-
PDF
- Split View
-
Views
-
Cite
Cite
Anton Sabashnikov, Alexander Weymann, Prashant N. Mohite, Bartlomiej Zych, Nikhil P. Patil, Diana García Sáez, Aron-Frederik Popov, Mohamed Zeriouh, Thorsten Wahlers, Thorsten Wittwer, Jens Wippermann, Fabio De Robertis, Toufan Bahrami, Mohamed Amrani, André R. Simon, Risk factors predictive of one-year mortality after lung transplantation, European Journal of Cardio-Thoracic Surgery, Volume 46, Issue 6, December 2014, Pages e82–e88, https://doi.org/10.1093/ejcts/ezu383
Close - Share Icon Share
Lung transplantation (LTx) is a life-saving therapy for patients with end-stage lung disease. However, there remains a significant postoperative complication rate and mortality in this extreme patient group. The aim of the present study was to identify donor, recipient and perioperative risk factors for one-year mortality after LTx.
A total of 252 LTxs were performed in our institution between 2007 and 2013. Donor and recipient demographics and clinical characteristics of 1-year survivors and non-survivors were collected and compared retrospectively. Multivariate logistic regression analysis was performed on univariate predictors for 1-year mortality with an entry criterion of P < 0.05.
Multivariate analysis revealed female-to-male transplantation (95% CI: 0.088–0.767; P = 0.015), lower pO2/FiO2-ratio at 72 h postoperatively (95% CI: 0.988–0.999; P = 0.024), need for postoperative extracorporeal membrane oxygenation (ECMO) support (95% CI: 0.035–0.658; P = 0.012) and on-pump technique (95% CI: 0.007–0.944; P = 0.045) as the only independent predictors for 1-year mortality. Mainly unplanned intraoperative conversion to cardiopulmonary bypass contributed to poorer survival in patients who underwent LTx using cardiopulmonary bypass (P < 0.001).
Our results show that the unplanned use of CPB (conversion from off- to on-pump) might adversely affect outcome after LTx. Also, the negative impact of female-to-male transplantation should not be underestimated during recipient selection. Furthermore, poor early postoperative oxygenation, particularly with the need for extracorporeal oxygenation, might be a very strong negative prognostic factor after LTx.
INTRODUCTION
Lung transplantation (LTx) provides an effective treatment for end-stage lung disease and remains the gold standard therapy in this extreme patient cohort [1]. However, steadily increasing shortage of organ-donors results in a significant waiting-list mortality [2, 3]. To overcome this problem, certain techniques such as donor management optimization, use of extended donor criteria, donors with history of cardiac arrest, donors after cardiac death (DCD) as well as ex vivo lung perfusion were adopted in the last decade [1, 4]. Furthermore, various strategies of mechanical circulatory assistance as a bridge to LTx have been implemented as a life-saving therapy before a suitable organ becomes available [5]. Despite these efforts in addition to continuous refinement of surgical technique and improvement in transplant immunology and microbiology, patients after LTx experience significant postoperative morbidity and mortality [6].
In order to improve survival after LTx, it is mandatory to have an in-depth knowledge of the variety of factors that may affect outcome in this distinct patient group. Previous reports have documented a significant influence of pretransplant patient characteristics [7], surgical risk factors [8] as well as patients postoperative course after LTx on survival [9]. Particularly, postoperative factors such as primary graft dysfunction (PGD), bronchiolitis obliterans syndrome (BOS) and acute allograft graft rejection may significantly influence the overall survival after LTx [10, 11]. So far, only a few studies have investigated factors influencing mortality in patients after LTx; however, the results of those studies vary significantly [9, 12, 13]. The objective of this study is to report our recent results from a large single-centre cohort over a 6-year period and to highlight prognostic factors. We analysed pretransplant donor and recipient characteristics, as well as intra- and postoperative factors in order to determine the cumulative predictors for one-year mortality after LTx.
METHODS
We included data of 252 consecutive LTxs that were performed at Harefield Hospital during the last 6 years. Recipients were divided into two groups according to overall 1-year survival: 214 (84.9%) survivors and 38 (15.1%) non-survivors. All the patients were eligible for LTx and were on our institutional transplant waiting list. The patients who were supported with extracorporeal membrane oxygenation (ECMO) and who underwent a redo transplantation were also included in the study in order to increase the number of possible risk factors in the analysis. The primary end point was the overall survival to 365 days after LTx. Secondary end points were perioperative clinical characteristics and adverse events, which could have an impact on early and mid-term postoperative mortality. All survivors completed a follow-up period of at least 365 days in order to be included in the present study. Detailed donor data, such as demographic parameters, cause of death, current clinical status, past social and medical history were analysed. Also, the demographics and preoperative recipient data as well as intra- and postoperative variables were compared with to identify predictors of 1-year mortality.
Definitions
Smoking history was defined as temporary or permanent smoking habit at the time of organ donation or in the past. One pack-year was defined as 20 cigarettes (one pack) smoked per day for 1 year. The total ischaemic time was defined as the time between cardiac arrest in DCD donors or aortic cross-clamp in donation after brain dead (DBD) donors and reperfusion of the second implanted lung. BOS was diagnosed when post-transplant fraction of expired volume in 1 s (FEV1) measured on the regularly basis after LTx permanently dropped >20% of the best FEV1 achieved after LTx. The grade of PGD was defined based on International Society for Heart and Lung Transplantation Working Group on Primary Dysfunction Report [14]. The pO2/FiO2 ratio < 200 was considered as PGD grade 3 independent of findings on the chest X-ray. Extended donor criteria were defined as the pO2/FiO2 ratio <300 (pO2 measured in mmHg) and/or age over 55 years and/or history of smoking >20 pack-years.
Organ assessment and organ procurement protocol
Donor organ assessment performed at donor hospitals included radiological assessment, fibre-optic bronchoscopy, gross organ inspection and palpation, assessment of compliance using deflation test and selective blood-gas analysis from each pulmonary vein. In order to improve donor gas exchange, we adopted an aggressive policy for therapeutic manipulation of potential donors. This included in particular antibiotic therapy in suspected sepsis, close scrutiny and adaptation of fluid balance, increase in tidal volume and PEEP and bronchial toilet to remove secretions and reduce atelectasis. The final decision on proceeding with organ procurement and transplantation was taken by the implanting surgeon. The standard preservation solution used was low potassium dextran (Perfadex, Medisan, Uppsala, Sweden) solution augmented with CaCl2, 3.6% tromethamine (THAM, Hospira, Inc., Lake Forest, IL, USA) and epoprostenol sodium 2.5 ml/l. For DBD donors, 4 l of the solution was usually administered antegradely through a Medtronic 24-Fr single-stage venous cannula and 1 l retrogradely through a Medtronic 15 Fr retrograde cannula with self-inflating balloon. For DCD donors, 3 l of pneumoplegia was administered antegradely and 2 l retrogradely. During the pulmonary artery flush, a flushing pressure between 10 and 15 mmHg was maintained. Once the organs were removed from the chest, they were inspected and then packed for storage on ice and transported half inflated with FiO2 0.5. Samples of donor main bronchi were taken during organ implantation and sent for histopathological assessment. Also, intrabronchial swabs were collected for microbiological analysis.
Statistical analysis
All the data were presented as continuous or categorical variables. The continuous data were evaluated for normality using histograms created for each group and confirmed by statistically stronger one sample Kolmogorov–Smirnov test. Univariate analysis was performed using either Student's t or Mann–Whitney U-test for normally and non-normally distributed continuous variables, respectively. Pearson's χ² or Fisher's exact tests were used for categorical data dependent on the minimum expected count in each cross tab. Multivariate logistic regression analysis was performed on univariate predictors for 1-year mortality with an entry criterion of P < 0.05. Kaplan–Meier actuarial survival estimate was generated to analyse post-transplant survival of the entire cohort. All the data were analysed using Statistical Package for Social Sciences, version 21.0 (SPSS, Inc., Chicago, IL, USA) and were expressed as the mean ± standard deviation (SD) for normally distributed or median (interquartile range) for non-normally distributed continuous variables. The categorical data are expressed as total numbers and percentages.
RESULTS
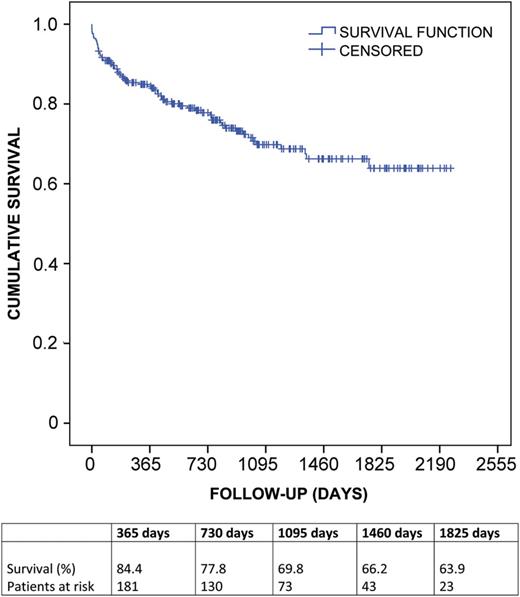
Kaplan–Meier survival estimate for patients after lung transplantation.
Univariate analysis
A subgroup analysis of 1-year survivors and non-survivors is presented in Tables 123–4. The survivors received organs from the donors with significantly higher rate of abnormal chest X-rays (P = 0.042) and significantly higher rate of smoking history (P = 0.012). Other donor variables, including demographics, type of donation, clinical characteristics obtained during organ assessment, cause of death and histological and microbiological findings did not reveal any further statistical differences (Table 1). There were significantly more female recipients (P = 0.039) in the non-survivor group with significantly higher rate of pulmonary fibrosis (P = 0.001) and pulmonary hypertension (P = 0.001) as primary diagnosis. Other preoperative demographic and clinical characteristics in recipient were comparable between survivors and non-survivors (Table 2). Analysing the donor-recipient mismatch data, we found that non-survivors had a significantly higher number of donor organs with smaller TLC transplanted in recipients with larger TLC (P = 0.025). Also, non-survivors were associated with a significantly higher rate of gender mismatch in terms of female-to-male donation (P = 0.001). There were no statistically significant differences in the number of acute rejection grade A0 (P = 0.378), Grade A1 (P = 0.455), Grade A2 (P = 0.135) and Grade A3 (P = 0.921) events calculated per month between in survivors and non-survivors. Further univariate predictors of 1-year mortality were intraoperative use of cardiopulmonary bypass (CPB) (P = 0.024), lower postoperative pO2/FiO2-ratio on arrival to intensive care unit (ICU) (P = 0.008) as well as at 24, 48 and 72 h after surgery (P < 0.001, respectively), longer mechanical ventilation, longer ICU stay, need for postoperative extracorporeal life support (ECLS), PGD on arrival to ICU, at 24, 48 and 72 h after surgery (P < 0.001, respectively) and lower post-transplant fraction of forced expiratory volume in 1 s (FEV1) 6 month after surgery (P = 0.001) with higher percentage of FEV1 deterioration from the best FEV1 achieved after LTx (P = 0.039). Other intra- and postoperative variables were comparable between the groups (Table 4).
| . | Survivors (n = 214) . | Non-survivors (n = 38) . | P-value . |
|---|---|---|---|
| Age (years) | 45 (36; 52) | 41 (28; 54) | 0.678 |
| Female | 130 (60.7%) | 26 (68.4%) | 0.369 |
| Height (cm) | 169.3 ± 10.3 | 167.7 ± 9.3 | 0.377 |
| Weight (kg) | 73.6 ± 14 | 72.5 ± 13.6 | 0.670 |
| Blood group | 0.630 | ||
| O | 94 (43.9%) | 15 (39.5%) | |
| A | 95 (44.4%) | 20 (52.6%) | |
| B | 19 (8.9%) | 3 (7.9%) | |
| AB | 6 (2.8%) | 0 | |
| pO2/FiO2 ratio preretrieval | 433 ± 99 | 402 ± 11.75 | 0.087 |
| TLC (l) | 5.91 ± 1.11 | 5.75 ± 0.98 | 0.427 |
| Ventilation duration (days) | 2 (1; 3) | 2 (1; 4) | 0.437 |
| Total ischaemic time (min) | 316 (253;437) | 373 (290;499) | 0.060 |
| Use of EVLP | 9 (4.2%) | 1 (2.6%) | 1.000 |
| DCD | 40 (18.7%) | 7 (18.4%) | 0.969 |
| Agonal time (min) | 17.4 ± 2.3 | 16.2 ± 7.8 | 0.783 |
| Time from cardiac arrest to preservation commencement (min) | 17.2 ± 2.3 | 17.7 ± 8.2 | 0.866 |
| History of cardiac arrest | 46 (21.5%) | 6 (16.7%) | 0.509 |
| Cardiac arrest duration (min) | 16.3 ± 12.8 | 23.1 ± 17.6 | 0.367 |
| Abnormal CXR | 50 (23.7%) | 14 (40.0%) | 0.042 |
| Abnormal bronchoscopy | 51 (24.5%) | 15 (39.5%) | 0.056 |
| Positive culture (bronchial) | 90 (57.7%) | 21 (75%) | 0.085 |
| Smoking history | 108 (50.5%) | 10 (27.8%) | 0.012 |
| Extent of smoking (pack-years) | 16.44 ± 10.6 | 13.4 ± 8.5 | 0.380 |
| Extended donor criteriaa | 66 (30.8%) | 13 (34.2%) | 0.680 |
| Other institute retrieval teams | 78 (39%) | 12 (32.4%) | 0.450 |
| Abnormal histology (bronchial) | 131 (61.2%) | 22 (57.8%) | 0.779 |
| Inflammation | 130 (60.7%) | 21 (55.3%) | 0.551 |
| Metaplasia | 5 (2.3%) | 2 (5.3%) | 0.276 |
| Cause of death/event | 0.943 | ||
| ICH | 139 (65%) | 27 (71.1%) | |
| HBI | 24 (11.2%) | 4 (10.5%) | |
| Trauma | 21 (9.8%) | 2 (5.3%) | |
| CVA | 17 (7.9%) | 3 (7.9%) | |
| Meningitis | 10 (4.7%) | 1 (2.6%) | |
| Other | 3 (1.4%) | 1 (2.6%) |
| . | Survivors (n = 214) . | Non-survivors (n = 38) . | P-value . |
|---|---|---|---|
| Age (years) | 45 (36; 52) | 41 (28; 54) | 0.678 |
| Female | 130 (60.7%) | 26 (68.4%) | 0.369 |
| Height (cm) | 169.3 ± 10.3 | 167.7 ± 9.3 | 0.377 |
| Weight (kg) | 73.6 ± 14 | 72.5 ± 13.6 | 0.670 |
| Blood group | 0.630 | ||
| O | 94 (43.9%) | 15 (39.5%) | |
| A | 95 (44.4%) | 20 (52.6%) | |
| B | 19 (8.9%) | 3 (7.9%) | |
| AB | 6 (2.8%) | 0 | |
| pO2/FiO2 ratio preretrieval | 433 ± 99 | 402 ± 11.75 | 0.087 |
| TLC (l) | 5.91 ± 1.11 | 5.75 ± 0.98 | 0.427 |
| Ventilation duration (days) | 2 (1; 3) | 2 (1; 4) | 0.437 |
| Total ischaemic time (min) | 316 (253;437) | 373 (290;499) | 0.060 |
| Use of EVLP | 9 (4.2%) | 1 (2.6%) | 1.000 |
| DCD | 40 (18.7%) | 7 (18.4%) | 0.969 |
| Agonal time (min) | 17.4 ± 2.3 | 16.2 ± 7.8 | 0.783 |
| Time from cardiac arrest to preservation commencement (min) | 17.2 ± 2.3 | 17.7 ± 8.2 | 0.866 |
| History of cardiac arrest | 46 (21.5%) | 6 (16.7%) | 0.509 |
| Cardiac arrest duration (min) | 16.3 ± 12.8 | 23.1 ± 17.6 | 0.367 |
| Abnormal CXR | 50 (23.7%) | 14 (40.0%) | 0.042 |
| Abnormal bronchoscopy | 51 (24.5%) | 15 (39.5%) | 0.056 |
| Positive culture (bronchial) | 90 (57.7%) | 21 (75%) | 0.085 |
| Smoking history | 108 (50.5%) | 10 (27.8%) | 0.012 |
| Extent of smoking (pack-years) | 16.44 ± 10.6 | 13.4 ± 8.5 | 0.380 |
| Extended donor criteriaa | 66 (30.8%) | 13 (34.2%) | 0.680 |
| Other institute retrieval teams | 78 (39%) | 12 (32.4%) | 0.450 |
| Abnormal histology (bronchial) | 131 (61.2%) | 22 (57.8%) | 0.779 |
| Inflammation | 130 (60.7%) | 21 (55.3%) | 0.551 |
| Metaplasia | 5 (2.3%) | 2 (5.3%) | 0.276 |
| Cause of death/event | 0.943 | ||
| ICH | 139 (65%) | 27 (71.1%) | |
| HBI | 24 (11.2%) | 4 (10.5%) | |
| Trauma | 21 (9.8%) | 2 (5.3%) | |
| CVA | 17 (7.9%) | 3 (7.9%) | |
| Meningitis | 10 (4.7%) | 1 (2.6%) | |
| Other | 3 (1.4%) | 1 (2.6%) |
TLC: total lung capacity; EVLP: ex vivo lung perfusion; DCD: donors after cardiac death; CXR: chest X-ray; ICH: intracranial haemorrhage; HBI: hypoxic brain injury; CVA: cerebrovascular accident.
aDonors outside standard criteria: PaO2/FiO2 ratio <300, age over 55 years and history of smoking >20 pack-years.
| . | Survivors (n = 214) . | Non-survivors (n = 38) . | P-value . |
|---|---|---|---|
| Age (years) | 45 (36; 52) | 41 (28; 54) | 0.678 |
| Female | 130 (60.7%) | 26 (68.4%) | 0.369 |
| Height (cm) | 169.3 ± 10.3 | 167.7 ± 9.3 | 0.377 |
| Weight (kg) | 73.6 ± 14 | 72.5 ± 13.6 | 0.670 |
| Blood group | 0.630 | ||
| O | 94 (43.9%) | 15 (39.5%) | |
| A | 95 (44.4%) | 20 (52.6%) | |
| B | 19 (8.9%) | 3 (7.9%) | |
| AB | 6 (2.8%) | 0 | |
| pO2/FiO2 ratio preretrieval | 433 ± 99 | 402 ± 11.75 | 0.087 |
| TLC (l) | 5.91 ± 1.11 | 5.75 ± 0.98 | 0.427 |
| Ventilation duration (days) | 2 (1; 3) | 2 (1; 4) | 0.437 |
| Total ischaemic time (min) | 316 (253;437) | 373 (290;499) | 0.060 |
| Use of EVLP | 9 (4.2%) | 1 (2.6%) | 1.000 |
| DCD | 40 (18.7%) | 7 (18.4%) | 0.969 |
| Agonal time (min) | 17.4 ± 2.3 | 16.2 ± 7.8 | 0.783 |
| Time from cardiac arrest to preservation commencement (min) | 17.2 ± 2.3 | 17.7 ± 8.2 | 0.866 |
| History of cardiac arrest | 46 (21.5%) | 6 (16.7%) | 0.509 |
| Cardiac arrest duration (min) | 16.3 ± 12.8 | 23.1 ± 17.6 | 0.367 |
| Abnormal CXR | 50 (23.7%) | 14 (40.0%) | 0.042 |
| Abnormal bronchoscopy | 51 (24.5%) | 15 (39.5%) | 0.056 |
| Positive culture (bronchial) | 90 (57.7%) | 21 (75%) | 0.085 |
| Smoking history | 108 (50.5%) | 10 (27.8%) | 0.012 |
| Extent of smoking (pack-years) | 16.44 ± 10.6 | 13.4 ± 8.5 | 0.380 |
| Extended donor criteriaa | 66 (30.8%) | 13 (34.2%) | 0.680 |
| Other institute retrieval teams | 78 (39%) | 12 (32.4%) | 0.450 |
| Abnormal histology (bronchial) | 131 (61.2%) | 22 (57.8%) | 0.779 |
| Inflammation | 130 (60.7%) | 21 (55.3%) | 0.551 |
| Metaplasia | 5 (2.3%) | 2 (5.3%) | 0.276 |
| Cause of death/event | 0.943 | ||
| ICH | 139 (65%) | 27 (71.1%) | |
| HBI | 24 (11.2%) | 4 (10.5%) | |
| Trauma | 21 (9.8%) | 2 (5.3%) | |
| CVA | 17 (7.9%) | 3 (7.9%) | |
| Meningitis | 10 (4.7%) | 1 (2.6%) | |
| Other | 3 (1.4%) | 1 (2.6%) |
| . | Survivors (n = 214) . | Non-survivors (n = 38) . | P-value . |
|---|---|---|---|
| Age (years) | 45 (36; 52) | 41 (28; 54) | 0.678 |
| Female | 130 (60.7%) | 26 (68.4%) | 0.369 |
| Height (cm) | 169.3 ± 10.3 | 167.7 ± 9.3 | 0.377 |
| Weight (kg) | 73.6 ± 14 | 72.5 ± 13.6 | 0.670 |
| Blood group | 0.630 | ||
| O | 94 (43.9%) | 15 (39.5%) | |
| A | 95 (44.4%) | 20 (52.6%) | |
| B | 19 (8.9%) | 3 (7.9%) | |
| AB | 6 (2.8%) | 0 | |
| pO2/FiO2 ratio preretrieval | 433 ± 99 | 402 ± 11.75 | 0.087 |
| TLC (l) | 5.91 ± 1.11 | 5.75 ± 0.98 | 0.427 |
| Ventilation duration (days) | 2 (1; 3) | 2 (1; 4) | 0.437 |
| Total ischaemic time (min) | 316 (253;437) | 373 (290;499) | 0.060 |
| Use of EVLP | 9 (4.2%) | 1 (2.6%) | 1.000 |
| DCD | 40 (18.7%) | 7 (18.4%) | 0.969 |
| Agonal time (min) | 17.4 ± 2.3 | 16.2 ± 7.8 | 0.783 |
| Time from cardiac arrest to preservation commencement (min) | 17.2 ± 2.3 | 17.7 ± 8.2 | 0.866 |
| History of cardiac arrest | 46 (21.5%) | 6 (16.7%) | 0.509 |
| Cardiac arrest duration (min) | 16.3 ± 12.8 | 23.1 ± 17.6 | 0.367 |
| Abnormal CXR | 50 (23.7%) | 14 (40.0%) | 0.042 |
| Abnormal bronchoscopy | 51 (24.5%) | 15 (39.5%) | 0.056 |
| Positive culture (bronchial) | 90 (57.7%) | 21 (75%) | 0.085 |
| Smoking history | 108 (50.5%) | 10 (27.8%) | 0.012 |
| Extent of smoking (pack-years) | 16.44 ± 10.6 | 13.4 ± 8.5 | 0.380 |
| Extended donor criteriaa | 66 (30.8%) | 13 (34.2%) | 0.680 |
| Other institute retrieval teams | 78 (39%) | 12 (32.4%) | 0.450 |
| Abnormal histology (bronchial) | 131 (61.2%) | 22 (57.8%) | 0.779 |
| Inflammation | 130 (60.7%) | 21 (55.3%) | 0.551 |
| Metaplasia | 5 (2.3%) | 2 (5.3%) | 0.276 |
| Cause of death/event | 0.943 | ||
| ICH | 139 (65%) | 27 (71.1%) | |
| HBI | 24 (11.2%) | 4 (10.5%) | |
| Trauma | 21 (9.8%) | 2 (5.3%) | |
| CVA | 17 (7.9%) | 3 (7.9%) | |
| Meningitis | 10 (4.7%) | 1 (2.6%) | |
| Other | 3 (1.4%) | 1 (2.6%) |
TLC: total lung capacity; EVLP: ex vivo lung perfusion; DCD: donors after cardiac death; CXR: chest X-ray; ICH: intracranial haemorrhage; HBI: hypoxic brain injury; CVA: cerebrovascular accident.
aDonors outside standard criteria: PaO2/FiO2 ratio <300, age over 55 years and history of smoking >20 pack-years.
| . | Survivors . | Non-survivors . | P-value . |
|---|---|---|---|
| Age (years) | 46 (29; 55) | 46 (37; 56) | 0.221 |
| Female | 112 (52.3%) | 13 (34.2%) | 0.039 |
| Height (cm) | 168 ± 8.8 | 168.2 ± 9.5 | 0.913 |
| Weight (kg) | 61 (53; 76) | 69 (54; 83) | 0.202 |
| TLC (l) | 5.87 ± 1.08 | 6.08 ± 1.07 | 0.286 |
| Blood group | 0.712 | ||
| O | 70 (32.7%) | 12 (31.6%) | |
| A | 108 (50.5%) | 22 (57.9%) | |
| B | 22 (10.3%) | 3 (7.9%) | |
| AB | 14 (6.5%) | 1 (2.6%) | |
| ECLS | 8 (3.7%) | 2 (5.3%) | 0.650 |
| Primary diagnosis | <0.001 | ||
| CF | 82 (38.3%) | 11 (28.9%) | 0.270 |
| Emphysema | 70 (32.7%) | 7 (18.4%) | 0.078 |
| α-1 antitrypsin deficiency | 31 (14.5%) | 1 (2.6%) | 0.060 |
| PF | 9 (4.2%) | 8 (21.1%) | 0.001 |
| PH | 6 (2.8%) | 7 (18.4%) | 0.001 |
| LAM | 7 (3.3%) | 0 | 0.599 |
| Sarcoidosis | 5 (2.3%) | 1 (2.6%) | 1.000 |
| BO | 3 (1.4%) | 1 (2.6%) | 0.482 |
| Bronchiectasis | 1 (0.5%) | 2 (5.3%) | 0.060 |
| . | Survivors . | Non-survivors . | P-value . |
|---|---|---|---|
| Age (years) | 46 (29; 55) | 46 (37; 56) | 0.221 |
| Female | 112 (52.3%) | 13 (34.2%) | 0.039 |
| Height (cm) | 168 ± 8.8 | 168.2 ± 9.5 | 0.913 |
| Weight (kg) | 61 (53; 76) | 69 (54; 83) | 0.202 |
| TLC (l) | 5.87 ± 1.08 | 6.08 ± 1.07 | 0.286 |
| Blood group | 0.712 | ||
| O | 70 (32.7%) | 12 (31.6%) | |
| A | 108 (50.5%) | 22 (57.9%) | |
| B | 22 (10.3%) | 3 (7.9%) | |
| AB | 14 (6.5%) | 1 (2.6%) | |
| ECLS | 8 (3.7%) | 2 (5.3%) | 0.650 |
| Primary diagnosis | <0.001 | ||
| CF | 82 (38.3%) | 11 (28.9%) | 0.270 |
| Emphysema | 70 (32.7%) | 7 (18.4%) | 0.078 |
| α-1 antitrypsin deficiency | 31 (14.5%) | 1 (2.6%) | 0.060 |
| PF | 9 (4.2%) | 8 (21.1%) | 0.001 |
| PH | 6 (2.8%) | 7 (18.4%) | 0.001 |
| LAM | 7 (3.3%) | 0 | 0.599 |
| Sarcoidosis | 5 (2.3%) | 1 (2.6%) | 1.000 |
| BO | 3 (1.4%) | 1 (2.6%) | 0.482 |
| Bronchiectasis | 1 (0.5%) | 2 (5.3%) | 0.060 |
TLC: total lung capacity; ECLS: extracorporeal life support; CF: cystic fibrosis; PF: pulmonary fibrosis; PH: pulmonary hypertension; LAM: lymphangiomyomatosis; BO: bronchiolitis obliterans.
| . | Survivors . | Non-survivors . | P-value . |
|---|---|---|---|
| Age (years) | 46 (29; 55) | 46 (37; 56) | 0.221 |
| Female | 112 (52.3%) | 13 (34.2%) | 0.039 |
| Height (cm) | 168 ± 8.8 | 168.2 ± 9.5 | 0.913 |
| Weight (kg) | 61 (53; 76) | 69 (54; 83) | 0.202 |
| TLC (l) | 5.87 ± 1.08 | 6.08 ± 1.07 | 0.286 |
| Blood group | 0.712 | ||
| O | 70 (32.7%) | 12 (31.6%) | |
| A | 108 (50.5%) | 22 (57.9%) | |
| B | 22 (10.3%) | 3 (7.9%) | |
| AB | 14 (6.5%) | 1 (2.6%) | |
| ECLS | 8 (3.7%) | 2 (5.3%) | 0.650 |
| Primary diagnosis | <0.001 | ||
| CF | 82 (38.3%) | 11 (28.9%) | 0.270 |
| Emphysema | 70 (32.7%) | 7 (18.4%) | 0.078 |
| α-1 antitrypsin deficiency | 31 (14.5%) | 1 (2.6%) | 0.060 |
| PF | 9 (4.2%) | 8 (21.1%) | 0.001 |
| PH | 6 (2.8%) | 7 (18.4%) | 0.001 |
| LAM | 7 (3.3%) | 0 | 0.599 |
| Sarcoidosis | 5 (2.3%) | 1 (2.6%) | 1.000 |
| BO | 3 (1.4%) | 1 (2.6%) | 0.482 |
| Bronchiectasis | 1 (0.5%) | 2 (5.3%) | 0.060 |
| . | Survivors . | Non-survivors . | P-value . |
|---|---|---|---|
| Age (years) | 46 (29; 55) | 46 (37; 56) | 0.221 |
| Female | 112 (52.3%) | 13 (34.2%) | 0.039 |
| Height (cm) | 168 ± 8.8 | 168.2 ± 9.5 | 0.913 |
| Weight (kg) | 61 (53; 76) | 69 (54; 83) | 0.202 |
| TLC (l) | 5.87 ± 1.08 | 6.08 ± 1.07 | 0.286 |
| Blood group | 0.712 | ||
| O | 70 (32.7%) | 12 (31.6%) | |
| A | 108 (50.5%) | 22 (57.9%) | |
| B | 22 (10.3%) | 3 (7.9%) | |
| AB | 14 (6.5%) | 1 (2.6%) | |
| ECLS | 8 (3.7%) | 2 (5.3%) | 0.650 |
| Primary diagnosis | <0.001 | ||
| CF | 82 (38.3%) | 11 (28.9%) | 0.270 |
| Emphysema | 70 (32.7%) | 7 (18.4%) | 0.078 |
| α-1 antitrypsin deficiency | 31 (14.5%) | 1 (2.6%) | 0.060 |
| PF | 9 (4.2%) | 8 (21.1%) | 0.001 |
| PH | 6 (2.8%) | 7 (18.4%) | 0.001 |
| LAM | 7 (3.3%) | 0 | 0.599 |
| Sarcoidosis | 5 (2.3%) | 1 (2.6%) | 1.000 |
| BO | 3 (1.4%) | 1 (2.6%) | 0.482 |
| Bronchiectasis | 1 (0.5%) | 2 (5.3%) | 0.060 |
TLC: total lung capacity; ECLS: extracorporeal life support; CF: cystic fibrosis; PF: pulmonary fibrosis; PH: pulmonary hypertension; LAM: lymphangiomyomatosis; BO: bronchiolitis obliterans.
| . | Survivors . | Non-survivors . | P-value . |
|---|---|---|---|
| Δ Height (cm) | 1.28 ± 7.2 | −0.45 ± 8.05 | 0.184 |
| Δ TLC (l) | 0.04 ± 0.72 | −0.33 ± 0.90 | 0.025 |
| Gender mismatch | |||
| Female-to-male-transplantation | 32 (15%) | 14 (36.8%) | 0.001 |
| Male-to-female-transplantation | 14 (6.5%) | 1 (2.6%) | 0.707 |
| . | Survivors . | Non-survivors . | P-value . |
|---|---|---|---|
| Δ Height (cm) | 1.28 ± 7.2 | −0.45 ± 8.05 | 0.184 |
| Δ TLC (l) | 0.04 ± 0.72 | −0.33 ± 0.90 | 0.025 |
| Gender mismatch | |||
| Female-to-male-transplantation | 32 (15%) | 14 (36.8%) | 0.001 |
| Male-to-female-transplantation | 14 (6.5%) | 1 (2.6%) | 0.707 |
TLC: total lung capacity.
| . | Survivors . | Non-survivors . | P-value . |
|---|---|---|---|
| Δ Height (cm) | 1.28 ± 7.2 | −0.45 ± 8.05 | 0.184 |
| Δ TLC (l) | 0.04 ± 0.72 | −0.33 ± 0.90 | 0.025 |
| Gender mismatch | |||
| Female-to-male-transplantation | 32 (15%) | 14 (36.8%) | 0.001 |
| Male-to-female-transplantation | 14 (6.5%) | 1 (2.6%) | 0.707 |
| . | Survivors . | Non-survivors . | P-value . |
|---|---|---|---|
| Δ Height (cm) | 1.28 ± 7.2 | −0.45 ± 8.05 | 0.184 |
| Δ TLC (l) | 0.04 ± 0.72 | −0.33 ± 0.90 | 0.025 |
| Gender mismatch | |||
| Female-to-male-transplantation | 32 (15%) | 14 (36.8%) | 0.001 |
| Male-to-female-transplantation | 14 (6.5%) | 1 (2.6%) | 0.707 |
TLC: total lung capacity.
| . | Survivors . | Non-survivors . | P-value . |
|---|---|---|---|
| Use of CPB | 162 (75.7%) | 35 (92.1%) | 0.024 |
| SLTx | 19 (8.9%) | 4 (10.5%) | 0.760 |
| Retransplantation | 2 (0.9%) | 0 | 1.000 |
| pO2/FiO2 ratio on arrival | 333.5 ± 123.4 | 245.8 ± 166.1 | 0.008 |
| pO2/FiO2 ratio 24 h | 353.2 ± 103 | 246.8 ± 113.5 | <0.001 |
| pO2/FiO2 ratio 48 h | 360.9 ± 105.6 | 253.2 ± 117.8 | <0.001 |
| pO2/FiO2 ratio 72 h | 360.9 ± 107.1 | 253.4 ± 114 | <0.001 |
| Ventilation (h) | 31 (15; 111) | 576 (90; 971) | <0.001 |
| ICU stay (days) | 5 (3; 17) | 28 (10; 43) | <0.001 |
| Hospital stay (days) | 33 (23; 51) | 40 (19; 70) | 0.376 |
| ECLS | 8 (3.7%) | 14 (37.8%) | <0.001 |
| PGD on arrival | 25 (11.9%) | 16 (51.6%) | <0.001 |
| PGD 24 h | 14 (16.7%) | 9 (32.1%) | <0.001 |
| PGD 48 h | 9 (4.6%) | 10 (35.7%) | <0.001 |
| PGD 72 h | 9 (4.9%) | 10 (37%) | <0.001 |
| FEV1 after 3 months (%) | 71.9 ± 29.7 | 53.6 ± 15.6 | 0.085 |
| FEV1 after 6 months (%) | 76 ± 21.8 | 39.7 ± 12 | <0.001 |
| FEV1 deterioration (%) | 8 (1; 21) | 28 (11; 45) | 0.039 |
| . | Survivors . | Non-survivors . | P-value . |
|---|---|---|---|
| Use of CPB | 162 (75.7%) | 35 (92.1%) | 0.024 |
| SLTx | 19 (8.9%) | 4 (10.5%) | 0.760 |
| Retransplantation | 2 (0.9%) | 0 | 1.000 |
| pO2/FiO2 ratio on arrival | 333.5 ± 123.4 | 245.8 ± 166.1 | 0.008 |
| pO2/FiO2 ratio 24 h | 353.2 ± 103 | 246.8 ± 113.5 | <0.001 |
| pO2/FiO2 ratio 48 h | 360.9 ± 105.6 | 253.2 ± 117.8 | <0.001 |
| pO2/FiO2 ratio 72 h | 360.9 ± 107.1 | 253.4 ± 114 | <0.001 |
| Ventilation (h) | 31 (15; 111) | 576 (90; 971) | <0.001 |
| ICU stay (days) | 5 (3; 17) | 28 (10; 43) | <0.001 |
| Hospital stay (days) | 33 (23; 51) | 40 (19; 70) | 0.376 |
| ECLS | 8 (3.7%) | 14 (37.8%) | <0.001 |
| PGD on arrival | 25 (11.9%) | 16 (51.6%) | <0.001 |
| PGD 24 h | 14 (16.7%) | 9 (32.1%) | <0.001 |
| PGD 48 h | 9 (4.6%) | 10 (35.7%) | <0.001 |
| PGD 72 h | 9 (4.9%) | 10 (37%) | <0.001 |
| FEV1 after 3 months (%) | 71.9 ± 29.7 | 53.6 ± 15.6 | 0.085 |
| FEV1 after 6 months (%) | 76 ± 21.8 | 39.7 ± 12 | <0.001 |
| FEV1 deterioration (%) | 8 (1; 21) | 28 (11; 45) | 0.039 |
CPB: cardiopulmonary bypass; SLTx: single lung transplant; ICU: intensive care unit; ECLS; extracorporeal life support; PGD: primary graft dysfunction; FEV1: forced expiratory volume in 1 s.
| . | Survivors . | Non-survivors . | P-value . |
|---|---|---|---|
| Use of CPB | 162 (75.7%) | 35 (92.1%) | 0.024 |
| SLTx | 19 (8.9%) | 4 (10.5%) | 0.760 |
| Retransplantation | 2 (0.9%) | 0 | 1.000 |
| pO2/FiO2 ratio on arrival | 333.5 ± 123.4 | 245.8 ± 166.1 | 0.008 |
| pO2/FiO2 ratio 24 h | 353.2 ± 103 | 246.8 ± 113.5 | <0.001 |
| pO2/FiO2 ratio 48 h | 360.9 ± 105.6 | 253.2 ± 117.8 | <0.001 |
| pO2/FiO2 ratio 72 h | 360.9 ± 107.1 | 253.4 ± 114 | <0.001 |
| Ventilation (h) | 31 (15; 111) | 576 (90; 971) | <0.001 |
| ICU stay (days) | 5 (3; 17) | 28 (10; 43) | <0.001 |
| Hospital stay (days) | 33 (23; 51) | 40 (19; 70) | 0.376 |
| ECLS | 8 (3.7%) | 14 (37.8%) | <0.001 |
| PGD on arrival | 25 (11.9%) | 16 (51.6%) | <0.001 |
| PGD 24 h | 14 (16.7%) | 9 (32.1%) | <0.001 |
| PGD 48 h | 9 (4.6%) | 10 (35.7%) | <0.001 |
| PGD 72 h | 9 (4.9%) | 10 (37%) | <0.001 |
| FEV1 after 3 months (%) | 71.9 ± 29.7 | 53.6 ± 15.6 | 0.085 |
| FEV1 after 6 months (%) | 76 ± 21.8 | 39.7 ± 12 | <0.001 |
| FEV1 deterioration (%) | 8 (1; 21) | 28 (11; 45) | 0.039 |
| . | Survivors . | Non-survivors . | P-value . |
|---|---|---|---|
| Use of CPB | 162 (75.7%) | 35 (92.1%) | 0.024 |
| SLTx | 19 (8.9%) | 4 (10.5%) | 0.760 |
| Retransplantation | 2 (0.9%) | 0 | 1.000 |
| pO2/FiO2 ratio on arrival | 333.5 ± 123.4 | 245.8 ± 166.1 | 0.008 |
| pO2/FiO2 ratio 24 h | 353.2 ± 103 | 246.8 ± 113.5 | <0.001 |
| pO2/FiO2 ratio 48 h | 360.9 ± 105.6 | 253.2 ± 117.8 | <0.001 |
| pO2/FiO2 ratio 72 h | 360.9 ± 107.1 | 253.4 ± 114 | <0.001 |
| Ventilation (h) | 31 (15; 111) | 576 (90; 971) | <0.001 |
| ICU stay (days) | 5 (3; 17) | 28 (10; 43) | <0.001 |
| Hospital stay (days) | 33 (23; 51) | 40 (19; 70) | 0.376 |
| ECLS | 8 (3.7%) | 14 (37.8%) | <0.001 |
| PGD on arrival | 25 (11.9%) | 16 (51.6%) | <0.001 |
| PGD 24 h | 14 (16.7%) | 9 (32.1%) | <0.001 |
| PGD 48 h | 9 (4.6%) | 10 (35.7%) | <0.001 |
| PGD 72 h | 9 (4.9%) | 10 (37%) | <0.001 |
| FEV1 after 3 months (%) | 71.9 ± 29.7 | 53.6 ± 15.6 | 0.085 |
| FEV1 after 6 months (%) | 76 ± 21.8 | 39.7 ± 12 | <0.001 |
| FEV1 deterioration (%) | 8 (1; 21) | 28 (11; 45) | 0.039 |
CPB: cardiopulmonary bypass; SLTx: single lung transplant; ICU: intensive care unit; ECLS; extracorporeal life support; PGD: primary graft dysfunction; FEV1: forced expiratory volume in 1 s.
Independent predictors of 1-year mortality
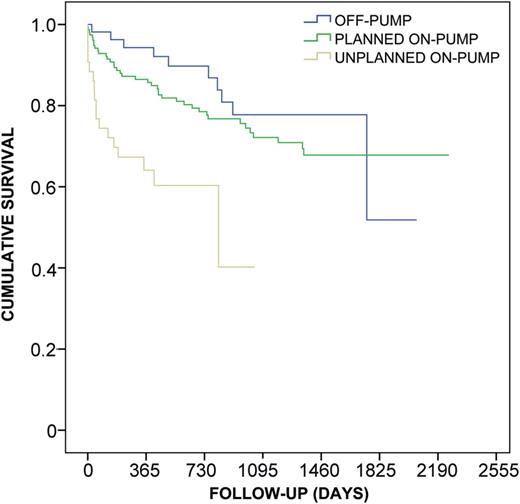
Kaplan–Meier survival estimate for patients undergoing lung transplantation with intraoperative use of CPB (planned vs converted) or off-pump. Unplanned intraoperative conversion to CPB is associated with a significantly poorer survival (log-rank P < 0.001, generalized Wilcoxon P < 0.001, Tarone Ware P < 0.001) compared with planned CPB use and off-pump procedure, whereas elective use of CPB compared with off-pump strategy is only associated with non-significantly poorer survival (log-rank P = 0.265, generalized Wilcoxon P = 0.174, Tarone Ware P < 0.235).
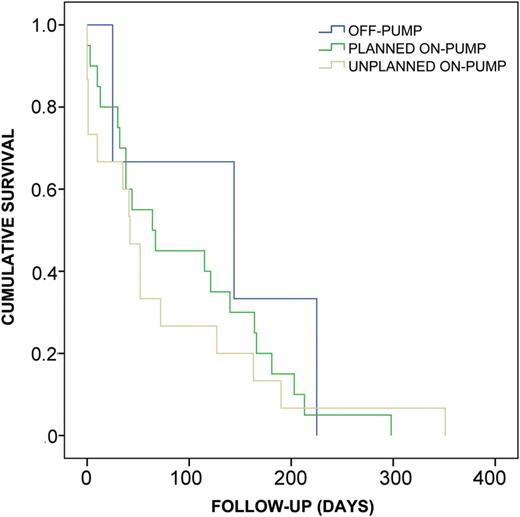
Kaplan–Meier survival estimate for patients undergoing lung transplantation with intraoperative use of CPB (planned vs converted) or off-pump who died within 1 year after transplantation. There were no statistically significant differences in terms of the time-course among the three groups (log-rank P < 0.702, generalized Wilcoxon P < 0.447, Tarone Ware P < 0.506).
DISCUSSION
One of the main strengths of this study is that no patients were excluded from our analysis representing a large real-world ‘all comers' cohort from an experienced LTx centre. Our patient group included recipients with end-stage lung disease of various aetiology, age and severity of pretransplant condition, whereas some of them were bridged to transplantation using ECLS. Furthermore, we analysed all main donor parameters including all currently available organ assessment criteria before organ procurement as well as histological results from the donor's bronchial mucosa. Also, the highly debated impact of non-heart beating donors and implementation of EVLP were analysed in this observation. Additionally, we included intra- and postoperative variables in order to provide a full spectrum of possible factors that may influence 1-year survival, as the previous research highlighted a significant influence of pretransplant patient characteristics [7], surgical risk factors [8] as well as patient's postoperative course [9] on survival after LTx. The postoperative risk factors for mortality seem to be particularly important for high volume centres as it was previously shown that they are best able to minimize the adverse effects of postoperative complications on short- and long-term survival.
The importance of the present observation might also be underlined by the fact that most previous research on risk factors in LTx focused on factors influencing post-transplant complications [10, 11, 15], quality of life or exercise performance [16] or risk factors of mortality awaiting LTx [17]. However, few currently available publications on risk factors of mortality after LTx are associated with the restricted categories of variables [7, 8, 18], small patient cohorts [9, 19–21], older era analyses [12] or are related to single recipient diagnosis [20] or paediatric transplants [19]. The smaller patient cohorts might compromise statistical analysis, particularly the multivariate approach. Also, it was previously reported that the differences in the complications and long-term survival show the important contribution of the recipient diagnosis to the success of LTx [22].
The principal finding of this study was that the unplanned conversion to CPB should be avoided if possible as it might adversely affect outcome after LTx. For that reason, particularly cases with a high risk of haemodynamic instability should be meaningfully planned in terms of intraoperative strategy. Particularly, the access to the hilum of the left lung, especially pulmonary veins may be associated with compromised circulation as the heart is lifted up and pulled rightward because of its position anterior to the left hilum. Nevertheless, cardiac luxation techniques to facilitate uncompromised off-pump bilateral LTx are described in previous literature [23].
A number of previous reports indicate negative influence of CPB in LTx. Paradela et al. [8] showed that the use of CPB represents one of the significant surgical risks in LTx and is associated with greater perioperative mortality. Machuca et al. [9] found intraoperative use of CPB to be one of the negative prognostic factors in terms of survival. Gómez et al. [21] also reported significant association of CPB during LTx with increased incidence of mortality in the postoperative recovery unit. Ferrer et al. [20] confirmed this finding and reported on significantly higher 1-month mortality after bilateral LTx with CPB in emphysema patients. Moreover, Diamond et al. [10] identified the use of CPB in LTx as one of the independent risk factors for Grade 3 PGD. On the other hand, de Boer et al. [24] demonstrated a survival benefit of CPB support during bilateral LTx in emphysema patients. However, the survival benefit was only shown in the group with two HLA-DR mismatches whereas this association was not present in the group with 0 or 1 HLA-DR mismatches, indicating the possibility of immunosuppressive effect of CPB support being responsible for this effect. However, all these studies attributed this fact to detrimental effects of CPB and failed to provide a subgroup analysis of patients with the elective use of CPB and the unplanned intraoperative conversion to CPB. This may lead to a possible misperception that even elective CPB might be associated with significantly poorer survival. As shown in our results, compared with the intraoperative conversion to CPB, survival after the elective use of CPB or the off-pump technique in LTx is significantly better.
Likewise, the use of ECLS after LTx was inferior in terms of survival in our study and might be related not only to the detrimental effects of mechanical circulatory support but also to the lower pO2/FiO2-ratio, which represents one of the diagnostic characteristics of PGD and one of the main indication for ECLS. Not surprisingly, our results also indicate lower pO2/FiO2-ratio at 72 h postoperatively as one of the independent predictors for one-year mortality after LTx. These findings corroborate the results by Diamond et al. [10] who identified PGD as a factor associated with 90-day and one-year mortality after LTx. Moreover, Gómez et al. [21] found pO2/FiO2 ratio <150 during the first 24 h following transplantation as one of the variables showing the highest correlations with mortality during the course in the postoperative recovery unit. However, compared with that study, the pO2/FiO2 ratio threshold predictive of 1-year mortality was higher in our investigation pleading for the fact that survival duration may correlate with early postoperative pO2/FiO2 ratio.
The problem of donor-recipient gender mismatch in LTx is highly debated as the results from previous research vary. Lindsey et al. [15] identified a benefit of female donor status in terms of long-term graft survival, whereas Alvarez et al. [25] indicated no negative impact of gender mismatch on early graft function and mortality following LTx. Roberts et al. found a significant improvement in overall survival for gender-mismatched donor-recipient pairs and a significantly shorter BOS-free period for male donor and female recipient pairs. In contrast, according to our results, female-to-male transplantation should be viewed with a degree of caution, which was also found as an independent risk factor for 1-year mortality in our study corroborating the results by Russo et al. [7]. This is also consistent with the fact that non-survivors had a significantly higher number of donor lungs with smaller TLC transplanted in recipients with larger TLC in our univariate analysis, which might be related to higher proportion of female-to-male transplantation in this group. This theory is supported by Eberlein et al. [18] who showed similar results regarding donor-recipient TLC mismatch as a predictor of death after LTx and highlighted the association of sex with survival.
Huppman et al. [13] have recently identified risk factors of long-term post-transplant mortality in their single-centre study taking donor, recipient and postoperative variables into account. Interestingly, compared with the factors predictive of 1-year mortality identified in our study, their multivariate analysis revealed acute rejection, lymphocytic bronchiolitis, donor age ≥55 years and HLA-A ≥ 2-/DR ≥ 2 mismatch and single LTx as independent negative predictors for 10-year survival. Similarly, another study on risk factors for 10-year mortality after LTx indicated that the bilateral LTx and fewer hospitalizations for rejection may portend an improved long-term survival [12]. This fact underlines perceptible difference in the factors predictive of short- and long-term mortality after LTx and can help the clinicians to improve survival at different stages during the follow-up. However, in order to identify the risk factors for long-term mortality, older era patients had to be included in those studies. Thus, it might have an impact on the results, as the continuous refinement of surgical technique and the improvement in transplant immunology and microbiology over the last decade might have changed the distribution of survivors and non-survivors.
We also did not identify any risk factors for mortality associated with donor organ function. The use of organs from extended criteria donors was not significantly different between survivors and non-survivors. Moreover, the survivors received organs from the donors with significantly higher rate of smoking history. This finding supports the theory that accepting ‘marginal’ or less than ideal donors might be appropriate and is not associated with inferior outcome in selected recipients. Furthermore, using the organs from such donors would dramatically impact the available pool of lung allografts and potentially the life expectancy of the patients with end-stage lung disease awaiting transplantation [1].
LIMITATIONS
These data have several limitations. This study is a retrospective analysis of data from a single institution. However, data collection in a single centre does not often suffer from variability in data entry, some grade of inconsistency and missing data could not be ruled out. Although the data analysis supports associations between variables and outcomes, causal relationships cannot be determined.
CONCLUSION
In summary, we demonstrated that the unplanned use of CPB should be avoided in LTx, as it is associated with higher mortality. Furthermore, the negative impact of female-to-male donation should not be underestimated during recipient selection. These factors can be potentially avoided at the stage of donor–recipient matching and planning surgical strategy. Furthermore, early postoperative deterioration in oxygenation particularly with the need for extracorporeal oxygenation might help clinicians in terms of prognosis.
Conflict of interest: none declared.




