lack of impact on hospital stay may be explained by the vagaries of reimbursement in the German medical system, complicating comparisons of hospital stays to those from other countries.
The most interesting observation in our study was the significantly reduced in-hospital and long-term mortality rate in MIS AVR patients. MIS was associated with an absolute increase in survival of 7.5 and 4.9% 5- and 8-years postoperatively, when compared with conventional AVR surgery. Although the MIS group had a significantly lower risk profile prior to matching, the improved survival rates persisted after careful propensity matching and multivariable analysis. The precise reason for our observed improved survival is unknown, but may be related to decreased surgical trauma or faster postoperative recovery with less perioperative complications. In addition, the fact that MIS AVR surgery tends to be performed by more experienced surgeons in our centre may also play an important role.
Although our improved survival rate in MIS patients was quite pronounced, other investigators have observed similar findings. Milhaljevic et al. noted a reduced mortality for patients undergoing MIS AVR [10]. Glauber et al. [6] also demonstrated excellent survival in MIS AVR patients 3 years postoperatively (96 vs 88% for conventional AVR), but this difference did not reach statistical significance. In addition, the above-mentioned meta-analysis revealed a statistically significant reduction in mortality for MIS AVR in over 4000 patients studied (OR: 0.72, 95% CI: 0.51–1.00) [2]. Indeed, the multiple clinical benefits demonstrated for MIS AVR surgery in this meta-analysis makes the very ambivalent title and conclusions of this manuscript rather difficult to interpret.
In addition to MIS surgery, our Cox multivariate logistic regression model identified other independent risk factors for long-term mortality. The risk factor with the highest HR was preoperative dialysis, followed by liver failure, age and logistic EuroSCORE. Multiple previous reports have documented that preoperative dialysis negatively influences short- and long-term outcomes of patients undergoing aortic valve or any cardiac surgery [18]. Although preoperative liver disease is a well-recognized risk factor for cardiac surgery [19, 20], precise estimates of its risk are lacking because of its relatively low prevalence. Although EuroSCORE is a frequently described risk factor in cardiac surgery, it is important to note that this risk predictive tool was not explicitly developed for AVR patients [21]. Indeed, Leontyev et al. [22] showed no significant difference in outcomes of octogenarian AVR patients stratified by logistic EuroSCORE, but these investigators were able to demonstrate good prediction of medium-term survival in this patient group.
Study limitations
The main limitation of our study is the fact that it is a retrospective, single-centre experience. We attempted to limit the inherent bias that can occur in a retrospective study with the use of a robust propensity matching analysis, but such methods remain inferior to a large, randomized trial. The single-centre nature of our study may bring into question its generalizability, but it is important to note that a large number of surgeons performed the AVR procedures at our centre and therefore the results should be generalizable to the cardiac surgery community. Finally, our analysis lacked information on postoperative quality of life, which would be interesting to compare in MIS and conventional AVR patients, particularly during the follow-up.
CONCLUSIONS
MIS is associated with excellent early and long-term survival when compared with conventional AVR surgery. Despite our observed longer myocardial ischaemic times and a higher re-exploration rate for bleeding, MIS AVR can be performed safely with very good early and long-term results that are at least equivalent to those achieved through a conventional sternotomy approach.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr M. Glauber(Massa, Italy): You are reporting a lower mortality and a better survival at follow-up. But you have a higher incidence of bleeding, which is something that is contrary to what is normally reported in the literature. We also report a lower incidence of bleeding and a lower rate of transfusion and that's an important concept.
One other aspect is related to the fact that propensity scoring is a very important way of analysing different groups of patients. Out of 2,000 patients operated in that period of time, 4 or 5 years if I remember correctly, you excluded only two patients from the minimally invasive group. So from 179 you go down to 177, and in your analysis you include only 12 of the many other factors shown in your preoperative patient characteristics. So why did you exclude some of these factors and cut down to only 12 factors?
Dr Merk: I would like to start with the last comment. When we did the comparison to propensity score match, we based it on our whole group, and we found that those 12 factors had the highest risk in our unmatched group. And then, because we did the matching on the nearest neighbour, for those two patients we couldn't find any neighbour. One of the patients was really young, so she was not able to be matched to another patient with a full sternotomy. And I can't remember the other patient. We have to look in detail; I was not able to find it and unfortunately I can't remember. So that was the main reason why we couldn't find a matched person or a matched case for those two.
Regarding the bleeding, we were surprised also that we had a higher transfusion rate and a higher re-exploration rate for our MIS patients. What we did, we looked up those patients in whom we had to reoperate or re-explore and then we found that in most cases, we had severe bleeding out of the sternum, something which might be due to the fact that initially when we closed it, we just didn't look carefully enough at the sternum and because of the limited view we had on MIS patients; we tried to do our skin incision around 5 cm.
Dr Glauber: I would also point out another aspect related to bleeding. I have another explanation which is coming from the analysis of the propensity score that shows that the two groups were not well-balanced in terms of preoperative creatinine and body surface area. So probably you have a different population in which, probably due to a higher preoperative creatinine, renal insufficiency, and a higher body surface area, you have a different philosophy in transfusion. So I would suggest recalculating this paying attention to these factors.
Dr Merk: That's a good suggestion.
Dr M. Zembala(Zabrze, Poland): We have the same experience with over 250 minimally invasive cases. Now, you mentioned bleeding. What about early and late tamponade, did you have any of these events? And if so, do you have any tips or tricks for us on how to put the chest tube in to prevent that?
Dr Merk: We didn't have a lot of late or early tamponade. But what we do is we go under the xiphoid to put in the drainage tube. And normally we're pretty fast taking it out. Our MIS patients are not going into the ICU. We have a fast track recovery room, and they have the chest tube removed after the first or second day and we didn't have a lot of problems with that.
Dr Zembala: So when did you observe that increased bleeding?
Dr Merk: The increased bleeding was in the first 12 h after surgery.
Dr S. Salizzoni(Turin, Italy): How did you define bleeding?
Dr Merk: For bleeding, I think there are different strategies. In our hospital, bleeding is if it's more than 100 ml/hour, and if the drainage bottle is filled with a litre in less than 4 or 5 h, we have to re-explore at that time.
Dr Salizzoni: So you didn't define bleeding on transfusions and drop in haemoglobin?
Dr Merk: No.
Dr Glauber: I have another comment related to the survival which is different for an isolated aortic valve replacement, and whether you calculate only the cardiac-related mortality or all-cause mortality?
Dr Merk: This is all-cause mortality. And this is done by our study centre. They call our patients once a year to check them out. Sometimes they find out what it is, but most of the time it's all-cause mortality.
Author notes
Presented at the 27th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 5–9 October 2013.
The first two authors contributed equally to this paper.
- aorta
- myocardial ischemia
- intra-aortic balloon pumping
- surgical procedures, minimally invasive
- perioperative cardiovascular risk
- hemorrhage
- aortic valve replacement
- bioprosthesis
- follow-up
- objective (goal)
- hospital mortality
- preoperative care
- surgical procedures, operative
- cox proportional hazards models
- european system for cardiac operative risk evaluation




